Abstract
Cocaine use disorder (CUD) remains a debilitating health problem in the United States for which there are no FDA-approved treatment options. Accumulating anatomical and electrophysiological evidence indicates that the muscarinic acetylcholine receptor (mAChR) subtype 5 (M5) plays a critical role in the regulation of the mesolimbic dopaminergic reward circuitry, a major site of action for cocaine and other psychostimulants. In addition, M5 KO mice exhibit reduced cocaine self-administration behaviors with no differences in sugar pellet-maintained responding relative to wildtype mice. These findings suggest that selective inhibition of M5 mAChR may provide a novel pharmacological approach for targeting CUD. Recently, we reported the synthesis and characterization of ML375, a selective negative allosteric modulator (NAM) for the rat and human M5 mAChR with optimized pharmacokinetic properties for systemic dosing in rodents. In the present study, male Sprague-Dawley rats were trained to self-administer intravenous cocaine (0.1–0.75 mg/kg/injection) under a 10-response, fixed ratio (FR10) or a progressive ratio (PR) schedule of reinforcement. Under both schedules of reinforcement, ML375 produced dose-related reductions in cocaine self-administration. ML375 also modestly reduced sugar pellet-maintained responding on the FR10 schedule, but had no effect under a PR schedule of reinforcement. Further, ML375 did not affect general motor output as assessed by a rotarod test. Collectively, these results provide the first demonstration that selective inhibition of M5 using the M5 NAM ML375 can attenuate both the reinforcing effects and the relative strength of cocaine and suggest that M5 NAMs may represent a promising, novel treatment approach for CUD.
Introduction
Cocaine use disorder (CUD) is a major ongoing health problem within the United States and characterized by compulsive cocaine use, dependence, and repeated relapse after periods of abstinence (SAMHSA 2015). Based on the 2014 National Survey on Drug Use and Health, it is estimated that approximately 1.5 million individuals 12 years or older are current cocaine users (SAMHSA 2015). Despite the considerable negative impact of CUD on individuals and their families, there remains no FDA-approved pharmacological treatment for CUD (Shorter et al., 2015; Czoty et al., 2016).
Previous studies have shown that the reinforcing effects of cocaine are attributed to acute and long-term pathophysiological adaptations in the canonical mesocorticolimbic dopamine (DA) reward circuitry that persist into abstinence and contribute to the high rates of drug craving and relapse (Korpi et al., 2015; Nestler 2005). The mesocorticolimbic reward circuitry consists of midbrain dopamine (DA) cell bodies located in the ventral tegmental area (VTA) and their respective projection targets, including the nucleus accumbens (NAc) and prefrontal cortex (PFC) (Nestler 2005). To date, several pharmacological strategies targeting modulation of mesolimbic DA circuitry have been under investigation in clinical populations of cocaine addicts, including indirect DA agonists and selective D2 DA receptor antagonists as substitution or detoxification approaches, respectively (Castells et al., 2016; Shorter et al., 2015). Unfortunately, these therapeutic strategies have not resulted in successful treatments in part due to the risk of abuse liability and/or cardiovascular effects with indirect DA agonists and the dose-limiting adverse effects on motor function associated with D2 DA receptor antagonists (Castells et al., 2016; Shorter et al., 2015; Negus and Henningfield 2015; Platt et al., 2002; Verrico et al., 2013). Thus, identification and validation of novel therapeutic targets that can modulate aspects of the mesocorticolimbic reward circuitry to selectively reduce cocaine-taking behaviors without unwanted side effects represents a critical unmet need.
One such alternative approach for the treatment of CUD may involve the selective modulation of the muscarinic acetylcholine receptor (mAChR) subtype 5 (M5) (Langmead et al., 2008). Of the five mAChR subtypes (termed M1-M5), only M5 is expressed at high levels in ventral midbrain structures, including the ventral tegmental area (VTA) and substantia nigra (Vilaro et al., 1990; Weiner et al., 1990; Yasuda et al., 1993). Previous studies have reported increased VTA DA cell firing and extracellular DA release in the NAc following electrical stimulation of cholinergic neurons of the laterodorsal tegmental and pedunculopontine tegmental nuclei that project to the VTA (Blaha et al., 1996; Forster and Blaha 2000) are absent in M5 knockout (KO) mice (Forster et al., 2002; Steidl et al., 2011). Moreover, the reinforcing effects and the relative strength of cocaine were robustly reduced in the M5 KO mice (Fink-Jensen et al., 2003; Thomsen et al., 2005). Importantly, the M5 KO mice exhibited no deleterious effects on food-maintained behavior or basic behavioral and physiological measures such as body weight, sleep, and sensorimotor output (Fink-Jensen et al., 2003; Thomsen et al., 2005). Taken together, these data suggest that M5 is critically involved in the regulation of midbrain DA neuronal activity and that development of selective M5 antagonists might represent an important strategy for the attenuation of the acute and/or long-term pathophysiological adaptations induced by cocaine within the mesocorticolimbic reward circuitry thought to underlie CUD. However, further investigation into the role of M5 has been hindered by the lack of selective compounds targeting M5.
Recently, we reported the discovery and optimization of the first negative allosteric modulator (NAM) for the M5 mAChR, ML375 (Gentry el al., 2013). This compound is highly selective for the rat and human M5 mAChR receptor. Importantly, this compound does not bind to the highly conserved orthosteric binding site of acetylcholine and instead binds to an allosteric site (Gentry el al., 2013). Furthermore, ML375 has a favorable drug metabolism and pharmacokinetics (DMPK) profile across multiple species with high CNS penetration, representing the first in vivo compound to allow examination of the M5 receptor for addiction-related behaviors (Gentry el al., 2013). In the present study, we evaluated the effects of ML375 on cocaine self-administration and sugar pellet-maintained responding under fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement to examine the role for M5 mAChRs in modulating drug and non-drug-related behavior. We also utilized the rotarod test to examine effects on an unconditioned measure of motor function. ML375 reduced cocaine self-administration under FR and PR schedules of reinforcement, however ML375 did affect sugar pellet-maintained responding under a PR schedule nor unconditioned motor activity. These data support our hypothesis that inhibition of M5 can selectively affect cocaine-related behaviors without impairing behaviors not associated with drug use.
