Abstract
Human islet amyloid polypeptide or amylin (hA) is a 37- amino acid peptide hormone produced and co-secreted with insulin by pancreatic β-cells. Under physiological conditions hA regulates a broad range of biological processes including insulin release and slowing of gastric emptying, thereby maintaining glucose homeostasis. However, under the pathological conditions associated with type-2 diabetes mellitus (T2DM), hA undergoes a conformational transition from soluble random coil monomers to alpha-helical oligomers and insoluble β-sheet amyloid fibrils or amyloid plaques. There is positive correlation between hA oligomerization/aggregation, hA toxicity and diabetes progression. Because the homeostatic balance between hA synthesis, release and uptake is lost in diabetics, and hA aggregation is a hallmark of T2DM, this chapter focuses on the biophysical and cell biology studies investigating molecular mechanisms of hA uptake, trafficking and degradation in pancreatic cells and its relevance to h’s toxicity. We will also discuss the regulatory role of endocytosis and proteolytic pathways in clearance of toxic hA species. Finally, we will discuss potential pharmacological approaches for specific targeting of hA trafficking pathways and toxicity in islet β-cells as potential new avenues toward treatments of T2DM patients.
Keywords: human amylin, type-2 diabetes mellitus, islet amyloidosis, endocytosis, proteasome, proteotoxicity, aggregation
1. Physiological roles of hA and other amyloid proteins
In amyloidosis, which is an intrinsic property of all polypeptides (Chiti and Dobson 2006), soluble and functional proteins misfolds into insoluble, structurally conserved fibers that are characterized by resistance to proteinase K digestion, dye binding specificity, and ordered β-sheet-rich structure (Sipe et al. 2010). Amyloids can be broadly categorized into detrimental and functional. Functional amyloid proteins, such as human pancreatic islet amyloid polypeptide hormone or human amylin (hA), support diverse cellular functions both in higher and lower organisms. For example, Curli found in E. coli plays a role in biofilm formation and mediates infection. Chaplin found in Streptomyces plays a role in protection against water surface tension. URE2p found in S. Cerevisiae plays a role in nitrogen catabolism, and Pmel17 found in humans play a role in melanin synthesis (Granzotto et al. 2011; Lau et al. 2007; Rymer and Good 2000).
hA plays important regulatory role in food intake, energy and glucose homeostasis (Lutz 2006, 2010). hA primarily regulates nutrient fluxes by acting as a potent satiation signal that reduces secretion of gastric juices and the glucagon hormone, and also reduces the rate of gastric emptying (Young and Denaro 1998). In addition, hA is also involved in adiposity signaling and, similar to leptin, in body weight regulation all through adult life (Lutz 2010). Studies with animal and human subjects showed that combinational application of leptin and hA increases leptin responsiveness in anti-obesity treatments, which suggests the synergistic function of these hormons (Lutz 2010). Recent studies suggest that hA-mediated regulation of energy balance is not limited to the control of nutrient flux but also involves the body’s energy expenditure (Lutz 2010). However, the exact mechanism and the physiological relevance are still under scrutiny (Lutz 2010). hA also plays a developmental role by contributing to the development of bone, kidney and pancreas (Wookey et al. 2006). In addition to its hormonal role, hA also imposes important paracrine and autocrine signaling in islets by regulating glucagon and insulin release from α- and β-cells, respectively (Trikha and Jeremic 2013; Wagoner et al. 1993). Recent studies demonstrate that rodent amylin (rA) stimulates ERK kinase signaling and cellular proliferation in mouse pancreatic β-cells (Visa et al. 2015).
2. Synthesis and Biological Roles of hA
hA and insulin genes share common promoter elements, and the transcription factor PDX1 regulates glucose-stimulated secretion of both these genes (German et al. 1992). hA is synthesized in cells as an 89-residue pre-pro-protein (Nakazato et al. 1990; Nishi et al. 1989). The 22-residue signal peptide of immature form is cleaved off in the endoplasmic reticulum (ER). Further processing of pro-hA, along with pro-insulin, takes place in the Golgi and the secretory vesicles in a pH-dependent manner using two endoproteases: prohormone convertase 2 (PC2) and prohormone convertase 1/3 (PC1/3) (Westermark et al. 2011). PC2 and PC1/3 cleave pro-hA after Lys10 and/or Arg11 (Wang et al. 2001) and after Lys 50 and Arg51, respectively (Marzban et al. 2004). After PC1/3-mediated cleavage, the two C-terminal amino acid residues are then removed by carboxypeptidase E, which results in an exposed glycine residue at the C-terminus of pro-hA (Westermark et al. 2011). This glycine is used as a signal for C-terminal amidation; finally, a disulphide bridge is formed between Cys2 and Cys7. Both C-terminal amide and this disulphide bridge are important for full biological activity of hA (Westermark et al. 2011). Fully processed hA is a 37-residue polypeptide stored in secretory granules of pancreatic islet β-cells along with fully processed insulin. Upon physiological stimulation such as glucose increase in blood, insulin and hA are co-secreted at a molar ratio of 20:1 (Martin 2006).
hA belongs to large class of calcitonin gene-related peptides, each binding to specific receptor on the cell surface to mediate distinct biological functions. However, specific receptor for hA remained enigma for a long time until the identification of a family of single-domain proteins called “receptor activity-modifying proteins”, or RAMPs, which are principal components of calcitonin-receptor class (McLatchie et al. 1998). hA receptor utilizes a novel principle that has so far been detected only among the family of calcitonin receptors (CT-R): binding and hetrodimerization of RAMP with CT-R yields a unique high-affinity amylin receptor (AM-R) phenotype (Poyner et al. 2002). The three known AM-R isoforms discovered so far have been shown to exhibit distinct pharmacological and functional properties (Morfis et al. 2008). AM-R is ubiquitously expressed in organs and tissues, particularly in the brain and in the pancreas. Consistent with this, studies revealed a regulatory role of amylin in glucose homeostasis, hormone and neurotransmitter release and signaling (Martinez et al. 2000; Trikha and Jeremic 2013).
2. Pathology of islet amyloidosis and T2DM
Detrimental amyloids, which cause protein misfolding in amyloid diseases, include huntingtin implicated in Huntington’s disease, α-synuclein (α-syn) implicated in Parkinson’s disease, prion protein implicated in Creutzfield-Jacob’s disease, superoxide dismutase implicated in amyotrophic lateral sclerosis, and amyloid-β (Aβ) peptide implicated in Alzheimer’s disease, to name the few. Islet amyloid was first reported in 1901 (Opie 1901) as thick proteinaceous deposits in the pancreas of diabetics, and was initially named “islet hyalinization” because of its hyaline-like or glassy appearance. It was later renamed “amyloid”, which means “starch-like”, because islet amyloids were initially believed to be carbohydrates as they could take up dyes which are typically used to stain starch (Clark and Nilsson 2004). Despite numerous studies, the origin and nature of islet amyloid remained enigmatic for a long time. Purification and characterization of amyloid aggregates from the amyloid-rich insulinoma cells and islets of human origin identified hA as the main component (Cooper et al. 1987; Westermark et al. 1986). Studies show that hA-derived amyloid aggregates often associate with apolipoprotein E (apoE) and heparan sulphate proteoglycans (Ancsin 2003; Clark and Nilsson 2004; Hoppener et al. 2000).
Type 2 diabetes mellitus, (T2DM), one of the most prevalent metabolic diseases in the world, is characterized by insulin resistance in the target organs, mainly muscle and liver, and by the decline in the production and secretion of insulin, loss of β-cell mass and formation of islet amyloid (Clark and Nilsson 2004; Hoppener et al. 2000). The role of islet amyloidosis in the pathogenesis of T2DM is supported by several studies showing the presence of hA-derived amyloid plaques in over 90 % of diabetics (Clark and Nilsson 2004; Hoppener et al. 2000). While islet amyloid has been detected in monkeys and cats, species known to develop T2DM, it is absent in rodents and mice, species that do not develop T2DM (Clark and Nilsson 2004; Hoppener et al. 2000). This is strong yet indirect evidence correlating T2DM and islet amyloidosis. Whether islet amyloidosis is a cause or a consequence of the disease is still unclear. In vitro studies revealed that hA but not rA undergoes rapid aggregation in physiological buffers and that insulin, but not pro-insulin, inhibits hA aggregation by forming heteromolecular complexes (Clark and Nilsson 2004; Westermark et al. 1999; Kayed et al. 1999). Therefore, faulty insulin processing in diabetics could partially explain amylin aggregation in T2DM. Defective processing of pro-hA into hA is another candidate for hA aggregation in T2DM, as N-terminal intact pro-hA has been identified in islet β-cells of diabetics (Clark and Nilsson 2004). In fact, pro-peptides have strong self-association properties and are capable of forming amyloid aggregates (Krampert et al. 2000). However, compared to fully processed hA, pro-hA has less amyloidogenicity and less toxicity (Jha et al. 2009; Krampert et al. 2000). This suggests that pro region of pro-peptide may play a protective role in amyloidogenic and toxic potentials of fully processed hA (Krampert et al. 2000). Increased accumulation of amyloid aggregates inside and outside the cells accounts for downstream pathological events such as calcium overload, cell membrane disruption, ER stress, mitochondrial dysfunction, defects in autophagy, oxidative stress and activation of JNK and caspase-3 death signaling pathways (Abedini and Schmidt 2013; Cao et al. 2013a; Costes et al. 2014; Huang et al. 2011; Konarkowska et al. 2006; Rivera et al. 2014; Zhang et al. 2003). Since the ability of hA to penetrate through lipid membranes depends on the lipid-to-peptide ratio, the toxicity of hA is proposed to be enhanced by its plasma membrane association and local accumulation (Cao et al. 2013b; Clark and Nilsson 2004).
Both hA oligomerization and aggregation (fibrilization), in vitro and in vivo, were linked to hA’s toxicity in the pancreas and progression of T2DM in hA -transgenic animals and humans (Cao et al. 2013a; Engel et al. 2008; Lorenzo et al. 1994). Supporting this view, studies reveal that hA-treated or hA overexpressing rodent’s β-cells show intracellular accumulation of reactive oxygen species (ROS) such as hydrogen-peroxide as well as changes in redox enzymes profile and mitochondrial dysfunction, suggesting a role of oxidative stress in hA-mediated β-cell toxicity (Mattson and Goodman 1995; Lim et al. 2010). Chronic oxidative stress is a common pathological condition that has been implicated in the occurrence and progression of several amyloidogenic (protein misfolding) diseases such as T2DM, Parkinson’s and Alzheimer’s disease (Curtin et al. 2002; Konarkowska et al. 2005; Newsholme et al. 2007; Ueda et al. 2002; Zraika et al. 2009; Lim et al. 2010). Prolonged oxidative stress is detrimental to many cells and tissues including pancreatic islets as it induces activation of various pro-apoptotic enzymes such as caspases and stress-activated kinases namely apoptosis signal regulating kinase (ASK1), c-JUN N-terminal kinase (JNK), and p-38 mitogen-activated protein kinase (p38MAPK), all of which were previously implicated in the etiology of T2DM (Hsieh and Papaconstantinou 2006; Liu and Min 2002; Matsukawa et al. 2004; Shen and Liu 2006; Subramanian et al. 2012). For instance, all three major T2DM risk factors hyperlipidemia, hyperglycemia and hyperamylinemia, trigger β-cell apoptosis, at least in part, by activating p38MAP and/or JNK MAP kinase signaling cascades (Subramanian et al. 2012; Matsukawa et al. 2004). The JNK, is readily activated in β-cells by redox and ER stress inducing factors including hA, leading to activation of its downstream pro-apoptotic factors such as caspases and PARP (Matsukawa et al. 2004; Nadeau et al. 2009; Nishitoh et al. 2002; Nishitoh et al. 1998; Watanabe et al. 2015).
3. Molecular determinants of hA aggregation and toxicity
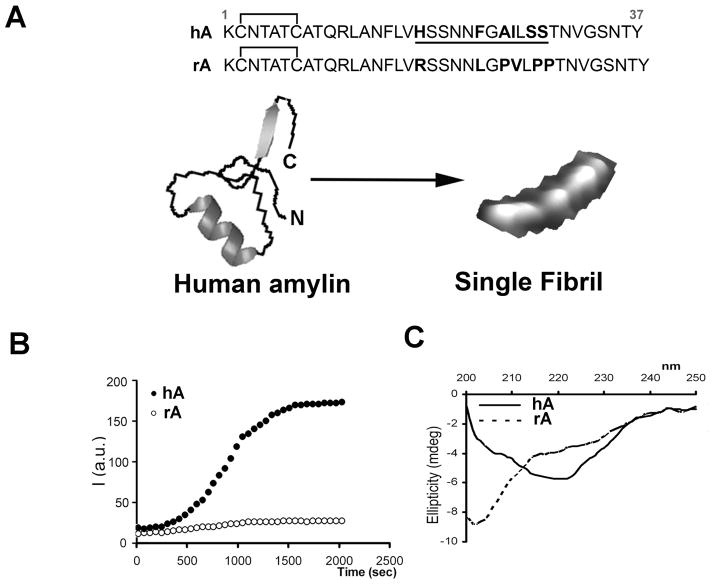
The primary sequences of mature (fully processed) rA and hA are depicted in Figure 1A. Although hA and rA share high sequence homology, the presence or absence of just a few key amino acids in the amyloidogenic region of the peptide (residues 18-29, Figure 1A) may drastically alter protein’s aggregation and cytotoxic properties. Computational and mutational studies confirmed that 18-29 aa segment of mature hA is highly amyloidogenic (Chiu et al. 2013; Moriarty and Raleigh 1999; Westermark et al. 1990). For instance, the presence of His at position 18 in hA is required for hA-plasma membrane interactions, aggregation and toxicity (Abedini and Raleigh 2005; Brender et al. 2008a; Tu and Raleigh 2013). The presence of three Pro residues in positions 25, 28 and 29 renders rA soluble (non-amyloidogenic) and non-toxic (Figures 1B, C) (Westermark et al. 2011). Likewise, substitutions of Asn22, Gly24, and residues 26-28 with Pro markedly reduced aggregation of 20-29 hA fragment (Moriarty and Raleigh 1999). Thus, an absence of His and the presence of Pro in the sensitive residue segment 18-29 of rA as compared to hA is believed to prevent its aggregation and toxicity in rodents.
Figure 1. Dynamics of hA aggregation and misfolding in solution.
(A) Primary structures of mature forms of hA and rA are shown. Species-specific amino-acids within the amyloidoigenic region (underlined) of the two polypeptide chains are bolded for clarity. (B) Kinetics and extent of aggregation of human and rA in buffer as a function of time. Thioflavin-T fluorescent assay reveals fibrilogenesis of 20μM hA in solution (closed circles) and lack of aggregation of non-amyloidogenic rA (20μM; open circles). (C) Far-UV CD spectra of hA (solid line) and rA (dashed line) taken after 20 min. in PBS solution in the presence of 2% HFIP. Note the absorption minimum at ~220nm for hA but not rA, typical for peptides and proteins adopting β-sheet conformation.
