Abstract
Social housing has been shown to attenuate the motivation for cocaine in female, but not male rats. Here we investigate the potential mechanisms mediating the effect of social housing on the response to methamphetamine (METH). Female rats were individually or socially (pair) housed. The dopamine (DA) response to an acute METH infusion (0.3 mg/kg, i.v.) was investigated using in vivo microdialysis in the nucleus accumbens with or without oxytocin (OT; 0.3 mg/kg, i.p.) 30 minutes prior to METH. The effects of social housing and OT on self-administered METH (0.06 mg/kg/inf) was investigated.
The METH-induced DA response was higher in individually housed compared to socially-housed females. On the other hand, individually housed females had a significantly higher breaking point (BP) than socially-housed females. Two weeks of OT treatment reduced BP in both groups. Reinstatement to METH was more pronounced in isolates compared to socially-housed females. More of the socially-housed females had very low BP than did the individually housed females. OT was most effective in reducing BP in females with moderate to high BP, irrespective of housing conditions.
These data show that social housing attenuates escalation of METH intake and reinstatement of METH seeking in female rats, and that chronic OT treatment can reduce motivation for METH.
Keywords: addiction, dopamine, microdialysis, social support
1.1 Introduction
Methamphetamine (METH) is a highly addictive psychomotor stimulant. Its abuse is associated with serious medical, social, and economic consequences In general women are younger when they start using METH and show higher rates of dependence compared to men (for review [1]). Female rats also show greater escalation of METH intake and METH seeking compared to males [2], suggesting a biological basis for the sex differences in METH use patterns.
A supportive social environment has been shown to have beneficial effects on a wide variety of disorders, like depression, psychological distress and drug dependence [3–6]. The presence of social support reduces drug use, decreases stress levels, and increases the chance of a favorable outcome after treatment [1,5,7,8].
Behavioral therapy, support groups and community-based programs are important components of successful treatment of drug dependence [2,9]. None of these can be mimicked exactly in preclinical studies of drug abuse, however, a social component can be implemented. Social- and group-housing can reduce the intake of heroin, morphine, and amphetamine in male rats [10–12]. Social-housing also reduces motivation for cocaine or morphine in females [10,13]. Interestingly, social housing in male rats does not decrease cocaine self-administration [12,13]. The different effects of cocaine and amphetamine may be due to different neural effect of the two drugs [14–16].
Escalation of drug use is an important characteristic of drug taking that leads to dependence and this can be modeled in self-administration models. Rats exposed to long-access cocaine or METH self-administration show escalation of drug intake compared to short-access animals, with females showing higher intake than males [2,17]. Cocaine self-administration on a progressive ratio schedule with limited training also results in an increase in the motivation to self-administer cocaine [13,18,19].
On the other side of the addiction problem, relapse after abstinence is a major problem for drug-dependent patients. Exposure to cues associated with drug-taking behavior or stress results in craving and increases the risk of relapse [3,4,20–22]. Preclinically, this is modeled using extinction and reinstatement of drug seeking after cues, drugs or stress [23–25]. Just as for self-administration behavior, the social environment has been shown to attenuate reinstatement of drug seeking after abstinence in rats [9,26,27].
The protective effect of a social environment on drug taking behavior is hypothesized to be mediated by oxytocin (OT). OT is released during positive social interactions and cuddling [28–32]. OT also attenuates responses to stress [28,29,33]. In preclinical studies OT reduces METH intake and reinstatement in male rats [34–37], although sex differences have also been reported, with OT being more effective in females [38,39]. OT induces neural activation differently in male and female rats and OT receptor binding also differs between the sexes [40,41]. These sex differences in the patterns of activation may mediate the sex differences in the behavioral effects of OT.
One of the mechanisms by which OT could affect drug-taking behavior is by modulating dopamine signaling in the mesolimbic reward system [42–45]. OT originating from the hypothamus projects to the ventral tegmental area and nucleus accumbens (NAc) and modulates dopamine (DA) release in the NAc [46–48]. Additionally, OT infused in NAc core reduces METH-induced conditioned place preference, indicating a possible interaction between OT and DA in mediating rewarding properties of drugs of abuse [34,36,49].
In the current experiments we investigated the effect of social (pair) housing of female rats on the DA response in the NAc to METH using in vivo microdialysis. We had previously shown that social housing decreases the motivation to self administer cocaine [13]. Here we determined if social housing would attenuate the motivation to self-administer METH and/or reduce METH and cue-induced reinstatement of drug taking. Finally, we assessed whether OT, after long-term METH self-administration, would reduce the motivation for METH in single housed vs. socially housed female rats.