Materials and Methods
Drugs
Cocaine hydrochloride (Sigma-Aldrich) was dissolved in 0.9% saline solution. ML375 was synthesized in house and was first dissolved in 100% DMSO and administered as an aqueous 20% (w/v) 2-hydroxypropyl-β-cyclodextrin, 5% DMSO microsuspension. Cocaine was administered intravenously (i.v.); ML375 and its vehicle were administered intraperitoneally (i.p.) in a volume of 2 mL/kg.
Subjects
20 male Sprague-Dawley rats (Envigo, Indianapolis, IN) were approximately 70 days old upon arrival at the facility, and weighed between 260 and 300 g at the onset of the study. Rats were single-housed under a 12/12-h light/dark cycle with ad libitum access to water. Rats were maintained at 85% of their free feeding weight for the duration of the study based on a standard growth curve and fed with standard rodent chow (Harlan Teklad, Madison, WI). Housing conditions and all experimental procedures and were approved by Vanderbilt University’s Institutional Animal Care and Use Committee and were conducted in accordance with the National Research Council’s Guide for Care and Use of Laboratory Animals (8th edition, 2011).
Apparatus
All behavioral testing was conducted during the first half of the light portion of the light/dark cycle, between 0900–1100 hrs. Sessions were run in modular operant chambers, equipped with two retractable levers, a stimulus light above each lever, and a house light located on the opposite wall (Med Associates, St. Albans, VT). Chambers were located in sound-attenuating cabinets equipped with fans to provide ventilation and mask external noise. For the cocaine self-administration sessions, a spring-leash tether connected to the vascular access buttons on the rat, housed the infusion line through which drug injections were delivered via an infusion pump (Razel Model A) located outside of the operant chamber.
Cocaine Self-administration
Surgical procedure
Rats were anesthetized with isoflurane, and a polyurethane catheter (Instech Laboratories, Plymouth Meeting, PA) was implanted into the right jugular vein. The catheter was connected to a vascular access button (Instech Laboratories), which was located on the back just caudal to the scapula. Rats were allowed to recover from surgery for 7 days and catheters were flushed with Baytril (0.23 mg/mL) followed by 0.1 mL of heparinized saline (30 IU/mL) daily to maintain catheter patency and prevent infection. Carprofen (5 mg/kg, s.c.) was given post-operatively for 7 days for analgesic support. Catheter patency was verified daily with pre-session and post-session flushing of catheters. The resistance to flow was used as the first indicator of possible catheter occlusion. If catheter occlusion was suspected, 0.1ml ketamine (15 mg/mL) was administered and loss of muscle tone within 5 sec was used to indicate a patent catheter. If catheter patency was lost that test day’s data point was excluded and the rat was excluded from self-administration studies until a left jugular catheter was implanted. Following 7 days of postoperative care, rats were returned to self-administration sessions. Reacquistion of stable cocaine self-administration on average occurred within a week. If the second catheter lost patency, the rat was removed from the study.
Experiment 1: Cocaine and sugar pellet self-administration maintained under a fixed ratio schedule of reinforcement
Self-administration procedures have been described elsewhere (Gould et al., 2016). Briefly, sessions started with a priming infusion of cocaine followed by extension of two levers, indicating the availability of drug. Initially, a single response on the active lever (fixed ratio 1; FR1) resulted in the retraction of both levers, illumination of the stimulus light above the active lever, and infusion of 0.5 mg/kg/infusion cocaine over a duration of time dependent upon the weight of the rat (3–4 sec). The stimulus light remained on during and for an additional second after the infusion, during which responses were recorded but had no consequences. After the light turned off, levers were extended again, signaling availability of cocaine. Once the rat received >10 infusions for 2 consecutive days, the response requirement was gradually increased to FR10. Sessions lasted 2 hr in length or until 60 reinforcers were earned, 5 days a week. Stable self-administration was defined as >10 infusions/day for 3 consecutive days and <25% variability in number of infusions with no upward or downward trends evident. All rats achieved these criteria within 14 sessions. The location (left or right) of the active and inactive lever was randomized for each rat. Once stability was obtained, test sessions commenced to examine the effects of ML375 (10 or 30 mg/kg) on the training dose of cocaine; administered 15 min prior to the start of the session.
Based on results from this first study, we next examined the effects of 30 mg/kg ML375 on multiple doses of the cocaine dose-response curve (0.1–0.75 mg/kg/injection cocaine or saline; dose order randomized per animal). The same cohort of rats as described above self-administered each cocaine dose until responding stabilized for three consecutive days followed by a test session in which rats were administered 30 mg/kg ML375 (i.p.) 15 min before the start of the session. The same dose of cocaine was always available 24 hr after each test session to assess any long-term effects on behavior. Animals were then returned to the maintenance dose of cocaine (0.5 mg/kg/injection) until responding stabilized (within 1–2 days), at which point another dose of cocaine was substituted.
To examine potential nonselective effects of ML375, we employed a similar research design to assess the effects of ML375 on sugar pellet-maintained responding. Rats were initially trained to make a single response (FR1) and then increased to an FR10 schedule with completion of each ratio resulting in delivery of a single 45 mg sugar pellet (Bio-Serve, Flemington, NJ) and illumination of a stimulus light above the active lever for 10 sec. After 10 seconds, the stimulus light turned off, signaling availability of the next reinforcer. Following stable responding, training session commenced during which rats received an i.p. injection of saline before the start of the session. Following stable responding (average number of reinforcers varied by less than 20% across the preceding 3 training sessions, with no upward or downward trend) test sessions were initiated. The test sessions were identical to training sessions except that rats received an i.p. injection of vehicle or ML375 (10, 30 mg/kg) 15 minutes before the start of the session. Once animals met stability criteria, another test session was conducted. Sessions lasted for 120 min or until 100 reinforcers were earned, 5 days a week with a minimum of 5 days between test sessions.
