Abstract
Rationale
Subanesthetic ketamine (KET) elicits rapid, robust, but transient antidepressant effects. KET’s antidepressant actions can be augmented and maintained for a longer duration when repeatedly delivered. However, KET is recreationally abused, raising long-term treatment safety concerns. Women are more likely than men to seek treatment for depression, escalate from casual to compulsive drug use, and are more sensitive to antidepressants. Similarly, female rodents are more sensitive than males to KET’s rapid antidepressant-like behavioral effects; dose-response thresholds in these assays equal 2.5 and 5.0 mg/kg (i.p.), respectively. This suggests the utility of preclinical rodent models in optimizing sex-differential KET therapy protocols and minimizing adverse drug reactions.
Objectives
Here, we assessed behavioral and biochemical correlates of abuse liability following six serial KET treatments on alternating days at three subanesthetic, antidepressant-like doses (2.5, 5.0, or 10 mg/kg, i.p.) in adult male and female rats. A potential role for ΔFosB-mediated transcription in the nucleus accumbens is outlined in the context of KET-mediated locomotor sensitization.
Results
Antidepressant-like threshold doses (2.5, 5.0 mg/kg KET) failed to evoke a conditioned place preference in all animals, but only males positively responded to a higher dose (10 mg/kg). Behavioral sensitization to 5.0 or 10 mg/kg KET’s locomotor-activating effects was established in both sexes, and females’ sensitized response to 5.0 mg/kg was greater than males’. KET-induced hyperlocomotion positively correlated with ΔFosB protein expression in the nucleus accumbens. rAAV-ΔJunD inhibition of ΔFosB-mediated transcription in the accumbens failed to block locomotor sensitization to 10 mg/kg KET.
Conclusions
These data suggest that in rats, six alternating-day treatments with 2.5 mg/kg KET do not induce apparent behavioral signatures of abuse liability despite accumulation of ΔFosB protein in the accumbens. Additionally, females are more sensitive than males to KET’s locomotor-stimulant properties, both acutely and after repeated treatments. More studies are needed to determine brain regions and neural mechanisms responsible for KET-induced behavioral adaptations and to extrapolate these data to inform sex-dependent strategies for long-term KET therapy protocols for depression.
Keywords: ketamine, sex differences, conditioned place preference, locomotor sensitization, deltaFosB, nucleus accumbens
1. Introduction
For decades, the N-methyl-D-aspartate receptor (NMDAR) antagonist drug ketamine (KET) has been utilized clinically as a dissociative anesthetic agent [1]. In more recent years, subanesthetic KET dosing has gained recognition as a promising rapid-acting antidepressant in clinical trials, eliciting robust effects within hours in a significant proportion of individuals experiencing unipolar, bipolar, or treatment-resistant depression [2–6]. However, KET’s antidepressant effects are generally short-lived, with a single infusion providing symptom remission for up to one week, on average [4,5,7]. Thus far, medium- to long-term maintenance regimens have only been partially developed. Clinical data supports the hypotheses that repeated infusions prevent depressive symptom relapse for longer than a single infusion and that a serial alternate-day dosing regimen (3x/week) is efficacious and well-tolerated [8–13]. Further, a six-treatment, thrice-weekly dosing schedule is capable of augmenting antidepressant response in treatment-resistant patients who did not achieve remission with their first infusion [13].
However, no drug is a panacea. KET is recreationally sought and abused worldwide for its dissociative and hallucinogenic properties [14–17], limiting its desirability and potential utility in clinic [1,6,18,19]. Indeed, major depressive disorder (MDD) is highly comorbid with substance use disorders [20], and two recent case studies have reported progression from outpatient antidepressant KET therapy to a full-blown KET use disorder [21,22].
There are documented sex differences in the prevalence and etiology of MDD: women are twice as likely as men to be diagnosed with and seek treatment for major depressive disorder, and depressed women are more likely than their male counterparts to endorse serious thoughts of suicide [23,24]. Additionally, women are more sensitive to antidepressants than men [25]. While there is currently limited clinical data on the topic, preclinical studies from our lab and others’ have demonstrated that the dose-response threshold for KET’s rapid antidepressant-like effects is lower in female rodents than in males in behavioral assays such as the forced swim test [26–30]. Gonadally-intact female rats display an antidepressive-like behavioral phenotype to a minimum dose of 2.5 mg/kg KET (i.p.), whereas males require 5.0 mg/kg KET to achieve the same effect. Furthermore, when quantity and frequency of intake are controlled for, women are likelier than men to escalate from casual to compulsive drug use [31]. Preclinical studies from our lab have suggested similar phenomena in rodents, highlighting a critical role for female gonadal hormones. For example, female rats will self-administer subanesthetic KET when offered on phase-locked proestrus (elevated hormone concentrations) days, but females in diestrus (low hormone concentrations) will not [32]. Additionally, female rats behaviorally sensitize to a lower dose of KET than males when treated on diestrus phase-locked days, an effect that is paired with changes in dendritic morphology and protein expression that are commonly associated with addiction [33]. Taken together, these lines of evidence suggest that women may be at an elevated risk for developing adverse drug reactions to repeated therapeutic KET treatments. Given the sex-dependent sensitivity to psychiatric drugs across species and behavioral domains, if subanesthetic KET is to become a viable long-term treatment option for MDD, the safety profile of repeated therapeutic dosing (ie: a maintenance regimen) demands urgent evaluation in both sexes independently.
Accordingly, here, we performed a dose-response study to model favorable clinical repeat-exposure studies. We aimed to assess behavioral and biochemical correlates of abuse liability in response to six alternating-day treatments with subanesthetic KET in adult male and female rats. A recent paper from our lab assessed the abuse potential of 2.5 and 5 mg/kg KET given approximately every four days for eight treatments in diestrus phase-locked female and time-matched male rats [33]. However, current clinical trials using repeated KET to treat depression do not control for women’s menstrual stage when determining treatment scheduling, instead assigning priority to inter-dose delay periods. To model recent repeat-dosing clinical trials, we opted for a serial alternating-day treatment schedule using low “threshold” antidepressant-like doses (2.5, 5.0 mg/kg) as well as a higher dose known to elicit a range of addictive-like behaviors in adult male rodents (10 mg/kg) [34–38]. To quantify KET-associated reward, or drug “liking”, animals were assayed on the conditioned place preference test (CPP), a passive learning paradigm that serves as a validated measure of drug-paired reward state [39–42]. The second behavioral paradigm assayed was locomotor sensitization, a phenomenon that emerges following repeated intermittent psychostimulant treatment, and has been characterized as an endophenotype of drug “craving” or “wanting” [42–44]. These two behavior sets are mediated by separate neural circuits and mechanisms [42]. We aimed to investigate a likely molecular mechanism for the observed behavioral effects: ΔFosB protein expression. The transcription factor ΔFosB is stably upregulated in the nucleus accumbens (NAc) following chronic drug treatment [45,46], and its role in addiction has been thoroughly documented [47,48]. We predicted that repeated KET would upregulate this addiction-related biomarker in the NAc as compared to the repeated VEH treatment condition. Finally, because ΔFosB accumulation in the NAc is known to mediate locomotor sensitization to psychostimulants and enhance antidepressant response [45,49,50], we examined the effects of antagonizing ΔFosB-mediated transcription by virally overexpressing ΔJunD, a dominant negative binding partner of ΔFosB [51,52], on locomotor response to repeated KET.
2. Materials and methods
2.1 Subjects
Adult male (n=50) and female (n=32) Sprague-Dawley rats aged eight weeks obtained from Charles River Laboratories (Raleigh, NC) were used. Animals were housed in same-sex pairs under controlled temperatures, 12-hr light/dark cycles (lights on at 0700), and were allowed ad libitum access to standard rat chow and water. Male and female rats were housed in separate rooms. Animals were allowed to acclimate to the housing conditions for ≥3 days prior to beginning the experiment. Animals were weighed daily prior to behavioral testing. Because experimenter sex is known to affect baseline responses in behavioral testing [53], the same female experimenter performed all handling and behavioral testing. All procedures were carried out under strict accordance to the NIH Guide for the Care and Use of Laboratory Animals [54], and the protocol was approved by the Florida State University Institutional Animal Care and Use Committee.