2.1 Methods
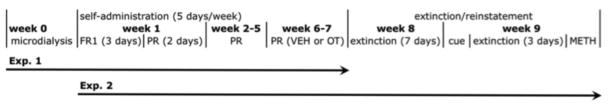
Female Long Evans rats (age 42 days; Charles Rivers, Portage, MI) were housed individually (n=48) or in pairs (n=48 pairs), on a 14:10 light:dark cycle (lights off at 7.00 hr). Food and water were available ad libitum. After 2 weeks of single or social housing, all of the individually housed animals and one of each of the pairs underwent surgery, the unoperated animals remained in the cages as social partners for the operated animals. All experiments were conducted in accordance with the National Institutes of Health (NIH) guidelines on laboratory animal use and care, using a protocol approved by the University Committee on Use and Care of Animals. The timeline for the experiments described below is illustrated in Figure 1,
Figure 1.
Time line for microdialysis, METH self-administration, extinction and reinstatement sessions.
2.2 Experiment 1
48 Rats received buprenorphine (0.02 mg/kg; s.c.) 30 minutes before anesthetization with ketamine/dexmedetomidine solution (60 and 0.25 mg/kg, resp., i.p.). Animals received indwelling intravenous jugular catheters connected to a back port with the outlet protected by a stainless steel cover (for details see [1]) and a microdialysis guide canula (SciPro, MAB 6.14 G) aimed at the NAc (from bregma; AP; +1.7 mm, ML; +0.8 mm, DV; −6.8 mm from top of skull), a dummy probe extending 2 mm below the guide with a dummy stylet was protected by a stainless steel cover (adapted from [1]).
After surgery, the animal’s intravenous catheter was flushed with bacteriostatic saline containing heparin (30 U/ml) and gentamicin (3 mg/ml) to prevent clotting and infection, respectively. Starting two days after surgery, catheters were flushed daily with bacteriostatic saline containing heparin (20 U/ml) and gentamicin (3 mg/ml).
2.2.1 Microdialysis
Animals (N=30) were allowed to recover for a week (animals were excluded if catheters were not patent, microdialysis probes leaked or the animals did not survive the two surgeries). The day before mircodialysis the dummy probe was removed and the microdialysis probe (SciPro MAB 6.14.2; 2 mm 15 kDalton’s cut-off PES membrane) was inserted and secured in place. The animal was placed in the microdialysis chamber (Med Associates) with food and water ad lib. The probe was flushed for 60 minutes with Ringers (1.5.ml/min; [50]). The animal was disconnected with the probe left inserted and covered with the protective stainless steel cover and returned to its home cage.
The next morning animals were reconnected to the microdialysis pump and swivel. Collection of baseline samples started 1 hour later. Six 10-minute baseline samples were collected, after which animals received OT (0.3 mg/kg ip.) or vehicle (sterile saline, 0.1 ml/kg, i.p.), 3 more 10-minute samples were collected followed by an infusion with METH (0.3 mg/kg, i.v.). Subsequently, six 2.5-minute samples were collected followed by six 10-minute samples. After collection of the last sample animals were removed from the chamber, the probe was removed and replaced by the dummy, and the rats were placed back in their home cage.
2.1.2 HPLC
DA content of the dialysate was determined using high performance liquid chromatography (HPLC) with electrochemical detection (C-18 ESA (ESA Biosciences, Chelmsford, MA) column (HR-80X3.2, 3 μm particle size, 80 mm length; mobile phase: 75 mM NaH2PO4, 0.2 mM EDTA, 2.0 mM octane sulfonic acid and 11% methanol (apparent pH 4.6–4.8), isocratic flow rate of 0.7ml/min). Potentials of +150 mV and −100 mV were applied to a dual coulometric analytical cell (ESA model #5014B) and the latter potential was used to determine DA (ESA Coulochem Detector II/III). A standard curve was run during each day of HPLC analysis and unknown sample values determined based on peak height determined as previously described [50].
2.3 Experiment 2a
48 rats (24 individually housed and 24 paired) received an injection of buprenorphine (0.02 mg/kg; s.c.) 30 minutes before they were anesthetized with isoflurane. Animals received indwelling intravenous jugular catheters connected to a back port. Surgical procedures were as described for experiment 1, except animals did not receive an intracranial guide implant for microdialysis.