Experiment 2: Cocaine and sugar pellet self-administration maintained under a progressive ratio schedule of reinforcement
Following completion of experiment 1 rats with patent catheters responded for cocaine (0.1–0.75mg/kg) under a progressive ratio schedule of reinforcement in which the response requirement for delivery of each reinforcer increased as determined by the exponential equation used by Richardson and Roberts (1996), ratio = [5 × e(R × 0.2)] – 5. The first 10 ratios given by this equation are 1, 2, 4, 6, 9, 12, 15, 20, 25, 32. Sessions terminated when 20 min had elapsed without completion of the current ratio requirement; the final completed ratio was termed the break point. Stable responding was defined as >10 reinforcers/session for 3 consecutive sessions and <25% variability in number of reinforcers with no upward or downward trends evident. Once stability had been achieved rats were injected i.p. with ML375 (10 or 30 mg/kg) or vehicle 15 min prior to the start of the session. Initially, responding was maintained by a 0.5 mg/kg/infusion of cocaine until responding was stable. The effects of ML 375 (30 mg/kg) were assessed at each dose of cocaine after responding was stable. A follow-up session at the same dose of cocaine 24 hr later was assessed to evaluate any long-term effects on behavior.
Lastly, we assessed effects of ML375 on sugar pellet-maintained responding under a progressive ratio schedule of reinforcement. Rats were initially trained to respond for delivery of a sugar pellet under an FR10 schedule before implementing the same PR schedule as described above. Stability criteria, training and testing conditions were identical to those described in the study assessing ML375 effects on sugar pellet-maintained responding under an FR schedule of reinforcement.
Rotarod
The effects of ML375 on motor coordination were tested using a rotarod apparatus (model 4600, Ugo Basile, Comerio, Italy) with a rod diameter of 3 cm. During two training sessions that were separated by at least 20 min, rats were trained to walk on a rotating rod maintained at a constant speed of 20 revolutions per minute (rpm). Only rats that walked on the rotarod for >100s during the second training session advanced to drug treatment, which commenced in the afternoon of the same day. Vehicle or ML375 (3–30 mg/kg i.p.) was administered 15 min prior to placing reach rat on the rotarod, and the latency to fall off the rod was measured. The maximum time allotted for each rat to remain on the rotorod was 120 sec. Previous studies have shown that these parameters are sensitive to D2-like dopamine receptor antagonist-induced disruptions in motor function (Kumar et al., 2009).
Data Analysis
The number of reinforcers/session and response rates (lever presses when a reinforcer was available) were examined for cocaine-dose response curves and sugar pellet-maintained responding. Group means (±SEM) were plotted as a function of ML375 dose and time (15 min or 24 hr after treatment) at one dose of cocaine (0.5mg/kg) or the effect of ML375 treatment (30 mg/kg) and time at each cocaine dose (0.1–0.75 mg/kg). Repeated measures two-way ANOVAs were used to test for main effects of ML375 treatment; main effects were followed by Dunnett’s post-hoc test comparing each test condition to respective vehicle treatment. Data from the rotarod test was analyzed using Kruskal-Wallis ANOVA followed by Dunn’s multiple comparisons test. In all cases p<0.05 was considered to be significant from respective vehicle conditions.
Results
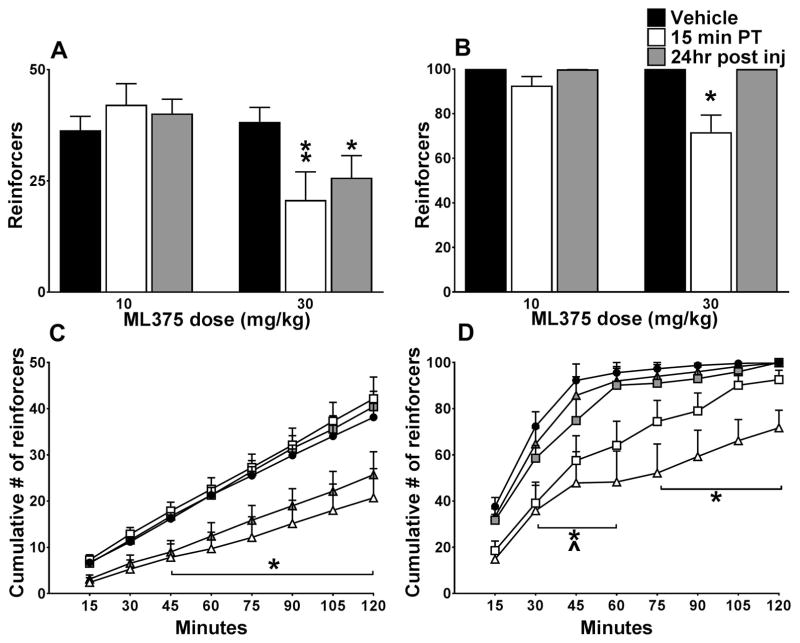
Figure 1A shows the number of reinforcers/session following administration of ML375 or vehicle when cocaine was self-administered at 0.5 mg/kg/infusion under a FR10 schedule of reinforcement. 30 mg/kg ML375 significantly reduced the number of reinforcers earned during cocaine self-administration sessions (Fdose (1,6) = 7.711, p=0.0321, Ftime (2,12) = 1.468, p=0.269, Fdose × time (2,12) = 10.02, p=0.0028) and this reduction persists 24 hours post injection (all p<0.05). As shown in Figure 1B, 30 mg/kg ML375 significantly reduced sugar pellet-maintained responding, (Fdose (1,6) = 10.43, p=0.0179, (Ftime (2,12) = 11.88, p= 0.0014, Fdose × time (2,12) = 10.72, p=0.0021) following a 15 min pretreatment (p<0.05) but this effect did not persist 24 hrs later. Only rats that received both doses of ML375 were included in these analyses. Importantly, ML375 had no effect on inactive lever responses in cocaine self-administration sessions or in sugar pellet-maintained responding sessions (data not shown) suggesting reductions in cocaine-lever or sugar pellet -maintained responding were not due to non-specific effects of ML375 (see Table S1 for response rate data). Figure 1C shows the cumulative number of cocaine injections earned in 15 min bins following treatment with vehicle or ML375 across the 120-min session. 30 mg/kg ML375 significantly reduced the number of reinforcers earned 45–120 min after the start of the session on the day of ML375 administration and 24 hrs later (Ftime(7,42) = 137.6, p < 0.001, Fdose (4,24) = 5.846, p=0.002; both p<0.05, Fdose × time (28, 168) = 3.67, p<0.0001). Figure 1D shows the cumulative number of sugar pellets earned after treatment with vehicle or ML375 across the 120-min session. 10 mg/kg ML375 significantly reduced the number of cumulative reinforcers earned during the 30–60 min time points, while 30 mg/kg ML375 significantly reduced cumulative reinforcers from 30–120 min (Ftime (7, 210) = 223.4, p < 0.0001; Fdose (4,30) = 5.588, p=0.0017; Fdose × time (28, 210) = 2.532, p<0.0001).
