Abstract
Oxytocin (OT) and arginine-vasopressin (AVP) act in the brain to regulate social cognition/social behavior and in the periphery to influence a variety of physiological processes. Although the chemical structures of OT and AVP as well as their receptors are quite similar, OT and AVP can have distinct or even opposing actions. Here, we review the increasing body of evidence that exogenously administered and endogenously released OT and AVP can activate each other’s canonical receptors (i.e., cross-talk) and examine the possibility that receptor cross-talk following the synaptic and non-synaptic release of OT and AVP contributes to their distinct roles in the brain and periphery. Understanding the consequences of cross-talk between OT and AVP receptors will be important in identifying how these peptides control social cognition and behavior and for the development of drugs to treat a variety of psychiatric disorders.
Keywords: G protein-coupled receptors, social cognition, intranasal administration, social communication, social recognition, social reward, pair bonding, prosocial behavior, social behavior, autism spectrum disorder
1. Introduction
Oxytocin (OT) and arginine-vasopressin (AVP) are important chemical signals that act in the brain to regulate a large number of adaptive social behaviors and in the periphery to coordinate a diverse group of physiological functions [1; 2]. Although the chemical structures of OT and AVP as well as their receptors are quite similar, there is substantial evidence that these nonapeptides (i.e., nine amino acid peptides) can have distinct or even opposing roles in the regulation of many behavioral and physiological functions [3; 4; 5; 6; 7]. Recently, however, there has been increasing evidence that OT and AVP can act on each other’s canonical receptors in potentially important ways. This review examines the potential interactions among OT, AVP and their receptors with an emphasis on the central role of these interactions in the regulation of social cognition and social behavior.
Much of the data discussed here has been collected in males despite the fact that there are major sex differences in OT and AVP systems [8; 9]. Indeed, the first demonstration of a sex difference in a neuropeptide system was identification of a sexually dimorphic innervation of the lateral septum by AVP fibers [10]. Subsequent work has found numerous examples of quantitative sex differences in nonapeptide-containing fibers and receptors in various brain regions. More recently, however, the existence of significant qualitative sex differences has also been identified. Activation of V1a receptors by AVP within the hypothalamus has opposite effects on the induction of aggression in males and females [11; 12; 13; 14].
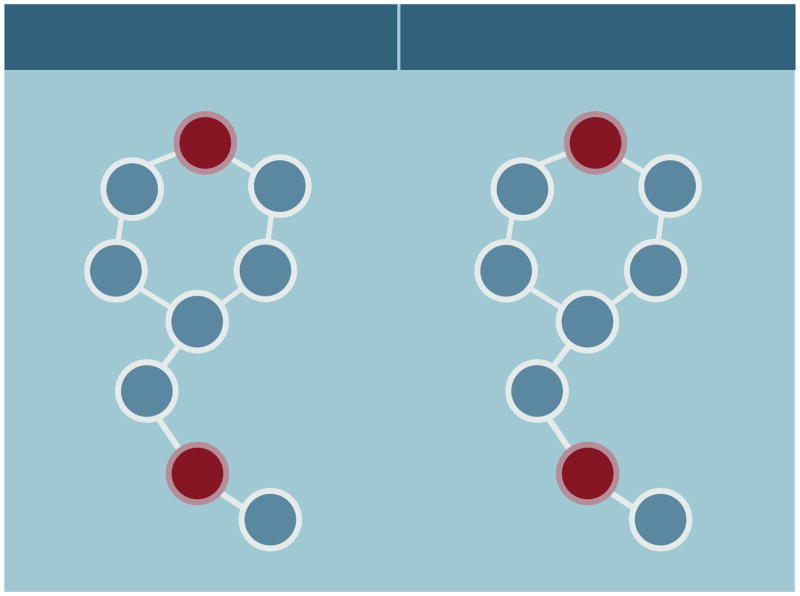
OT and AVP evolved from a common precursor through gene duplication more than 600 million years ago [15]. OT and AVP share seven of nine amino acid sequences in their primary structure, differing only in the third and eighth positions (Figure 1). OT and AVP are found in extensive neural networks that innervate many regions in the mammalian brain (Figure 2). In mammals, OT is considered to have only one canonical receptor (i.e., OTR) while AVP is thought to have three: V1aR, V1bR and V2R [16; 17; 18]. These evolutionarily ancient receptors belong to the G protein-coupled receptor superfamily that have seven putative transmembrane domains and are found extensively throughout the body. Around 25% of the amino acid sequences in human OTR, V1aR, V1bR, and V2R are the same [19]. OT/AVP receptors are found in many different peripheral tissues [2; 20]. In mammalian brain OTRs and V1aRs are robustly expressed in many regions but their distributions often differ [9; 21; 22] (Figure 3). V1bRs appear to have a much more restricted distribution, although they are expressed prominently in the hippocampus and at lower levels in the hypothalamus and amygdala [23; 24]. Although V2Rs have also been reported in the mammalian brain, it seems unlikely that they play a significant role in mediating the central effects of these peptides so they will be mentioned only briefly in discussions of peripheral AVP receptors [25; 26; 27].
Figure 1.
Schematic diagram of the primary structure of oxytocin and arginine-vasopressin. Seven of the nine amino acid sequences of these peptides are identical. Amino acids in the third and eighth positions differ (in red). Both peptides contain disulphide bridges between Cysteine (Cys) residues at positions one and six.
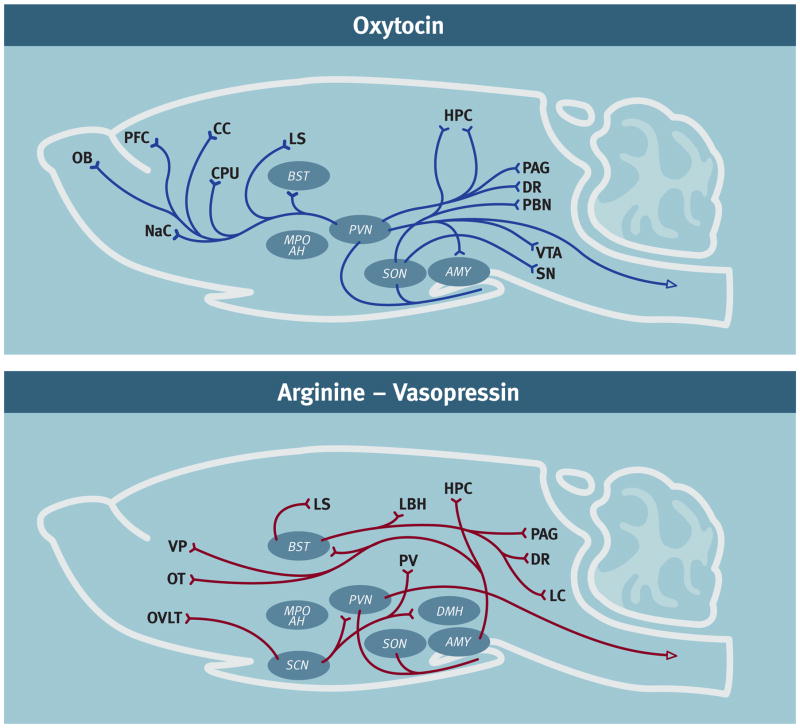
Figure 2.