In addition to His and Pro, other polar amino acids from the amyloidogenic region (Figure 1A), such as Ser20, may also play a regulatory role in hA aggregation and islet amyloid formation. In fact, Ser20 to Gly mutation in mature hA was observed in a small subset of Chinese and Japanese populations who are at an increased risk of developing T2DM. Interestingly, in vitro studies revealed that Ser20Gly substitution accelerated hA aggregation in solution (Cao et al. 2012), which may help explain increased incidence of diabetes in these two ethnic groups. Notably, hA has a characteristic intramolecular disulphide bond between Cys2 and Cys7, which does not initially contribute to the aggregation (nucleation) process, although its absence reduces fibril formation (Khemtemourian et al. 2008; Koo and Miranker 2005). The rate of hA fibrillization parallels the onset and the extent of membrane damage in vitro (Engel et al. 2008). These findings support the fibril hypothesis of hA’s toxicity in pancreatic islets. However, recent studies point to an important role of pre-fibrillar, low-MW oligomeric species in hA-induced membrane damage and β-cell death, commonly referred to as toxic oligomer hypothesis (Cao et al. 2013a; Janson et al. 1999; Konarkowska et al. 2006; Ritzel et al. 2007; Trikha and Jeremic 2011; Zhang et al. 2014). Below we will explain main postulates of hA oligomerization and aggregation.
4. Changes in secondary conformation drive hA aggregation in solution and on membranes
Recent biochemical, biophysical and microscopy studies revealed important details of the dynamics and the mechanism of hA oligomerization and aggregation in solution and native and cellular membranes (Cao et al. 2013a; Engel et al. 2008; Ritzel et al. 2007). Such high-resolution studies are essential to understand hA aggregation at the molecular level. This in turn may help us to understand how certain cellular processes such as secretion, trafficking and degradation may regulate turnover of amyloid proteins and contribute or prevent the formation of amyloid plaques in the pancreas and other organs.
Thioflavin (ThT) fluorescence assay (Figure 1B) is a commonly used method to monitor the extent and the kinetics of aggregation of various amyloid peptides and proteins in vitro in cell-free environment (Munishkina and Fink 2007). In the absence of amyloid, the ThT is weakly fluorescent in solution. However, in the process of aggregation, the ThT molecules intercalate into the growing amyloid fibers, rendering the probe more fluorescent. Thus, increase in ThT fluorescence over time reflects the kinetics and extent of protein aggregation in solution that is amenable for experimental manipulations. Lag (nucleation) phase followed by sigmoidal (fibril growth) phase are two common traits shared by amyloid proteins undergoing aggregation (Figure 1B). For example, increasing the salt concentrations in the incubation medium to screen out electrostatic interactions in solution decreased both the rate and the extent of hA aggregation. (Cho et al. 2008). Thus, hA aggregation inversely correlates to the solvent ionic strength, which suggests that intra- and inter-molecular non-covalent interactions among certain residues play a major role in self-association and polymerization of hA in solution. Aromatic and hydrophobic interactions were proposed to play a major role in hA polymerization in solution (Gazit 2002; Tu and Raleigh 2013). These two non-covalent interactions also play an important role in self-assembly (oligomerization) of peptides into channel-like structures in the membrane, the efficacy of which inversely correlates with the ionic strength of the solution (Zhao et al, 2008). In this study, formation of protein pores was inhibited when ionic strength of solution increased, whereas both hydrophobic and aromatic interactions were retarded with the increase of salt concentration (Zhao et al., 2008). Thus, it is highly conceivable that hA oligomerization, the first step in hA aggregation, is retarded in solution with increased ionic strength due to the inhibitory effect of salts on aromatic and hydrophobic interactions, two major driving forces in hA polymerization (Gazit, 2002; Tu & Raleigh, 2013). This eventually would diminish aggregation of hA, as shown recently (Cho et al, 2008).
Together with ThT assay, the structural studies revealed a causal link between conformational changes in hA and its propensity to aggregate (Figure 1C) (Brender et al. 2008b; Cho et al. 2008; Cho et al. 2009; Wiltzius et al. 2008). Similar to many other small proteins and peptides, hA is natively unfolded in solution. However, hA can polymerize in a cross-β-sheet conformation upon aggregation in amyloid fibers. CD analysis revealed that aggregation of hA is accompanied by secondary structural changes, from random coil in the monomeric from to the β-sheet-enriched fibrillar form characterized by a single minimum at ~ 220 nm (Figure 1C). In contrast, rA retains its random coil conformation in solution, characterized by a minimum at 202 nm (Figure 1C), which prevents its aggregation (Figure 1B). The likely reason for this difference is that rA contains three structure-breaking prolines, Pro25, Pro28 and Pro29, in the residue segment that probably initiates amyloid formation of hA (Figure 1A); these three prolines are expected and observed to prevent β-aggregation (Figure 1B, C) (Moriarty and Raleigh 1999). Inhibition of hA transition towards β-sheet conformation by certain inhibitors (divalent metals, insulin or cholesterol) also prevents its aggregation (Cho et al. 2008; Cho et al. 2009; Salamekh et al. 2011; Susa et al. 2014). Collectively, these biophysical studies reveal that aggregation of hA, like other amyloid proteins, is strongly conformation-dependent and that transition to β-sheet is a requirement for the formation of fibrils.
5. High resolution microscopy analysis of hA deposition on synthetic and plasma membranes
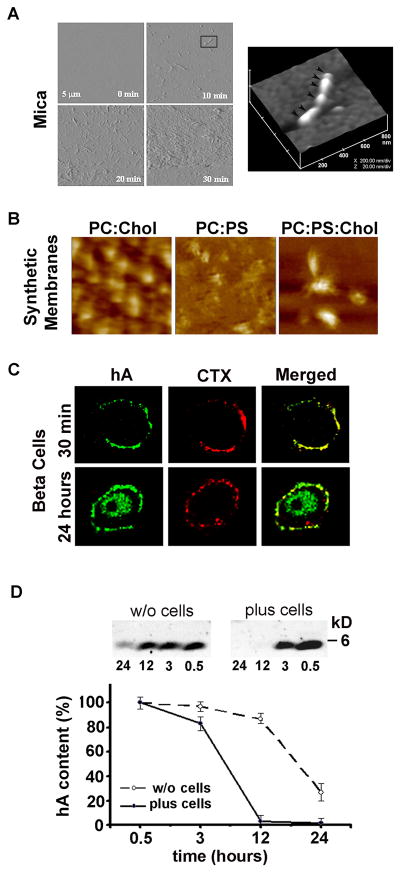
Although the aforementioned bulk spectroscopy studies provided important information on the dynamics and conformational changes associated with protein misfolding and aggregation, they could neither provide information on the nature and architecture of pre-aggregated species, nor explain how they assemble into fibrils. Without this information, the process of hA aggregation and amyloid formation in tissues cannot be fully understood. Therefore, visualization of hA aggregation became imperative. Given the small size of aggregated species, and in order to visualize peptide/protein transition from monomers to oligomers to large aggregates, a new real-time imaging tool capable of imaging at nm-resolution was needed. The development of atomic force microscope (AFM), a 3D lens imaging instrument, allowed investigators to examine, for the first time, the process of amyloid formation with unprecedented clarity and specificity. Formation and growth (extension) of a single fibril has been monitored using this technology (Figure 2A) (Cho et al. 2008; Goldsbury et al. 1999; Green et al. 2004). The unique capability of AFM to directly monitor changes in the conformation or aggregation state of macromolecules, and to study dynamic aspects of molecular interactions in their physiological buffer environment has allowed examination of hA aggregates at ~5 nm lateral and ≤1 nm vertical resolution (Figure 2 A, B) (Cho et al. 2008; Cho et al. 2009; Green et al. 2004). This novel imaging technology has provided new insights into the molecular mechanism of amyloid assembly.
Figure 2. High resolution microscopic analysis of hA aggregation on solid surfaces and membranes.
(A) Tapping mode time-lapse AFM was used to capture structural intermediates, oligomers and fibrils, during hA aggregation on mica. Note a time-dependent structural transition of hA from small fibrils (early stage of hA aggregation, 10 min) to amyloid-like dense deposits (late stage of hA aggregation, 20–30 min). All micrographs on the left panel are 5×5 μm. 3-D AFM image of a single full-grown fibril on mica (inset, 10 min) reveals linear alignment of several hA oligomers and their bi-directional extension into a fibril (depicted by arrowheads). Micrograph is 800 × 800 nm scale. (B) AFM analysis of membrane-directed hA self-assembly. High-resolution 2D AFM analysis revealed distinct deposition pattern and morphology of hA aggregates on synthetic lipid membranes. Note the clustering of hA aggregates on cholesterol-containing membranes, PC:Chol (3.2:0.8 mol:mol) and PC:PS:Chol (2.3:1:0.8 mol:mol:mol). In contrast, hA aggregates were less compact and homogenously distributed across cholesterol-free membranes-PC:PS (2.8:1.2 mol:mol). In contrast to mica (Figure 2A), no fibrils were detected on either membranes (Figure 2B). Micrographs are 2×2 μm. (C) Confocal microscopy analysis of binding and clustering of hA on the β-cell PM. Rinm5F cells were exposed to hA (20 μM) for 30 min or 24h. Cells were then washed and fixed prior to immunochemical analysis. hA specific antibody (green) was used to analyze peptide’s accumulation on the plasma membrane and intracellularly. Fluorescently-labeled lipid-raft marker cholera toxin (CTX, red) was added to cell during last 30 min. of hA incubations to localize lipid rafts micro domains on the cell plasma membrane. Note hA and CTX co-clustering on the cell plasma membrane (yellow puncta) and time-dependent hA internalization in a single β-cell indicating hA extracellular clearance. (D) Clearance of extracellular hA by pancreatic β-cell revealed by western blot. hA (20 μM) was added to Rinm5F cells or cell-free buffer and the changes in hA content in solution were analyzed over 24 by western blot approach. hA (4 kDa) was detected using amylin-specific antibody. Note the accelerated clearance of hA from solution containing cells. The slow decrease in hA content in cell-free solution is due to delayed hA aggregation and precipitation from solution. Due to its toxicity, peptide solvent HFIP was omitted from these studies.
In our studies, time-lapse AFM operating either in contact or tapping mode was used to investigate the organization of hA aggregates on solid surface such as mica (Figure 2A) and on planar lipid membranes (Figure 2B), two surfaces bearing distinct physicochemical properties. With the scanner speed set at 1 Hz and image acquisition time of ~5 min/image, and using high-resolution scanning parameters (512 × 512 lines per image), the dynamics, polymorphism and the extent of hA fibrillization can be obtained. Time-lapse amplitude AFM micrographs revealed structural transitions of hA on mica, from small spherical oligomers to extended fibrils, over a 30 min time period (Figure 2A).
After acquiring micrographs, the size of individual fibrils and oligomers (i.e. radius, length and height) that were deposited on mica (Figure 2A) or on planar membranes (Figure 2B) could be determined using a section analysis tool (Veeco, Santa Barbara, CA). Cross-sectional analysis revealed that hA fibrils varied by length and consistently measured 90–110 nm in width and 5–6 nm in height (Figure 2A) (Cho et al. 2008). Some fibrils were relatively short (less than 200 nm), whereas others extended over 500 nm in length (Figure 2A, inset). In the presence of 1–2 % hexafluoride isopropanol, which accelerates hA aggregation, massive amyloid-like hA deposits generally developed after 30 min of incubation (Figure 2A, 30–35 min). AFM studies revealed that hA fibrillization depends on formation of “building block” oligomers, or nuclei, measuring approximately ~6 nm in height and ~90 nm in diameter. Once formed, these nuclei align and elongate into a fibril (Figure 2A), a scenario originally proposed by Aebi and co-workers (Green et al. 2004).
The above-mentioned spectroscopy and microscopy studies revealed species and molecular mechanism of hA aggregation in solution and on solid surface. However, hA aggregates were also found in close proximity to islet β-cells, with some fibrils integrated into the β–cell plasma membranes (PM) (MacArthur et al. 1999). This finding suggests that hA-membrane interactions may be important for both hA aggregation on the cell surface and for the integrity and function of the β-cell PM. The regulatory role and involvement of the membrane’s main constituents, phospholipids and cholesterol, in hA aggregation were explored during the last decade, prompted by findings that hA toxicity stems, at least in part, from its ability to disrupt fluidity and organization of cellular membranes (Brender et al. 2008a; Brender et al. 2008b; Khemtemourian et al. 2008). Thus, understanding the process of hA aggregation on membranes has a direct implication for the etiology of islet amyloidosis and T2DM. We used AFM to investigate the supramolecular organization and dynamics of hA aggregates on model membranes (Cho et al. 2009) that resemble the cell PM in composition and fluidity. hA aggregation on neutral (PC) and negatively-charged (PC:PS) planar membranes that contained or lacked cholesterol was investigated by time-lapse AFM (Figure 2B). The large clustering effect of cholesterol was quite obvious in both neutral (PC:Chol, Figure 2B) and negatively charged membranes (PC:PS:Chol, Figure 2B). Cholesterol stimulated a significant increase in the height of hA aggregate as compared to cholesterol-lacking (PC) membranes. This was accompanied by an overall decrease in hA deposition across the planar membranes (Figure 2B) (Cho et al. 2009). As hA aggregated and accumulated in some membrane areas, other regions of the membrane were virtually devoid of the protein aggregates (PC:Chol, PC:PS:Chol, Figure 2B). Consequently, hA’s capacity to form an extensive network of amyloid aggregates on the membrane was diminished in membranes that contained cholesterol (Cho et al. 2009).
Experiments performed with planar membranes provided important although indirect evidence for the role of membranes in aggregation of hA and other amyloid proteins. To confirm that native membranes modulate hA’s turnover and toxicity in situ, we resorted to cellular studies (Trikha and Jeremic 2011, 2013). In our experimental setup, initially monomeric hA was added to pancreatic rat and human islet cells in which we systematically varied plasma membrane cholesterol levels using cholesterol biosynthesis inhibitor lovastatin (Lov) and/or the cholesterol-depleting agent, beta-cyclodextrin (BCD). The extent of hA aggregation in cholesterol-containing and cholesterol-depleted cells was assessed over 24 hours by confocal microscopy (Trikha and Jeremic 2011). To detect hA monomer distribution on the PM and inside the cells, we used a human-specific amylin antibody that does not cross-react with the rat isoform or large oligomers/aggregates (Trikha and Jeremic 2011). In addition to hA, we used the lipid raft marker, cholera toxin (CTX), and the clathrin endocytotic marker, transferrin (see below), to determine the specificity of hA monomer and oligomer binding to the cell PM and subsequent internalization routes (as described below). hA and CTX were sequentially (Trikha and Jeremic 2011) incubated with cultured pancreatic insulinoma RIN-m5F cells for the indicated periods of time, fixed and processed for immunochemical analysis. In experiments in which hA and CTX were concurrently incubated with cells, immuno-confocal microscopy revealed a clustering of CTX and hA on the cell PM, exhibiting high spectral overlap (yellow) and a high co-localization coefficient (R=0.74±0.09) in discrete membrane regions [Figure 2C, 30 min, (Trikha and Jeremic 2011)]. CTX and hA clusters were also observed at 24 h following addition to cells (Figure. 2C), indicating long-lasting regulatory effect of phospholipids and cholesterol on hA accumulation on cell PM. In analogy with results obtained on synthetic membranes, cholesterol was primary regulator of hA deposition on PM because hA oligomer clustering was diminished in cells pre-treated with cholesterol-depleting agent, beta-methyl-cyclodextrin (Trikha and Jeremic 2011). Confocal microscopy also revealed gradual binding and internalization of hA in pancreatic β cells: intracellular hA was not observed during the first 30 min of hA addition to cultures. However, at 24 hours, a significant intracellular accumulation of hA was observed (Figure 2B). This result suggests that pancreatic cells can sense and regulate extracellular hA concentration. In line with this idea, western blot analysis revealed accelerated decomposition of hA in media supplemented with β-cells as compared to cell-free media (Figure 2D).