2.3.1 Self-administration
Self-administration was performed in standard operant chambers (Med Associates, Inc., St. Albans, VT) with 2 nose poke holes. The active hole was lit, indicating availability of METH. A nose poke in the active hole resulted in a 50-μl infusion of METH delivered over 2.8 seconds accompanied by inactivation of the stimulus light in the active hole. Each infusion was followed by a 5-second timeout period, during which time nose pokes were recorded yet had no consequences. Catheters were flushed with 0.1 ml of sterile saline prior to each session and with Gentamicin/Heparin flushing solution following each self-administration session and on weekends. Animals were weighed daily. Estrous cycle was monitored via daily vaginal lavage. Catheter patency was checked weekly using a solution of 0.05–0.1 ml of Methohexital sodium (Brevital) (7.5 mg/ml) in sterile saline.
All self-administration sessions occurred daily for 5 days a week between 9.00 and 18.00 hr. Fixed Ratio (FR) 1: The week after microdialysis, animals were transported to the SA chambers and allowed to nose-poke for METH (0.06 mg/kg/50 μl inf,) using a FR1 schedule of reinforcement for 3 hours, or a maximum of 15 infusions. The number of infusions was limited to reduce individual differences in exposure prior to the start of the Progressive Ratio (PR) schedule. Animals were subjected to the FR1 schedule for 3 consecutive days. PR: After 3 days on the FR1, animals were transferred to a PR schedule of reinforcement. The PR schedule escalated through an exponential series of response ratios: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208…. [51]. The number of infusions, nose pokes in the active, inactive hole, and Breaking Point (highest number of responses after 4 hours or if 1 hour elapsed without an infusion) were recorded. During weeks 6 and 7 of self-administration animals received OT (0.3 mg/kg, ip.) or vehicle (sterile saline; 0.1 ml/kg, i.p.) daily 30 minutes prior to the start of self-administration on the PR schedule (Figure 1).
2.3.2 Experiment 2b: Extinction/reinstatement
Following self-administration all rats underwent 7 days of 1 hour extinction (active and inactive nose-pokes were recorded but had no consequences). For cue-induced reinstatement, the same schedule was used as for the PR, the active hole was lit, and when the requirement for an infusion was reached, the nose-poke light went out for 5 seconds, and the animals received an infusion with saline. For METH-induced reinstatement, at the start of the session the animals received an i.v. infusion with 0.18 mg/kg. Nose-pokes had no consequences.
2.4 Statistics
Statistical analyses were performed with SPSS (version 21). Normality was examined via normal Q-Q plots and the Shapiro-Wilk test and homoscedasticity via Levene’s test, Microdialysis and weight gain data were analyzed with repeated measures ANOVA with sample as repeated measure and housing (individually housed or paired) and treatment (VEH or OT) as between subject variables.
Self-administration data from experiment 1 and 2 were pooled after determining that there were no differences between the groups. Since the data did not show a normal distribution, groups were compared using Kruskal-Wallis tests, conducted separately for each week or day. Friedman’s tests for related samples were conducted separately within each housing and treatment group to identify changes in behavior over time.
Rats from both the individually housed and paired group showed a wide range of rates of self-administration, therefore rats were also assigned to 3 different categories based on BP during week 5. Rats in the upper (≥ 133.25) and lower (≤17) quartile range of the median BP for all animals during week 5 were assigned to the ‘HIGH and ‘LOW’ group respectively, this strategy provided the most obvious split for the top and bottom clusters of animals. The rest were labeled intermediate (INT). A Chi-square test was used to evaluate group differences.
3.1 Results
3.2 DA measured during Microdialysis
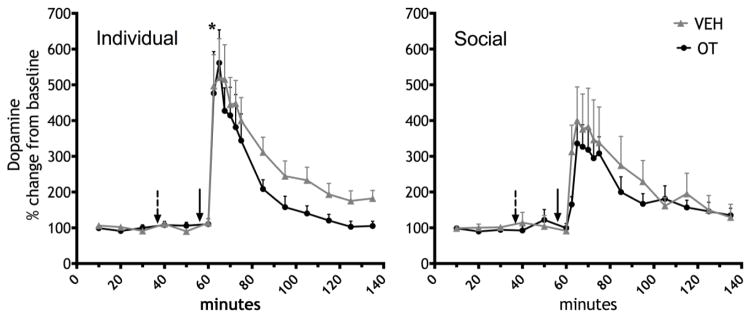
As shown in Figure 2, socially housed females showed a significantly lower increase in DA in dialysate from the NAc compared to individually housed females during the first sample after METH treatment (F1,25=6.951, p=0.014). Baseline DA values did not differ between individually housed and socially housed females (individually housed: 0.059 ± 0.019 pg/μl; socially housed: 0.059 ± 0.013 pg/μl). Following METH delivery there was a significant effect of time and a time by housing interaction (F11,275 =28.685, p≤0.001 and F11,275 =3.004, p=0.04 resp.; Figure 2). There was no effect of OT on either baseline or METH-induced DA in dialysate.