2.2 Drugs
Ketamine hydrochloride (Ketathesia®1, racemic, Henry Schein Animal Health Inc.) in an injectable solution (100 mg/mL) was diluted to concentrations of 2.5, 5.0, and 10.0 mg/mL in sterile saline vehicle (VEH). All injections were delivered intraperitoneally (i.p.) at a volume of 1.0 mL/kg body mass.
2.3 Conditioned place preference test (CPP)
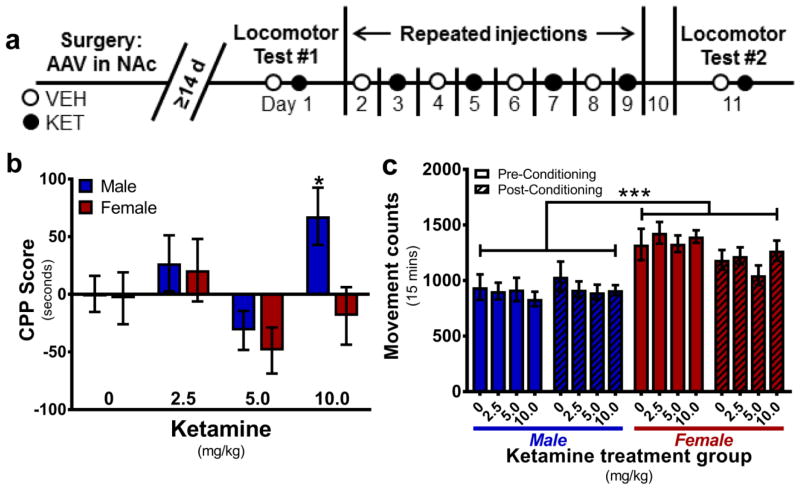
Animals were acclimated to the quiet dimly-lit behavioral testing room for 30 mins prior to all CPP testing and conditioning sessions. Using a 3-compartment apparatus (Med Associates Inc., St. Albans, VT, USA), the unbiased CPP experimental design was adapted from a previous study with some modifications [34] (Fig. 1a). Briefly, on experimental day 1, animals were placed in the gray center compartment of the 3-chamber apparatus, automated guillotine doors were raised, and animals were allowed to freely explore all zones for 15 mins to determine a baseline side preference. Animals spending greater than 300 sec between the black and white sides were considered to have a side preference and drug conditioning was counter-balanced to the non-preferred side; non-side-preferring animals were conditioned with drug to the white chamber. On alternating days for experimental days 3–10, animals were either injected with VEH and placed on the preferred side (or black in the absence of side preference), or with their assigned KET dose (0, 2.5, 5.0, or 10 mg/kg) and placed in the non-preferred (or white) side for 45 mins, then returned to their home cage. Experimental day 11 was performed exactly as experimental day 1, without injection and 15 mins free roam between all CPP chambers. Automated software (MED-PC, Med Associates Inc.) recorded time spent in each chamber and movement counts (photobeam breaks) during both pre- and post-conditioning tests. Test equipment was cleaned with 70% ethanol after each use. The CPP Score for each animal was calculated by subtracting seconds spent in the drug-paired chamber during the pre-conditioning test (experimental day 1) from the post-conditioning test (experimental day 11).
Fig. 1.
Contextual cues paired with low-dose ketamine are not rewarding. a Experimental timeline for behavioral assessment of repeated KET exposure. Animals received a total of six KET injections given every other day. On locomotor testing days (2 and 12), animals were given both VEH and their assigned KET dose with 1 hr separation. b Low doses of KET (2.5, 5.0 mg/kg) do not elicit a conditioned place preference in either sex. Males developed a conditioned place preference to 10 mg/kg, but females did not (*p<0.05 vs 0 CPP Score). c There were no locomotor differences between treatment groups in the CPP chamber during injection-free testing days 1 and 11 (***p<0.001 main effect of sex). Data are presented as mean ± SEM. n = 8/group
2.4 Locomotor testing
To control for individual differences in stress response and psychostimulant response [55,56], a standard novel environment locomotor test was performed on all animals one day prior to experimental behavioral testing (see Supplemental Methods). No analyses were done utilizing high/low novelty response as a factor; this preliminary measure was only used to balance treatment group assignments. After CPP pretest and test days (Fig. 1a, experimental days 1 and 11), animals were assessed for locomotor response to VEH and KET (Fig. 1a, experimental days 2 and 12). On each of the two locomotor testing days, animals were allowed to acclimate to the quiet dimly-lit testing room in their home cage for 30 mins. Then, animals were injected with VEH (1.0 mL/kg body weight, i.p.), placed into the circular runway chamber (Med Associates Inc.), and allowed to run freely for 60 mins. Immediately thereafter, animals were injected with their assigned KET dose, placed back into the locomotor chamber, and allowed to run freely for an additional 60 mins. Horizontal locomotion was quantified via photobeam breaks recorded in 10-min time bins by a computer fitted with software designed at Florida State University. Locomotor tests were performed between 0900–1300 hr. Testing equipment was cleaned with 70% ethanol after each use. Immediately following the final locomotor testing session, animals were terminated via rapid decapitation under stress-free conditions. Brain tissue was flash-frozen in dry ice-cooled 2-methylbutane and stored at −80°C until further processing.
2.5 Western immunoblotting
Frozen brains were coronally sectioned at −20°C into 200 μm-thick slices using a cryostat (Leica VT1000S). Bilateral tissue punches including NAc core and shell subregions were taken using 2mm diameter disposable biopsy punchers (Integra Miltex). Proteins were isolated from whole-cell lysates using a Trizol extraction and manufacturer’s protocol (ThermoFisher Scientific). Standard fluorescent Western immunoblotting procedures were performed as previously described [26]. Primary antibodies against ΔFosB (1:250; Cell Signaling Technology #14695S) and Dynamin I/II (1:1,000; Cell Signaling Technology #2342S) were used. Fluorescent secondary antibody against the primary antibody host (Donkey anti-rabbit (680nm), 1:10,000, LI-COR Biosciences #926-68023) was used. Relative protein abundance per sample was quantified against the adult neuronal housekeeping gene Dynamin I/II [57] using optical density readouts obtained from ImageJ software (National Institutes of Health).
2.6 Viral-mediated gene transfer
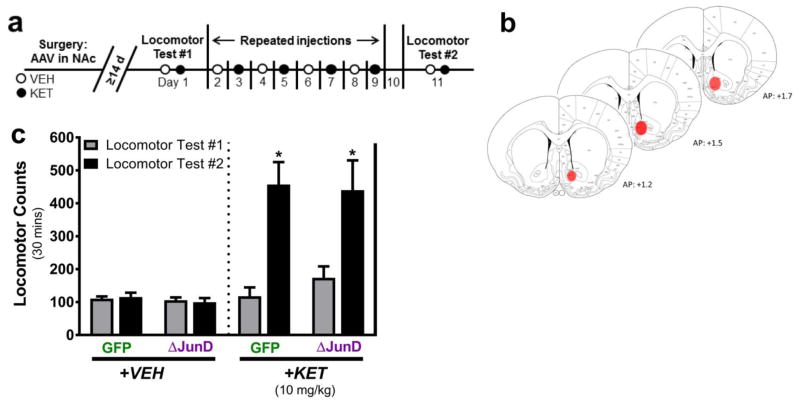
A total of 18 adult male rats (aged 8 weeks) were anesthetized with 4% isoflurane gas and prepared for stereotaxtic surgery under standard sterile conditions. Once a surgical plane of anesthesia was confirmed via toe pinch reflex failure, animals were maintained on 1–2% isoflurane gas. 24-gauge Hamilton syringes (Hamilton Company) were used to infuse purified high-titer recombinant adeno-associated virus serotype 2(rAAV2) expressing either green fluorescent protein (GFP) or ΔJunD under a 3X FLAG reporter (vectors generously donated by Dr. Eric J. Nestler; see Supplemental Fig. S1 for additional information) into bilateral NAc (1.0 μL per hemisphere, coordinates: AP +1.5mm, ML ±1.2mm from bregma; DV -7.6mm from skull surface) [58] at a rate of 0.2 μL/min, followed by an additional 5 min pause. Animals recovered in their home cages for ≥14 days prior to beginning behavioral experiments (Fig. 4a). This AAV vector has previously been shown to be nontoxic to neurons, maximally expressed after 10 days in rodents, and persistent for at least 6 months [52,59–62]. Immediately following the final locomotor testing session (experimental day 11), animals were terminally overdosed with sodium pentobarbital (100 mg/kg, i.p.) (Henry Schein Animal Health Inc.) and transcardially perfused with ice-cold 0.2M phosphate-buffered saline (PBS) pH 7.4, followed by ice-cold 4% paraformaldehyde in 0.2M PBS. Brains were post-fixed in 4% paraformaldehyde in 0.2M PBS at 4°C for 48 hrs. Fixed brain tissue was processed for verification of virus spread via fluorescent immunohistochemistry to visualize expression of GFP or FLAG reporter within NAc (Supplemental Methods, Supplemental Fig. S2). The rAAVs used in the current study were determined to have spread to both NAc core and shell subregions in all animals at the termination of the experiment (Fig. 4b, Supplemental Fig. 2b).