Diagrammatic representations of the oxytocin- (OT) and arginine-vasopressin- containing (AVP) neural network in rodents. It is noteworthy that OT and AVP immunoreactivity can vary by species, sex, age and social experience [8; 127]. These diagrams represent a compilation of the major OT and AVP projections from several rodent species. In addition to the cell bodies indicated in the diagram there are also accessory nuclei that likely also play an important role. A. OT network: [97; 128; 129; 130; 131; 132]. B. AVP network: [97; 114; 131; 133; 134; 135; 136] Abbreviations: Amygdala (AMY), Bed nucleus of the stria terminalis (BST), Caudate-putamen (CPU), Cingulate cortex (CC), Dorsal raphe (DR), Hippocampus (HPC), Lateral septum (LS), Locus coeruleus (LC), Medial preoptic area – anterior hypothalamus (MPO AH), Nucleus Accumbens (NaC), Olfactory bulb (OB), Olfactory tubercle (OT), Organum vasculosum laminae teriminalis (OVLT), Parabrachial nucleus (PBN), Paraventricular nucleus (PVN), Periaqueductal grey (PAG), Periventricular nucleus hypothalamus (PV), Prefrontal cortex (PFC), Substantia nigra (SN), Suprachiasmatic nucleus (SCN), Supraoptic nucleus (SON), Ventral pallidum (VP), Ventral tegmental area (VTA)
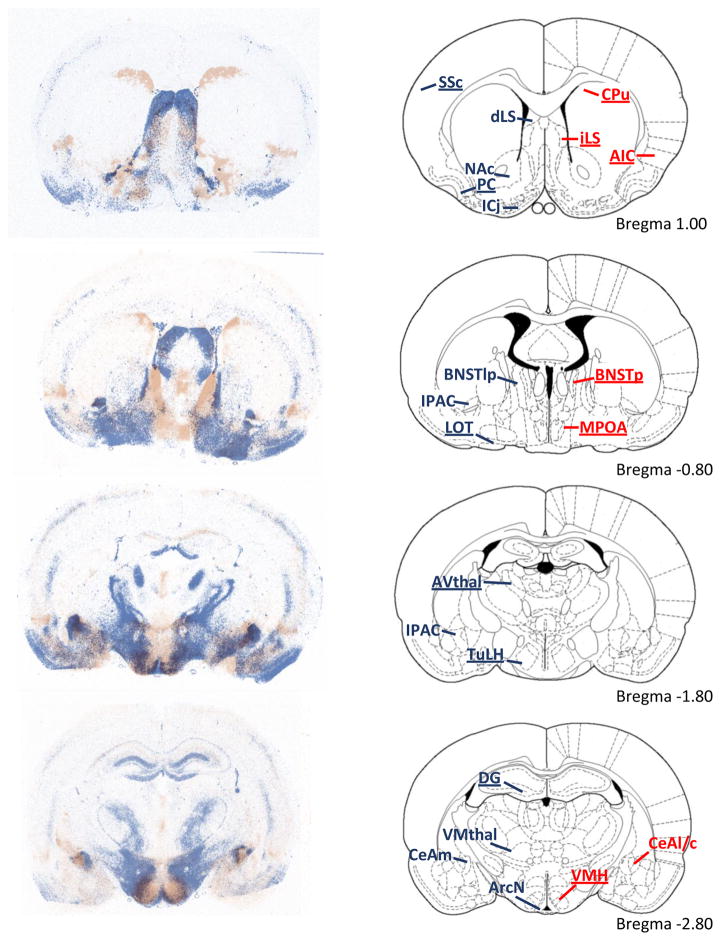
Figure 3.
The distribution of V1aR (blue) and OTR (red) binding in male rats. Overall, there is little overlap between the patterns of V1aR and OTR binding. The right column illustrates rat brain images adapted from The Rat Brain Atlas [137]. Dense V1aR binding was found in somatosensory cortex (SSc), piriform cortex (PC), Islands of Calleja (ICj), nucleus accumbens (NAc), lateral septum (LS), lateral posterior bed nucleus of the stria terminalis (BNST) (BNSTlp), nucleus of the lateral olfactory tract (LOT), dentate gyrus (DG), tuberal lateral hypothalamus (TuLH), anteroventral thalamus (AVthal), interstitial nucleus of the posterior limb of the anterior commissure (IPAC), arcuate nucleus of the hypothalamus (ArcN), ventromedial thalamus (VMthal), and medial central amygdala (CeAm). Dense OTR binding was found in the dorsal caudate putamen (CPu), agranular insular cortex (AIP), posterior BNST (BNSTp), medial preoptic area (MOPA), ventromedial hypothalamus (VMH), and later and capsular central amygdala (CeAl/c). Regions where sex differences were identified are underlined. Taken from Dumais and Veenema (2016) with permission.
The selectivity of OT and AVP for OTRs, V1aRs, V1bRs, and V2Rs has been examined using a variety of in vitro and in vivo assays ranging from measurements of cellular activity to overt behavior. It is important to point out that there is no “gold standard” that necessarily applies to defining the selectivity of OT and AVP for OTRs, V1aRs, V1bRs, and V2Rs. Indeed, many factors including species differences, differences in the assays employed, and differences in the tissues examined need to be considered when drawing conclusions from data on the selectivity of these receptors. Nevertheless, it appears that there can be differences of around 10–100 fold in the selectivity of OTRs and AVP receptors for OT and AVP. AVP has a similar affinity for OTRs, V1aRs, and V1bRs while OT may have a higher affinity for OTRs than V1aRs or V1bRs [28; 29; 30; 31; 32; 33]. For example, studies employing recombinant expression systems to examine selectivity of human, rat, and mouse OT and AVP receptors have shown that only the human OTR is selective for OT using a standard criterion [34; 35] (Table 1). This overall lack of receptor selectivity is not surprising when one considers that these receptors have a high degree of structural homology (e.g., 85% between V1aR and OTR) (Figure 4). Although their structural similarity and their lack of selectivity has been known for many years, only recently has the potential functional significance of the cross-talk between OT and AVP and their receptors been considered [36]. This emerging literature has come primarily from studies employing highly selective OT and AVP agonists and antagonists (see Table 2 for some commonly used and commercially available agonists and antagonists) as well as targeted genetic manipulations of OT and AVP as well as their receptors.
Table 1.
Selectivity of arginine-vasopressin (AVP) V1aR, AVP V1bR and oxytocin receptors (OTR) to AVP and OT.
| AVP | OT | |||||
|---|---|---|---|---|---|---|
| Receptor | V1aR | V1bR | OTR | V1aR | V1bR | OTR |
| Humans | 1.1 | 0.7 | 1.7 | 120 | >1000 | 0.8* |
| Rats | 2.6 | 0.3 | 1.7 | 71 | 294 | 1.0 |
| Mice | 1.3 | 0.3 | 1.8 | 46.1 | 494 | 0.6 |
Figure 4.
Diagrammatic representations comparing the primary structures of human OTRs, V1aRs and V1bRs. Amino acid residues conserved between receptors are indicated by dark circles: (A) OTRs and V1aRs, (B) V1aRs and V1bRs, and (C) V1aRs and V1bRs. See [19; 20]
Table 2.
Commonly used commercially available oxytocin (OT) and arginine-vasopressin (AVP) receptor agonists and antagonists.
| Category | Compound | Name/acronym | Supplier |
|---|---|---|---|
| OTR agonist | [Thr4, Gly7]-OT | TGOT | Abgent; Bachem |
| WAY267, 464*# | N/A | Tocris | |
| OTR antagonist | d(CH2)5-Tyr(Me)-[Orn8]-vasotocin | N/A | Bachem |
| V1aR antagonist | d(CH2)5[Try(Me)2]AVP | Manning Compound | Sigma-Aldrich |
| SR49059* | N/A | Tocris; Sigma-Aldrich | |
| V1bR antagonist | SSR149415* | Nelivaptan | Sigma-Aldrich |
indicates non-peptide agonists/antagonists.
is also a V1aR antagonist [126].
2. Cross-talk between OT and AVP administered peripherally
2.1 OT acting via V1aRs
Peripherally administered OT and AVP have a wide range of behavioral and physiological actions. Peripherally administered OT and AVP produce similar effects in reducing heart rate and body temperature, inducing contractions in ejaculatory tissues, and in promoting seizure susceptibility in male rats and rabbits [37; 38; 39]. These effects of OT and AVP can be inhibited by selective V1aR but not OTR antagonists. Other studies on pain perception also support the hypothesis that the effects of OT and AVP can be mediated by peripheral V1aRs. Peripheral administration of OT or AVP induces analgesia in OTR but not in V1aR knock-out male and female mice [40]. In addition, selective V1aR but not OTR antagonists inhibit both OT- and AVP- induced analgesia in wild-type mice. These analgesic effects of peripheral activation of V1aRs by OT and AVP may be mediated by V1aR modulation of ion channels in primary sensory neurons [41; 42]. Although both OT and AVP inhibit neuronal signaling in dorsal root ganglia, these effects can be inhibited by a selective V1aR antagonist but not by an OTR antagonist.
Some of the behavioral actions of peripheral administration of OT also appear to be mediated by V1aRs. The ability of peripheral administration of OT to reduce locomotor activity in male rats is reduced by a selective V1aR antagonist [43]. Both OT and AVP administered peripherally increase a form of social interaction in male rats referred to as “adjacent lying” in which rats maintain passive side-by-side contact during their first interaction [44]. A selective V1aR, but not an OTR antagonist, inhibits adjacent lying following peripheral administration of OT or AVP. Peripheral administration of either OT or AVP also increases social huddling in response to threatening stimuli (i.e., cat fur) and these effects are inhibited by a selective V1aR antagonist [45]. Taken together, these data suggest that peripherally administered OT and AVP can act through V1aRs to alter physiology and behavior.