Figure 2.
Dopamine response to an i.v. infusion of METH (0.3 mg/kg in 100 μl) after VEH or OT (0.3 mg/kg, ip.). The broken arrow depicts the time point of the VEH or OT injection, the solid arrow shows the time point of the METH infusion. (*p<0.05, difference between individual (left) and social (right) housing conditions for that time point).
3.2.1 METH Self-Administration
3.1.2.1 FR1
No significant effects of housing were found in number of infusions, active, inactive nose pokes, and duration on the FR1 schedule. Estrous cycle did not significantly affect FR1 responding on any of the measured parameters.
3.2.2 Breaking point
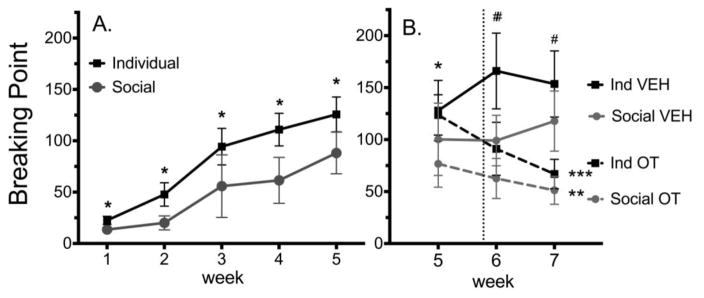
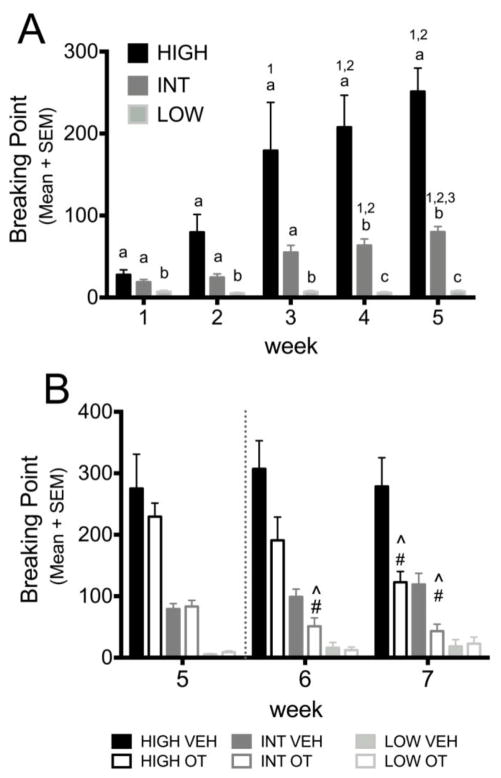
As illustrated in Figure 3A, BP increased over time for both individually housed and socially housed females (k=5, n=40, F=65.260, p≤0.001 and k=5, n= 39, F=38.394, p≤0.001 resp.; (Figure 3A). Individually housed females showed significantly higher breaking points than socially-housed females during the first 5 weeks (n=79) (week 1: U=573, p=0.042; week 2: U=374.5, p≤0.001; week 3: U=380.5, p≤0.001; week 4: U=386, p≤0.001; week 5: U=487.5, p=0.004; Figure 3A).
Figure 3.
The effect of social housing (A) and OT (B) on breaking point (BP) for METH (0.06 mg/kg/inf).
A. BP +/− SEM *p<0.05, difference between Individually and Socially housed animals for that week.
B. BP +/− SEM OT treatment was initiated at the dashed line. There were no differences in the groups that received OT vs. Vehicle (VEH). *p<0.05, difference between individually housed and socially housed animals, #p<0.05, difference between VEH and OT within housing conditions,** p<0.05, different from week 5 during week 7, *** p<0.05, different from week 5 during weeks 6 & 7.