Figure 1.
A, The highest dose of ML375 administered reduces cocaine self-administration (0.5 mg/kg) under a FR10 schedule of reinforcement and this reduction persists 24 hours post injection, n=7. B, ML375 reduces the number of sugar pellets earned under the same schedule but the effect does not persist 24 hours post injection, n =7. C, The cumulative reduction in cocaine infusions after treatment with ML375 (30 mg/kg, triangle symbols) is significant from 45 minutes after the start of the session and continuing to the end of the self-administration session, and this effect is similar 24 hours post injection. D, ML375 (10 mg/kg, square symbols) reduces the cumulative number of sugar pellets 30 minutes after the start of the session to 60 minutes, while the highest dose (30 mg/kg) reduces the number of sugar pellets from 30 minutes to the end of the session. All comparisons are to sessions with vehicle pretreatment (circle symbols). ^ ( 10 mg/kg 15 min PT) *( 30 mg/kg 15 min & 24 hr post inj), p < 0.05.
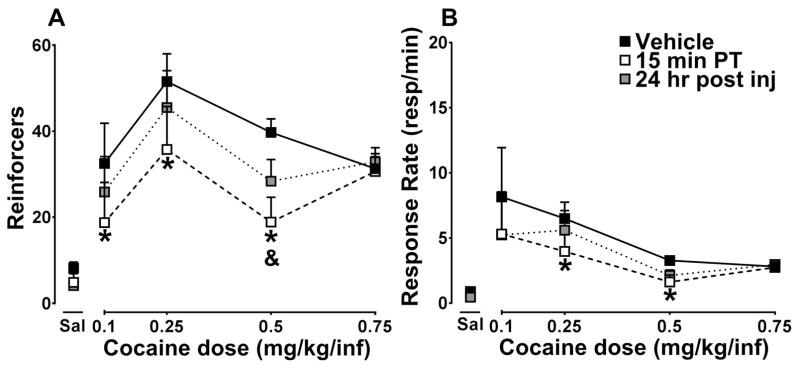
Figure 2A shows the number of reinforcers/session after pretreatment with 30 mg/kg ML375 or vehicle as a function of cocaine dose under a FR10 schedule of reinforcement. Rats exhibited a typical biphasic dose-response curve and ML375 acutely reduced the number of reinforcers earned during the session (Fcocdose (4,28) = 10.13, p<0.0001; FML375treatment (2,14) = 4.702, p=0.03; Fcocdose × ML375treatment (8, 56) = 1.593, p=0.15) specifically ML375 reduced the number of reinforcers at the 0.1, 0.25 and 0.5 mg/kg doses of cocaine acutely (p < 0.05). However at 24 hr after administration, reinforcers were significantly reduced only at the 0.5 mg/kg dose of cocaine (p<0.05). Figure 2B shows the response rates during cocaine self-administration sessions following acute vehicle or 30 mg/kg ML375 administration and 24 hr post injection. Response rates decreased as a function of cocaine dose; ML375 significantly altered response rates for cocaine (Fcocdose (4,28) = 2.76, p =0.0472; FML375treatment (2,14) = 4.561, p=0.03; Fcocdose × ML375treatment (8, 56) = 1.2, p=0.32), Post-hoc analysis revealed effects at 0.25 and 0.5 mg/kg doses of cocaine (p < 0.05). Only rats with full dose response curves were included in these analyses. The group dose-response curve does not appear as a typical biphasic dose-response curve as one rat had significantly higher rates of responding (30.41 resp/min) after vehicle pretreatment at 0.1 mg/kg cocaine than the other rats (mean = 4.98 resp/min). This rat achieved the maximum number of infusions at this dose of cocaine within 45 min for each 0.1 mg/kg cocaine self-administration session.
Figure 2.
A, ML375 (30 mg/kg) reduces the number of reinforcers earned during cocaine self-administration sessions at the lower doses of cocaine, n=8. B, Response rates (resp/sec) during cocaine self-administration sessions were reduced after treatment with ML375 compared to vehicle. Vehicle pretreatment (black squares), ML375 pretreatment (open squares), 24 hr post ML375 pretreament (gray squares). *( 15 min PT), & (24 hr post inj), p < 0.05.