6. Role of hA receptor in hA turnover in pancreatic cells
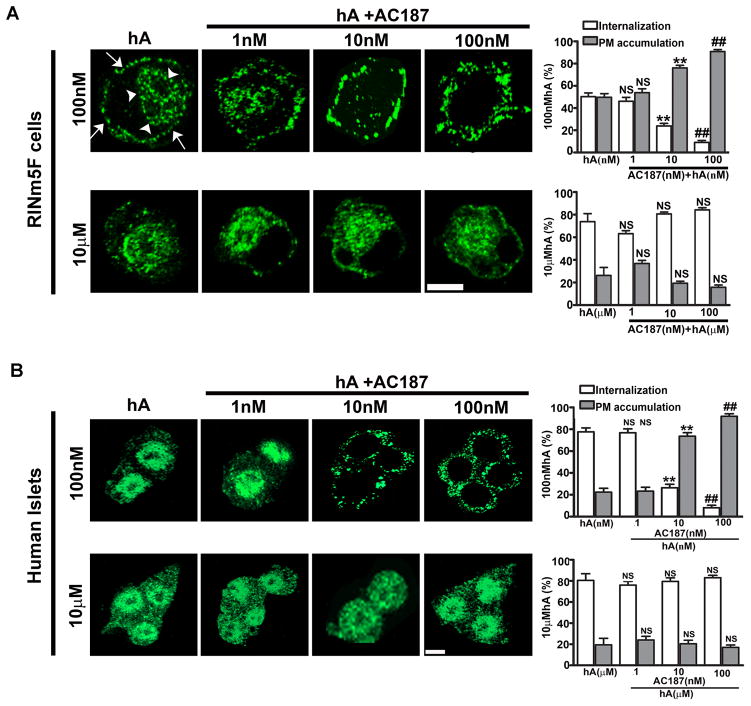
Next, we sought to determine mechanism of hA clearance by pancreatic cells and possible involvement of hA-receptor (AM-R) in hA internalization in β-cells (Figure 3). Using immuno-confocal microscopy, we investigated the roles of AM-R and endocytosis on the uptake and toxicity of hA in cultured pancreatic RIN-m5F and human islet cells. As hA is non-toxic at low (nM) concentrations and cytotoxic at higher (μM) concentrations, we examined the mechanism of hA monomer and oligomer internalization at these two distinct concentrations, aiming to understand how cells deal with hA overload. Hence, cells were incubated for 24 hours with low (100 nM) or high (10 μM) concentrations of freshly prepared hA and its intracellular/PM accumulation determined by quantitative immunoconfocal analysis (Figure 3), (Trikha and Jeremic 2013). Prolonged incubation of cells with 100 nM hA allowed hA accumulation both on the PM and in the perinuclear compartments (Figure 3A, top panel). Whole cell analysis (Figure 3A top panel, graph) revealed that monomers were equally distributed between PM and intracellular compartments. Incubations of cells with 10 μM hA increased intracellular accumulations of monomeric hA by ~20 % (Figure 3A bottom panel, graph), indicating a saturable and possibly receptor dependent mechanism for hA uptake. To confirm or refute a receptor dependent mechanism for hA monomer uptake, cells were co-incubated with hA (100 nM or 10 μM) and the selective AM-R antagonist, AC-187 (Jhamandas et al. 2011; Jhamandas and MacTavish 2004; Bailey et al. 2012; Reidelberger et al. 2004; Jhamandas and Mactavish 2012) (1–100 nM) for 24 hours. Immunocytochemistry revealed a dose-dependent inhibition of hA monomer uptake and its concomitant accumulation on the PM in RIN-m5F β-cells at low (100 nM) hA concentration (Figure 3A, top panel and graph) and in human islets (Figure 3B top panel, graph) indicating a receptor-dependent mechanism in both cell types. When a high 10 μM hA concentration was used, the extent of hA monomer internalization was not significantly changed by AC-187 in RIN-m5F β-cells (Figure 3A bottom panel, graph) nor in human islets (Figure 3B bottom panel, graph), suggesting an AM-R-independent uptake mechanism. Thus, our results indicate that the mechanism of hA monomer internalization is dependent on its concentration. We also tested the involvement of the AM-R in internalization of hA oligomers. Interestingly, in both pancreatic cell types, AC-187 failed to prevent hA oligomer internalization indicating that the AM-R is not involved in uptake of these toxic species (Trikha and Jeremic 2013).
Figure 3. High affinity AM-R-dependent and low affinity AM-R-independent hA transport operate in pancreatic cells.
Rin-m5F (A) and human islet cells (B) were incubated with 100 nM or 10 μM hA either in the presence or absence of the AM-R antagonist, AC-187 (1–100 nM) for 24 hours. hA accumulation on the cell PM and subsequent internalization was concurrently assessed with quantitative confocal microscopy analysis. (A) Confocal microscopy analysis of hA uptake in Rin-m5F cells is shown. When low concentration of hA (100 nM) was used, hA monomer internalization was significantly inhibited with increasing concentrations of AC-187 (top panel, graph). A corresponding increase in hA accumulation on cell PM was observed. In contrast to this high affinity uptake process, hA monomer/oligomer uptake at high (10 μM) was not affected with increasing concentrations of AC-187 (bottom panel, graph). (B) Confocal microscopy analysis of hA uptake in cultured human islet cells is depicted. Note a dose-dependent inhibition of hA uptake by AC-187 at lower (100 nM) but not higher (10 μM) hA concentrations, revealing high- and low-affinity hA transport mechanism in β-cells, respectively. Significance established at p<0.05 by ANOVA followed by Dunnett-Square test. Bar 5μm.
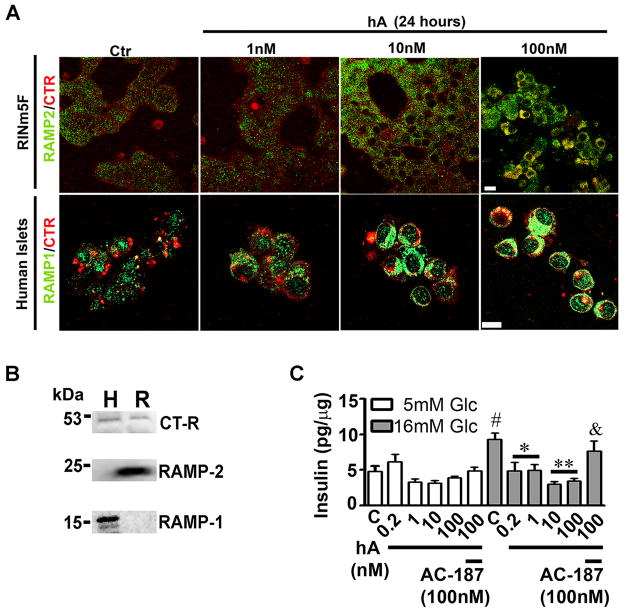
Although, the receptor for hA has been identified and cloned (Tilakaratne et al. 2000; Bailey et al. 2012; Christopoulos et al. 1999; Morfis et al. 2008; Poyner et al. 2002), its expression in pancreatic cells and its contribution to hA signaling and toxicity remains enigmatic. Using western blot analysis and isotype specific antibodies, we detected co-expression of RAMP2 and CT-R in RIN-m5F β-cells and RAMP1 and CT-R in human islets, reflecting expressions of type-2 and type-1 AM-R respectively (Figure 4B). However, no signal was detected using the RAMP3 antibody, indicating no or low expression of the type-3 AM-R isoform in these two cell types (data not shown). In a concentration-dependent manner (1–100 nM), hA stimulated expression of type-2 AM-R in RIN-m5F β-cells as evident by increased co-trafficking of AM-R constituents, RAMP2 and CT-R to the PM (Figure 4A, top panel). Similarly, upon addition of hA (1–100 nM), there was an increased expression of type-1 AM-R on the PM of human islets (Figure 4A, bottom panel), indicating hA -evoked AM-R turnover in these cells (Trikha and Jeremic 2013). Insulin release assay by ELISA further revealed a functional coupling between hA and AM-R in pancreatic cells as supplementation of AC-187 to the culturing medium revoked the inhibitory effect of hA on glucose-evoked insulin release in a dose-dependent manner from RIN-m5F β-cells (data not shown) and from human islets (Figure 4C), (Trikha and Jeremic 2013). In contrast to its modulatory effect on hA-mediated glucose-evoked insulin release or AM-R trafficking in islet cells (Figure 4), AC-187 did not show any significant effect on hA toxicity in either rat or human pancreatic cells (Trikha and Jeremic 2013), indicating an AM-R-independent mechanism of hA toxicity in these cells.
Figure 4. hA regulates trafficking of AM-R and insulin release in β-cells.
(A) Immunoconfocal microscopy analysis revealed expression and location of RAMP2 (green)/CT-R (red) in RINm5F β-cells (top panel) and RAMP1 (green)/CT-R (red) in human islet cells (bottom panel). Note increased recycling on AM-R components, CT-R and RAMPs, to the plasma membranes (yellow puncta) following exposure to increasing hA concentration (1–100 nM). Bar 10 μm. (B) Western blot analysis shows expression of CT-R and two RAMPs isoforms RAMP1 in human islets (H) and RAMP2 in RINm5F β-cells (R). (C) The inhibitory effect of hA on glucose-evoked insulin release from human islets was reversed by addition of AM-R antagonist, AC-187, indicating an AM-R mediated process. Intact human islets were exposed to normal (5mM) or high (16mM) glucose (Glc) concentrations in the presence or absence of hA (0.2–100 nM) and/or AC-187 (100 nM) for 30 minutes, following which insulin content in the samples (release) was analyzed by ELISA. Data was normalized to total protein content in samples. #p<0.05, 5mM Glc vs. 16mM Glc, n=6, **p<0.01, control vs. hA 0.2–100nM; and &p<0.05, hA 100nM vs. hA 100nM +AC-187 100nM, n=6. Significance established by ANOVA followed by Dunnett-Square test.
7. Micropinocytosis drives hA uptake in pancreatic cells
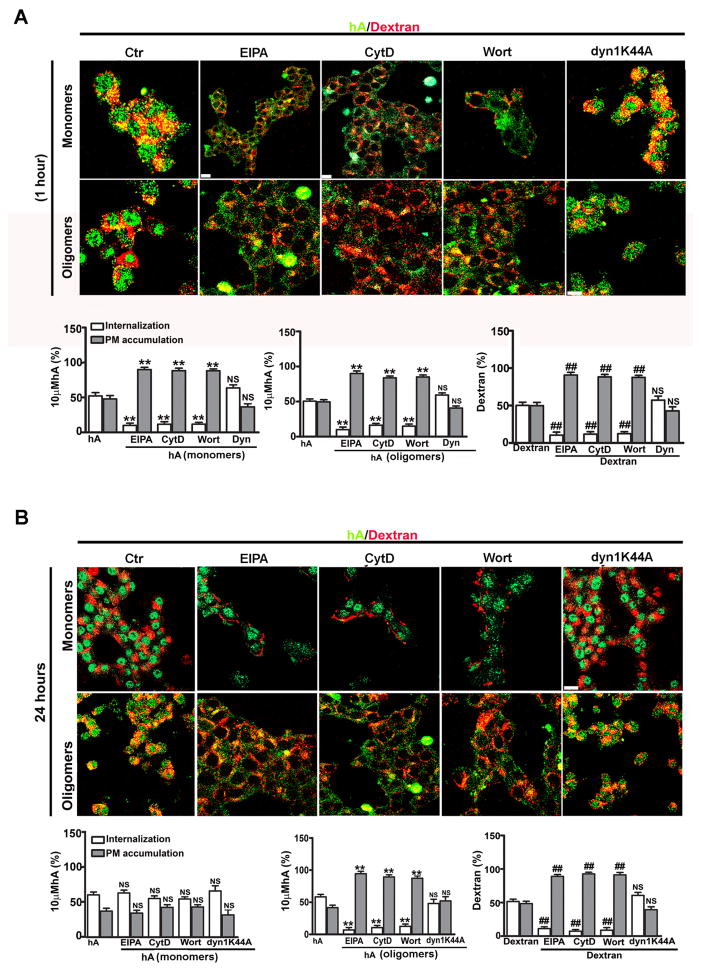
We recently used confocal microscopy along with specific fluorescent endocytotic markers and pharmacological inhibitors to further dissect the molecular mechanism of hA monomer and oligomer internalization (Trikha and Jeremic 2013). We first examined the mechanism that operates during an early phase (1 hour) of hA internalization (Figures 5A and 6A). It was previously shown that the small and soluble oligomeric forms of brain derived β-amyloid peptide were avidly taken up by microglia cells through fluid phase-macropinocyotsis (Mandrekar et al. 2009) which may also play a role in the initial uptake of hA in RINm5F β-cells. To test this hypothesis, cells were pre-treated 30 min with the macropinocytotic inhibitors, 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), Cytochalasin-D (CytD) or Wortmannin (Wort) prior to addition of hA or macropinocytotic marker, dextran for 1h or 24h. To detect hA monomer distribution on the PM and inside the cells, we used a human-specific hA antibody that does not cross-react with the rat isoform or large oligomers/aggregates (Trikha and Jeremic 2011). Oligomers were detected with the oligomer-specific A11 antibody that does not react with either monomers or fibrils (Kayed et al. 2003). Under control conditions (no inhibitors), a sizable fraction (52±5%) of hA monomers internalized in these cells (Figure 5A), a portion of which trafficked to dextran-positive intracellular compartments in RINm5F β-cells as evident by their partial co-localization (R=0.48±0.02), (Trikha and Jeremic 2013). This suggests a common macropinocytotic dependent internalization mechanism for dextran and hA monomers. To further confirm that macropinocytosis is involved in the uptake of hA monomers, macropinocytotic inhibitors were used (Figure 5). Whole cell analyses revealed that 52±5% of cell-associated hA monomers and 50±4% dextran (Figure 5A, graphs) accumulated intracellularly in control (vehicle-treated) cells under control conditions, the rest being associated with the PM. Inhibition of macropinocytosis by EIPA (Gold et al. 2010; Khalil et al. 2006; Sandgren et al. 2010), markedly decreased the internalization of monomers to 10±3% and dextran to 10±4%, and in turn increased their PM accumulation to ≥90% (Figure 5A, top panel). Consequently, a significant decrease in intracellular co-localization between hA monomers and dextran was observed (R=0.07±0.01) (Trikha and Jeremic 2013). Macropinocytosis is known to be dependent on actin polymerization. The latter is required for PM ruffling and subsequent formation of macropinosomes (Gold et al. 2010; Khalil et al. 2006; Mandrekar et al. 2009; Trikha and Jeremic 2011). Consistent with fluid-phase uptake mechanism, an inhibitor of actin polymerization, cytochalasin-D (cytD) inhibited internalization of both monomers and dextran by ~40% causing a comparable increase in their PM accumulation (Figure 5A top panel, and graphs hA monomers/dextran). Other than actin, phosphatidylinositide-3-kinase (PI 3-kinase) is required for macropinocytosis by directing the proper closure of membrane ruffles that leads to the formation of macropinosomes (Araki et al. 1996; Sandgren et al. 2010; Amyere et al. 2000; Kruth et al. 2005). Hence, cells were pre-incubated with a specific inhibitor of PI3-kinase, wortmannin (Araki et al. 1996; Sandgren et al. 2010; Amyere et al. 2000; Kruth et al. 2005) and its effect on hA internalization was examined. As with other macropinocytotic inhibitors, wortmannin significantly reduced internalization of both monomers and dextran, thereby stimulating their PM accumulation (Figure 5, top panel and graphs hA monomers/dextran). It has been widely debated whether dynamin is required for macropinocytosis. Both dynamin dependent and independent macropinocytosis were found to operate in cells (Gold et al. 2010). To investigate the possible involvement of dynamin in hA internalization, we overexpressed a dominant negative mutant of dynamin, dyn1K44, or pretreated cells with the dynamin inhibitor, dynasore (Gold et al. 2010; Sandgren et al. 2010). Confocal microscopy revealed that neither dynasore nor dyn1K44 prevented or reduced the internalization of hA or dextran (Figure 5A, top panel, graphs) in RINm5F β-cells. However, dynasore effectively blocked internalization of Cholera Toxin (CTX) and Transferrin (Trf) (Figure 6A), known endocytotic markers of dynamin-dependent pathways (Gold et al. 2010; Khalil et al. 2006; Lai and McLaurin; Sandgren et al. 2010; Yu et al. 2010). Thus, these results suggest that hA monomers, at higher (10 μM) concentration, initially (1 hour) internalize in RINm5F β-cells by a dynamin-independent fluid phase-macropinocytotic pathway.