3.2.3 Effect of Oxytocin on METH self-administration
As illustrated in Figure 3B, OT treatment of individually housed females significantly decreased BP’s compared with VEH-treated individual females during weeks 6 and 7 (n=40) (U=293, p=0.011 and U=294, p=0.01 resp.). Socially housed VEH and OT treated females did not differ from each other in BP’s in weeks 6 or 7
The 2 weeks of treatment with VEH did not change BP’s in individually housed or socially housed females. In contrast, OT treated females did show a change in BP over time (individually housed OT: k=3, n=21, F=21.434, p≤0.001; paired OT: k=3, n=19, F=7.684, p=0.021), with individually housed OT-treated females showing an attenuated BP during week 6 and 7 compared to week 5 (p=0.03 and p≤0.001 resp.) and socially housed females showing a decrease in BP from week 5 to 7 (p=0.017; Figure 3B).
3.2.4 Extinction and reinstatement of METH self-administration
There was no significant effect of prior treatment with OT on extinction or reinstatement so VEH and OT treated animals were pooled and analyses are by housing condition only.
3.2.5 Extinction
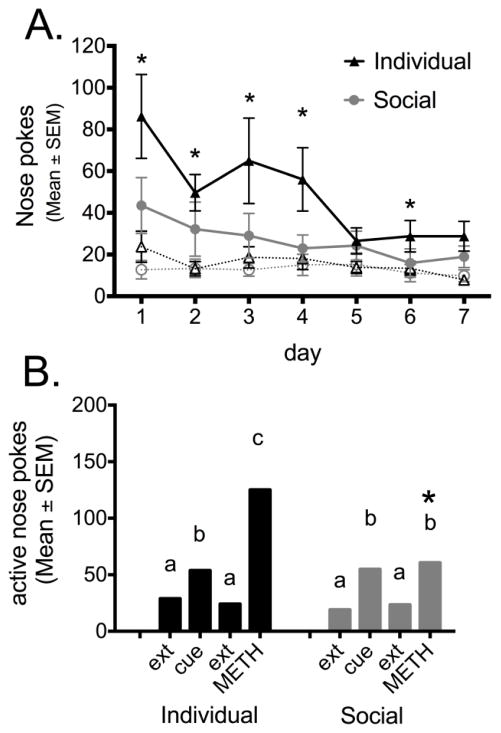
Individually housed females had significantly more active pokes than socially housed rats during the first 4 days of extinction, and again on day 6 (day 1: U=102, p=0.008; day 2: U=105.5, p=0.01; day 3: U=91.5, p=0.003; day 4: U=125 p=0.044; day 6: U=126.5, p=0.047). The number of active pokes decreased during the 7 days of extinction for both individually housed and socially housed rats (k=7, n=19, F=51.536, p≤0.001 and k=7, n=21, F=17.853, p=0.007 resp; Figure 4A).
Figure 4.
Extinction and Reinstatement of Responding for METH. (A) The number of nose-pokes over 7 days of extinction training (solid line: active nose pokes, dotted line: inactive nose pokes). (*p<0.05, difference between individually and socially housed females). B) Cue and METH-induced (0.18 mg/kg) reinstatement of drug seeking measured with the number of active nose pokes. Different letters above the bars indicate a significant difference between days, within each housing condition (a,b,c) *p<0.05: difference between individually and socially housed females.
3.2.6 Reinstatement
The cue significantly increased nose-poking in the cue-lit hole in both individually housed and socially housed rats when compared to the last day of extinction (k=2, n=19, F=162, p=0.007 and k=2, n=21, F=187, p≤0.001 resp.; Figure 4B).
A non-contingent METH infusion significantly increased active NP’s in both individually housed and socially housed females compared to the extinction session of the day before (k=2, n=19, F=183.5, p≤0.001 and k=2, n=21, F=210.5, p=0.001 resp.). Individually housed, but not socially housed rats, showed a significantly higher level of reinstatement in response to METH compared to the cue (k=2, n=19, F=184, p≤0.001 and k=2, n=21, F=74, p=0.397; Figure 4B).
Individually housed and socially housed females did not differ in number of active nose-pokes during the last day of extinction and cue-induced reinstatement, however, individually housed females showed a significant higher level of poking after a infusion with METH compared to socially housed females (U=112.5, p=0.017; Figure 5B).