Fig. 4.
Virally overexpressing ΔJunD in the nucleus accumbens does not block locomotor sensitization to 10 mg/kg ketamine. a Experimental timeline for behavioral assessment of ΔJunD overexpression in NAc on repeated KET exposure. b Coronal sections representing regional rAAV spread to the NAc shell and core in all animals. c Summed locomotor activity for thirty minutes following the first and final VEH and KET injections. Animals infected with rAAV-GFP or rAAV-ΔJunD in the NAc behaviorally sensitized to the same degree following six repeated injections of 10 mg/kg KET. No differences in locomotion were observed following repeated VEH injections. Data are presented as mean ± SEM. *p<0.001 main effect of time, n = 8–10/group
2.7 Statistical analysis
Data were analyzed using GraphPad Prism 7.0 software and are represented as mean ± SEM. To examine within-subject effects of drug conditioning, CPP scores were analyzed using one-sample t tests (vs. theoretical mean = 0). To compare the sexes given each dose level, CPP scores were analyzed using two-way ANOVA (Sex x Dose) with post-hoc testing. CPP movement counts were analyzed using three-way ANOVA (Sex x Dose x Time) followed up by two-way ANOVA with post-hoc testing to investigate factor interactions where appropriate. For locomotion data, within-subject locomotor sensitization was analyzed via two-way repeated measures ANOVA (Time x Dose). Between-subject locomotion was analyzed via one-way ANOVA (acute dose effect within each sex) or two-way ANOVAs (Sex x Dose within each testing session). For Western blot data, optical density readouts of protein abundance were quantified, background optical density signal was subtracted per lane, and the ratio of the protein of interest (ΔFosB) over the housekeeping protein (Dynamin I/II) was calculated for each sample. To determine within-sex effect of dose, protein values were normalized to same-sex VEH-treated control mean value and analyzed using one-way ANOVA with post-hoc analysis. To examine potential sex differences in protein expression at each dose level, all within-lane normalized values were compared against the male VEH-treated mean value and analyzed using two-way ANOVA (Sex x Dose) with post-hoc analysis. For correlations, differential locomotor counts were calculated for each animal as: [(KET locomotor test #2 30 mins sum) – (VEH locomotor test #2 30 mins sum)] – [(KET locomotor test #1 30 mins sum) – (VEH locomotor test #1 30 mins sum)]. Individual subjects’ mean non-normalized protein values were correlated to their differential locomotor counts using Pearson’s r. Alpha (p) was set to 0.05 for all analyses. To control for false discovery rate, p values from all aforementioned analyses were analyzed simultaneously via the two-stage linear step-up method of Benjamini, Krieger, and Yekutieli with the FDR(Q) value set to 5%. This analysis set an adjusted p value threshold to 0.03368. Analyses with a p value below the threshold were classified as true discoveries and are reported as such. All post-hoc tests were performed where appropriate using the same Benjamini two-stage linear step-up method and p value threshold.
3. Results
3.1 Repeated ketamine’s effects on conditioned place preference
One-sample two-tailed t-tests against a theoretical mean CPP Score of 0 indicated that male rats treated with 10 mg/kg KET spent significantly more time in the drug-paired chamber after conditioning (t=2.734, df=7, *P<0.05). Females treated with 5.0 mg/kg KET achieved statistical significance in the one-sample t-test suggesting less time spent in the drug-paired chamber after conditioning, but this p-value failed to meet the FDR adjusted p-value cutoff threshold, indicating a false-positive discovery (t=2.438, df=7, *P=0.0449). All other treatment groups had no significant difference in time spent on the drug-paired chamber after conditioning (P>0.05). To determine sex- and dose- dependent CPP outcomes, data were analyzed by two-way ANOVA (Dose x Sex). A main effect of dose was indicated (F(3,56)=3.69, *P<0.05) such that 5.0 mg/kg KET scores were significantly less than 2.5 and 10.0 mg/kg scores (2.5 vs 5.0: mean diff.=63.98±22.36, t=2.861, **P<0.01; 10 vs 5.0: mean diff.=64.54±22.36, t=2.887, **P<0.01). No effect of sex and no interaction were indicated (P>0.05) (Figure 1b). To ensure the observed effects were not due to Dose x Sex interactions confounding locomotor activity, movement counts within the CPP chamber during injection-free CPP testing days 1 and 11 were analyzed. Three-way ANOVA (Sex x Dose x Time) indicated no effects of dose assignment or time (conditioning) (P>0.05). A main effect of sex was indicated (F(1,112)=61.03, ***P<0.001) such that females moved significantly more than males (mean diff.=355.8±41.32, t=8.611, ***P<0.001). There were no significant interactions of Dose x Sex, Dose x Time, or Sex x Dose x Time. A Sex x Time interaction was indicated (F(1,3)=6.335, *P<0.05). To investigate the nature of this interaction, data were consolidated by Dose and analyzed via two-way repeated measures ANOVA (Sex x Time). Post-hoc analysis indicated that females, but not males, moved significantly less within the CPP testing box as a result of conditioning (time) (Females: mean diff.=189.2±49.46, t=3.825, ***P<0.001) (Figure 1c).
3.2 Repeated ketamine’s effects on locomotor sensitization
3.2.1 Males
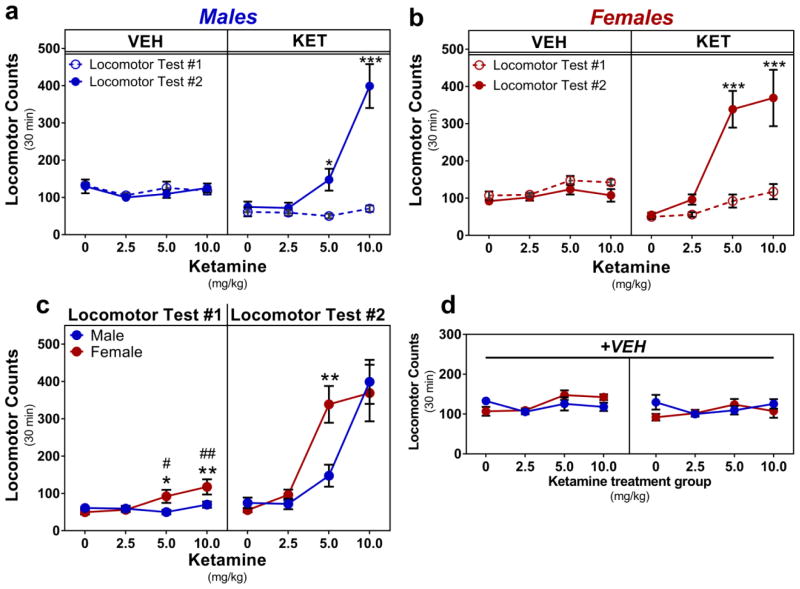
For males, upon summing locomotor counts for thirty minutes following the first and final VEH injections, two-way repeated measures ANOVA indicated no main effect of time, dose, or their interaction (P>0.05) (Fig. 2a, left). Upon summing locomotor counts for thirty minutes following the first and final KET injections, two-way repeated measures ANOVA indicated a main effect of time (F(1,26)=47.51, ***P<0.001), a main effect of dose (F(3,26)=20.93, ***P<0.001), and a significant interaction (F(3,26)=20.15, ***P<0.001). Post-hoc testing indicated that males repeatedly injected with 5.0 or 10 mg/kg KET ran significantly more during locomotor test #2 vs test #1 (5.0: mean diff.=97.43±33.92, t=2.872, *P<0.05; 10: mean diff.=329.1±33.92, t=9.702, ***P<0.001) (Fig. 2a, right).