Figure 5. Fluid phase uptake of hA monomers and oligomers by pancreatic cells.
(A) Initial entry (1h) of hA monomers (top panel) and oligomers (bottom panel) is through dynamin-independent macropinocytosis in RINm5F cells. Cells were treated with various endocytotic inhibitors EIPA, CytD, Wort or Dyn for 1 hour followed by hA (green) (10 μM) for an additional 1 hour at 37°C. Dextran (red) at 40μg/ml was finally added for 30 minutes. Confocal microscopy revealed a significant reduction in internalization and an increase in PM accumulation of hA monomers (green) and dextran (red) in the presence EIPA, CytD or Wort but not Dyn when compared to controls. (A, top panel and graph). Similar internalization pathway was also demonstrated for hA oligomers and dextran within the first hour (A, bottom panel and graph). (B) Late entry (24h) of hA oligomers but not monomers is through dynamin-independent macropinocytosis in RINm5F cells. Note no significant change in the cellular distributions of hA monomers (top panel, graph) in the presence of EIPA, CytD, Wort or DN dyn1K44A when compared to controls. On the contrary, dextran internalization was completely blocked with these macropinocytotic inhibitors but not with DN dyn1K44A (A, top panel and graph). Marked inhibition in internalization of hA oligomers and dextran was observed following treatments with EIPA, CytD or Wort but not with DN dyn1K44A (A, bottom panel and graph). Bar 10μm. **p<0.01, hA vs. hA plus inhibitors, ##p<0.01, dextran vs. dextran plus inhibitors, NS p>0.05, n=9. Significance established by ANOVA followed by Dunnett-Square test.
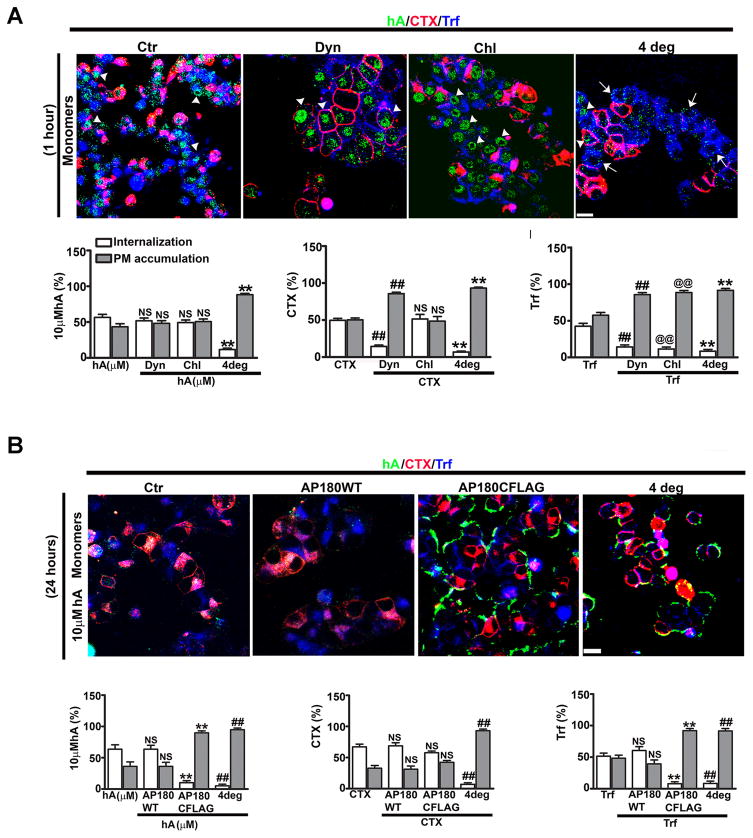
Figure 6. Clathrin-dependent and independent endocytotic pathways regulate hA internalization in pancreatic cells.
(A) hA monomer internalization is independent of clathrin and dynamin at 1 hour in RINm5F cells. Cells were treated with dynamin inhibitor dynasore (Dyn) or clathrin inhibitor chlorpromazine (Chl) for 1 hour followed by hA (green) (10 μM) for an additional 1 hour at 37°C. In parallel, cells were incubated with hA (10 μM) for 1 hour at 4°C. CTX (red) (20 μg/ml) and Trf (blue) (50 μg/ml) were finally added for 30 minutes at 37°C or 4°C. Whole cell analysis (graph) demonstrated no noticeable difference in cellular distributions of monomers at 1 hour when treated with Dyn or Chl. However, lowering temperature to 4°C blocked monomer internalization as well as CTX and Trf (A). Arrowheads and arrows denote cells with internalized and PM associated hA monomers, respectively. (B) Late phase of hA monomer internalization requires clathrin in RINm5F cells. Cells were first transfected with wild type (wt-AP180) or dominant negative clathrin adaptor AP180 protein, containing clathrin binding domain at its C-terminus (DN AP180CFLAG) for 16–18 hours. Following transfections, cells were incubated with 10 μM hA (green) for an additional 24 hours at 37°C. Cells were also treated with hA at the indicated concentrations for 24 hours at 4°C. CTX (red) (20 μg/ml) and Trf (blue) (50 μg/ml) were finally added for 30 minutes at 37°C or 4°C after incubating the cells with hA. Confocal microcopy and whole cell analysis revealed a significant reduction in internalization and an increase in PM accumulation of hA when transfected with DN AP180CFLAG or when incubated at 4°C. In contrast, there was no change in their cellular distributions in wt-AP180 expressed cells and controls (B). Transferrin but cholera toxin internalization was blocked in cells transfected with DN AP180CFLAG construct (B). Internalization of all three cargoes were effectively blocked at 4°C (B). **p<0.01, hA vs. hA plus inhibitors, ##p<0.01, hA vs. hA/4°C, ##p<0.01, CTX vs. CTX/4°C, and Trf vs. Trf/wt-AP180, NS p>0.05, n=9. Significance established by ANOVA followed by Dunnett-Square test. Bar 10μm.
To determine if hA oligomers follow the same internalization route as monomers, we studied trafficking of oligomers and dextran in RINm5F β-cells with immuno-confocal microscopy (Figure 5A, bottom panel). Under control conditions, All positive oligomers partially co-localized with dextran-positive intracellular compartments (R=0.45±0.03) (Trikha and Jeremic 2013), indicating a common internalization mechanism (macropinocytosis) for these two cargos. Like monomers (Figure 5A, top panel), initial oligomer internalization was diminished to 10±3% with EIPA, 16±3% with cytD and 15±3% with Wort, which in turn increased their accumulation (~85–90%) on the PM of the RINm5F β-cells (Figure 5A, bottom panel, graph hA oligomers). Dextran internalization was also significantly reduced to 8–13% by the same inhibitors (Figure 5A, bottom panel), suggesting a common internalization mechanism. As shown for monomers, internalization of hA oligomers and dextran was unchanged by dynasore or dyn1K44A, indicating a dynamin-independent uptake mechanism for these two cargos.
We further investigated if macropinocytosis also plays a role in hA internalization at later times (24 hours). The cells were pre-incubated with EIPA, CytD or Wort for 1 hour and then incubated with low (100 nM) or high (10 μM) hA for additional 24 hours. This procedure minimizes the toxic effects of these inhibitors, which may interfere with hA uptake. Following the treatments, dextran was added to the cells. Under control conditions, 55±4% of the cell-associated hA monomers accumulated inside the cells when incubated with the 100 nM hA concentration (Trikha and Jeremic 2013), whereas 62±5% of monomers internalized when challenged with high (10 μM) hA concentration (Figure 5B, top panel). This result indicates a saturable uptake mechanism for hA, not a characteristic of fluid phase endocytosis (Mandrekar et al. 2009). Furthermore, the macropinocytotic inhibitors did not prevent or reduced hA monomer internalization or PM accumulation respective to controls at 24 hours (Figure 5B, top panel). In contrast to hA monomers, EIPA, CytD and Wort treatments blocked dextran uptake (Figure 5B, top panel and graph). A very low co-localization value (R=0.05±0.01) was obtained between hA monomers and dextran either in the absence or presence of these inhibitors (Trikha and Jeremic 2013). Thus, hA monomers and dextran follow distinct internalization pathways at later, 24h time point. In contrast to monomers, hA oligomers uptake at 24h was significantly reduced in cells pretreated with macropinocytotic inhibitors (Figure 5B, bottom panel).
We previously reported that the endocytotic protein, clathrin is implicated in the later stage (24 hours) of hA monomer internalization in pancreatic cells (Trikha and Jeremic 2011). To determine if clathrin is also required for initial entry of monomers, RINm5F β-cells were first pre-treated with a specific clathrin inhibitor, chlorpromazine (Trikha and Jeremic 2011; Wang et al. 1993; Yu et al. 2010), followed by addition of hA for 1 hour. CTX and Trf were then added. Trf but not CTX follows clathrin-mediated pathway (Gold et al. 2010; Khalil et al. 2006; Lai and McLaurin; Sandgren et al. 2010; Yu et al. 2010; Kandimalla et al. 2009). Chlorpromazine reduced Trf internalization but had no significant effect on internalization of either hA monomers or CTX (Figure 6A) during the first hour. Interestingly, small fractions of hA monomers (12±2%, Figure 6A) and oligomers (Trikha and Jeremic 2013) were internalized even when the cells were incubated at low temperatures (≤ 4°C). By contrast, both CTX and Trf internalization were almost completely blocked (≥ 92%) at ≤ 4°C (Figure 5A). The confocal microscopy also revealed a ~5–6 fold decrease in the number of cells with internalized hA monomers and oligomers at ~4°C (Trikha and Jeremic 2013). Therefore, our results demonstrate that hA monomers and oligomers initially internalize in pancreatic cells through clathrin/dynamin-independent fluid phase macropinocytosis and to a lesser extent (10–15%) by a non-endocytotic (translocation) mechanism.
8. hA internalization requires clathrin but not dynamin
As mentioned earlier, clathrin inhibitor chlorpromazine effectively blocked hA internalization in pancreatic cells at 24h (Trikha and Jeremic 2011). These results implicate clathrin-mediated endocytosis in the later stage (24 hours) of hA monomer internalization in pancreatic cells. To confirm this, cells were transfected with a full length wild type clathrin construct, wt-AP180 or dominant-negative DN AP180CFLAG mutant construct containing a clathrin binding domain at the C-termini region of AP180, which specifically inhibits clathrin-mediated endocytosis (Schneider et al. 2008; Stavrou and O’Halloran 2006; Yu et al. 2010). The cells were sequentially incubated with hA for 24 hours. Fluorescently tagged CTX and Trf were then added to the cells to label compartments involved in hA turnover. Expression of wt-AP180 did not significantly change the extent of hA monomer internalization as compared to its uptake in non-transfected cells (Figure 6B). Transfection with the DN AP180CFLAG mutant reduced hA monomer internalization with a concomitant increase in their PM accumulations at 24 hours at both hA concentrations (Figure 6B). Clathrin dependent Trf internalization was significantly reduced in the cells transfected with DN AP180CFLAG (Figure 6B). hA monomer internalization was also blocked at low temperature (≤4°C) (Figure 6B) as was uptake of CTX and Trf (Figure 6B). All these observations support the view that hA monomers at 24 hours are taken in by clathrin dependent endocytosis.
To probe whether dynamin is involved in hA monomer internalization at these later times, a plasmid encoding the DN dynamin mutant form (dyn1K44A), deficient in its GTP binding and GTPase activity (Damke et al. 2001; Llorente et al. 1998; Schneider et al. 2008; Yu et al. 2010), was used to transfect RINm5F β-cells. Internalization of hA monomers and dextran were not significantly reduced with respect to the controls in cells expressing the DN dynamin form, while CTX and Trf internalization were almost completely blocked (Figure 5B) (Trikha and Jeremic 2013), indicating a dynamin-independent pathway for hA. Taken together, our biochemical and immunoconfocal studies suggest that at later times (24 hours), when their concentration drops to sub-μM range, hA monomers change their internalization pathway from dynamin-independent macropinocytosis to clathrin-dependent endocytosis.
Collectively, our studies revealed that AM-R and endocytosis are essential for internalization of hA monomers and oligomers in pancreatic cells. hA uptake was both time and concentration dependent, both factors dictating the mechanism of peptide’s entry into these cells. Although minor fraction of extracellular hA was able to translocate by a non-endocytotic mechanism, in our studies the majority of the monomers and oligomers entered β-cells via bulk fluid uptake-macropinocytosis. The significant increase in hA toxicity in macropinocytosis-impaired cells (Trikha and Jeremic 2013) suggests a cyto-protective mechanism operating in pancreatic cells. Thus, pharmacological approaches that activate macropinocytosis such as ligand-induced dimerization of receptor tyrosine kinases may be beneficial against extracellular hA accumulation and oligomerization in the pancreas as well as hA-induced β-cell toxicity. These pharmacological interventions may delay the onset and/or halt progression of T2DM, which remains to be confirmed.