2.2 AVP acting via OTRs
There is a limited amount of evidence that peripherally administered AVP can act via OTRs. Uterine contractions induced by OT or AVP are largely mediated by OTRs [46; 47; 48]. Both OT and AVP administered peripherally also increase social investigation in male rats. Peripheral administration of a selective OTR antagonist, but not a selective V1aR antagonist, inhibits OT- and AVP-induced increases in social investigation suggesting that both peptides are acting through the OTR [49].
2.3 OT acting via V1bRs
In the pituitary gland OT induces release of adrenocorticotropin via both OTRs and V1bRs in male mice and female rats [50; 51]. There is also evidence to suggest that OT can act on V1bRs as well as OTRs and that AVP can act on OTRs as well as V1bRs to induce glucagon secretion from pancreatic islet cells in male mice [52].
3.0 Cross-talk between OT and AVP administered in the brain
3.1 OT acting via V1aRs
While there are many examples of centrally administered OT and AVP influencing behavior, emerging evidence shows that receptor cross-talk may be involved in mediating at least some of these effects. Central administration of OT or AVP induces scratching behavior in wild-type and OTR knock-out male and female mice but not in V1aR knock-out mice [40]. There are also studies that have found that OT can act via V1aRs to influence a range of social behaviors [53]. In OTR null mice social exploration and social recognition are reduced, while aggression is increased when compared to wild-type mice. While intracerebroventricular (ICV) injection of OT or AVP can restore wild-type levels of these behaviors, the effects of OT and AVP are inhibited by a selective V1aR antagonist. Another example comes from studies of peer affiliation in female meadow voles [54]. When housed in winter-like conditions female voles form partner preferences for other females. Injection of OT into the lateral septum prevents the formation of these partner preferences, an effect that is blocked by co-administration of OT with a V1aR but not an OTR antagonist.
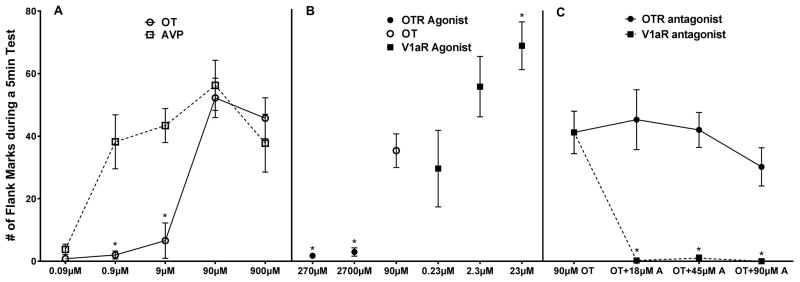
Another social behavior that can be elicited by central administration of OT or AVP is a form of social communication in Syrian hamsters called flank marking. Injection of AVP into several brain sites including the anterior hypothalamus (AH), the lateral septum (LS), and the periaqueductal gray (PAG), induce robust bouts of flank marking [55; 56; 57; 58]. Structure-activity studies as well as the central injection of highly selective V1a agonists and antagonists indicates that AVP acts on V1aRs to stimulate flank marking [59; 60]. More recently, ICV administration of OT has also been shown to induce flank marking in male hamsters [33]. Both OT and AVP induce flank marking in a dose-dependent manner, although AVP is around 100-fold more potent than OT in inducing the behavior (Figure 5). To determine whether OT also acts on V1aRs to induce flank marking highly selective V1aR and OTR agonists and antagonists were given ICV. A highly selective OTR agonist failed to induce flank marking and a highly selective OTR antagonist failed to inhibit the induction of flank marking by OT. In contrast, not only did a selective V1aR agonist induce flank marking, a selective V1aR antagonist inhibited the ability of OT to induce flank marking.
Figure 5.
Effects of OT, AVP and highly selective OTR and V1aR agonists and antagonists on a form social communication called flank marking in hamsters. (A) introcerebroventricular injections of both OT and AVP induced flank marking, but AVP is about 100 times more potent than OT (* indicates a significant difference between OT and AVP). (B) Effects of OTR and V1aR agonists on flank marking. The V1aR agonist but not the OTR agonist induced high levels of flank marking (* indicates a signficant difference compared to OT). (C) Effects of OTR and V1aR antagonists on OT-induced flank marking. All three concentrations of the V1aR antagonist completely blocked OT-induced flank marking while the OTR antagonist did not significantly affect flank marking (* indicates a significant difference compared to OT).
3.2 AVP acting via OTRs
There is considerable evidence that central administration of OT and AVP significantly influences the duration of social recognition in rats and mice [61; 62]. For example, social recognition is enhanced by optogenetically-induced OT release in the brain in female rats [63], and administration of AVP into the septum improves social recognition in male Brattleboro rats, a mutant rat strain that naturally lacks AVP [64]. A majority of the studies examining the roles of OT and AVP in social recognition have used relatively short tests of social memory, ranging from 10 min to 2 hrs, and “neutral” social stimuli such as juvenile conspecifics or ovariectomized females. Recently we used a different approach to study social recognition [65]. In these studies odors from the flank glands of adult male hamsters were used as the social stimulus. Flank gland odors are very potent social stimuli that serve to communicate a variety of different types of social information (e.g., dominance status) [66]. Indeed, flank gland odors were found to be recognized for at least 24 hrs after a brief 3 min exposure. As with other tests of social recognition, ICV injection of OT or AVP enhances social recognition; it increased social memory of flank gland odors from 24 to 48 hrs. Further, using selective OTR and V1aR agonists and antagonists these studies supported the hypothesis that OT and AVP act on OTRs to influence social recognition. ICV injection of an OTR but not a V1aR agonist increased the duration of social recognition in a manner that mimicked the effects of OT and AVP. A highly selective OTR but not a highly selective V1aR antagonist significantly reduced the duration of social recognition suggesting that OT and AVP regulate social recognition by acting on OTRs.
Several lines of evidence provide further support for the hypothesis that OTRs are essential for the expression of social recognition (for reviews see [62; 67]). For example, social recognition in OT knock-out male mice is severely impaired [68; 69]. Similarly, OTR knock-out mice also display significant deficits in social recognition [69; 70; 71; 72]. There is also evidence, however, that V1aR activation contributes to social recognition in rats and mice. Antagonism of V1aRs in several different CNS sites impairs social recognition [73; 74] and as discussed above, a V1aR antagonist inhibits the ability of OT to increase social recognition in OTR knock-out mice [53]. Conflicting results have come from studies employing the same strains of V1aR knock-out mice [75; 76; 77]. As a result, the data examining the hypothesis that OTRs and not V1aRs are essential for social recognition are not entirely consistent.
In another series of studies the roles of OTRs and V1aRs in mediating the rewarding properties of social interactions were examined [78]. Both OT and AVP injected into the ventral tegmental area (VTA), a key element in the mesolimbic reward network, enhanced social reward in male Syrian hamsters tested using the conditioned place preference (CPP) apparatus. Social reward can be measured as a preference for the chamber of the CPP apparatus where individuals are allowed to socially interact with a non-aggressive male conspecific. Hamsters injected with OT or AVP had a larger increase in the time spent in the chamber associated with social-interaction after conditioning compared to those injected with saline. A highly selective OTR but not a highly selective V1aR agonist mimicked the reward-enhancing effects of OT and AVP. Importantly, these studies also showed that an OTR, but not a V1aR antagonist injected into the VTA inhibits social reward. These data support the possibility that OT and AVP act on OTRs in the VTA, to regulate the expression of social reward.
4. Receptor cross-talk in response to exogenously administered peptides
Because OT and AVP as well as their canonical receptors display strikingly similar chemical structures, it is not surprising that there can be substantial cross-talk between these peptides and OTRs and V1aRs [79]. Crosstalk may also occur among all OT/AVP receptors particularly when these peptides are administered at high concentrations. Existing data suggest that there are 10–100 fold differences in the selectivity of OT and AVP for OTRs, V1aRs, and V1bRs, and that AVP has similar affinities for OT and AVP receptors while OT has a higher affinity for OT receptors than AVP receptors. As a result, very high levels of OT and AVP would likely result in the activation of all OT/AVP receptors. It must be remembered, however, that these estimates of receptor selectivity come from studies using different in vitro and in vivo assays in a limited number of tissues taken from only a very few species. As a result, our understanding of the selectivity of OT/AVP receptors may change as additional data become available.