Fig. 2.
Effects of repeated ketamine on locomotor sensitization. a Repeated treatment with 5.0 or 10 mg/kg KET elicited a locomotor sensitization in males, b and in females. No change in locomotor activity was observed following repeated VEH injections. c Female locomotor response to an acute dose of 5.0 or 10 mg/kg KET is greater than that of female VEH-treated controls and greater than that of males given the same dose (left). Female locomotor sensitization to repeated 5.0 mg/kg KET was significantly greater than that of males given the same dose (right). d No significant differences in locomotion were observed between sexes following repeated VEH injections. Data are presented as summed locomotor activity for 30 minutes following the first and the final VEH and KET injections, mean ± SEM. a–b display within-subject comparisons: *p<0.05, ***p<0.001 vs Locomotor Test #1. c–d display between-subject comparisons: #p<0.05, ##p<0.01 vs female VEH-treated mean, *p<0.05, **p<0.01 vs comparable male value. n = 7–8/group
3.2.2 Females
For females, upon summing locomotor counts for thirty minutes following the first and final VEH injections, two-way repeated measures ANOVA indicated main effects of time and dose assignment group, but not their interaction (P>0.05) (Fig. 2b, left). Upon summing locomotor counts for thirty minutes following the first and final KET injections, two-way repeated measures ANOVA indicated a main effect of time (F(1,27)=34.79, ***P<0.001), a main effect of dose (F(3,27)=18.53, ***P<0.001), and a significant interaction (F(3,27)=8.128, ***P<0.001). Post-hoc testing indicated that females repeatedly injected with 5.0 or 10 mg/kg KET ran significantly more during locomotor test #2 vs test #1 (5.0: mean diff.=246.5±45.30, t=5.442, ***P<0.001; 10: mean diff.=251.6±48.43, t=5.195, ***P<0.001) (Fig. 2b, right).
3.3 Sex-differential locomotor response to ketamine
3.3.1 Acute drug response
Upon summing locomotor counts for thirty minutes following the first KET injection, one-way ANOVA indicated no effect of dose on locomotion in males (P>0.05). In females, one-way ANOVA indicated an effect of dose on locomotion (F(3,27)=5.292, **P<0.01). Post-hoc testing indicated that females treated with 5.0 or 10 mg/kg KET ran significantly more than VEH-treated females (5.0: mean diff.=42.5±18.96, t=2.242, #P<0.05; 10: mean diff.=67.80±19.62, t=3.455, ##P<0.01) (Fig. 2c, left). To compare the effects of acute KET on locomotion between the sexes, data from locomotor test #1 were analyzed via two-way ANOVA, indicating statistically significant main effects of dose (F(3,53)=4.446, **P<0.01), sex (F(1,53)=5.228, *P<0.05), and their interaction (F(3,53)=3.395, *P<0.05). Post-hoc testing indicated that females treated with 5.0 or 10 mg/kg KET ran significantly more than males given the same dose (5: mean diff.=42.13±16.58, t=2.54, *P<0.05; 10.0: mean diff.= 47.57±17.13, t=2.778, **P<0.01) (Fig. 2c, left).
3.3.2 Repeated dosing response
To compare the effects of repeated KET on locomotion between the sexes, data from locomotor test #2 were analyzed via two-way ANOVA, indicating a statistically significant main effect of dose (F(3,53)=29.67, ***P<0.001) and a significant Dose x Sex interaction (F(3.53)=3.491, *P<0.05). No effect of sex was noted (P>0.05). Post-hoc testing indicated that females treated with 5.0 mg/kg KET ran significantly more than their male counterparts (mean diff.=191.2±54.83, t=3.487, **P<0.01) (Fig. 2c, right). Upon summing locomotor counts for thirty minutes following the first VEH injection, two-way ANOVA indicated a main effect of dose assignment group (F(3,53)=3.353, *P<0.05), but post-hoc testing indicated that no KET dose assignment group ran significantly more or less than VEH-treated controls (0 mg/kg vs each group: P>0.05). There was no main effect of sex and no Sex x Dose interaction (P>0.05). Upon summing locomotor counts for thirty minutes following the final VEH injection, two-way ANOVA indicated no effects of sex, dose, or their interaction (P>0.05) (Fig. 2d).
3.4 Repeated ketamine’s effects on ΔFosB protein expression in NAc
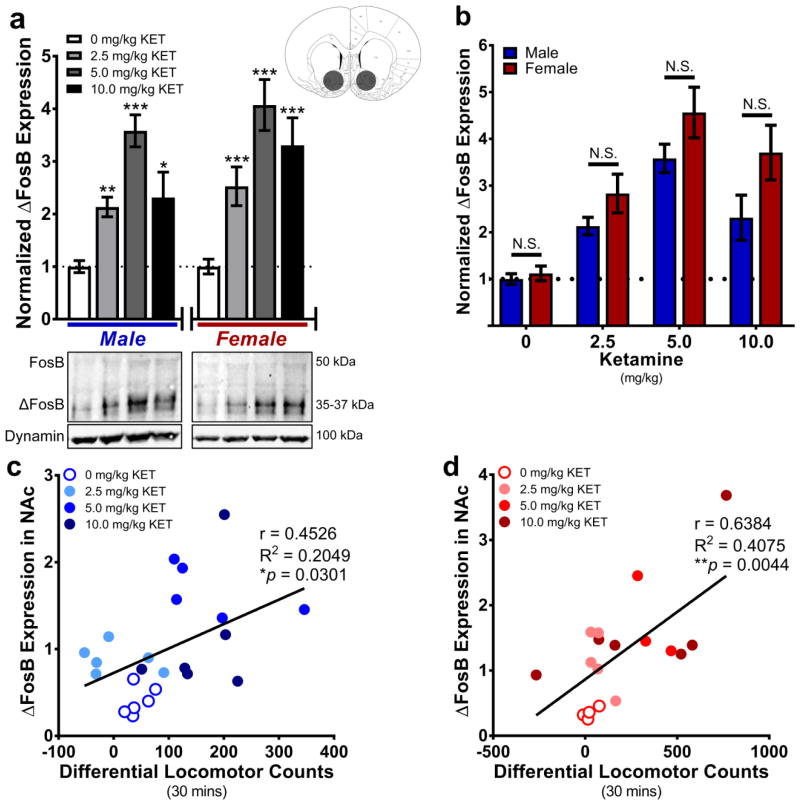
ΔFosB abundance in rat whole NAc tissue was quantified via Western immunoblot analysis. In males, one-way ANOVA indicated a main effect of drug treatment (F(3,88)=26.52, ***P<0.001). Post-hoc testing indicated that ΔFosB expression was greater in samples from all KET doses as compared to the VEH-treated control mean (2.5: mean diff.=2.085±0.2761, t=3.93, ***P<0.001; 5.0: mean diff.=2.582±0.2924, t=8.83, ***P<0.001; 10.0: mean diff.=1.313±0.3618, t=3.631, ***P<0.001). In females, one-way ANOVA indicated a main effect of drug treatment (F(3,80)=16.39, ***P<0.001). Post-hoc testing indicated that ΔFosB expression was significantly greater in all doses of KET as compared to the VEH-treated control mean (2.5: mean diff.=1.545±0.4686, t=3.297, **P<0.01; 5.0: mean diff.=3.072±0.4555, t=6.745, ***P<0.001; 10.0: mean diff.=2.307±0.5734, t=4.023, ***P<0.001) (Fig 3a). Next, all protein values were normalized to the male VEH-treated mean value. 2-way ANOVA indicated a main effect of sex (F(1, 170)=11.11, **P<0.01), such that ΔFosB expression was greater in females than in males (mean diff.= 0.798±0.207). A main effect of dose was also indicated (F(3,170)=38.05, ***P<0.001), such that ΔFosB expression from all KET treatment groups were greater than the VEH mean (2.5: mean diff.=1.397±0.2807; 5.0: mean diff.=3.041±0.2885; 10.0: mean diff.=1.955±0.3606). However, the Dose x Sex interaction was not statistically significant (P>0.05), indicating no sex differences in protein expression within each dose level (Fig. 3b). Within subjects, non-normalized ΔFosB expression values positively correlated with differential locomotor counts in males (r=0.4526, R2=0.2049, *P<0.05) (Fig. 3c) and in females (r=0.6384, R2=0.4075, **P<0.01) (Fig. 3d).