9. Role of endocytotic proteins and endocytosis in turnover and toxicity of other amyloid proteins
Alzheimer’s disease (AD) and Parkinson disease (PD) are progressive neurodegenerative disorders characterized by the cognitive dysfunctions, accumulation of intracellular tau enriched neurofibrillary tangles, extracellular β-amyloid derived amyloid plaques (AD), and appearance of α-synuclein (α-Syn) derived perinuclear aggregates or Lewy bodies in PD. Several independent studies reported the involvement of various endocytotic pathways in uptake of the extracellular Aβ and α-Syn in various cell and tissue types (Goncalves et al. 2016; Volpicelli-Daley et al. 2014; Hansen et al. 2011). Over dozen of neuronal receptors, such as glutamate and acetylcholine receptors, integrins and soluble receptors like apolipoprotein E were implicated in the uptake of extracellular Aβ in neuronal cells (Lai and McLaurin 2010). Association of APP within clathrin-coated vesicles and accumulation of Aβ in the endocytotic compartments led researchers to investigate the role of dynamin and clathrin in APP processing and internalization. Dynamin is a large molecular weight GTPase and a crucial regulatory element of clathrin dependent and independent endocytosis. Studies showed that over-expression of dominant negative mutated form of dynamin (dyn I K44A), resulted in increased processing of APP via non-amyloidogenic pathways and decreased production of toxic β amyloid species (Aβ1-40) in HEK and mouse neuroblasmoma cells (Carey et al. 2005; Ehehalt et al. 2003). These results suggested the importance of dynamin in regulating Aβ toxicity. Further studies by Yu et al., (2010), demonstrated that oligomeric Aβ42 neuronal toxicity and intracellular levels remained unchanged following downregulation of clathrin’s expression and function, indicating the importance of clathrin-independent endocytotic pathways in Aβ turnover and toxicity, similar to hA (Trikha and Jeremic 2011, 2013; Yu et al. 2010). Analogous to uptake mechanisms of nontoxic Aβ monomers, the primary mechanism of extracellular α-Syn uptake is clathrin-mediated endocytosis (Ben Gedalya et al. 2009; Goncalves et al. 2016; Sung et al. 2001; Trikha and Jeremic 2011, 2013). This is in good agreement with hA monomer uptake studies showing macropinocytosis (within the first few hours of internalization) and clathrin-dependent endocytosis [at later stage (>12 hours)] as main internalization routes for hA in pancreatic β–cells (Trikha and Jeremic 2013). Further insights about the cellular uptake mechanism of different processing variants of Aβ came from the study by Wesen et al., (2017). Using flow cytometry and confocal microscopy in combination with pharmacological and genetic manipulation of various endocytotic pathways, the authors dissected the uptake mechanism of these two predominant variants of β amyloid monomers: Aβ1-40 and Aβ1-42 in cultured human neuroblastoma cells. Results showed constitutive uptake of both the monomeric variants in exclusively an endocytosis-dependent manner. However, authors did not find any reduction in the uptake of Aβ variants following perturbation of clathrin mediated and dynamin dependent endocytosis. Instead, there results showed reduced uptake of Aβ1-40 and Aβ1-42 following disruption of actin polymerization and inhibition of micropinocytosis, indicating a clathrin and dynamin independent but macropinocytosis dependent uptake of Aβ variants in the early phases of AD (Wesen et al. 2017). In contrast to these reports, studies by Kandimalla et al., (2009) demonstrated strikingly different uptake mechanisms of Aβ in neuronal vs endothelial cells. The authors showed that fluorescein labeled Aβ40 and Aβ42 primarily accumulates outside of the endosomal/lysosomal compartments of primary hippocampal neurons using energy independent, non-endocytotic pathways (Kandimalla et al. 2009).
10. The intracellular fate of hA following internalization
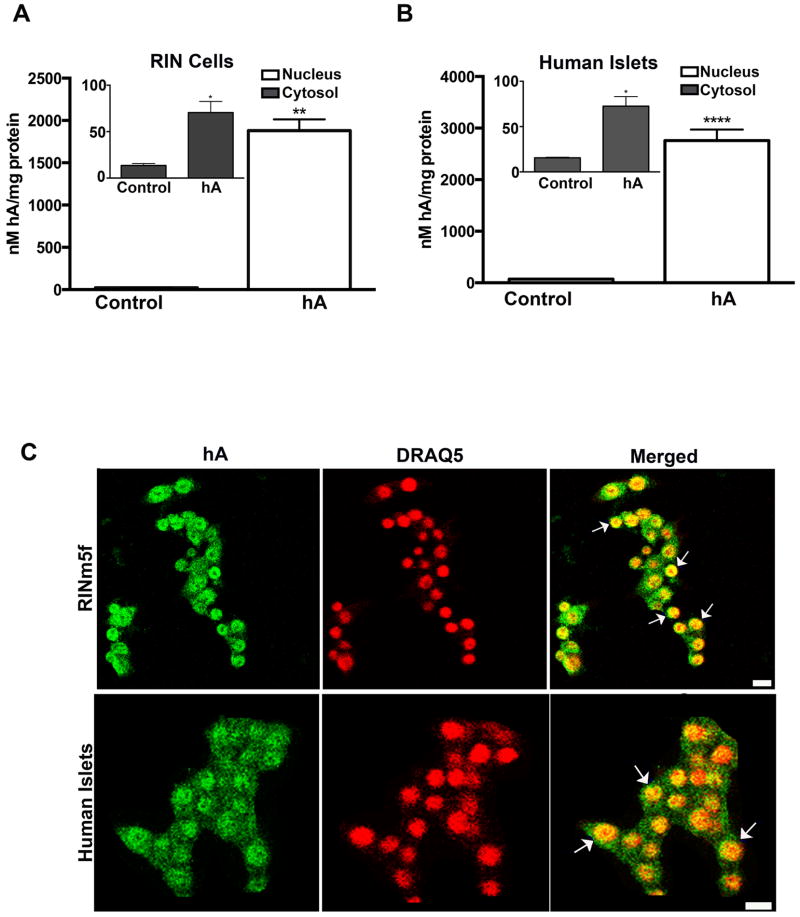
In sequel studies, we explored the fate of hA post internalization, including its intracellular trafficking routes. We treated RIN-m5F cells and human islets with cytotoxic hA concentrations for 24 hours, and intracellular hA redistribution was determined by ELISA (Figure 7A, B) and confocal immune confocal microscopy (Figure 7C). At this toxic (10–30 μM) concentrations and prolonged time exposures (0–24 hours), hA readily oligomerizes and aggregates (Figure 8A, B). By using this experimental set up we examined how cells defend themselves against high molecular weight toxic hA species. Following hA’s internalization into the cells, nuclear, cytosolic and organelle fractions were isolated using detergent-based approach (Singh et al. 2016). Fraction purity and redistribution of organelles and other cellular components within fractions were determined by western blot analysis (Singh et al. 2016). Interestingly, hA specific ELISA revealed a large twenty-fold increase in absolute hA levels in the nucleus and to a much smaller extent (three-fold increase) in the cytosol of hA -treated (for 24h) RIN-m5F cells as compared to control rA-producing cells (Figure 8A). Similar results were obtained in human islets (Figure 8B). Because in both these fractions the externally applied hA dwarfs the production of a native rA, this allowed us to study turnover of internalized hA in β-cells. hA accumulation in the large-organelle fraction of RIN-m5F cells was comparable to the cytosol, ~ 3% of the total internalized hA (Singh et al. 2016). This biochemical finding was reconfirmed by indirect immunocytochemistry (Figure 7C). Immunoconfocal microscopy, using hA specific polyclonal antibody (Trikha and Jeremic 2011), showed a high colocalization value (R>0.6) between nuclear marker DRAQ5 and hA, demonstrating its predominantly nuclear accumulation in RIN-m5F cells (Figure 7C, top panel). In contrast to the nucleus, immunoconfocal data showed relatively lower levels of hA in the cytosol, mostly perinuclear, in agreement with biochemical data (Figure 7C, top panel). To determine if hA trafficking is cell specific, we incubated partially dissociated human islet cells with hA for 24 hours and intracellular hA redistribution was again analyzed by immunoconfocal and biochemical approaches (Figure 7C, bottom panel). Similar to RIN-m5F cells, internalized hA predominantly localized in the nucleus of human islet cells (Figure 7C, bottom panel). Biochemical analysis confirmed that cytosolic and organelle enriched fractions together accumulated less than 10% of the total internalized hA in both cell types (Singh et al. 2016).
Figure 7. hA accumulates in cytosol and nucleus of pancreatic cells.
Cells were treated with hA for 24 hours and intracellular redistribution of hA in intact cells and cell fractions determined. (A) Analysis of hA accumulation in cytosolic and nuclear fractions revealed by ELISA in RIN-m5F cells. (B) Redistribution of internalized hA between the nucleus and cytosol examined by ELISA. Note accumulation of hA in the nucleus and to a lesser extent in cytosol following its uptake in RIN-m5F cells (A) and human islets (B). Significance established at * p<0.05,** p<0.01 and *** p<0.001, n=6, Student’s t-test. (C) Confocal microscopy analysis of hA localization in pancreatic cells. Nuclear marker DRAQ5 colocalizes with hA in RIN-m5F (top panel) and human islets (bottom panel) as indicated by arrows in merged images. Bars, 10μm.
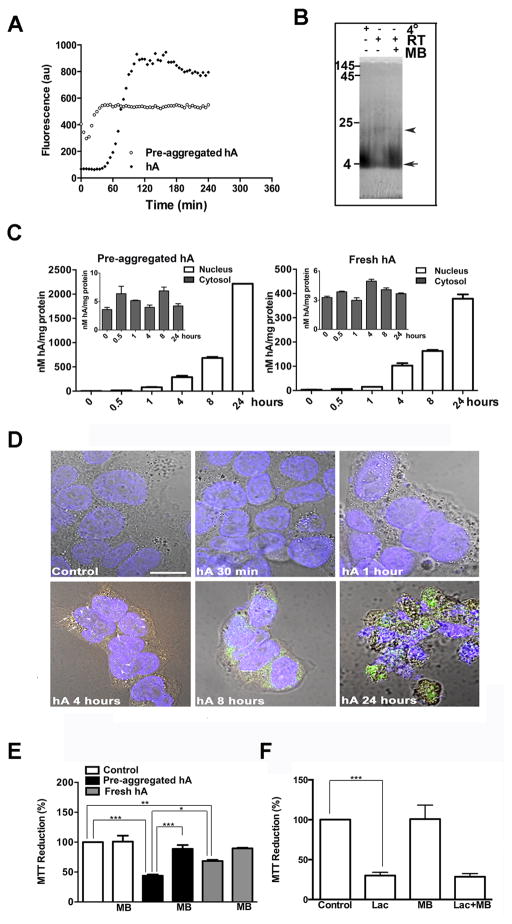
Figure 8. Dynamics of intracellular accumulation and aggregation of hA.
(A) Time course and extent of aggregation of hA (30μM) at RT, prepared by two distinct methods is shown. Note immediate increase in ThT fluorescence, reflecting hA fibrilization in preaggregated sample (circles). In contrast, freshly prepared equimolar samples lacking aggregates (black diamonds) show delayed hA aggregation (lag phase > 1 hour). (B) Characterization of hA oligomeric state by native PAGE. Freshly prepared hA was incubated at +4°C, room temperature (RT) or in the presence of amyloid-inhibitor methylene blue (MB, 500 μM) for 4 hours. Arrow denotes monomeric hA, whereas arrowhead denotes oligomers. (C) Dynamics of hA internalization in RIN-m5F cells examined by ELISA. (D) Confocal microscopy was used to assessed kinetics and location of hA in these cells. (E) MTT cellular stress assay was used to evaluate toxicity of hA in the absence or presence of oligomeric inhibitor methylene blue (MB). (F) Effect of protein stress inducer Lactacystin (Lac, 10μM) on mitochondrial activity in the presence or absence of MB is shown. Significance was established at * p<0.05, ** p<0.01 and *** p<0.001, n=6, ANOVA followed by Tukey’s post hoc comparison test.
Next, we investigated the dynamics of hA internalization (Figure 8C, D) of freshly prepared and pre-aggregated hA featuring high oligomeric and fibril content (Figure 8A, B). Cells were treated with two distinct hA preparations (having small or large oligomeric/fibrilar content) for increasing periods of time (0 to 24h), cell fractions prepared as described previously (Singh et al. 2016), and hA content in both nuclear and cytosolic fractions analyzed by ELISA. Biochemical analysis demonstrated that irrespective of the method of hA preparation, hA progressively translocates in the nucleus over a period of 24 hours, with a lag phase of ~4 hours (Figure 8C, D). In contrast, hA content in the cytosolic fractions spiked after 30 minutes without further significant accumulation after 4 hours (Figure 8C, inset). In both preparations, the cytosolic influx of hA preceded its accumulation in the nucleus. Thus, translocation of hA into the nucleus (Figures 7, 8D), and to a lesser extent into mitochondria accounts, at least in part, for the small hA accumulation in the cytosol. The time course confocal analysis of hA internalization (Figure 8D) confirmed ELISA experiments by showing visible hA accumulation in the perinuclear (arrows) and nuclear (arrowheads) regions of the cell as early as 4h following the peptide addition, and thereafter. Prolonged and excessive intracellular accumulation of hA (24h, Figure 8D) induced nuclear condensation and cell shrinkage indicating apoptosis, as previously reported (Trikha and Jeremic 2011). If aggregation state of hA matters for its enhanced accumulation and/or toxicity, we would expect method one (featuring aggregating species) to be more cytotoxic than fresh samples initially lacking larger aggregates (Figure 8A, B). 24 hours after hA treatment, a five-fold increase in nuclear but not cytosolic hA content is seen when hA is added in its pre-aggregated form as compared to fresh hA (Figure 8C), consistent with the ability of amyloid oligomers to incorporate into and penetrate membranes (Friedman et al. 2009; Gurlo et al. 2010; Jang et al. 2013; Tofoleanu and Buchete 2012). In accordance with the aggregation (oligomeric) hypothesis (Haataja et al. 2008), MTT metabolic stress assay revealed that pre-aggregated hA was significantly more stressful to cells as compared to fresh hA preparation (Figure 8E), linking nuclear accumulation of hA and its toxicity. Amyloid oligomeric inhibitor and antioxidant methylene blue (MB) prevented hA oligomerization (Figure 8B) and specifically reversed hA’s toxicity (Figure 8E) but not toxicity due protein stress (Figure 8F) in RINm5F cells, further demonstrating the detrimental effect of oligomers, and redox sensitive mechanism of hA -evoked β-cell death.
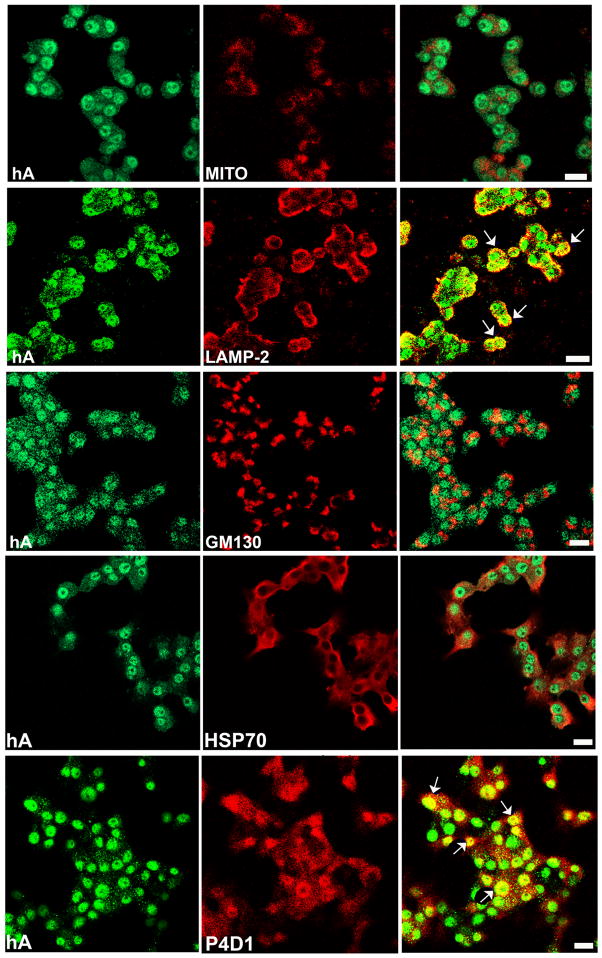
Both biochemical (Singh et al. 2016) and microscopy (Figure 9) data are consistent with a rather minor accumulation of internalized hA in the mitochondria (hA/MITO) in our model cells indicating largely indirect effects of hA on mitochondrial dysfunction. We also investigated if lytic cytoplasmic compartments such as lysosomes accumulate internalized (cytosolic) hA. A partial (R~0.5) and mostly perinuclear colocalization (arrows) of hA and lysosomes was observed at 24 hours (Figure 9, hA/LAMP2). This result is in agreement with lysosomal accumulation of endogenous hA (Rivera et al. 2011), suggesting a similar but not identical recycling mechanism for extracellular and intracellular hA. Under certain conditions, endocytotic vesicles can merge with biosynthetic compartments like Golgi complex, thus routing internalized cargos and recycling vesicles away from lytic compartments, lysosomes and autophagosomes (Proux-Gillardeaux et al. 2005). To explore this possibility, we examined the extent to which internalized hA co-localizes with Golgi complex by confocal microscopy. Pancreatic cells were exposed to unlabeled hA, and then co-stained with Golgi marker, GM130 and anti- hA antibody. However, very low co-localization values between GM130 and hA were detected in both RIN-m5F cells (R<0.3, Figure 9) and human islets (data not shown), refuting a major involvement of Golgi complex in the turnover of internalized hA in these two cell types.