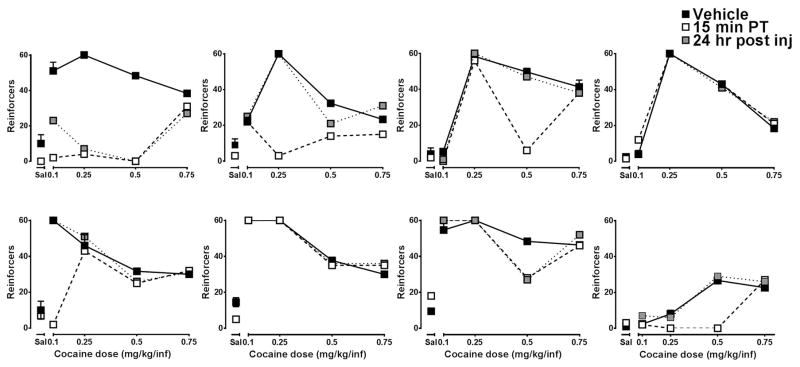
Although group data showed that ML375 reduced the number of cocaine infusions across multiple unit doses of the dose-response curve, variability attributed to individual sensitivity to cocaine, ML375 or a combination thereof was present. Due to the heterogeneity associated with human and nonhuman primate drug self-administration, data are often examined on an individualized basis (e.g. Freeman and Woolverton 2011; Shoptaw et al., 2003, Gould et al., 2017) and by patient stratification in clinical studies (Simpson et al 1999, Shoptaw et al., 2003); we sought to take a similar approach with the present rodent data. Examination of individual dose-response curves as shown in Figure 3 revealed several important points. First, the dose response curves of 4 rats resembled the classic inverted-U-shaped curve and mirrors the group data shown in figure 2. However, in the other four rats, the dose response curves demonstrated only the ascending or descending limb; moreover the dose that engendered the peak number of infusions varied (0.1–0.5) as did the maximum number of infusions at the peak. Based on these observations alone, it is not surprising that a single dose of a pharmacological agent would not engender the same effect in all rats. However, ML375 reduced cocaine self-administration in at least one dose in 6 of 8 rats.
Figure 3.
Representative individual cocaine dose-response curves after vehicle and ML375 (30 mg/kg) administration. Note the variable cocaine dose that elicited maximum responding, and the individual sensitivities to ML375.
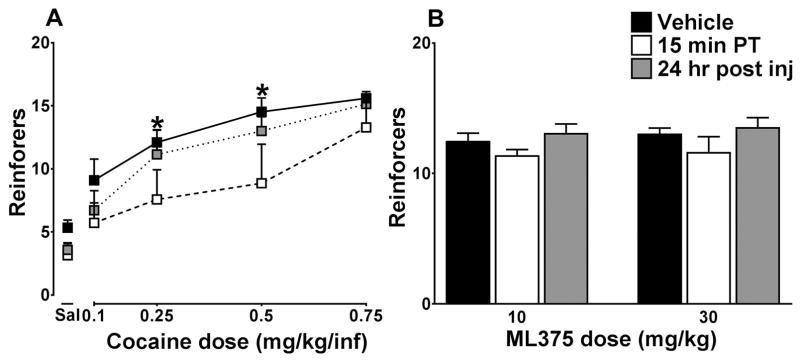
Figure 4A shows the cocaine dose-response curve under a PR schedule of reinforcement. ML375 significantly reduced breakpoints (Fcocdose (4,24) = 29.43, p<0.0001), (FML375treatment (2,12) = 14.72, p= 0.0006); Fcocdose × ML375treatment (8, 48) = 0.68, p=0.71), specifically at 0.25 and 0.5 mg/kg cocaine compared to vehicle (p < 0.05). To determine the selectivity of these effects for drug versus non-drug related reinforcers, the effects on ML375 were also assessed in rats responding under a PR schedule maintained by delivery of sugar pellets. As shown in Figure 4B, ML375 did not significantly reduce breakpoints for sugar pellets.
Figure 4.
A, ML375 (30 mg/kg, open squares) reduces breakpoints of the lower doses of cocaine compared to vehicle (black squares) sessions, n=7. This effect is not seen 24 hr post ML375 ( 30 mg/kg) injection (gray squares). B, ML375 does not reduce breakpoints for sugar pellets compared to vehicle at either 10 or 30 mg/kg, n=10. *, p < 0.05.
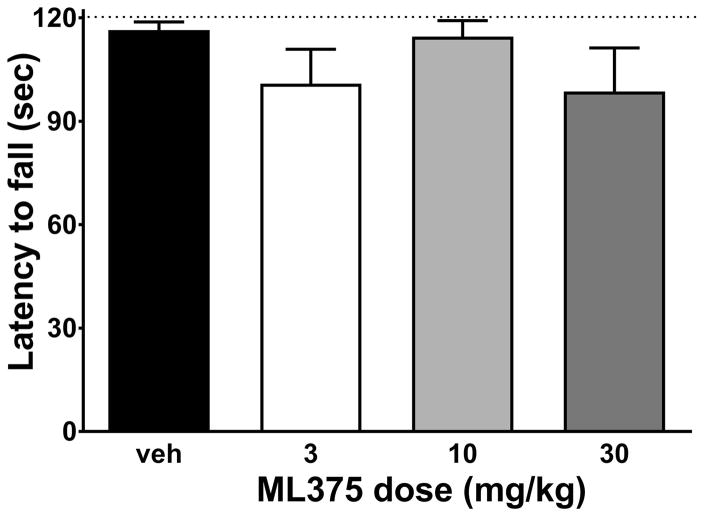
To determine whether reductions in responding may be due non-specific sedative/motor effects we evaluated motor function using the rotarod test following ML375 administration. As shown in Figure 5, ML375 had no effect on latency to fall off the rotating rod (Ftreatment (3, 28) = 1.298, p=0.7296; n=7–8 per treatment group).
Figure 5.
ML375 does not impair performance on the rotarod test in rats. Latency to fall off the rotarod turning at 20 rpm was not affected by ML375. Data are represented as mean ± SEM of 7–8 rats per treatment group.
Discussion
The present study provides the first demonstration of the effects of the highly selective, brain penetrant M5 NAM ML375 on cocaine self-administration in rats. As described, ML375 reduced cocaine self-administration at lower unit doses of cocaine when responding was maintained under both FR and PR schedules of reinforcement. Importantly, the modest reductions in sugar pellet-maintained responding observed with ML375 under a FR schedule were absent under a PR schedule and it did not affect unconditioned motor behavior suggesting that selective negative allosteric modulation (or functional antagonism) of M5 produces relatively selective effects on cocaine-related behaviors supporting further examination for the treatment of multiple aspects of CUD.