There is considerable evidence that exogenously administered OT can act on V1aRs in the periphery and several examples that exogenously administered AVP can act on peripheral OTRs to influence both physiological and behavioral endpoints. There is also some evidence to suggest that OT can act on V1bRs to influence endocrine activity. In the brain, there is strong support for the hypothesis that OT can act on V1aRs and that AVP can act on OTRs to influence several different behaviors. There is also some evidence to suggest that OT and AVP act exclusively on V1aRs to influence some behaviors (e.g., social communication) and that OT and AVP act exclusively on OTRs to influence other behaviors (e.g., social reward). As a result, it is clear that the actions of exogenously administered OT and AVP in both the periphery and the brain cannot be assumed to be the sole result of activation of their canonical receptors. These and other studies illustrate the importance of employing selective OT and AVP receptor agonists and antagonists [80].
The potential for cross-talk between OT and AVP receptors should be a consideration in all situations where these peptides are exogenously administered, particularly when given in high concentrations. Studies in humans have employed intranasal administration to investigate the role of OT and AVP in both basic mechanisms of social cognition as well as in treatment for various psychiatric disorders including autism, anxiety and schizophrenia [81; 82; 83]. The commonly used intranasal concentrations of OT and AVP (i.e., 20 IU to 40 IU) produce high, supraphysiological levels of peptide in the periphery, and these concentrations are likely high enough to also produce biologically active levels in the brain [2]. The high levels of peptide produced within the periphery by intranasal administration likely results in activation of peripheral OTRs, V1aRs, V1bRs, and V2Rs. Because OTRs, V1aRs, V1bRs, and V2Rs are found on peripheral tissues of virtually every major physiological system of the body, the impact of these peptides is likely to be very widespread [2; 20]. The potential consequences of the simultaneous activation of OTRs, V1aRs, V1bRs and V2Rs are not understood. Although these receptors are contained on systems that have the potential to impact cognitive functioning indirectly (e.g., adrenal gland), it does not appear that they alter body temperature, heart rate or blood pressure [84]. It is clear, however, that peptides administered via the intranasal route produce significant alterations in cognition and neuronal activity whether they are acting peripherally and/or centrally.
Because the brain levels of peptide produced by intranasal administration are not well understood [2; 85], it is not clear if the central levels of these peptides induced by this form of administration could be sufficiently high to produce cross-talk across OT/AVP receptors in the brain. Cross-talk between OT and AVP receptors may be more limited in humans when the peptides are given at more physiological levels because in humans, OT appears to have a higher affinity for its receptors than for AVP receptors. The few studies that have compared the effects of intranasally administered OT and AVP in humans have reported that these peptides do not have opposing roles. These data have, however, found OT and AVP can have differential effects on neural activity (i.e., BOLD fMRI responses) and cognition, at least in some cases although the effects of OT are more robust [84; 86; 87; 88; 89; 90]. It is not possible to compare the effects of OT and AVP in these studies because the peptides are consistently administered in different doses. Thus, differences in the effects of OT and AVP could simply be the result of different doses and/or differences in the selectivity of OTRs for OT in humans. As such, it is important to consider the possibility that intranasal administration of OT or AVP produces a global activation of both OT and AVP receptors. Therefore, the use of intranasal administration in studies of the specific roles of OTRs, V1aRs or V1bRs in social cognition or for therapeutic purposes will require the administration of selective OT or AVP receptor agonists and antagonists, many of which are currently under development [80; 91; 92].
5. Is there cross-talk in response to endogenously released OT and AVP?
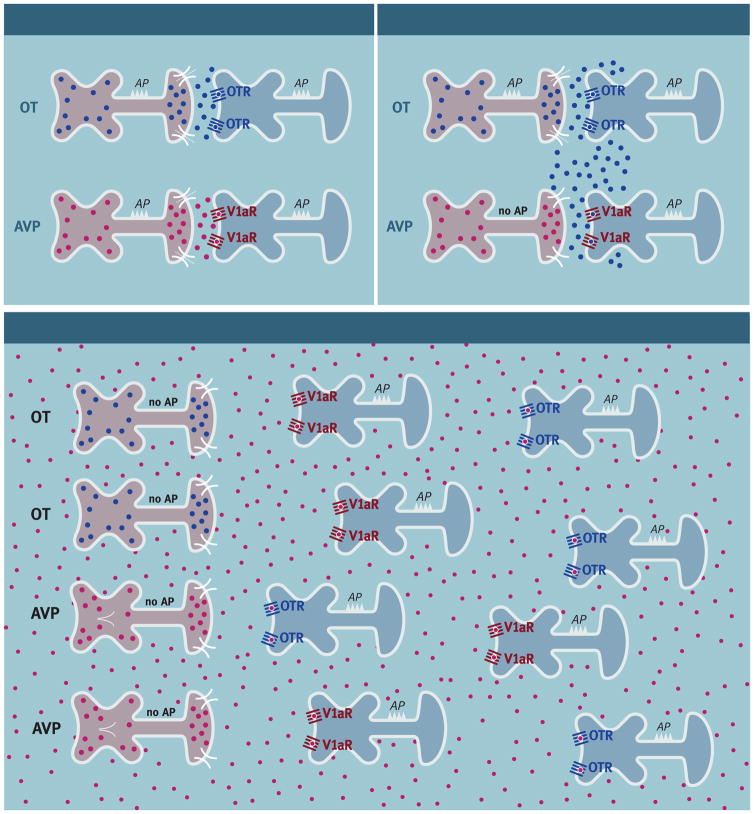
The endogenous release of OT and AVP in the brain may be the result of neuropeptide release from synaptic and/or non-synaptic regions of neurons, although the relative roles of these forms of release in different populations of OT and AVP-containing cells are controversial [93; 94; 95; 96]. Several lines of evidence support the possibility of synaptic release of OT and AVP (for a review see [97]). For example, electron microscopic analysis has revealed dense core vesicles containing AVP- and OT-ir in terminals located in synaptic regions of brain areas such as the habenula, lateral septum, amygdala, supraoptic nuclei, ventromedial hypothalamus and the nucleus of the solitary tract [98; 99; 100; 101; 102; 103; 104]. In addition, depolarizing stimuli can induce OT and AVP release in a calcium-dependent manner in brain regions where AVP and OT fibers terminate synaptically [105; 106; 107]. OT or AVP release from synaptic terminals or release from axons in passage could therefore produce a focal activation of local receptors (Figure 6). The potential for receptor cross-talk would likely be reduced by local synaptic release of neuropeptide and the anatomical segregation of OT and AVP receptors. That being said, however, comparatively little is known about the fate of endogenously released neuropeptides because the dynamics of peptide degradation in the brain are not well understood. Therefore, the possibility that synaptically released neuropeptides could spread beyond the synaptic space (e.g., synaptic spillover) cannot be excluded (Figure 6).
Figure 6.
Diagrammatic representation of synaptic release with no cross-talk, synaptic release with cross-talk, and non-synaptic release with volume transmission and cross-talk in neurons that produce oxytocin (OT) or arginine-vasopressin (AVP). Synaptic release of OT or AVP is produced by action potentials (AP) that activate an influx of extracellular calcium (Ca2+) through voltage sensitive Ca2+ channels. Non-synaptic release of OT or AVP is produced by the release of Ca2+ from endogenous stores (e.g., endoplasmic reticulum (ER)).
A more global form of OT and AVP release can occur when these neuropeptides are released from non-synaptic regions of neurons (e.g., dendrites) (Figure 6) [108; 109; 110]. Non-synaptic neuropeptide release is stimulated by Ca2+ coming from intracellular stores (e.g., endoplasmic reticulum) as opposed to synaptic release that is mediated by the influx of extracellular Ca2+ through voltage-gated channels [105]. Because non-synaptic release does not require a change in membrane voltage, synaptic release and non-synaptic release can occur independently of each other. A major source of non-synaptically released OT and AVP is the magnocellular neurons in the hypothalamus, although there is also evidence that hypothalamic parvocellular neurons can also release neuropeptides from dense core vesicles non-synaptically [111]. The distance that non-synaptically released neuropeptides travel is uncertain, but is the subject of active investigation [96; 109]. The further that non-synaptically released neuropeptides spread at high concentrations the greater potential for receptor cross-talk despite the anatomical segregation of OT and AVP receptors.