Figure 9. Confocal microscopy analysis of hA trafficking in pancreatic cells.
hA was incubated with cells for 24 hours, cells fixed and its trafficking and association with cellular organelles, cytosolic and nuclear proteins was analyzed by indirect immunocytochemistry. Association of hA or lack of it with lysosomes (LAMP1), mitochondria (Mitotracker-MITO), Golgi (GM130), heat shock protein (HSP70) and ubiquitin (PD41) is shown. Representative cells in which hA accumulates in LAMP2 positive perinuclear compartments and interacts with ubiquitin in cytosol and nucleus are indicated by arrows.
11. hA interacts with 26S proteasome complex in pancreatic cells
We extended our studies by investigating whether internalized hA, in addition to mitochondria and lysosomes, interacts with aggresomes in cultured pancreatic RIN-m5F and human islet cells. Aggresomes, a protein complex consisting of proteasome, ubiquitin, heat shock proteins (HSP) and γ-tubulin, serve as alternative degrading/sequestering center to lysosomes for many misfolded and potentially toxic cytosolic proteins (Blair et al. 2014; Bonanomi et al. 2014; Junn et al. 2002). To determine if the cytosolic pool of internalized hA is targeted to aggresomes for degradation, immuno-confocal approach was again employed. A low co-localization was observed between hA and HSP70 in RIN-m5F cells (R<0.3, Figure 9) and human islets (data not shown), while a high co-localization of the peptide was detected upon co-staining with antibody against ubiquitin (P4D1) in RIN-m5F β-cells (R>0.6, Figure 9) and human islets (data not shown). Aggresomes are usually formed around the microtubule organizing center (MTOC) in the cells, which requires the presence of polymerized γ-tubulin (Shimohata et al. 2002). Similar to HSP70 very low co-localization values (R<0.3) between hA monomers and γ-tubulin was obtained (Singh et al, 2016) suggesting that internalized hA is not associated with the MTOC.
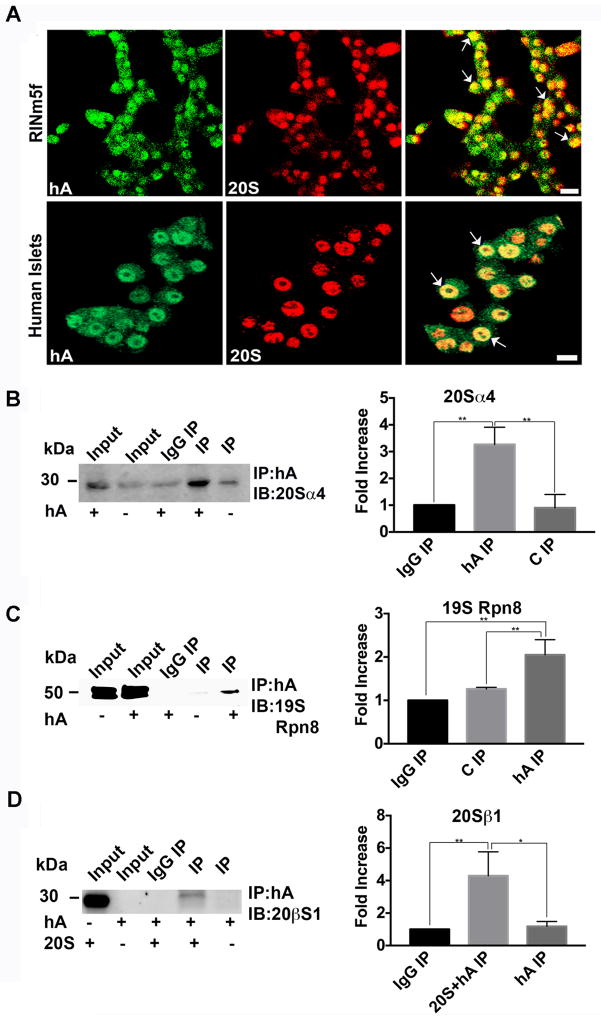
In contrast to HSP70, the internalized hA co-localized with the α-4 subunit of the 20S proteasome complex in the nucleus (denoted by arrows) and to a lesser extent, in the cytosol of RIN-m5F β-cells and human islets (R>0.6, Figure 10A), suggesting their possible interaction in these cells. This idea was further tested in immunoprecipitation studies in which hA served as bait (Singh et al. 2016). In agreement with confocal microscopy studies (Figure 10A), 20S α4 proteasome subunit was detected in the immunoprecipitated hA complex isolated from nuclear fraction (Figure 10B). Thus, hA forms a tight complex with 20S proteasome catalytic subunit in the nucleus of hA treated RIN-m5F cells (Figure 10A, B). In addition, Rpn8 an important regulatory subunit of the proteasome 19S complex co-precipitated with hA (Figure 10C) and 20Sα4 subunit (Figure 10B) from nuclear extracts. Presence of both regulatory (lid) and catalytic (core) subunits in hA -immunoprecipitated samples (Figure 10B, C) suggests formation of hA/26S proteasome complex in pancreatic β-cells.
Figure 10. hA interacts with the catalytic and regulatory components of the 26S proteasome complex.
hA was incubated with in RIN-m5F cells and human islets for 24 hours and its interaction with 20s proteasome was assessed by confocal microscopy and immunoprecipitation. (A) hA colocalizes (arrows) with 20S proteasome in the nucleus of RIN-m5F cells (top panel) and human islets (bottom panel) as confirmed by indirect immunocytochemistry. Bars,10 μm. (B–C) hA interacts with the catalytic and lid components of 26S proteasome complex in RINm5F cells. hA was pulled down using hA specific antibody from the nuclear fraction (B) or whole cell extract (C) of hA -treated RIN-m5F cells and immunoblotted with antibodies against 20Sα4 subunit (B) and 19SRpn8 (C) subunits of the 26S proteasome complex. (D) hA interacts with 20Sβ1 in vitro to form a heterocomplex. Synthetic hA and purified 20S complex were co-incubated and immunoprecipitated using anti- hA antibody as bait. Anti-20Sβ1 antibody was used to confirm pull down of hA/20S immunocomplex. Significance was established at * p<0.05,** p<0.01 n=3, ANOVA followed by Tukey’s post hoc comparison test (B–D, histograms).
To examine the role of aggregation and ubiquitination in hA -proteasome interactions, in vitro IP studies using synthetic hA and 20S purified complex were performed at low temperature (+4°C), a condition that efficiently abolishes hA oligomerization (Figure 8B) and aggregation (Singh et al. 2016). Our in vitro pull-down studies revealed that hA co-imunoprecipitated with the 20S catalytic core subunit, 20S β1 (Figure 10D). This finding suggests that ubiquitination or aggregation of hA is not essential for its interaction with 20S proteasome. However, this result does not exclude the possibility that a portion of the internalized hA interacts with proteasomes via ubiquitin dependent step. In accordance with a recent study showing ubiquitination of internally expressed hA (Rivera et al. 2014), our IP studies reveal physical interactions between hA and 19S rpn8 subunit in β-cells, suggesting that ubiquitination of hA may indeed play a role in their mutual interactions. Collectively, these results suggest that hA interacts with the catalytic and regulatory subunits of the proteasome complex in the pancreatic cells, and that aggresomes do not serve as major hubs for turnover of internalized hA in pancreatic cells, analogous to aggresome-independent β-amyloid clearance in neuronal cells (Buckig et al. 2002).
12. The proteasome complex regulates hA turnover and toxicity in pancreatic cells
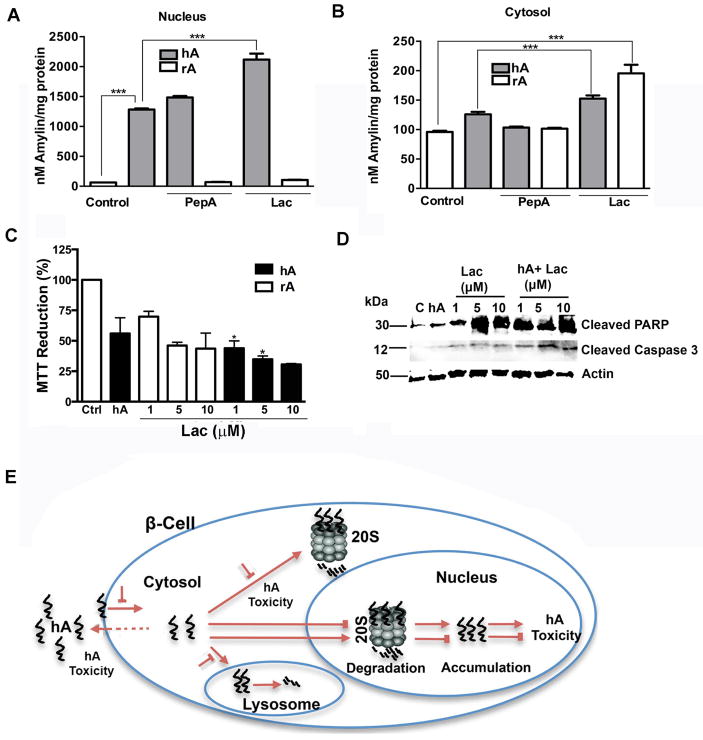
Previous studies showed that proteasomes and lysosomes serve as degradation centers for many amyloid proteins (Rubinsztein 2006; Webb et al. 2003; David et al. 2002), including possibly hA. This hypothetical but plausible scenario is suggested from our confocal and immunoprecipitation studies (Figure 10). Hence, we reasoned that if lysosomes and proteasomes are involved in hA degradation, then inhibition of their proteolytic function should enhance hA accumulation in cells. To test this idea, cells were incubated with hA for 24 hours, in the absence (control) or presence of selective lysosomal inhibitor, pepstatin A (PepA) or proteasomal inhibitor lactacystin (Lac) and nuclear and cytosolic hA accumulation in response to these treatments was determined by hA ELISA (Figure 11A, B). Addition of hA to cells induced a marked accumulation of hA in the nucleus after 24 hours (Figure 11A) and to a much smaller extent in the cytosol (Figure 11B) as compared to control cells. In agreement with our proteasome hypothesis, we observed a substantial (~70%) increase in nuclear (Figure 11A), and smaller (~20%) but significant cytosolic accumulation of the peptide in response to Lac (Figure 11B). In contrast, we did not observe any change in hA content in response to lysosome inhibitor PepA in either compartment (Figure 11A, B). ELISA also revealed that RIN-m5F cells express endogenous rA in small quantities (Figure 11B, control) consistent with previous reports (Clark et al. 1997). We explored if the proteasome also regulates homeostatic balance of rA. As demonstrated for hA, Lac but not PepA significantly augmented endogenous rA levels (Figure 11B). This study reestablished the important and more general role of proteasomes in the clearance of different hA isoforms. Together, these findings suggest that 20/26S proteasome complex in the nucleus, and to a lesser extent cytosolic proteasome complex function as major hubs for turnover (degradation) of internalized hA and endogenous rA in pancreatic cells, respectively. Interestingly, the major contribution of lysosomes to the degradation of overexpressed pre-pro hA was previously demonstrated (Rivera et al. 2011), suggesting that lysosomes could serve as alternative proteolytic centers to proteasomes for hA degradation in pancreatic cells.
Figure 11. Inhibition of proteasome proteolytic function accelerates nuclear accumulation and toxicity of hA in pancreatic cells.
(A–B) hA was incubated with cells in the presence or absence of lactacystin for 24 hours. The extent of hA accumulation in the presence and absence of lactacystin (1uM) or pepstatin A (1μM) in (A) nucleus and (B) cytosol is revealed by ELISA. Significance was established at * p<0.05,** p<0.01 and *** p<0.001, n=3–6, ANOVA followed by Tukey’s post hoc comparison test. (C–D) Analysis of hA cytotoxicity in response to proteasomal inhibition. Dose dependent effect of lactacystin (1–10μM) on hA toxicity was analyzed by (C) MTT stress assay and (D) PARP/Caspase 3 cleavage assay. Significance was established at * p<0.05, n=3–6, ANOVA followed by Tukey’s post hoc test, hA vs Lac treatments. (E) A schematic representation of endocytotic-regulated hA internalization followed by proteasome-mediated degradation and detoxicification of hA in pancreatic cells is depicted. Conversely, inhibition of hA internalization and proteasome functions can lead to excessive accumulation of hA on the plasma membrane and intracellularly leading to its aggregation and toxicity.
To further infer about the pathophysiological significance of the proteasome-mediated hA degradation in cells, we assessed the toxic potential of hA (Figure 11C, D) under conditions of both mild and severe protein stress induced by1 μM or 10 μM Lac respectively (Singh et al. 2016). The lower (1 μM) Lac concentration was used to avoid a direct cytotoxic effect of Lac on cell viability as seen with higher (5 and 10 μM) Lac (Singh et al. 2016). Lac (1μM) potentiated hA toxicity as indicated by a significant decrease in mitochondrial metabolic activity (Figure 11C) and concomitant increase in levels of cleaved PARP in the nucleus, a known stress marker (Soldani et al. 2001) as compared to hA alone (Figure 11D). Western blot analysis confirmed an increase in hA -evoked PARP and caspase-3 cleavage in cells concurrently exposed to 1μM Lac, as compared to Lac-lacking cells (Figure 11D). A further potentiation by Lac was also observed at higher 5 and 10 μM inhibitor concentrations (Figure 11C, D). In line with this gradual stimulatory effect on hA toxicity, Lac also enhanced intracellular hA accumulation in a dose-dependent manner (Singh et al. 2016).
13. Role of proteasomes in turnover and toxicity of other amyloid proteins
Cellular protein homeostasis is largely dependent on tight regulation between production and elimination of proteins. Elimination of unwanted or damaged proteins encompasses several cellular processes like proteolytic degradation, secretion, passive and active transport, intracellular clustering or sequestration, and the aggregation and deposition of proteins into insoluble aggregates (Saido and Leissring 2012). A growing body of evidence indicates that the pathological overproduction and defective clearance of Aβ and α-Syn in the brain triggers various forms of neurodegeneration (Saido and Leissring 2012; Lopes da Fonseca et al. 2015). The important link in this pathological process is the main intracellular proteolytic complex, the proteasome. Studies using nonfibrillar, oligomeric and fibrillar forms of Aβ, showed significant reduction in the chymotrypsin like activity of proteasome in SH-SY5Y cells (Cecarini et al. 2008). Detail structure function study revealed that the most potent inhibitor of proteolytic function of proteasome is Aβ40 variant (Gregori et al. 1995). Interestingly, despite the ability of Aβ40 to impair proteasome function, it is not a direct substrate of UPS (Gregori et al. 1995). Instead, studies showed that proteasome regulates intracellular concentration of APP processing proteins: presenilins 1 and 2. Thus pharmacological inhibition of proteasome function increases the level of Aβ by increasing the processing of APP (Ciechanover 1998; Cheng et al. 2011). Similar to Aβ, numerous independent studies using purified proteins and cell culture systems demonstrated that mutant α–Syn, particularly in soluble oligomeric and small aggregated forms, downregulate the proteolytic function of proteasome (Petrucelli et al. 2002; Snyder et al. 2003; Stefanis et al. 2001; Xilouri et al. 2013). The exact mechanism of α–Syn mediated impairment of proteasomal function is still unclear. Some studies point to direct interactions between α–Syn oligomers and its bulky aggregates with the active sites of proteasomal subunits, thereby preventing binding and degradation of proteasome’s clients (Ghee et al. 2000; Lindersson et al. 2004; Snyder et al. 2003; Xilouri et al. 2013). In addition to proteasome, the other members of ubiquitin proteasome system (UPS) such the ubiquitin E3 ligase and UCHL1 play prominent roles in the Aβ and α–Syn aggregation and neurodegeneration (Burns et al. 2009; Kitada et al. 1998; Leroy et al. 1998; Maraganore et al. 2004; Hong et al. 2014; Tramutola et al. 2016; M. Zhang et al. 2012).