By utilizing both FR and PR schedules of reinforcement, we determined that selective inhibition of M5 by ML375 acutely attenuates both the reinforcing effects and relative reinforcing strength of cocaine as measured by reduced breakpoints. Our findings are consistent with previous M5 KO mouse studies in which these mice self-administered less cocaine than wildtype mice under a FR schedule of reinforcement (Fink-Jensen et al., 2003). At the single dose of 30 mg/kg, ML375 appeared to reduce responding across the cocaine dose-response curve when cocaine was available under the FR10 schedule of reinforcement. In contrast, previous studies using D2 DA receptor KO mice or D2 DA receptor antagonists reported rightward shifts the cocaine dose-response curves implying that the effects on D2 DA receptors are surmountable with higher doses of cocaine (Caine et al., 2002; Bari and Pierce 2005). Compared to a simple FR schedule that only provides information regarding the ability to increase or decrease the reinforcing effects of a stimulus, a PR schedule provides a measure of the reinforcing efficacy of a stimulus, and may provide a more relevant measure for the pharmacological augmentation of the reinforcing strength of cocaine (Arnold and Roberts 1997). In the current study, ML375 reduced breakpoints at lower doses of cocaine under a PR schedule, while having no effects on breakpoints for sugar pellets, a finding consistent with previous studies M5 KO studies (Thomsen et al., 2005). Thus, these data suggest a therapeutic profile that blocks motivation for cocaine potentially without affecting motivation for natural rewards. While our present data are promising, there are also important caveats. First, our study framework was based on data using genetically modified M5 KO mice for which there is always the potential for confounding, compensatory changes in gene expression that may contribute to the observed drug-seeking behaviors in these animals (Fink-Jensen et al., 2003; Thomsen et al., 2005). Second, the effects of ML375 were only determined after acute administration once stable self-administration behavior was acquired. Thus, in future studies, it will be important to compare the effects of acute and chronic administration of ML375 on all phases of cocaine self-administration in order to understand the effects of repeated dosing on behavior and underlying changes in mesocorticolimbic reward circuitry. Finally, since the current study focused on evaluating the effects of a single dose of ML375 on the cocaine dose-response curve, it will be important in future studies to investigate the effects of a range of doses of ML375 on the cocaine dose-response curve to confirm the magnitude of any potential downward shift in the FR dose-response curves or whether these effects may be surmounted.
While the specific site of action for the observed effects of the M5 NAM ML375 on cocaine-related behaviors remains unknown, one important site involves regulation of potential acute and/or long-term pathophysiological adaptations induced by cocaine within the mesocorticolimbic reward circuitry thought to underlie CUD (Nestler 2005). In particular, anatomical studies have revealed that M5 expression is largely confined to midbrain DA neurons of the VTA, as well as the substantia nigra, within the central nervous system (Weiner et al., 1990; Yasuda et al., 1993). Moreover, electrophysiological studies have shown that increased VTA DA cell firing and extracellular DA release in the NAc following electrical stimulation of the cholinergic projections from the laterodorsal tegmental and pedunculopontine tegmental nuclei to the VTA (Blaha et al., 1996; Forster and Blaha 2000, 2003) are absent in M5 KO mice (Forster et al., 2002; Steidl et al., 2011). These studies suggest that inhibition of M5 may be able to reduce cocaine-induced elevations in DA levels in the NAC by blocking acetylcholine-induced activation of VTA DA cell firing. Consistent with this, we found the effects of the M5 NAM were similar to previous studies showing that the reinforcing effects and the relative strength of cocaine were decreased in the M5 KO mice (Fink-Jensen et al., 2003; Thomsen et al., 2005). Importantly, we saw no effects of the M5 NAM on behavior maintained by sugar pellets or general motoric output that would be indicative of actions of M5 expressed on nigrostriatal neurons (Fink-Jensen et al., 2003; Thomsen et al., 2005).
Since mesolimbic DA circuitry mediates not only the reinforcing effects of drugs of abuse, but also natural rewards such as sugar pellets, (Salamone et al., 2016; Wise 2006) it is possible that modulation of this circuit with a M5 NAM could adversely affect motivation for sugar pellets. Interestingly, ML375 subtly and transiently affected sugar pellet-maintained responding under an FR but not a PR schedule of reinforcement. Moreover, effects of ML375 on cocaine self-administration persisted 24 hr after administration although effects of sugar pellet-maintained responding returned to normal by the 24 hr assessment. While selective effects on drug-related behaviors are more desirable, this transient nature regarding effects on sugar pellet-maintained responding is not novel. In fact, D-amphetamine, a DA releaser that has gained strong support as an treatment for CUD from preclinical and clinical studies (Negus and Henningfield 2015), acutely disrupts sugar pellet-maintained responding on an FR schedule but not a PR schedule (Czoty et al., 2011, Hutsell et al., 2016). Further, tolerance rapidly develops to the acute effects on sugar pellet-maintained responding while reductions in cocaine-maintained responding persist (Czoty et al., 2011, Hutsell et al., 2016). D2 DA receptor antagonists such as haloperidol have also been proposed as possible therapeutics for CUD as they reduce cocaine intake, however these compounds also negatively affect food reinforcement thus limiting their therapeutic profile (Platt et al., 2002).
As previously discussed, a number of different pharmacological treatment strategies targeting the direct or indirect modulation of mesocorticolimbic reward circuitry have been investigated in clinical populations of cocaine addicts. For example, indirect DA agonists and selective D2 DA receptor antagonists have been developed as potential substitution or detoxification approaches, respectively, for cocaine addiction (Castells et al., 2016; Platt et al., 2002; Shorter et al., 2015). However to date, these DA receptor mediated approaches have failed in clinical development due to dose-limiting adverse effects (Shorter et al., 2015). Alternatively, indirect modulation of mesocorticolimbic reward circuitry by the GABAB receptor agonist Baclofen and the anti-epileptic agent Topiramate with actions at sodium channels and GABAA receptors have both produced promising reductions in cocaine seeking behaviors in clinical populations and animal models across a broad therapeutic index (Johnson et al., 2005; Roberts 2005; Fadda et al., 2003; Hutsell et al., 2016). Based on the present preclinical studies, selective inhibition of M5 by ML375 appears to also provide a broader therapeutic profile in reducing cocaine-taking without eliciting adverse side effects in line with these other treatments that indirectly modulate mesocorticolimbic DAergic activity.