Fig. 3.
Repeated ketamine upregulates ΔFosB protein expression in the nucleus accumbens. a As compared to same-sex VEH-treated controls, ΔFosB protein expression was upregulated in the NAc of all KET-treated males (left) and females (right). Brain atlas inset indicates location of whole-NAc tissue punches. Below: Representative Western blots of FosB, ΔFosB, and Dynamin expression in male and female NAc tissue. b There were no sex differences in ΔFosB expression when all protein values were compared to the male control (VEH) mean value. c Differential (KET-evoked) locomotion positively correlated with NAc ΔFosB expression in males; d and in females. Data are presented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 vs same-sex VEH mean value, N.S. = not significant. n = 4–8/group
3.5 Effects of rAAV-ΔJunD in NAc on locomotor response to repeated 10 mg/kg KET
The experimental timeline was identical to that of the previous experiments, except for the addition of stereotaxic surgery to virally express rAAV-GFP or rAAV-ΔJunD in bilateral NAc 14 days prior to behavioral testing and withdrawal of CPP testing (Fig. 4a). rAAV spread within NAc was verified postmortem in all animals via fluorescent immunohistochemistry (Supplemental methods). Virus reliably spread to both NAc core and shell (Fig. 4b) and no animals were excluded from the study due to off-target injections. Upon summing locomotor counts for thirty minutes following the first and final VEH injections, two-way repeated measures ANOVA revealed no effect of time, rAAV, or their interaction on locomotion (P>0.05) (Fig. 4c, left). Upon summing locomotor counts for thirty minutes following the first and final KET injections, two-way repeated measures ANOVA revealed no main effect of rAAV or rAAV x Time interaction (P>0.05). A main effect of time was indicated (F(1,16)=21.78, ***P<0.001) such that locomotion was significantly greater during test #2 than test #1 (mean diff.=302.7±36.41) (Fig. 4c, right).
4. Discussion
Although subanesthetic KET promises outstanding antidepressant potential, its short-lived therapeutic benefits and documented abuse liability may hinder incorporation of a repeated-treatment regimen into a common MDD treatment protocol [6,18]. Previous findings have demonstrated female rodents’ heightened sensitivity to KET’s rapid antidepressant-like effects (as compared to males’) [26,27,29]. However, a dose-response study investigating alternating-day treatments to mimic favorable clinical protocols [7] has not yet been identified or contrasted between the sexes. Here, we treated adult male and female rats with a circa-threshold antidepressant-like dose of KET (2.5, 5.0, or 10 mg/kg) on alternating days for six treatments and assayed indicators of drug reward and craving.
CPP is an passive learning paradigm that assesses reward status to drug-paired contextual cues [39–41]. We found that 2.5 and 5.0 mg/kg KET failed to evoke a place preference in either sex when administered on alternating days, mirroring the same effect found with an intermittent (every 3–4 days) dosing schedule [33]. Taken together, these data suggest that doses of KET which approximate antidepressant-like response thresholds [26] are not rewarding when delivered repeatedly on multiple timescales. As a positive control, we found that 10 mg/kg KET, a supra-threshold dose for antidepressant-like responses, elicited a place preference in adult male rats, matching data reported in previous studies [34,36–38]. Interestingly, this effect was absent in females, suggesting a role for sex and/or gonadal hormones in mediating reward to 10 mg/kg KET. A recent study demonstrated that female mice treated daily with low-dose KET displayed more anxiety-like and less antidepressant-like behaviors than their male counterparts [63], further supporting that repeated KET modulates affect in a sex-dependent manner. Sex differences in subjective reward to drugs of abuse and the responsible functions of the mesolimbic dopamine system have been established [64–66], and estrogen is known to play a critical role within and outside of this circuit [65,67]. Two recent studies from our lab have suggested a role for circulating gonadal hormones in females to enhance pharmacological reward to subanesthetic KET: 1. Cyclic estrogen and progesterone are required for an acute pro-hedonic response to 2.5 mg/kg KET in the sucrose preference test [68], and 2. Naturally-cycling rats will intravenously self-administer low-dose KET when offered on phase-locked proestrus, but not diestrus days [32]. Given that the estrous cycle of a rat is approximately 4 days long, we did not control for estrous stage in the present study, as priority was placed on the alternating-day dosing regimen. Nonetheless, it is reasonable to speculate that female gonadal hormone status may tune the reward system’s response to subanesthetic KET. In support of this, women report more psychotropic effects than men given KET anesthesia [69], women report greater negative symptoms upon discontinuation of recreational KET [70], and female rats sleep longer than males given the same anesthetic dose of KET [71]. Despite its known reinforcing properties in humans [14,15,72], repeated antidepressant KET treatment seems to be well-tolerated overall in depressed patients [8,10,73–75], suggesting the long-term feasibility of repeated treatment protocols. However, additional research is needed to fully elucidate a role for gonadal hormones on the rewarding properties of repeated low-dose KET.
Locomotor sensitization (or “reverse tolerance”) is defined as increased responsiveness to the same concentration of drug after repeated exposure [43]. In rodents, locomotor sensitization indicates mesocorticolimbic dopamine reward system plasticity and increased motivation for drug-seeking [43,76–78]. Locomotor sensitization to KET has been established in male rodents at higher subanesthetic doses (12.5 – 50 mg/kg, i.p.) at longer treatment intervals (every 4–7 days) [79,80]. We recently demonstrated that female rats behaviorally sensitize to intermittent treatments with 2.5 mg/kg KET, but males require 5.0 mg/kg KET to display a sensitized response [33]. Here, we aimed to determine the nature of locomotor adaptation following six alternating-day treatments with antidepressant-like KET dosing in both sexes of rat in order to mimic common clinical treatment protocols [7]. In rodents, behavioral sensitization following repeated intermittent drug exposure is a well-documented mechanism implicated with increased motivation for drugs and escalation into drug addiction [43,44]. Intermittent repeated treatment with higher subanesthetic doses of KET is known to elicit robust locomotor sensitization in male rodents [79,80], but a similar effect is lacking in the human literature [81,82]. Because female rats are more sensitive than males to the acute locomotor-activating properties of NMDAR antagonists [83] as well as other behavioral outputs from low-dose KET [26,28], we analyzed locomotor responses to matched dose concentrations of KET in males and females. We found that when delivered every other day for six treatments, the threshold dose of KET required for rapid antidepressant-like response in female rats (2.5 mg/kg) [26] did not promote locomotor sensitization in either sex, suggesting this concentration may be safe to administer repeatedly to both sexes for medium- to long-term treatment regimens. However, the dose-response threshold for KET’s rapid antidepressant-like effects is elevated in male rats (≥5.0 mg/kg) [26]. We found that when given repeatedly, this concentration elicited a robust locomotor sensitization in both sexes, indicating the potential for adverse drug reactions. This finding is interesting when compared with recent data demonstrating that female rats given eight intermittent treatments with 2.5 or 5.0 mg/kg KET displayed a locomotor sensitization after a challenge dose of 5.0 mg/kg KET, but this same effect was found only in males given repeated 5.0 mg/kg KET [33]. Although the Strong study used a different dosing regimen and locomotor sensitization paradigm than the present experiment, both studies suggest that female rats are more sensitive than males to repeated KET’s locomotor-stimulant properties in a dose-dependent manner (Fig. 2C).