In summary, recent studies unraveled a novel link between endocytotic-mediated hA uptake, proteasome-mediated hA degradation and proteasome-regulated hA toxicity in pancreatic β-cells, which may protect these cells from harmful extracellular and intracellular hA oligomers and aggregates (Figure 11E). Pharmacological approaches that stimulate proteasome and/or lysosome proteolytic function(s) may be cyto-protective against toxic hA aggregates and hA-induced β cell mass loss observed in T2DM. Conversely, agents that inhibit proteasome function will likely accelerate and enhance disease severity by stimulating extracellular and/or intracellular accumulation of toxic hA aggregates in the pancreas (Figure 11E). Future clinical and animal studies will be needed to validate the efficacy and safety of pharmacological modulators of endocytosis and proteasome function against hA toxicity and islet amyloid-induced diabetes.
Acknowledgments
This work was supported by the NIH grant RO1DK091845 and the ICR Basic Science Islet Distribution Program (to A.J.).
Abbreviations
- AFM
atomic force microscopy
- CTX
cholera toxin
- CD
circular dichroism
- EM
electron microscopy
- hA
human amylin
- HFIP
hexafluoride isopropanol
- HSP
heat shock protein
- Lac
lactacystin
- MB
methylene blue
- MT
mitotracker
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide
- PepA
pepstatin A
- rA
rodent amylin
- ThT
thioflavin-T
- T2DM
type two diabetes mellitus
- Trf
transferrin
- PM
plasma membrane
- Ub
ubiquitin
References
- Abedini A, Raleigh DP. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44(49):16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]
- Abedini A, Schmidt AM. Mechanisms of islet amyloidosis toxicity in type 2 diabetes. FEBS Lett. 2013;587(8):1119–1127. doi: 10.1016/j.febslet.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11(10):3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancsin JB. Amyloidogenesis: historical and modern observations point to heparan sulfate proteoglycans as a major culprit. Amyloid. 2003;10(2):67–79. doi: 10.3109/13506120309041728. [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135(5):1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RJ, Walker CS, Ferner AH, Loomes KM, Prijic G, Halim A, et al. Pharmacological characterization of rat amylin receptors: implications for the identification of amylin receptor subtypes. Br J Pharmacol. 2012;166(1):151–167. doi: 10.1111/j.1476-5381.2011.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Gedalya T, Loeb V, Israeli E, Altschuler Y, Selkoe DJ, Sharon R. Alpha-synuclein and polyunsaturated fatty acids promote clathrin-mediated endocytosis and synaptic vesicle recycling. Traffic. 2009;10(2):218–234. doi: 10.1111/j.1600-0854.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair LJ, Sabbagh JJ, Dickey CA. Targeting Hsp90 and its co-chaperones to treat Alzheimer’s disease. Expert Opin Ther Targets. 2014;18(10):1219–1232. doi: 10.1517/14728222.2014.943185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanomi M, Mazzucchelli S, D’Urzo A, Nardini M, Konarev PV, Invernizzi G, et al. Interactions of ataxin-3 with its molecular partners in the protein machinery that sorts protein aggregates to the aggresome. Int J Biochem Cell Biol. 2014;51:58–64. doi: 10.1016/j.biocel.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Brender JR, Hartman K, Reid KR, Kennedy RT, Ramamoorthy A. A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry. 2008a;47(48):12680–12688. doi: 10.1021/bi801427c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brender JR, Lee EL, Cavitt MA, Gafni A, Steel DG, Ramamoorthy A. Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide. J Am Chem Soc. 2008b;130(20):6424–6429. doi: 10.1021/ja710484d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckig A, Tikkanen R, Herzog V, Schmitz A. Cytosolic and nuclear aggregation of the amyloid beta-peptide following its expression in the endoplasmic reticulum. Histochem Cell Biol. 2002;118(5):353–360. doi: 10.1007/s00418-002-0459-2. [DOI] [PubMed] [Google Scholar]
- Burns MP, Zhang L, Rebeck GW, Querfurth HW, Moussa CE. Parkin promotes intracellular Abeta1-42 clearance. Hum Mol Genet. 2009;18(17):3206–3216. doi: 10.1093/hmg/ddp258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Abedini A, Wang H, Tu LH, Zhang X, Schmidt AM, et al. Islet amyloid polypeptide toxicity and membrane interactions. Proc Natl Acad Sci U S A. 2013a;110(48):19279–19284. doi: 10.1073/pnas.1305517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Marek P, Noor H, Patsalo V, Tu LH, Wang H, et al. Islet amyloid: from fundamental biophysics to mechanisms of cytotoxicity. FEBS Lett. 2013b;587(8):1106–1118. doi: 10.1016/j.febslet.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Tu LH, Abedini A, Levsh O, Akter R, Patsalo V, et al. Sensitivity of amyloid formation by human islet amyloid polypeptide to mutations at residue 20. J Mol Biol. 2012;421(2–3):282–295. doi: 10.1016/j.jmb.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Balcz BA, Lopez-Coviella I, Slack BE. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid beta protein. BMC Cell Biol. 2005;6:30. doi: 10.1186/1471-2121-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecarini V, Bonfili L, Amici M, Angeletti M, Keller JN, Eleuteri AM. Amyloid peptides in different assembly states and related effects on isolated and cellular proteasomes. Brain Res. 2008;1209:8–18. doi: 10.1016/j.brainres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Cheng B, Maffi SK, Martinez AA, Acosta YP, Morales LD, Roberts JL. Insulin-like growth factor-I mediates neuroprotection in proteasome inhibition-induced cytotoxicity in SH-SY5Y cells. Mol Cell Neurosci. 2011;47(3):181–190. doi: 10.1016/j.mcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- Chiu CC, Singh S, de Pablo JJ. Effect of proline mutations on the monomer conformations of amylin. Biophys J. 2013;105(5):1227–1235. doi: 10.1016/j.bpj.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WJ, Jena BP, Jeremic AM. Nano-scale imaging and dynamics of amylin-membrane interactions and its implication in type II diabetes mellitus. Methods Cell Biol. 2008;90:267–286. doi: 10.1016/S0091-679X(08)00813-3. [DOI] [PubMed] [Google Scholar]
- Cho WJ, Trikha S, Jeremic AM. Cholesterol regulates assembly of human islet amyloid polypeptide on model membranes. J Mol Biol. 2009;393(3):765–775. doi: 10.1016/j.jmb.2009.08.055. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56(1):235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17(24):7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47(2):157–169. doi: 10.1007/s00125-003-1304-4. [DOI] [PubMed] [Google Scholar]
- Clark SA, Quaade C, Constandy H, Hansen P, Halban P, Ferber S, et al. Novel insulinoma cell lines produced by iterative engineering of GLUT2, glucokinase, and human insulin expression. Diabetes. 1997;46(6):958–967. doi: 10.2337/diab.46.6.958. [DOI] [PubMed] [Google Scholar]
- Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KB. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes S, Gurlo T, Rivera JF, Butler PC. UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in beta-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy. Autophagy. 2014;10(6):1004–1014. doi: 10.4161/auto.28478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Donovan M, Cotter TG. Regulation and measurement of oxidative stress in apoptosis. J Immunol Methods. 2002;265(1–2):49–72. doi: 10.1016/s0022-1759(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Damke H, Binns DD, Ueda H, Schmid SL, Baba T. Dynamin GTPase domain mutants block endocytic vesicle formation at morphologically distinct stages. Mol Biol Cell. 2001;12(9):2578–2589. doi: 10.1091/mbc.12.9.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG. Proteasomal degradation of tau protein. J Neurochem. 2002;83(1):176–185. doi: 10.1046/j.1471-4159.2002.01137.x. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160(1):113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel MF, Khemtemourian L, Kleijer CC, Meeldijk HJ, Jacobs J, Verkleij AJ, et al. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc Natl Acad Sci U S A. 2008;105(16):6033–6038. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R, Pellarin R, Caflisch A. Amyloid aggregation on lipid bilayers and its impact on membrane permeability. J Mol Biol. 2009;387(2):407–415. doi: 10.1016/j.jmb.2008.12.036. [DOI] [PubMed] [Google Scholar]
- Gazit E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16(1):77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- German MS, Moss LG, Wang J, Rutter WJ. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical beta-cell nuclear complexes. Mol Cell Biol. 1992;12(4):1777–1788. doi: 10.1128/mcb.12.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghee M, Fournier A, Mallet J. Rat alpha-synuclein interacts with Tat binding protein 1, a component of the 26S proteasomal complex. J Neurochem. 2000;75(5):2221–2224. doi: 10.1046/j.1471-4159.2000.0752221.x. [DOI] [PubMed] [Google Scholar]
- Gold S, Monaghan P, Mertens P, Jackson T. A clathrin independent macropinocytosis-like entry mechanism used by bluetongue virus-1 during infection of BHK cells. PLoS One. 2010;5(6):e11360. doi: 10.1371/journal.pone.0011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbury C, Kistler J, Aebi U, Arvinte T, Cooper GJ. Watching amyloid fibrils grow by time-lapse atomic force microscopy. J Mol Biol. 1999;285(1):33–39. doi: 10.1006/jmbi.1998.2299. [DOI] [PubMed] [Google Scholar]
- Goncalves SA, Macedo D, Raquel H, Simoes PD, Giorgini F, Ramalho JS, et al. shRNA-Based Screen Identifies Endocytic Recycling Pathway Components That Act as Genetic Modifiers of Alpha-Synuclein Aggregation, Secretion and Toxicity. PLoS Genet. 2016;12(4):e1005995. doi: 10.1371/journal.pgen.1005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzotto A, Suwalsky M, Zatta P. Physiological cholesterol concentration is a neuroprotective factor against beta-amyloid and beta-amyloid-metal complexes toxicity. J Inorg Biochem. 2011;105(8):1066–1072. doi: 10.1016/j.jinorgbio.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Green JD, Goldsbury C, Kistler J, Cooper GJ, Aebi U. Human amylin oligomer growth and fibril elongation define two distinct phases in amyloid formation. J Biol Chem. 2004;279(13):12206–12212. doi: 10.1074/jbc.M312452200. [DOI] [PubMed] [Google Scholar]
- Gregori L, Fuchs C, Figueiredo-Pereira ME, Van Nostrand WE, Goldgaber D. Amyloid beta-protein inhibits ubiquitin-dependent protein degradation in vitro. J Biol Chem. 1995;270(34):19702–19708. doi: 10.1074/jbc.270.34.19702. [DOI] [PubMed] [Google Scholar]
- Gurlo T, Ryazantsev S, Huang CJ, Yeh MW, Reber HA, Hines OJ, et al. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176(2):861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev. 2008;29(3):303–316. doi: 10.1210/er.2007-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Liu J, Zhu G, Zhuang Y, Suo H, Wang P, et al. Parkin overexpression ameliorates hippocampal long-term potentiation and beta-amyloid load in an Alzheimer’s disease mouse model. Hum Mol Genet. 2014;23(4):1056–1072. doi: 10.1093/hmg/ddt501. [DOI] [PubMed] [Google Scholar]
- Hoppener JW, Ahren B, Lips CJ. Islet amyloid and type 2 diabetes mellitus. N Engl J Med. 2000;343(6):411–419. doi: 10.1056/NEJM200008103430607. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006;20(2):259–268. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, Gurlo T, Haataja L, Costes S, Daval M, Ryazantsev S, et al. Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J Biol Chem. 2011;285(1):339–348. doi: 10.1074/jbc.M109.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Connelly L, Arce FT, Ramachandran S, Kagan BL, Lal R, et al. Mechanisms for the Insertion of Toxic, Fibril-like beta-Amyloid Oligomers into the Membrane. J Chem Theory Comput. 2013;9(1):822–833. doi: 10.1021/ct300916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48(3):491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- Jha S, Sellin D, Seidel R, Winter R. Amyloidogenic propensities and conformational properties of ProIAPP and IAPP in the presence of lipid bilayer membranes. J Mol Biol. 2009;389(5):907–920. doi: 10.1016/j.jmb.2009.04.077. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Li Z, Westaway D, Yang J, Jassar S, MacTavish D. Actions of beta-amyloid protein on human neurons are expressed through the amylin receptor. Am J Pathol. 2011;178(1):140–149. doi: 10.1016/j.ajpath.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas JH, MacTavish D. Antagonist of the amylin receptor blocks beta-amyloid toxicity in rat cholinergic basal forebrain neurons. J Neurosci. 2004;24(24):5579–5584. doi: 10.1523/JNEUROSCI.1051-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhamandas JH, Mactavish D. beta-Amyloid protein (Abeta) and human amylin regulation of apoptotic genes occurs through the amylin receptor. Apoptosis. 2012;17(1):37–47. doi: 10.1007/s10495-011-0656-3. [DOI] [PubMed] [Google Scholar]
- Junn E, Lee SS, Suhr UT, Mouradian MM. Parkin accumulation in aggresomes due to proteasome impairment. J Biol Chem. 2002;277(49):47870–47877. doi: 10.1074/jbc.M203159200. [DOI] [PubMed] [Google Scholar]
- Kandimalla KK, Scott OG, Fulzele S, Davidson MW, Poduslo JF. Mechanism of neuronal versus endothelial cell uptake of Alzheimer’s disease amyloid beta protein. PLoS One. 2009;4(2):e4627. doi: 10.1371/journal.pone.0004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, Bernhagen J, Greenfield N, Sweimeh K, Brunner H, Voelter W, et al. Conformational transitions of islet amyloid polypeptide (IAPP) in amyloid formation in vitro. J Mol Biol. 1999;287(4):781–796. doi: 10.1006/jmbi.1999.2646. [DOI] [PubMed] [Google Scholar]
- Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300(5618):486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- Khemtemourian L, Killian JA, Hoppener JW, Engel MF. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in beta-cell death in type 2 diabetes mellitus. Exp Diabetes Res. 2008;2008:421287. doi: 10.1155/2008/421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. Thiol reducing compounds prevent human amylin-evoked cytotoxicity. FEBS J. 2005;272(19):4949–4959. doi: 10.1111/j.1742-4658.2005.04903.x. [DOI] [PubMed] [Google Scholar]
- Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. Febs J. 2006;273(15):3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- Koo BW, Miranker AD. Contribution of the intrinsic disulfide to the assembly mechanism of islet amyloid. Protein Sci. 2005;14(1):231–239. doi: 10.1110/ps.041051205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampert M, Bernhagen J, Schmucker J, Horn A, Schmauder A, Brunner H, et al. Amyloidogenicity of recombinant human pro-islet amyloid polypeptide (ProIAPP) Chem Biol. 2000;7(11):855–871. doi: 10.1016/s1074-5521(00)00034-x. [DOI] [PubMed] [Google Scholar]
- Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, et al. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280(3):2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- Lai AY, McLaurin J. Mechanisms of amyloid-Beta Peptide uptake by neurons: the role of lipid rafts and lipid raft-associated proteins. Int J Alzheimers Dis. 2011:548380. doi: 10.4061/2011/548380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, McLaurin J. Mechanisms of amyloid-Beta Peptide uptake by neurons: the role of lipid rafts and lipid raft-associated proteins. Int J Alzheimers Dis. 2010;2011:548380. doi: 10.4061/2011/548380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau TL, Gehman JD, Wade JD, Perez K, Masters CL, Barnham KJ, et al. Membrane interactions and the effect of metal ions of the amyloidogenic fragment Abeta(25-35) in comparison to Abeta(1-42) Biochim Biophys Acta. 2007;1768(10):2400–2408. doi: 10.1016/j.bbamem.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Leroy E, Boyer R, Auburger G, Leube B, Ulm G, Mezey E, et al. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395(6701):451–452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- Lim YA, Rhein V, Baysang G, Meier F, Poljak A, Raftery MJ, et al. Abeta and human amylin share a common toxicity pathway via mitochondrial dysfunction. Proteomics. 2010;10(8):1621–1633. doi: 10.1002/pmic.200900651. [DOI] [PubMed] [Google Scholar]