There are anatomical regions where OT or AVP have not been detected and yet their receptors are present at high levels. For example, in hamsters the lateral septum contains large numbers of V1aRs but few, if any, AVP containing fibers or cell bodies have been identified with immunohistochemistry [112; 113; 114; 115]. Although “isolated” receptors appear to be a common phenomenon in the brain [116], the number of mismatches between the presence of neuropeptides and their receptors may be at least partially the result of a lack of sensitivity of the techniques used to localize neuropeptides [98; 117]. Nevertheless, when isolated receptors do occur more global forms of neuropeptide release, such as non-synaptic release, would be required for their activation. In any case, non-synaptic release of OT and AVP has the potential to result in substantial cross-talk among OT and AVP receptors across many brain regions.
There is only a limited amount of evidence that endogenously released OT or AVP can produce functionally significant responses by acting on each other’s receptors. One approach has been to stimulate the endogenous release of OT by the central administration of melanocyte stimulating hormone (α-MSH) and to determine whether the effects of the endogenously released OT are mediated by V1aRs. α-MSH acts on melanocortin 4 receptors to induce non-synaptic release of OT but not AVP from hypothalamic neurons [118; 119]. Studies in hamsters have found that ICV or hypothalamic injection of α-MSH induces flank marking behavior and that α-MSH-induced flank marking is blocked by central administration of a selective V1a antagonist [33]. These data provide support for the possibility that endogenously released OT can act on V1aRs to induce social behavior.
6. Conclusions
Untangling which receptors mediate the actions of exogenously administered and endogenously released OT and AVP is critically important for understanding the central and peripheral actions of these peptides. It is clear, however, that the actions of OT and AVP cannot be assumed to be the sole result of activation of their canonical receptors. Rather, OT and AVP can activate each other’s receptors, particularly when present at high levels, resulting in the potential for substantial cross-talk between the OT and AVP systems. Of course, cross-talk comes into play only when OT interacts with AVP receptors and vice versa. When they do it is likely that OT and AVP will produce similar effects. Therefore, a simple notion that OT and AVP always have diametrically opposed actions is incorrect. When considering the evidence that OT and AVP can produce distinct and in some cases opposite effects it is important to consider the effects of these peptides in the context of their ability to activate each other’s receptors. OT and AVP receptors are imbedded in neural circuits that are frequently anatomically separate so it is likely that the opposite effects of these peptides are more the result of which receptor is activated rather than which peptide was responsible for the activation.
Unfortunately, little is known about how frequently synapses containing OT or AVP also contain OT receptors or AVP receptors because it has not been possible to identify OT or AVP receptors with immunohistochemistry. Based on approaches with less anatomical resolution (i.e., receptor autoradiography) OT and AVP receptors appear to be anatomically segregated in many areas of the brain (e.g., Figure 3). If the presence of both OT and AVP receptors in the same synaptic regions is rare then the possibility of cross-talk following synaptic release of these neuropeptides would seem unlikely, with the exception of when these neuropeptides spillover beyond synaptic regions (i.e., synaptic spillover).
Cross-talk is likely to occur when neuropeptides are released non-synaptically and spread more globally due to volume transmission. Substantial non-synaptic release of either OT or AVP could activate a large number of OT and AVP receptors indiscriminately, thereby overriding the “wiring” diagrams of OT and AVP networks. Therefore, OT and AVP receptors within these networks could be activated in very different patterns as the result of different combinations of synaptic and non-synaptic release. Different patterns of OT and AVP receptor activation across structures within these networks could contribute to the regulation of the complex behaviors and physiological processes under their control (see [8] for a review). As discussed above, however, it remains important to confirm that cross-talk can occur in response to endogenously released OT and AVP and to have a better understanding of the dynamics of non-synaptic release of these neuropeptides. It is clear that dose-response studies and the use of selective OTR, V1aR, and V1bR agonists and antagonists as well as animal studies employing gene targeting techniques will be important for understanding the ligand/receptor interactions of OT and AVP.
Several different social behaviors can be influenced by both OT and AVP, but this influence appears to be mediated by only one of their receptors. For example, although OT and AVP increase social communication, social recognition, and social reward, the effects of these neuropeptides on social communication are mediated by V1aRs and their effects on social recognition and social reward are mediated by OTRs. Although much more work needs to be done, these data suggest that certain classes of social behavior are mediated by OTRs while other classes of social behavior are mediated by V1aRs. Exogenous administration of OT, AVP and selective agonists and antagonists of their receptors have considerable potential as new drugs to treat a number of different psychiatric disorders. Understanding cross-talk has the potential for substantial translational relevance that could lead to important clinical breakthroughs. Dysfunctions in the actions of OT and AVP have been linked to psychiatric disorders such as autism, anxiety and schizophrenia. As such, a better understanding of how these signals exert their effects could potentially lead to important new treatments of these disorders. For example, perhaps deficits in social communication would be most effectively treated with drugs that target V1aRs, while deficits in social recognition and social reward would be most effectively treated with drugs targeting OTRs. These data are also potentially important for the understanding of the actions of intranasally administered OT and AVP in humans. Intranasal OT and AVP administration, which is currently in use in clinical trials, produces supraphysiological levels of these peptides in peripheral tissues. These high levels of peptide have the potential to result in significant amounts of receptor cross-talk in the periphery as well as in the brain resulting in the activation of large numbers of OT and AVP receptors. Therefore, the use of intranasal administration in studies of the specific roles of OT and AVP receptors in social cognition/social behavior or in studies of the efficacy of these peptides for therapeutic purposes likely require the administration of selective OT or AVP receptor agonists and antagonists. As such, the development of highly selective agonists and antagonists will be particularly important for future clinical applications targeting OT or AVP receptors.
Highlights.