The transcription factor ΔFosB is stably upregulated in the NAc following chronic treatment with various drugs of abuse, where, as a component of the AP-1 complex with JunD, it is transcriptionally active and regulates relevant behavioral plasticities [45,46,48,50,84]. In the NAc, ΔFosB expression is known to mediate both natural and drug reward [51,60,85] as well as permit appropriate response to chronic antidepressant treatment [49]. We found that KET-sensitized hyperlocomotion positively correlated with NAc ΔFosB protein expression in both sexes. However, ΔFosB protein expression was also upregulated in the NAc of 2.5 mg/kg KET-treated rats who did not develop a locomotor sensitization. This suggests that unlike other drugs of abuse [60,87], KET-induced upregulation of this protein in the NAc is not sufficient to alter locomotor behavioral output. Additionally, an intriguing difference between the present experiment and data from Strong et al. [33] deserves recognition. When injected on diestrus phase-locked days (every ~4 days) for eight treatments, Strong et al. found no change in ΔFosB expression in the NAc of females given 2.5 or 5.0 mg/kg KET; males on a time-matched intermittent treatment paradigm had increased ΔFosB expression given 5.0 but not 2.5 mg/kg KET [33]. Taken together with the present data, this may suggest that alternating-day repeated KET dosing regimens are capable of increasing ΔFosB expression in the NAc whereas dose- by sex-specific intermittent dosing regimens are not. However, more research is needed to more fully elucidate roles for repeated KET dosing regimens, gonadal steroid hormones, and underlying neural molecular mechanisms on sex differences in KET-induced behavioral plasticity.
ΔJunD acts as a dominant-negative binding partner for ΔFosB, preventing AP-1-mediated transcription under control of this specific dimer [86]. Viral-mediated antagonism of ΔFosB in the NAc reduces behavioral sensitivity to various drugs of abuse, natural reward, and the antidepressant drug fluoxetine [49–51,59], whereas overexpression of ΔFosB in the NAc enhances locomotor response to acute cocaine, opiate reward, and promotes resilience against stress (akin to antidepressant treatment) [49,60,87]. Interestingly, we found that virally overexpressing ΔJunD in the NAc failed to block locomotor sensitization to repeated 10 mg/kg KET, suggesting ΔFosB in NAc may not be responsible for locomotor sensitization to KET. This finding may suggest that KET’s stimulant-like attributes may be mediated by some mechanism independent from the canonical ΔFosB/JunD binding in NAc. However, ΔFosB is known to differentially modify transcriptional activity patterns in D1- and D2-type medium spiny neurons in the striatum, as well as in the NAc shell and core subregions [84,88–90]. In the present study, it is unclear which cell types or NAc subregions upregulated their ΔFosB expression in response to KET treatment. Additionally, the ΔJunD virus utilized here is not cell-type-specific and spread to both the NAc core and shell, preventing a more thorough analysis of intra-NAc specificity. Future studies should investigate potential cell-type specificity of repeated KET’s effects on ΔFosB expression and whether a more targeted antagonist is capable of preventing the locomotor effects observed here. It is also entirely possible that one or more brain regions outside the NAc may house responsible mechanisms for the observed behavioral adaptations to repeated KET. Because ΔFosB expression increased in the NAc of 2.5 mg/kg KET-treated rats (absent of locomotor sensitization), one or more alternative molecular mechanisms or transcription factors within the NAc may be responsible for mediating KET’s addictive-like effects. Therefore, the present data do not rule out the possibility that KET-related locomotor sensitization can occur independently of AP-1-mediated transcription within the NAc. Further studies are needed to elucidate this and other mechanisms more clearly.
It is of importance to note that while previous studies have demonstrated a single acute dose of 2.5 mg/kg KET (i.p.) is not sufficient to elicit rapid antidepressant-like behavioral responses in male rats [26,28,68], it yet remains unclear whether this dose could achieve antidepressant-like efficacy when delivered repeatedly to males. Additionally, the estrous stage of female rodents in this study was not tracked; future studies of alternating-day treatment paradigms should utilize estrous stage information for valuable post-hoc correlational analyses. It should be noted that the KET used in these experiments is a racemic mixture of both R- and S- isomers. Given that the R- isomer lacks psychomimetic side effects [91], it is presumed that the S- isomer is primarily responsible for eliciting the observed results in the present study. However, due to known sex differences in KET metabolism in rodents [29,30], further research is needed to investigate individual roles of R- and S- isomers and their various metabolites on neural and behavioral adaptation following repeated KET dosing in both sexes. Finally, the rodents in this study did not undergo any manipulations or procedures to model depression, potentially limiting how the present results might be accurately extrapolated to the target population of treatment-resistant individuals with major depression. In order to better model the medium-to-long-term effects of subanesthetic KET on treatment-resistant depression, future studies should aim to investigate how repeated low-dose KET affects addictive-like behaviors in a validated preclinical model of depression. To better inform the field about drug-seeking motivational behaviors, future studies should include a thorough analysis of low-dose KET self-administration in both sexes.
5. Conclusions
Taken together, these results suggest the identification of a dose of KET (2.5 mg/kg, i.p.) that is safe to deliver up to six times on alternating days without apparent rewarding or sensitizing behavioral effects in both sexes of rat. Given that the primary pushback for utilizing KET as a first-choice treatment option for MDD has relied heavily upon the drug’s abuse liability and related adverse drug reactions at higher subanesthetic doses [1,92,93], this finding brings promise to the future of KET as a viable long-term treatment option. In line with modern healthcare’s growing advocacy of individualized treatment options for MDD [94], we propose the concept of titrating antidepressant KET concentration between the sexes as a sustainable form of putative a priori therapeutic drug monitoring. Given the sex-differential response to low-dose KET across multiple behavioral domains in rodents [30], future pre-clinical studies should focus on identifying relevant molecular adaptations following repeated treatment in both sexes independently to better inform and navigate potential pitfalls the drug may encounter in long-term clinical applications. Additionally, a precise role for the interaction between circulating female gonadal hormones and reward to subanesthetic KET warrants further investigation. As the present data in rodents suggests prudent sex-differential KET therapy protocols, future clinical studies should thoroughly examine the effects of repeated infusions within a range of established outpatient doses [2–4,8–10] to contrast male and female responses across affective, psychomotor, cognitive, and motivational domains.
Supplementary Material
Highlights.
Alternating-day low-dose ketamine is not rewarding.
Dose-dependent repeated ketamine sensitizes locomotor activity.
Females are more sensitive than males to ketamine’s stimulant properties.
ΔFosB expression in the nucleus accumbens correlates with sensitized locomotion.
Acknowledgments
Authors would like to thank Drs. Eric J. Nestler, Hossein Aleyasin, and Ezekiell Mouzon for their generous donation of rAAV vectors. Thanks to Ruth Didier and the FSU Confocal Microscopy Laboratory for assistance with confocal microscopy. KJS and MK designed the experiments. KJS carried out the data collection. KJS and SKS performed the data analysis. KJS and MK wrote the manuscript. KNW, CES, and MK proofread and provided editorial support for the article.
Funding This work was supported by the National Institutes of Health [grant number 5R01MH099085-05] (to MK).
Footnotes
Ketathesia® is comprised of ketamine in solution with ≤0.1% benzethonium chloride added as a preservative. Ketathesia® is supplied as ketamine hydrochloride in solution in a concentration equivalent to the mass of ketamine base rather than ketamine salt. While unlikely, these two features have the possibility of introducing a small margin of error when diluting Ketathesia® stock solution to the described concentrations as compared to concentrations made by research groups utilizing ketamine (salt) powder.