- Lindersson E, Beedholm R, Hojrup P, Moos T, Gai W, Hendil KB, et al. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279(13):12924–12934. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90(12):1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K. Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. J Cell Biol. 1998;140(3):553–563. doi: 10.1083/jcb.140.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes da Fonseca T, Villar-Pique A, Outeiro TF. The Interplay between Alpha-Synuclein Clearance and Spreading. Biomolecules. 2015;5(2):435–471. doi: 10.3390/biom5020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89(4):465–471. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Lutz TA. The role of amylin in the control of energy homeostasis. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1475–1484. doi: 10.1152/ajpregu.00703.2009. [DOI] [PubMed] [Google Scholar]
- MacArthur DL, de Koning EJ, Verbeek JS, Morris JF, Clark A. Amyloid fibril formation is progressive and correlates with beta-cell secretion in transgenic mouse isolated islets. Diabetologia. 1999;42(10):1219–1227. doi: 10.1007/s001250051295. [DOI] [PubMed] [Google Scholar]
- Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29(13):4252–4262. doi: 10.1523/JNEUROSCI.5572-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraganore DM, Lesnick TG, Elbaz A, Chartier-Harlin MC, Gasser T, Kruger R, et al. UCHL1 is a Parkinson’s disease susceptibility gene. Ann Neurol. 2004;55(4):512–521. doi: 10.1002/ana.20017. [DOI] [PubMed] [Google Scholar]
- Martin C. The physiology of amylin and insulin: maintaining the balance between glucose secretion and glucose uptake. Diabetes Educ. 2006;32(Suppl 3):101S–104S. doi: 10.1177/0145721706288S237. [DOI] [PubMed] [Google Scholar]
- Martinez A, Kapas S, Miller MJ, Ward Y, Cuttitta F. Coexpression of receptors for adrenomedullin, calcitonin gene-related peptide, and amylin in pancreatic beta-cells. Endocrinology. 2000;141(1):406–411. doi: 10.1210/endo.141.1.7261. [DOI] [PubMed] [Google Scholar]
- Marzban L, Trigo-Gonzalez G, Zhu X, Rhodes CJ, Halban PA, Steiner DF, et al. Role of beta-cell prohormone convertase (PC)1/3 in processing of pro-islet amyloid polypeptide. Diabetes. 2004;53(1):141–148. doi: 10.2337/diabetes.53.1.141. [DOI] [PubMed] [Google Scholar]
- Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136(3):261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y. Different amyloidogenic peptides share a similar mechanism of neurotoxicity involving reactive oxygen species and calcium. Brain Res. 1995;676(1):219–224. doi: 10.1016/0006-8993(95)00148-j. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Morfis M, Tilakaratne N, Furness SG, Christopoulos G, Werry TD, Christopoulos A, et al. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology. 2008;149(11):5423–5431. doi: 10.1210/en.2007-1735. [DOI] [PubMed] [Google Scholar]
- Moriarty DF, Raleigh DP. Effects of sequential proline substitutions on amyloid formation by human amylin20-29. Biochemistry. 1999;38(6):1811–1818. doi: 10.1021/bi981658g. [DOI] [PubMed] [Google Scholar]
- Munishkina LA, Fink AL. Fluorescence as a method to reveal structures and membrane-interactions of amyloidogenic proteins. Biochim Biophys Acta. 2007;1768(8):1862–1885. doi: 10.1016/j.bbamem.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Nadeau PJ, Charette SJ, Landry J. REDOX reaction at ASK1-Cys250 is essential for activation of JNK and induction of apoptosis. Mol Biol Cell. 2009;20(16):3628–3637. doi: 10.1091/mbc.E09-03-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato M, Asai J, Miyazato M, Matsukura S, Kangawa K, Matsuo H. Isolation and identification of islet amyloid polypeptide in normal human pancreas. Regul Pept. 1990;31(3):179–186. doi: 10.1016/0167-0115(90)90004-g. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, et al. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Sanke T, Seino S, Eddy RL, Fan YS, Byers MG, et al. Human islet amyloid polypeptide gene: complete nucleotide sequence, chromosomal localization, and evolutionary history. Mol Endocrinol. 1989;3(11):1775–1781. doi: 10.1210/mend-3-11-1775. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16(11):1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, et al. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol Cell. 1998;2(3):389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- Opie EL. On the Relation of Chronic Interstitial Pancreatitis to the Islands of Langerhans and to Diabetes Melutus. J Exp Med. 1901;5(4):397–428. doi: 10.1084/jem.5.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucelli L, O’Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36(6):1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54(2):233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Proux-Gillardeaux V, Rudge R, Galli T. The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways? Traffic. 2005;6(5):366–373. doi: 10.1111/j.1600-0854.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- Reidelberger RD, Haver AC, Arnelo U, Smith DD, Schaffert CS, Permert J. Amylin receptor blockade stimulates food intake in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R568–574. doi: 10.1152/ajpregu.00213.2004. [DOI] [PubMed] [Google Scholar]
- Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes. 2007;56(1):65–71. doi: 10.2337/db06-0734. [DOI] [PubMed] [Google Scholar]
- Rivera JF, Costes S, Gurlo T, Glabe CG, Butler PC. Autophagy defends pancreatic beta cells from human islet amyloid polypeptide-induced toxicity. J Clin Invest. 2014;124(8):3489–3500. doi: 10.1172/JCI71981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera JF, Gurlo T, Daval M, Huang CJ, Matveyenko AV, Butler PC, et al. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic beta-cells: protective role of p62-positive cytoplasmic inclusions. Cell Death Differ. 2011;18(3):415–426. doi: 10.1038/cdd.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443(7113):780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Rymer DL, Good TA. The role of prion peptide structure and aggregation in toxicity and membrane binding. J Neurochem. 2000;75(6):2536–2545. doi: 10.1046/j.1471-4159.2000.0752536.x. [DOI] [PubMed] [Google Scholar]
- Saido T, Leissring MA. Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med. 2012;2(6):a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamekh S, Brender JR, Hyung SJ, Nanga RP, Vivekanandan S, Ruotolo BT, et al. A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc. J Mol Biol. 2011;410(2):294–306. doi: 10.1016/j.jmb.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandgren KJ, Wilkinson J, Miranda-Saksena M, McInerney GM, Byth-Wilson K, Robinson PJ, et al. A differential role for macropinocytosis in mediating entry of the two forms of vaccinia virus into dendritic cells. PLoS Pathog. 2010;6(4):e1000866. doi: 10.1371/journal.ppat.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Rajendran L, Honsho M, Gralle M, Donnert G, Wouters F, et al. Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J Neurosci. 2008;28(11):2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40(6):928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Shimohata T, Sato A, Burke JR, Strittmatter WJ, Tsuji S, Onodera O. Expanded polyglutamine stretches form an ‘aggresome’. Neurosci Lett. 2002;323(3):215–218. doi: 10.1016/s0304-3940(02)00162-3. [DOI] [PubMed] [Google Scholar]
- Singh S, Trikha S, Sarkar A, Jeremic AM. Proteasome regulates turnover of toxic human amylin in pancreatic cells. Biochem J. 2016;473(17):2655–2670. doi: 10.1042/BCJ20160026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17(3–4):101–104. doi: 10.3109/13506129.2010.526812. [DOI] [PubMed] [Google Scholar]
- Snyder H, Mensah K, Theisler C, Lee J, Matouschek A, Wolozin B. Aggregated and monomeric alpha-synuclein bind to the S6′ proteasomal protein and inhibit proteasomal function. J Biol Chem. 2003;278(14):11753–11759. doi: 10.1074/jbc.M208641200. [DOI] [PubMed] [Google Scholar]
- Soldani C, Lazze MC, Bottone MG, Tognon G, Biggiogera M, Pellicciari CE, et al. Poly(ADP-ribose) polymerase cleavage during apoptosis: when and where? Exp Cell Res. 2001;269(2):193–201. doi: 10.1006/excr.2001.5293. [DOI] [PubMed] [Google Scholar]
- Stavrou I, O’Halloran TJ. The monomeric clathrin assembly protein, AP180, regulates contractile vacuole size in Dictyostelium discoideum. Mol Biol Cell. 2006;17(12):5381–5389. doi: 10.1091/mbc.E06-06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21(24):9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian SL, Hull RL, Zraika S, Aston-Mourney K, Udayasankar J, Kahn SE. cJUN N-terminal kinase (JNK) activation mediates islet amyloid-induced beta cell apoptosis in cultured human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2012;55(1):166–174. doi: 10.1007/s00125-011-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Kim J, Paik SR, Park JH, Ahn YS, Chung KC. Induction of neuronal cell death by Rab5A-dependent endocytosis of alpha-synuclein. J Biol Chem. 2001;276(29):27441–27448. doi: 10.1074/jbc.M101318200. [DOI] [PubMed] [Google Scholar]
- Susa AC, Wu C, Bernstein SL, Dupuis NF, Wang H, Raleigh DP, et al. Defining the Molecular Basis of Amyloid Inhibitors: Human Islet Amyloid Polypeptide-Insulin Interactions. J Am Chem Soc. 2014 doi: 10.1021/ja504031d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther. 2000;294(1):61–72. [PubMed] [Google Scholar]
- Tofoleanu F, Buchete NV. Alzheimer Abeta peptide interactions with lipid membranes: fibrils, oligomers and polymorphic amyloid channels. Prion. 2012;6(4):339–345. doi: 10.4161/pri.21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramutola A, Di Domenico F, Barone E, Perluigi M, Butterfield DA. It Is All about (U)biquitin: Role of Altered Ubiquitin-Proteasome System and UCHL1 in Alzheimer Disease. Oxid Med Cell Longev. 2016;2016:2756068. doi: 10.1155/2016/2756068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha S, Jeremic AM. Clustering and internalization of toxic amylin oligomers in pancreatic cells require plasma membrane cholesterol. J Biol Chem. 2011;286(41):36086–36097. doi: 10.1074/jbc.M111.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha S, Jeremic AM. Distinct internalization pathways of human amylin monomers and its cytotoxic oligomers in pancreatic cells. PLoS One. 2013;8(9):e73080. doi: 10.1371/journal.pone.0073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LH, Raleigh DP. Role of aromatic interactions in amyloid formation by islet amyloid polypeptide. Biochemistry. 2013;52(2):333–342. doi: 10.1021/bi3014278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4(3):405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- Visa M, Alcarraz-Vizan G, Montane J, Cadavez L, Castano C, Villanueva-Penacarrillo ML, et al. Islet amyloid polypeptide exerts a novel autocrine action in beta-cell signaling and proliferation. FASEB J. 2015;29(7):2970–2979. doi: 10.1096/fj.15-270553. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Lee VM. Addition of exogenous alpha-synuclein preformed fibrils to primary neuronal cultures to seed recruitment of endogenous alpha-synuclein to Lewy body and Lewy neurite-like aggregates. Nat Protoc. 2014;9(9):2135–2146. doi: 10.1038/nprot.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagoner PK, Chen C, Worley JF, Dukes ID, Oxford GS. Amylin modulates beta-cell glucose sensing via effects on stimulus-secretion coupling. Proc Natl Acad Sci U S A. 1993;90(19):9145–9149. doi: 10.1073/pnas.90.19.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xu J, Finnerty J, Furuta M, Steiner DF, Verchere CB. The prohormone convertase enzyme 2 (PC2) is essential for processing pro-islet amyloid polypeptide at the NH2-terminal cleavage site. Diabetes. 2001;50(3):534–539. doi: 10.2337/diabetes.50.3.534. [DOI] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123(5):1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sekine S, Naguro I, Sekine Y, Ichijo H. Apoptosis Signal-regulating Kinase 1 (ASK1)-p38 Pathway-dependent Cytoplasmic Translocation of the Orphan Nuclear Receptor NR4A2 Is Required for Oxidative Stress-induced Necrosis. J Biol Chem. 2015;290(17):10791–10803. doi: 10.1074/jbc.M114.623280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003;278(27):25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Wesen E, Jeffries GDM, Matson Dzebo M, Esbjorner EK. Endocytic uptake of monomeric amyloid-beta peptides is clathrin- and dynamin-independent and results in selective accumulation of Abeta(1-42) compared to Abeta(1-40) Sci Rep. 2017;7(1):2021. doi: 10.1038/s41598-017-02227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark G, Westermark P, Eizirik DL, Hellerstrom C, Fox N, Steiner DF, et al. Differences in amyloid deposition in islets of transgenic mice expressing human islet amyloid polypeptide versus human islets implanted into nude mice. Metabolism. 1999;48(4):448–454. doi: 10.1016/s0026-0495(99)90102-6. [DOI] [PubMed] [Google Scholar]
- Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91(3):795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990;87(13):5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- Wiltzius JJ, Sievers SA, Sawaya MR, Cascio D, Popov D, Riekel C, et al. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17(9):1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wookey PJ, Lutz TA, Andrikopoulos S. Amylin in the periphery II: An updated mini-review. Scientific World Journal. 2006;6:1642–1655. doi: 10.1100/tsw.2006.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Brekk OR, Stefanis L. alpha-Synuclein and protein degradation systems: a reciprocal relationship. Mol Neurobiol. 2013;47(2):537–551. doi: 10.1007/s12035-012-8341-2. [DOI] [PubMed] [Google Scholar]
- Young A, Denaro M. Roles of amylin in diabetes and in regulation of nutrient load. Nutrition. 1998;14(6):524–527. doi: 10.1016/s0899-9007(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Yu C, Nwabuisi-Heath E, Laxton K, Ladu MJ. Endocytic pathways mediating oligomeric Abeta42 neurotoxicity. Mol Neurodegener. 2010;5:19. doi: 10.1186/1750-1326-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Deng Y, Luo Y, Zhang S, Zou H, Cai F, et al. Control of BACE1 degradation and APP processing by ubiquitin carboxyl-terminal hydrolase L1. J Neurochem. 2012;120(6):1129–1138. doi: 10.1111/j.1471-4159.2011.07644.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu H, Chuang CL, Li X, Au M, Zhang L, et al. The pathogenic mechanism of diabetes varies with the degree of overexpression and oligomerization of human amylin in the pancreatic islet beta cells. FASEB J. 2014 doi: 10.1096/fj.14-251744. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu J, Dragunow M, Cooper GJ. Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem. 2003;278(52):52810–52819. doi: 10.1074/jbc.M308244200. [DOI] [PubMed] [Google Scholar]
- Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52(4):626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]