The chemical structures of oxytocin and arginine-vasopressin are similar, and the structures of their receptors are also similar
Exogenously administered oxytocin and arginine-vasopressin can activate each other’s canonical receptors (i.e., cross-talk)
Receptor cross-talk can occur in the brain and the periphery
Understanding oxytocin and arginine-vasopressin receptor cross-talk will be an important consideration in drug development
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caldwell HK. Neuroscientist. 2017. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. 1073858417708284. [DOI] [PubMed] [Google Scholar]
- 2.Leng G, Ludwig M. Intranasal Oxytocin: Myths and Delusions. Biol Psychiatry. 2016;79:243–50. doi: 10.1016/j.biopsych.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35:649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Legros JJ. Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology. 2001;26:649–655. doi: 10.1016/s0306-4530(01)00018-x. [DOI] [PubMed] [Google Scholar]
- 5.Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–6. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 8.Albers HE. Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network. Front Neuroendocrinol. 2015;36:49–71. doi: 10.1016/j.yfrne.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Research. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 11.Terranova JI, Song Z, Larkin TE, 2nd, Hardcastle N, Norvelle A, Riaz A, Albers HE. Serotonin and arginine-vasopressin mediate sex differences in the regulation of dominance and aggression by the social brain. Proc Natl Acad Sci U S A. 2016;113:13233–13238. doi: 10.1073/pnas.1610446113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. Journal of Neuroscience. 1997;17:4331–4340. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus) Eur J Neurosci. 2010;31:1655–1663. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- 14.Caldwell HK, Albers HE. Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Hormones and Behavior. 2004;46:444–449. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Acher R, Chauvet J. The neurohypophysial endocrine regulatory cascade: precursors, mediators, receptors, and effectors. Front Neuroendocrinol. 1995;16:237–89. doi: 10.1006/frne.1995.1009. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell HK, Young WS., III . Oxytocin and Vasopressin: Genetics and behavioral implications. In: Lim R, editor. Neuroactive Proteins and Peptides. Springer; New York: 2006. pp. 573–607. [Google Scholar]
- 17.Barberis C, Tribollet E. Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol. 1996;10:119–54. doi: 10.1615/critrevneurobiol.v10.i1.60. [DOI] [PubMed] [Google Scholar]
- 18.Hasunuma I, Toyoda F, Okada R, Yamamoto K, Kadono Y, Kikuyama S. Roles of arginine vasotocin receptors in the brain and pituitary of submammalian vertebrates. Int Rev Cell Mol Biol. 2013;304:191–225. doi: 10.1016/B978-0-12-407696-9.00004-X. [DOI] [PubMed] [Google Scholar]
- 19.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 20.Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiol Rev. 2012;92:1813–64. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- 21.Veinante P, Freund-Mercier MJ. Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study. J Comp Neurol. 1997;383:305–325. [PubMed] [Google Scholar]
- 22.Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct. 2017;222:981–1006. doi: 10.1007/s00429-016-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson EL, Caldwell HK. The vasopressin 1b receptor and the neural regulation of social behavior. Hormones and Behavior. 2012;61:277–282. doi: 10.1016/j.yhbeh.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirasawa A, Hashimoto K, Tsujimoto G. Distribution and developmental change of vasopressin V1A and V2 receptor mRNA in rats. Eur J Pharmacol. 1994;267:71–5. doi: 10.1016/0922-4106(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 26.Kato Y, Igarashi N, Hirasawa A, Tsujimoto G, Kobayashi M. Distribution and developmental changes in vasopressin V2 receptor mRNA in rat brain. Differentiation. 1995;59:163–169. doi: 10.1046/j.1432-0436.1995.5930163.x. [DOI] [PubMed] [Google Scholar]
- 27.Vargas KJ, Sarmiento JM, Ehrenfeld P, Anazco CC, Villanueva CI, Carmona PL, Brenet M, Navarro J, Muller-Esterl W, Gonzalez CB. Postnatal expression of V2 vasopressin receptor splice variants in the rat cerebellum. Differentiation. 2009;77:377–385. doi: 10.1016/j.diff.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chini B, Mouillac B, Ala Y, Balestre MN, Trumpp-Kallmeyer S, Hoflack J, Elands J, Hibert M, Manning M, Jard S. Tyr115 is the key residue for determining agonist selectivity in the V1a vasopressin receptor. EMBO J. 1995;14:2176–2182. doi: 10.1002/j.1460-2075.1995.tb07211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawtin SR, Wesley VJ, Parslow RA, Patel S, Wheatley M. Critical role of a subdomain of the N-terminus of the V1a vasopressin receptor for binding agonists but not antagonists; functional rescue by the oxytocin receptor N-terminus. Biochemistry. 2000;39:13524–33. doi: 10.1021/bi0013400. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Makino Y, Saji F, Takemura M, Inoue T, Kikuchi T, Kubota Y, Azuma C, Nobunaga T, Tokugawa Y, et al. Molecular characterization of a cloned human oxytocin receptor. Eur J Endocrinol. 1994;131:385–90. doi: 10.1530/eje.0.1310385. [DOI] [PubMed] [Google Scholar]
- 31.Lolait SJ, O’Carroll AM, Brownstein MJ. Molecular biology of vasopressin receptors. Ann N Y Acad Sci. 1995;771:273–92. doi: 10.1111/j.1749-6632.1995.tb44688.x. [DOI] [PubMed] [Google Scholar]
- 32.Thibonnier M, Coles P, Thibonnier A, Shoham M. The basic and clinical pharmacology of nonpeptide vasopressin receptor antagonists. Annu Rev Pharmacol Toxicol. 2001;41:175–202. doi: 10.1146/annurev.pharmtox.41.1.175. [DOI] [PubMed] [Google Scholar]
- 33.Song Z, McCann KE, McNeill JK, Larkin TE, Huhman KL, Albers HE. Oxytocin induces social communication by activating arginine-vasopressin V1a receptors and not oxytocin receptors. Psychoneuroendocrinology. 2014;50C:14–19. doi: 10.1016/j.psyneuen.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manning M, Stoev S, Chini B, Durroux B, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. In: Neumann ID, Landgraf R, editors. Advances in Vasopressin and Oxytocin: From genes to behavior. Elsevier; 2008. pp. 473–512. [DOI] [PubMed] [Google Scholar]
- 35.Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, Durroux T, Mouillac B, Corbani M, Guillon G. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. Journal of Neuroendocrinology. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young LJ, Flanagan-Cato LM. Editorial comment: Oxytocin, vasopressin and social behavior. Hormones and behavior. 2012;61:227–229. doi: 10.1016/j.yhbeh.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hicks C, Ramos L, Reekie T, Misagh GH, Narlawar R, Kassiou M, McGregor IS. Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: a biotelemetry study in rats. Br J Pharmacol. 2014;171:2868–87. doi: 10.1111/bph.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loyens E, Vermoesen K, Schallier A, Michotte Y, Smolders I. Proconvulsive effects of oxytocin in the generalized pentylenetetrazol mouse model are mediated by vasopressin 1a receptors. Brain Res. 2012;1436:43–50. doi: 10.1016/j.brainres.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 39.Gupta J, Russell R, Wayman C, Hurley D, Jackson V. Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br J Pharmacol. 2008;155:118–26. doi: 10.1038/bjp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, Crawley JN, Hu SB, Nishimori K, Young LJ, Tribollet E, Quirion R, Mogil JS. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–84. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu F, Qiu CY, Cai H, Liu TT, Qu ZW, Yang Z, Li JD, Zhou QY, Hu WP. Oxytocin inhibits the activity of acid-sensing ion channels through the vasopressin, V1A receptor in primary sensory neurons. Br J Pharmacol. 2014;171:3065–76. doi: 10.1111/bph.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubo A, Shinoda M, Katagiri A, Takeda M, Suzuki T, Asaka J, Yeomans DC, Iwata K. Oxytocin alleviates orofacial mechanical hypersensitivity associated with infraorbital nerve injury through vasopressin-1A receptors of the rat trigeminal ganglia. Pain. 2017;158:649–659. doi: 10.1097/j.pain.0000000000000808. [DOI] [PubMed] [Google Scholar]
- 43.Hicks C, Ramos L, Dampney B, Baracz SJ, McGregor IS, Hunt GE. Regional c-Fos expression induced by peripheral oxytocin administration is prevented by the vasopressin 1A receptor antagonist SR49059. Brain Res Bull. 2016;127:208–218. doi: 10.1016/j.brainresbull.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, McGregor IS. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38:2249–59. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowen MT, McGregor IS. Oxytocin and vasopressin modulate the social response to threat: a preclinical study. Int J Neuropsychopharmacol. 2014;17:1621–1633. doi: 10.1017/S1461145714000388. [DOI] [PubMed] [Google Scholar]