Conflict of interest The authors declare no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Domino EF. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 2.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 3.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate Carlos AJ. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605. doi: 10.4088/JCP.09m05327blu. http://www.ncbi.nlm.nih.gov/pubmed/20673547 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 5.Zarate CA, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanacora G, Heimer H, Hartman D, Mathew S, Frye M, Nemeroff C, Beale RR. Balancing the Promise and Risks of Ketamine Treatment for Mood Disorders. Neuropsychopharmacology. 2017;42:1179–1181. doi: 10.1038/npp.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB. A Consensus Statement on the Use of Ketamine in the Treatment of Mood Disorders. JAMA Psychiatry. 2017;74:399. doi: 10.1001/jamapsychiatry.2017.0080. [DOI] [PubMed] [Google Scholar]
- 8.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.03. [DOI] [PubMed] [Google Scholar]
- 9.Diamond PR, Farmery AD, Atkinson S, Haldar J, Williams N, Cowen PJ, Geddes JR, McShane R. Ketamine infusions for treatment resistant depression: a series of 28 patients treated weekly or twice weekly in an ECT clinic. J Psychopharmacol. 2014;28:536–544. doi: 10.1177/0269881114527361. [DOI] [PubMed] [Google Scholar]
- 10.Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol Psychiatry. 2013;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen KG, Lineberry TW, Galardy CW, Kung S, Lapid MI, Palmer BA, Ritter MJ, Schak KM, Sola CL, Hanson AJ, Frye MA. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27:444–450. doi: 10.1177/0269881113478283. [DOI] [PubMed] [Google Scholar]
- 12.Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry. 2016;173:816–826. doi: 10.1176/appi.ajp.2016.16010037. [DOI] [PubMed] [Google Scholar]
- 13.Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, Lim KO. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123–129. doi: 10.1016/j.jad.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction. 2000;95:575–590. doi: 10.1046/j.1360-0443.2000.9545759.x. [DOI] [PubMed] [Google Scholar]
- 15.Dillon P, Copeland J, Jansen K. Patterns of use and harms associated with non-medical ketamine use. Drug Alcohol Depend. 2003;69:23–28. doi: 10.1016/S0376-8716(02)00243-0. [DOI] [PubMed] [Google Scholar]
- 16.Morgan HL, Turner DC, Corlett PR, Absalom AR, Adapa R, Arana FS, Pigott J, Gardner J, Everitt J, Haggard P, Fletcher PC. Exploring the Impact of Ketamine on the Experience of Illusory Body Ownership. Biol Psychiatry. 2011;69:35–41. doi: 10.1016/j.biopsych.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan CJA, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 18.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Charney DS. Subanesthetic Effects of the Noncompetitive NMDA Antagonist, Ketamine, in Humans: Psychotomimetic, Perceptual, Cognitive, and Neuroendocrine Responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 20.C. for B.H.S. and Quality, Behavioral health trends in the United States: results from the 2014 National Survey on Drug Use and Health, 2015.
- 21.Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47:276–285. doi: 10.1080/02791072.2015.1072653. [DOI] [PubMed] [Google Scholar]
- 22.Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, Palmer BA, Frye MA. Potential risks of poorly monitored ketamine use in depression treatment. Am J Psychiatry. 2016;173:215–218. doi: 10.1176/appi.ajp.2015.15081082. [DOI] [PubMed] [Google Scholar]
- 23.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/S0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 24.Marcus SM, Young EA, Kerber KB, Kornstein S, Farabaugh AH, Mitchell J, Wisniewski SR, Balasubramani GK, Trivedi MH, Rush AJ. Gender differences in depression: Findings from the STARD study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Keers R, Aitchison KJ. Gender differences in antidepressant drug response. Int Rev Psychiatry. 2010;22:485–500. doi: 10.3109/09540261.2010.496448. [DOI] [PubMed] [Google Scholar]
- 26.Carrier N, Kabbaj M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 2013;70:27–34. doi: 10.1016/j.neuropharm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience. 2015;290:49–60. doi: 10.1016/j.neuroscience.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Sarkar A, Kabbaj M. Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biol Psychiatry. 2016;80:448–456. doi: 10.1016/j.biopsych.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang X-P, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Gould TD. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saland SK, Duclot F, Kabbaj M. Integrative analysis of sex differences in the rapid antidepressant effects of ketamine in preclinical models for individualized clinical outcomes. Curr Opin Behav Sci. 2017;14:19–26. doi: 10.1016/j.cobeha.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright KN, Strong CE, Addonizio MN, Brownstein NC, Kabbaj M. Reinforcing properties of an intermittent, low dose of ketamine in rats: effects of sex and cycle. Psychopharmacology (Berl) 2017;234:393–401. doi: 10.1007/s00213-016-4470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strong CE, Schoepfer KJ, Dossat AM, Saland SK, Wright KN, Kabbaj M. Locomotor sensitization to intermittent ketamine administration is associated with nucleus accumbens plasticity in male and female rats. Neuropharmacology. 2017;121:195–203. doi: 10.1016/j.neuropharm.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li F, Fang Q, Liu Y, Zhao M, Li D, Wang J, Lu L. Cannabinoid CB1 receptor antagonist rimonabant attenuates reinstatement of ketamine conditioned place preference in rats. Eur J Pharmacol. 2008;589:122–126. doi: 10.1016/j.ejphar.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 35.Rocha B, Ward A, Egilmez Y, Lytle D, Emmett-Oglesby M. Tolerance to the discriminative stimulus and reinforcing effects of ketamine. Behav Pharmacol. 1996;7:160–168. doi: 10.1097/00008877-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Kato H, Aoki T, Tsuda M, Narita M, Misawa M. Effects of the non-competitive NMDA receptor antagonist ketamine on morphine-induced place preference in mice. Life Sci. 2000;67:383–389. doi: 10.1016/S0024-3205(00)00639-1. [DOI] [PubMed] [Google Scholar]
- 37.Xu DD, Mo ZX, Yung KKL, Yang Y, Leung AWN. Individual and combined effects of methamphetamine and ketamine on conditioned place preference and NR1 receptor phosphorylation in rats. Neurosignals. 2006;15:322–331. doi: 10.1159/000127492. [DOI] [PubMed] [Google Scholar]
- 38.van der Kam EL, De Vry J, Tzschentke TM. 2-Methyl-6 (phenylethynyl)-pyridine (MPEP) potentiates ketamine and heroin reward as assessed by acquisition, extinction, and reinstatement of conditioned place preference in the rat. Eur J Pharmacol. 2009;606:94–101. doi: 10.1016/j.ejphar.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 39.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 40.Carlezon WA. Place conditioning to study drug reward and aversion. Humana Press; Totowa: 2003. 243. [DOI] [PubMed] [Google Scholar]
- 41.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/S0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 42.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71:670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247. doi: 10.1016/0165-0173(93)90013-p. http://www.ncbi.nlm.nih.gov/pubmed/8401595 . [DOI] [PubMed] [Google Scholar]
- 44.Kalivas PW. Recent understanding in the mechanisms of addiction. Curr Psychiatry Rep. 2004;6:347–351. doi: 10.1007/s11920-004-0021-0. [DOI] [PubMed] [Google Scholar]