In summary, the present findings are the first to report the behavioral effects of a highly selective M5 mAChR NAM in animal models of drug addiction. Ongoing efforts include the development of more optimized M5 mAChR NAMs to allow us to further unravel their specific role in modulating mesolimbic DA circuitry and allow us to assess the effects of repeated dosing on self-administration as well as utilizing a wider range of doses for testing. While we hypothesize that the present effects on cocaine self-administration are dependent upon M5-mediated effects on DA neurons in the VTA, it is possible that M5 may also be expressed on GABAergic interneurons in the VTA to exert effects on inhibitory signaling in the mesolimbic DA circuitry (Foster et al., 2014). Previous studies have shown reduced morphine conditioned place preference and less-pronounced morphine-induced withdrawal-like effects in M5 knockout mice (Basile et al., 2002). Thus, it will be important to further explore the role of M5 in other aspects of the addiction cycle (e.g. models of relapse and abstinence-related symptoms) as well as extend our behavioral characterizations to other classes of drugs of abuse (e.g. opioids). The present results suggest selective modulation of the M5 mAChRs may be an exciting novel therapeutic approach for treating CUD.
Supplementary Material
Responses rates during FR10 cocaine (0.5 mg/kg) self-administration (n=7) and sugar pellet-maintained responding sessions (n=7) after treatment with vehicle or ML375 (10 mg/kg & 30 mg/kg). *, p < 0.05.
Acknowledgments
The authors would like to acknowledge Peter Miller, Timothy Broughton, Erica Williams, Eunice Shin, Lee Schmidt, and Caroline Bertsch for their assistance with this study. This study was supported by NIH Grant R01DA37207.
Footnotes
Author Contributions
BWG, RWG, MB, CKJ designed the experiments and the wrote manuscript
BWG and RWG conducted the experiments
KMM and CWL contributed reagents
References
- 1.Arnold JM, Roberts D. A Critique of Fixed and Progressive Ratio Schedules Used to Examine the Neural Substrates of Drug Reinforcement. Pharmacology Biochemistry and Behavior. 1997;57(3):441–447. doi: 10.1016/s0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 2.Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135(3):959–68. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 3.Basile AS, Fedorova I, Zapata A, Liu X, Shippenberg T, Duttaroy A, Yamada M, Wess J. Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc Natl Acad Sci USA. 2002;99:11452–1145. doi: 10.1073/pnas.162371899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaha CD, Allen LF, Das S, Inglis WL, Latimer MP, Vincent SR, Winn P. Modulation of dopamine efflux in the nucleus accumbens after cholinergic stimulation of the ventral tegmental area in intact, pedunculopontine tegmental nucleus-lesioned, and laterodorsal tegmental nucleus-lesioned rats. J Neurosci. 1996;16:714–722. doi: 10.1523/JNEUROSCI.16-02-00714.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci. 2002 Apr 1;22(7):2977–88. doi: 10.1523/JNEUROSCI.22-07-02977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castells X, Cunill R, Pérez-Mañá C, Vidal X, Capellà D. Psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2016 Sep;27(9):CD007380. doi: 10.1002/14651858.CD007380.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration (SAMHSA), Center for Behavioral Health Statistics and Quality. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015 (HHS Publication No. SMA 15–4927, NSDUH Series H-50). Retrieved from http://www.samhsa.gov/data/
- 8.Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged Attenuation of the Reinforcing Strength of Cocaine by Chronic d-Amphetamine in Rhesus Monkeys. Neuropsychopharmacology. 2011 Jan;36(2):539–47. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czoty PW, Stoops WW, Rush CR. Evaluation of the “Pipeline” for Development of Medications for Cocaine Use Disorder: A Review of Translational Preclinical, Human Laboratory, and Clinical Trial Research. Pharmacological Reviews. 2016;68(3):533–562. doi: 10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Baclofen antagonizes nicotine-, cocaine-, and morphine-induced dopamine release in the nucleus accumbens of rat. Synapse. 2003 Oct;50(1):1–6. doi: 10.1002/syn.10238. [DOI] [PubMed] [Google Scholar]
- 11.Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. J Neurosci Res. 2003;74:91–96. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- 12.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. European Journal of Neuroscience. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 13.Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002 Jan 1;22(1):RC19. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003;17:751–762. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- 15.Foster DJ, Gentry PR, Lizardi-Ortiz JE, Bridges TM, Wood MR, Niswender CM, Sulzer D, Lindsley CW, Xiang Z, Conn PJ. M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor’s location. J Neurosci. 2014 Feb 26;34(9):3253–62. doi: 10.1523/JNEUROSCI.4896-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman KB, Woolverton WL. Self-administration of cocaine and remifentanil by monkeys: choice between single drugs and mixtures. Psychopharmacology (Berl) 2011 May;215(2):281–90. doi: 10.1007/s00213-010-2131-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentry PR, Kokubo M, Bridges TM, Kett NR, Harp JM, Cho HP, Smith E, Chase P, Hodder PS, Niswender CM, Daniels JS, Conn PJ, Wood MR, Lindsley CW. Discovery of the first M5-selective and CNS penetrant negative allosteric modulator (NAM) of a muscarinic acetylcholine receptor: (S)-9b-(4-chlorophenyl)-1-(3,4-difluorobenzoyl)-2,3-dihydro-1H-imidazo[2,1-a]isoindol-5(9bH)-one (ML375) Journal of Medicinal Chemistry. 2013 Nov 27;56(22):9351–5. doi: 10.1021/jm4013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould RW, Amato RJ, Bubser M, Joffe ME, Nedelcovych MT, Thompson AD, Nickols HH, Yuh JP, Zhan X, Felts AS, Rodriguez AL, Morrison RD, Byers FW, Rook JM, Daniels JS, Niswender CM, Conn PJ, Emmitte KA, Lindsley CW, Jones CK. Partial mGlu5 Negative Allosteric Modulators Attenuate Cocaine-Mediated Behaviors and Lack Psychotomimetic-Like Effects. Neuropsychopharmacology. 