- 46.Bossmar T, Akerlund M, Fantoni G, Szamatowicz J, Melin P, Maggi M. Receptors for and myometrial responses to oxytocin and vasopressin in preterm and term human pregnancy: effects of the oxytocin antagonist atosiban. Am J Obstet Gynecol. 1994;171:1634–42. doi: 10.1016/0002-9378(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 47.Akerlund M, Bossmar T, Brouard R, Kostrzewska A, Laudanski T, Lemancewicz A, Serradeil-Le Gal C, Steinwall M. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br J Obstet Gynaecol. 1999;106:1047–53. doi: 10.1111/j.1471-0528.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- 48.Kawamata M, Mitsui-Saito M, Kimura T, Takayanagi Y, Yanagisawa T, Nishimori K. Vasopressin-induced contraction of uterus is mediated solely by the oxytocin receptor in mice, but not in humans. Eur J Pharmacol. 2003;472:229–34. doi: 10.1016/s0014-2999(03)01914-9. [DOI] [PubMed] [Google Scholar]
- 49.Ramos L, Hicks C, Caminer A, Couto K, Narlawar R, Kassiou M, McGregor IS. MDMA (‘Ecstasy’), oxytocin and vasopressin modulate social preference in rats: A role for handling and oxytocin receptors. Pharmacol Biochem Behav. 2016;150–151:115–123. doi: 10.1016/j.pbb.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–63. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura K, Fujiwara Y, Mizutani R, Sanbe A, Miyauchi N, Hiroyama M, Yamauchi J, Yamashita T, Nakamura S, Mori T, Tsujimoto G, Tanoue A. Effects of vasopressin V1b receptor deficiency on adrenocorticotropin release from anterior pituitary cells in response to oxytocin stimulation. Endocrinology. 2008;149:4883–91. doi: 10.1210/en.2007-1528. [DOI] [PubMed] [Google Scholar]
- 52.Fujiwara Y, Hiroyama M, Sanbe A, Yamauchi J, Tsujimoto G, Tanoue A. Mutual regulation of vasopressin- and oxytocin-induced glucagon secretion in V1b vasopressin receptor knockout mice. J Endocrinol. 2007;192:361–9. doi: 10.1677/joe.1.06864. [DOI] [PubMed] [Google Scholar]
- 53.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, Finardi A, Donzelli A, Pattini L, Rubino T, Parolaro D, Nishimori K, Parenti M, Chini B. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 54.Anacker AM, Christensen JD, LaFlamme EM, Grunberg DM, Beery AK. Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology. 2016;68:156–62. doi: 10.1016/j.psyneuen.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hennessey AC, Whitman DC, Albers HE. Microinjection of Arginine-Vasopressin into the Periaqueductal Gray Stimulates Flank Marking in Syrian-Hamsters (Mesocricetus-Auratus) Brain Research. 1992;569:136–140. doi: 10.1016/0006-8993(92)90379-n. [DOI] [PubMed] [Google Scholar]
- 56.Ferris CF, Albers HE, Wesolowski SM, Goldman BD, Luman SE. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science. 1984;224:521–523. doi: 10.1126/science.6538700. [DOI] [PubMed] [Google Scholar]
- 57.Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–9. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- 58.Huhman KL, Babagbemi TO, Albers HE. Bicuculline Blocks Neuropeptide Y-Induced Phase Advances When Microinjected in the Suprachiasmatic Nucleus of Syrian-Hamsters. Brain Research. 1995;675:333–336. doi: 10.1016/0006-8993(95)00018-l. [DOI] [PubMed] [Google Scholar]
- 59.Albers HE, Pollock J, Simmons WH, Ferris CF. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J Neurosci. 1986;6:2085–9. doi: 10.1523/JNEUROSCI.06-07-02085.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferris CF, Singer EA, Meenan DM, Albers HE. Inhibition of vasopressin-stimulated flank marking behavior by V1-receptor antagonists. Eur J Pharmacol. 1988;154:153–9. doi: 10.1016/0014-2999(88)90092-1. [DOI] [PubMed] [Google Scholar]
- 61.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 62.Albers HE. The regulation of social recognition, social communication and aggression: vasopressin in the social behavior neural network. Horm Behav. 2012;61:283–92. doi: 10.1016/j.yhbeh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, Kelsch W. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron. 2016;90:609–21. doi: 10.1016/j.neuron.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55:145–9. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 65.Song Z, Larkin TE, Malley MO, Albers HE. Oxytocin (OT) and arginine-vasopressin (AVP) act on OT receptors and not AVP V1a receptors to enhance social recognition in adult Syrian hamsters (Mesocricetus auratus) Horm Behav. 2016;81:20–7. doi: 10.1016/j.yhbeh.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Johnston RE. Communication. In: Seigel HI, editor. The Hamster: Reproduction and Behavior. Plenum Press; New York: 1985. pp. 121–149. [Google Scholar]
- 67.Caldwell HK, Aulino EA, Freeman AR, Miller TV, Witchey SK. Oxytocin and behavior: Lessons from knockout mice. Dev Neurobiol. 2017;77:190–201. doi: 10.1002/dneu.22431. [DOI] [PubMed] [Google Scholar]
- 68.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 69.Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009;8:558–67. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, Yanagisawa T, Kimura T, Matzuk MM, Young LJ, Nishimori K. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choleris E, Little SR, Mong JA, Puram SV, Langer R, Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci U S A. 2007;104:4670–5. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–63. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, Noack J, Landgraf R, Onaka T, Leng G, Meddle SL, Engelmann M, Ludwig M. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–7. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012;61:50–6. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 76.Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–51. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 77.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–13. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 78.Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE. Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions. Psychoneuroendocrinology. 2016;74:164–172. doi: 10.1016/j.psyneuen.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ragnauth AK, Goodwillie A, Brewer C, Muglia LJ, Pfaff DW, Kow LM. Vasopressin stimulates ventromedial hypothalamic neurons via oxytocin receptors in oxytocin gene knockout male and female mice. Neuroendocrinology. 2004;80:92–9. doi: 10.1159/000081844. [DOI] [PubMed] [Google Scholar]
- 80.Chinia BBM, Burton O, Stoev S, Manning M. The renaissance of oxytocin: A new frontier in translational research. In: Beck-Sickinger A, Morl K, Bellmann-Sickert K, Els-Heindl S, editors. Proceedings of the 34th European Peptide Symposium/8th International Peptide Symposium; 2017; pp. 32–33. [Google Scholar]
- 81.Caldwell HK, Albers HE. Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. Curr Top Behav Neurosci. 2016;27:51–103. doi: 10.1007/7854_2015_390. [DOI] [PubMed] [Google Scholar]
- 82.Modi ME, Young LJ. The oxytocin system in drug discovery for autism: animal models and novel therapeutic strategies. Horm Behav. 2012;61:340–50. doi: 10.1016/j.yhbeh.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 84.Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37:447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Insel TR. Translating Oxytocin Neuroscience to the Clinic: A National Institute of Mental Health Perspective. Biol Psychiatry. 2016;79:153–4. doi: 10.1016/j.biopsych.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 86.Chen X, Hackett PD, DeMarco AC, Feng C, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. Effects of oxytocin and vasopressin on the neural response to unreciprocated cooperation within brain regions involved in stress and anxiety in men and women. Brain Imaging Behav. 2016;10:581–93. doi: 10.1007/s11682-015-9411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel N, Grillon C, Pavletic N, Rosen D, Pine DS, Ernst M. Oxytocin and vasopressin modulate risk-taking. Physiol Behav. 2015;139:254–60. doi: 10.1016/j.physbeh.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–48. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 2015;9:754–64. doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- 90.Li T, Chen X, Mascaro J, Haroon E, Rilling JK. Intranasal oxytocin, but not vasopressin, augments neural responses to toddlers in human fathers. Horm Behav. 2017 doi: 10.1016/j.yhbeh.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Busnelli M, Bulgheroni E, Manning M, Kleinau G, Chini B. Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J Pharmacol Exp Ther. 2013;346:318–27. doi: 10.1124/jpet.113.202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Busnelli M, Kleinau G, Muttenthaler M, Stoev S, Manning M, Bibic L, Howell LA, McCormick PJ, Di Lascio S, Braida D, Sala M, Rovati GE, Bellini T, Chini B. Design and Characterization of Superpotent Bivalent Ligands Targeting Oxytocin Receptor Dimers via a Channel-Like Structure. J Med Chem. 2016;59:7152–66. doi: 10.1021/acs.jmedchem.6b00564. [DOI] [PubMed] [Google Scholar]