- 45.Kelz MB, Chen J, Carlezon WA, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang Y-J, Marotti L, Self DW. Expression of the transcription factor ΔFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- 46.Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of ΔFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–1244. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 48.Nestler EJ, Barrot M, Self DW. ΔFosB: A Sustained Molecular Switch for Addiction. Colloq Pap. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vialou V, Robison AJ, LaPlant QC, Covington HE, III, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, III, Watts EL, Wallace DL. [Delta] FosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M, Wee S, Koob G, Turecki G, Neve R, Thomas M, Nestler EJ. Behavioral and structural responses to chronic cocaine require a feedforward loop involving ΔFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci. 2013;33:4295. doi: 10.1523/JNEUROSCI.5192-12.2013. http://www.ncbi.nlm.nih.gov/pubmed/23467346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J Neurosci. 2013;33:3434. doi: 10.1523/JNEUROSCI.4881-12.2013. http://www.ncbi.nlm.nih.gov/pubmed/23426671 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629–632. doi: 10.1038/nmeth.2935. [DOI] [PubMed] [Google Scholar]
- 54.N.R. Council. Guide for the care and use of laboratory animals. National Academies Press (US); Washington (DC): 2011. [DOI] [PubMed] [Google Scholar]
- 55.Kabbaj M. Neurobiological Bases of Individual Differences in Emotional and Stress Responsiveness: High Responders Low–Responders Model. Arch Neurol. 2004;61:1009–1012. doi: 10.1001/archneur.61.7.1009. [DOI] [PubMed] [Google Scholar]
- 56.Kabbaj M. Individual Differences in Vulnerability to Drug Abuse: The High Responders/Low Responders Model. CNS Neurol Disord - Drug Targets. 2006;5:513–520. doi: 10.2174/187152706778559318. [DOI] [PubMed] [Google Scholar]
- 57.Nakatax T, Iwamoto A, Noda Y, Takemura R, Yoshikura H, Hirokawa N. Predominant and developmentally regulated expression of dynamin in neurons. Neuron. 1991;7:461–469. doi: 10.1016/0896-6273(91)90298-E. [DOI] [PubMed] [Google Scholar]
- 58.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Acad. Press; San Diego: 1997. [u.a.] [DOI] [PubMed] [Google Scholar]
- 59.Been LE, Hedges VL, Vialou V, Nestler EJ, Meisel RL. ΔJunD overexpression in the nucleus accumbens prevents sexual reward in female Syrian hamsters, Genes. Brain Behav. 2013;12:666–672. doi: 10.1111/gbb.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ. An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- 61.Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/S0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCown TJ, Xiao X, Li J, Breese GR, Jude Samulski R. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 63.Thelen C, Sens J, Mauch J, Pandit R, Pitychoutis PM. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav Brain Res. 2016;312:305–312. doi: 10.1016/j.bbr.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 64.Becker JB. Gender Differences in Dopaminergic Function in Striatum and Nucleus Accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/S0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 65.Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22–28. doi: 10.1016/j.drugalcdep.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/S0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 67.Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, Martz JR, Nutsch VL, Dominguez JM. Estradiol in the preoptic area regulates the dopaminergic response to cocaine in the nucleus accumbens. Neuropsychopharmacology. 2016;41:1897–1906. doi: 10.1038/npp.2015.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saland SK, Schoepfer KJ, Kabbaj M. Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Sci Rep. 2016;6 doi: 10.1038/srep21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knox JW, Bovill JG, Clarke RS, Dundee JW. Clinical studies of induction agents. XXXVI: Ketamine. Br J Anaesth. 1970;42:875–885. doi: 10.1093/bja/42.10.875. [DOI] [PubMed] [Google Scholar]
- 70.Chen W-Y, Huang M-C, Lin S-K. Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst Abuse Treat Prev Policy. 2014;9:39. doi: 10.1186/1747-597X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Douglas BG, Dagirmanjian R. The effects of magnesium deficiency of ketamine sleeping times in the rat. Br J Anaesth. 1975;47:336–340. doi: 10.1093/bja/47.3.336. [DOI] [PubMed] [Google Scholar]
- 72.Morgan C, Mofeez A, Brandner B, Bromley L, Curran H. Ketamine impairs response inhibition and is positively reinforcing in healthy volunteers: a dose–response study. Psychopharmacology (Berl) 2004;172:298–308. doi: 10.1007/s00213-003-1656-y. [DOI] [PubMed] [Google Scholar]
- 73.Wan L-B, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry. 2015;76:247–252. doi: 10.4088/JCP.13m08852. [DOI] [PubMed] [Google Scholar]
- 74.Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blier J, Blier P, Zigman D. On the safety and benefits of repeated intravenous injections of ketamine for depression. Biol Psychiatry. 2012;72:e12. doi: 10.1016/j.biopsych.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 76.Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 77.Wolf ME. NMDA receptors and behavioural sensitization: beyond dizocilpine. Trends Pharmacol Sci. 1999;20:188–189. doi: 10.1016/S0165-6147(99)01356-5. [DOI] [PubMed] [Google Scholar]
- 78.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–65. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry. 2008;63:178–183. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 80.Uchihashi Y, Kuribara H, Morita T, Fujita T. The repeated administration of ketamine induces an enhancement of its stimulant action in mice. Jpn J Pharmacol. 1993;61:149–151. doi: 10.1254/jjp.61.149. [DOI] [PubMed] [Google Scholar]
- 81.Cho H-S, D’Souza DC, Gueorguieva R, Perry EB, Madonick S, Karper LP, Abi-Dargham A, Belger A, Abi-Saab W, Lipschitz D, Bennet A, Seibyl JP, Krystal JH. Absence of behavioral sensitization in healthy human subjects following repeated exposure to ketamine. Psychopharmacology (Berl) 2005;179:136–143. doi: 10.1007/s00213-004-2066-5. [DOI] [PubMed] [Google Scholar]
- 82.Perry EB, Cramer JA, Cho H-S, Petrakis IL, Karper LP, Genovese A, O’Donnell E, Krystal JH, D’Souza DC, Group YKS. Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 2007;192:253–260. doi: 10.1007/s00213-007-0706-2. [DOI] [PubMed] [Google Scholar]
- 83.Honack D, Loscher W. Sex differences in NMDA receptor mediated responses in rats. Brain Res. 1993;620:167–170. doi: 10.1016/0006-8993(93)90287-W. [DOI] [PubMed] [Google Scholar]
- 84.Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han M-H, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. ΔFosB Induction in Striatal Medium Spiny Neuron Subtypes in Response to Chronic Pharmacological, Emotional, and Optogenetic Stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jorissen HJMM, Ulery PG, Henry L, Gourneni S, Nestler EJ, Rudenko G. Dimerization and DNA-Binding Properties of the Transcription Factor ΔFosB. Biochemistry. 2007;46:8360–8372. doi: 10.1021/bi700494v. [DOI] [PubMed] [Google Scholar]
- 87.Colby CR, Whisler K, Steffen C, Nestler EJ, Self DW. Striatal Cell Type-Specific Overexpression of Delta FosB Enhances Incentive for Cocaine. J Neurosci. 2003;23:2488. doi: 10.1523/JNEUROSCI.23-06-02488.2003. http://www.jneurosci.org/cgi/content/abstract/23/6/2488 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandra R, Lobo MK. Beyond Neuronal Activity Markers: Select Immediate Early Genes in Striatal Neuron Subtypes Functionally Mediate Psychostimulant Addiction. Front Behav Neurosci. 2017;11:112. doi: 10.3389/fnbeh.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. ΔFosB differentially modulates nucleus accumbens direct and indirect pathway function. Pnas. 2013;110:1923–8. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nestler EJ. ΔFosB: a transcriptional regulator of stress and antidepressant responses. Eur J Pharmacol. 2015;753:66–72. doi: 10.1016/j.ejphar.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang C, Shirayama Y, Zhang J, Ren Q, Yao W, Ma M, Dong C, Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanacora G, Schatzberg AF. Ketamine: Promising Path or False Prophecy in the Development of Novel Therapeutics for Mood Disorders? Neuropsychopharmacology. 2015;40:259–267. doi: 10.1038/npp.2014.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang C, Hashimoto K. Rapid antidepressant effects and abuse liability of ketamine. Psychopharmacology (Berl) 2014;231:2041–2042. doi: 10.1007/s00213-014-3543-0. [DOI] [PubMed] [Google Scholar]
- 94.Niitsu T, Fabbri C, Bentini F, Serretti A. Pharmacogenetics in major depression: A comprehensive meta-analysis. Prog Neuro-Psychopharmacology Biol Psychiatry. 2013;45:183–194. doi: 10.1016/j.pnpbp.2013.05.011.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.