2016 Mar;41(4):1166–78. doi: 10.1038/npp.2015.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould RW, Czoty PW, Porrino LJ, Nader MA. Social Status in Monkeys: Effects of Social Confrontation on Brain Function and Cocaine Self-Administration. Neuropsychopharmacology. 2017 Jan 25; doi: 10.1038/npp.2016.285. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronier B, Rasmussen K. Activation of midbrain presumed dopaminergic neurones by muscarinic cholinergic receptors: an in vivo electrophysiological study in the rat. British Journal of Pharmacology. 1998;124:455–464. doi: 10.1038/sj.bjp.0701850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernández-López S, Góngora-Alfaro JL, Martínez-Fong D, Rosales MG, Aceves J. Cholinergic stimulation of rostral and caudal substantia nigra pars compacta produces opposite effects on circling behavior and striatal dopamine release measured by brain microdialysis. Neuroscience. 1994;62:441–447. doi: 10.1016/0306-4522(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 22.Hutsell BA, Negus SS, Banks ML. Effects of 21-day d-amphetamine and risperidone treatment on cocaine vs food-choice and extended-access cocaine intake in male rhesus monkeys. Drug Alcohol Depend. 2016 Nov 1;168:36–44. doi: 10.1016/j.drugalcdep.2016.08.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson BA. Recent Advances in the Development of Treatments for Alcohol and Cocaine Dependence: Focus on Topiramate and Other Modulators of GABA or Glutamate Function. CNS Drugs. 2005;19(10):873–96. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Kenneth Lloyd G, Mason BJ. Development of pharmacotherapies for drug addiction: a Rosetta Stone approach. Nature reviews Drug discovery. 2009;8(6):500–515. doi: 10.1038/nrd2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ, Hyytiä P, Dawe GS. Mechanisms of Action and Persistent Neuroplasticity by Drugs of Abuse. Pharmacol Rev. 2015 Oct;67(4):872–1004. doi: 10.1124/pr.115.010967. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Riddle LR, Griffin SA, Chu W, Vangveravong S, Neisewander J, Mach RH, Luedtke RR. Evaluation of D2 and D3 dopamine receptor selective compounds on L-dopa-dependent abnormal involuntary movements in rats. Neuropharmacology. 2009 May-Jun;56(6–7):956–69. doi: 10.1016/j.neuropharm.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117(2):232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Negus SS, Henningfield J. Agonist Medications for the Treatment of Cocaine Use Disorder. Neuropsychopharmacology. 2015;40(8):1815–25. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nestler EJ. The neurobiology of cocaine addiction. Sci Pract Perspect. 2005 Dec;3(1):4–10. doi: 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Platt DM, Rowlett JK, Spealman RD. Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 2002 Oct;163(3–4):265–82. doi: 10.1007/s00213-002-1137-8. [DOI] [PubMed] [Google Scholar]
- 31.Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005 Sep 15;86(1–2):18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Rudd RA, Seth P, David F, Scholl L. Increases in Drug and Opioid-Involved Overdose Deaths United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- 33.Salamone JD, Correa M, Yohn S, Lopez Cruz L, San Miguel N, Alatorre L. The pharmacology of effort-related choice behavior: Dopamine, depression, and individual differences. Behav Processes. 2016 Jun;127:3–17. doi: 10.1016/j.beproc.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Shorter D, Domingo CB, Kosten TR. Emerging drugs for the treatment of cocaine use disorder: a review of neurobiological targets and pharmacotherapy. Expert Opin Emerg Drugs. 2015;20:15–29. doi: 10.1517/14728214.2015.985203. [DOI] [PubMed] [Google Scholar]
- 35.Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD. A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 1999;56:507–514. doi: 10.1001/archpsyc.56.6.507. [DOI] [PubMed] [Google Scholar]
- 36.Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiatry. 2003 Dec;64(12):1440–8. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- 37.Steidl S, Miller AD, Blaha CD, Yeomans JS. M5 Muscarinic Receptors Mediate Striatal Dopamine Activation by Ventral Tegmental Morphine and Pedunculopontine Stimulation in Mice. PLoS One. 2011;6(11):e27538. doi: 10.1371/journal.pone.0027538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality. The DAWN Report: Highlights of the 2010 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. Rockville, MD: Jul 2, 2012. [PubMed] [Google Scholar]
- 39.Thomsen M, Woldbye D, Wörtwein G, Fink-Jensen A, Wess J, Caine SB. Reduced Cocaine Self-Administration in Muscarinic M5 Acetylcholine Receptor-Deficient Mice. J Neurosci. 2005 Sep 7;25(36):8141–9. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verrico CD, Haile CN, Newton TF, Kosten TR, De La Garza R., 2nd Pharmacotherapeutics for substance-use disorders: a focus on dopaminergic medications. Expert Opin Investig Drugs. 2013 Dec;22(12):1549–68. doi: 10.1517/13543784.2013.836488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vilaro MT, Palacios JM, Mengod G. Localization of M5 muscarinic receptor mRNA in rat brain examined by in situ hybridization histochemistry. Neurosci Lett. 1990;114:154–159. doi: 10.1016/0304-3940(90)90064-g. [DOI] [PubMed] [Google Scholar]
- 42.Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor messenger-RNAs in rat basal ganglia. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006 Jul 29;361(1471):1149–58. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB. Development of antisera selective for M4 and M5 muscarinic cholinergic receptors -distribution of M4 and M5 receptors in ratbrain. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Responses rates during FR10 cocaine (0.5 mg/kg) self-administration (n=7) and sugar pellet-maintained responding sessions (n=7) after treatment with vehicle or ML375 (10 mg/kg & 30 mg/kg). *, p < 0.05.