- 93.Johnson ZV, Young LJ. Oxytocin and vasopressin neural networks: Implications for social behavioral diversity and translational neuroscience. Neurosci Biobehav Rev. 2017;76:87–98. doi: 10.1016/j.neubiorev.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chini B, Verhage M, Grinevich V. The Action Radius of Oxytocin Release in the Mammalian CNS: From Single Vesicles to Behavior. Trends Pharmacol Sci. 2017 doi: 10.1016/j.tips.2017.08.005. [DOI] [PubMed]
- 97.Buijs RM. Vasopressin and oxytocin - their role in neurotransmission. Pharmacology Therapy. 1983;22:127–141. doi: 10.1016/0163-7258(83)90056-6. [DOI] [PubMed] [Google Scholar]
- 98.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 99.Theodosis DT. Oxytocin-immunoreactive terminals synapse on oxytocin neurones in the supraoptic nucleus. Nature. 1985;313:682–4. doi: 10.1038/313682a0. [DOI] [PubMed] [Google Scholar]
- 100.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci. 2008;28:11731–40. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Griffin GD, Ferri-Kolwicz SL, Reyes BA, Van Bockstaele EJ, Flanagan-Cato LM. Ovarian hormone-induced reorganization of oxytocin-labeled dendrites and synapses lateral to the hypothalamic ventromedial nucleus in female rats. J Comp Neurol. 2010;518:4531–45. doi: 10.1002/cne.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buijs RM, Swaab DF. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res. 1979;204:355–65. doi: 10.1007/BF00233648. [DOI] [PubMed] [Google Scholar]
- 103.Voorn P, Buijs RM. An immuno-electronmicroscopical study comparing vasopressin, oxytocin, substance P and enkephalin containing nerve terminals in the nucleus of the solitary tract of the rat. Brain Res. 1983;270:169–73. doi: 10.1016/0006-8993(83)90809-0. [DOI] [PubMed] [Google Scholar]
- 104.Buijs RM. Immunocytochemical demonstration of vasopressin and oxytocin in the rat brain by light and electron microscopy. J Histochem Cytochem. 1980;28:357–60. doi: 10.1177/28.4.6989899. [DOI] [PubMed] [Google Scholar]
- 105.Buijs RM, Van Heerikhuize JJ. Vasopressin and oxytocin release in the brain--a synaptic event. Brain Res. 1982;252:71–6. doi: 10.1016/0006-8993(82)90979-9. [DOI] [PubMed] [Google Scholar]
- 106.Neumann I, Ludwig M, Engelmann M, Pittman QJ, Landgraf R. Simultaneous microdialysis in blood and brain: oxytocin and vasopressin release in response to central and peripheral osmotic stimulation and suckling in the rat. Neuroendocrinology. 1993;58:637–45. doi: 10.1159/000126604. [DOI] [PubMed] [Google Scholar]
- 107.Landgraf R, Neumann I, Schwarzberg H. Central and peripheral release of vasopressin and oxytocin in the conscious rat after osmotic stimulation. Brain Research. 1988;457:219–225. doi: 10.1016/0006-8993(88)90689-0. [DOI] [PubMed] [Google Scholar]
- 108.Leng G, Ludwig M. Neurotransmitters and peptides: whispered secrets and public announcements. J Physiol. 2008;586:5625–32. doi: 10.1113/jphysiol.2008.159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Engelmann M, Wotjak CT, Ebner K, Landgraf R. Behavioural impact of intraseptally released vasopressin and oxytocin in rats. Exp Physiol. 2000;85(Spec No):125S–130S. doi: 10.1111/j.1469-445x.2000.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 111.Castel M, Morris J, Belenky M. Non-synaptic and dendritic exocytosis from dense-cored vesicles in the suprachiasmatic nucleus. Neuroreport. 1996;7:543–7. doi: 10.1097/00001756-199601310-00040. [DOI] [PubMed] [Google Scholar]
- 112.Johnson AE, Barberis C, Albers HE. Castration reduces vasopressin receptor binding in the hamster hypothalamus. Brain Res. 1995;674:153–8. doi: 10.1016/0006-8993(95)00010-n. [DOI] [PubMed] [Google Scholar]
- 113.Albers HE, Rowland CM, Ferris CF. Arginine-vasopressin immunoreactivity is not altered by photoperiod or gonadal hormones in the Syrian hamster (Mesocricetus auratus) Brain Res. 1991;539:137–142. doi: 10.1016/0006-8993(91)90696-s. [DOI] [PubMed] [Google Scholar]
- 114.Dubois-Dauphin M, Pevet P, Tribollet E, Dreifuss JJ. Vasopressin in the brain of the golden hamster: The distribution of vasopressin binding sites and of immunoreactivity to the vasopressin-related glycopeptide. Journal of Comparative Neurology. 1990;300:535–548. doi: 10.1002/cne.903000408. [DOI] [PubMed] [Google Scholar]
- 115.Young LJ, Wang Z, Cooper TT, Albers HE. Vasopressin (V1a) receptor binding, mRNA expression and transcriptional regulation by androgen in the Syrian hamster brain. J Neuroendocrinol. 2000;12:1179–85. doi: 10.1046/j.1365-2826.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- 116.Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- 117.Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, Chini B. Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biol Psychiatry. 2016;79:155–64. doi: 10.1016/j.biopsych.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 118.Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23:10351–8. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sabatier N. alpha-Melanocyte-stimulating hormone and oxytocin: a peptide signalling cascade in the hypothalamus. J Neuroendocrinol. 2006;18:703–10. doi: 10.1111/j.1365-2826.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- 120.Elands J, Barberis C, Jard S. [3H]-[Thr4, Gly7]OT: a highly selective ligand for central and peripheral OT receptors. Am J Physiol. 1988;254:E31–E38. doi: 10.1152/ajpendo.1988.254.1.E31. [DOI] [PubMed] [Google Scholar]
- 121.Mouillac B, Chini B, Balestre MN, Elands J, Trumpp-Kallmeyer S, Hoflack J, Hibert M, Jard S, Barberis C. The binding site of neuropeptide vasopressin V1a receptor. Evidence for a major localization within transmembrane regions. Journal of Biological Chemistry. 1995;270:25771–25777. doi: 10.1074/jbc.270.43.25771. [DOI] [PubMed] [Google Scholar]
- 122.Thibonnier M, Preston JA, Dulin N, Wilkins PL, Berti-Mattera LN, Mattera R. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signaling pathways. Endocrinology. 1997;138:4109–22. doi: 10.1210/endo.138.10.5432. [DOI] [PubMed] [Google Scholar]
- 123.Terrillon S, Cheng LL, Stoev S, Mouillac B, Barberis C, Manning M, Durroux T. Synthesis and characterization of fluorescent antagonists and agonists for human oxytocin and vasopressin V(1)(a) receptors. J Med Chem. 2002;45:2579–88. doi: 10.1021/jm010526+. [DOI] [PubMed] [Google Scholar]
- 124.Derick S, Cheng LL, Voirol MJ, Stoev S, Giacomini M, Wo NC, Szeto HH, Ben Mimoun M, Andres M, Gaillard RC, Guillon G, Manning M. [1-deamino-4-cyclohexylalanine] arginine vasopressin: a potent and specific agonist for vasopressin V1b receptors. Endocrinology. 2002;143:4655–64. doi: 10.1210/en.2002-220363. [DOI] [PubMed] [Google Scholar]
- 125.Serradeil-Le Gal C, Raufaste D, Derick S, Blankenstein J, Allen J, Pouzet B, Pascal M, Wagnon J, Ventura MA. Biological characterization of rodent and human vasopressin V1b receptors using SSR-149415, a nonpeptide V1b receptor ligand. Am J Physiol Regul Integr Comp Physiol. 2007;293:R938–R949. doi: 10.1152/ajpregu.00062.2007. [DOI] [PubMed] [Google Scholar]
- 126.Hicks C, Ramos L, Reekie TA, Narlawar R, Kassiou M, McGregor IS. WAY 267,464, a non-peptide oxytocin receptor agonist, impairs social recognition memory in rats through a vasopressin 1A receptor antagonist action. Psychopharmacology (Berl) 2015;232:2659–67. doi: 10.1007/s00213-015-3902-5. [DOI] [PubMed] [Google Scholar]
- 127.DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 2017 doi: 10.1002/cne.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whitman DC, Albers HE. Oxytocin immunoreactivity in the hypothalamus of female hamsters. Cell Tissue Res. 1998;291:231–7. doi: 10.1007/s004410050993. [DOI] [PubMed] [Google Scholar]
- 129.Otero-Garcia M, Agustin-Pavon C, Lanuza E, Martinez-Garcia F. Distribution of oxytocin and co-localization with arginine vasopressin in the brain of mice. Brain Struct Funct. 2016;221:3445–73. doi: 10.1007/s00429-015-1111-y. [DOI] [PubMed] [Google Scholar]
- 130.Hawthorn J, Ang VT, Jenkins JS. Effects of lesions in the hypothalamic paraventricular, supraoptic and suprachiasmatic nuclei on vasopressin and oxytocin in rat brain and spinal cord. Brain Res. 1985;346:51–7. doi: 10.1016/0006-8993(85)91093-5. [DOI] [PubMed] [Google Scholar]
- 131.Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–14. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- 132.Fuxe K, Borroto-Escuela DO, Romero-Fernandez W, Ciruela F, Manger P, Leo G, Diaz-Cabiale Z, Agnati LF. On the role of volume transmission and receptor-receptor interactions in social behaviour: focus on central catecholamine and oxytocin neurons. Brain Res. 2012;1476:119–31. doi: 10.1016/j.brainres.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 133.De Vries GJ. The vasopressinergic innervation of the brain in normal and castrated rats. Netherlands Instutite for Brain Research. 1985:81–108. [Google Scholar]
- 134.De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mahoney PD, Koh ET, Irvin RW, Ferris CF. Computer-Aided Mapping of Vasopressin Neurons in the Hypothalamus of the Male Golden Hamster: Evidence of Magnocellular Neurons that do not Project to the Neurohypophysis. Journal of Neuroendocrinology. 1990;2:113–122. doi: 10.1111/j.1365-2826.1990.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 136.Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol. 2011;519:2434–2474. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]