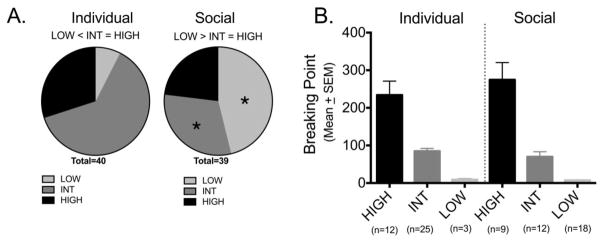
Figure 5.
Individual variability in BP varied with housing conditions. (A) Pie charts of the distribution of the number of animals within the individually and socially housed groups falling in the HIGH, INT (Intermediate) or LOW category. *p<0.05; significant difference between the number of individually housed and socially housed rats in the corresponding categories. (B) Group means (± SEM) of breaking points for individually housed and socially housed females within each category.
We did not observe an effect of estrous cycle on cue or METH induced reinstatement, however the number of animals for each stage of the cycle within each housing condition was limited.
3.3 Individual variability
One of the keys to making a difference in our understanding of addiction is to determine why it is that some individuals are more susceptible than others. Analysis of the data by housing group conditions indicated that the social housing group had a greater proportion of animals that did not escalate BP during extended METH self-administration, compared with the individually housed animals. We investigated the proportion of animals in the upper and lower quartiles for BP at week 5 (all animals combined) and found a significant overall Chi-square (χ2=17.93, DF=2, p=0.0001). There were more socially-housed rats than individually housed females were in the LOW category (18 vs. 3 rats respectively) and there were more individually housed than socially housed females in the Intermediate category (25 vs. 12 rats resp). As shown in Figure 5A, there was no difference in number of individually housed and socially housed animals in the HIGH category (12 vs. 9 rats).
As shown in Figure 5B, among individually housed females, there were significantly fewer females in the LOW category than the HIGH and Intermediate categories (low<intermediate=high). Among the paired females there were significantly more rats in the LOW category compared to the Intermediate and HIGH categories (LOW>Intermediate=HIGH). Within each category there were no differences between individually housed and pair-housed females (HIGH: n=21, U=72.50, p=0.193; INT: n= 37, U=107.5, p=0.170; LOW: n= 21, U= 19.5, p=0.471; Figure 5B), therefore they were pooled by category for further analyses.
3.3.1 Motivation for METH
Animals in the three motivational categories exhibited different trajectories for increasing BP over time, as well as differential sensitivity for reduced BP after OT treatment (Figure 6). As can be seen in Figure 6A, animals in the HIGH and INT categories exhibited an increase in BP over time (n=37, F=63.849, p≤0.001 and n=21, F=55.162, p≤0.001 resp.), while those in the LOW category showed no significant change over time (n=21 F=8.211, p=0.084). Breaking points differed between the 3 categories (week 1: H(2)=15.872,, week 2: H(2)=26.259, week 3: H(2)=27.548, week 4: H(2)=50.328, week 5: H(2)=65.552, p≤0.001 for all).
Figure 6.
Effect of Individual Variability on the effect of OT on BP. (A). Group means ± SEM for each week. Different letters above the bars indicate a significant difference between categories within a week (a,b,c p<0.005). 1,2 and 3 indicate a significant difference from week 1, 2 and 3 resp. within a category (1,2,3p<0.05). (B) Effects of OT treatment within that week and category (#p<0.005), ^ depict a significant difference from week 5 within that category and treatment group (^p<0.05).
3.3.2 Treatment effects
OT treatment decreased motivation for METH in the HIGH and INT groups of females (Figure 6B)). For the females in the HIGH category there was a significant effect of week (k=3, n=11, F=14.727, p=0.001) and a significant decrease in BP between week 5 and 7 (p≤0.001; Fig. 6B). In the OT treated INT group, a significant effect of week was also found (k=3, n=18, F=20.111, p≤0.001). OT decreased motivation during weeks 6 and 7, compared to week 5 (p=0.005 and p≤0.001 resp.). No effect of OT was found in the LOW category, no doubt due to the low BP prior to treatment.
OT treatment attenuated the motivation to self-administer METH in the HIGH females compared to VEH treated counterparts during week 7 (U=93, p=0.006. In the INT category animals, OT significantly attenuated BP during weeks 6 and 7 compared to VEH (U=271, p=0.002 and U=285, p≤0.001 resp.). In the LOW category no differences were found in BP between VEH and OT treated groups (Figure 7B). None of the VEH treated females, irrespective of category, showed a significant change in BP during week 6 and 7 compared to week 5.
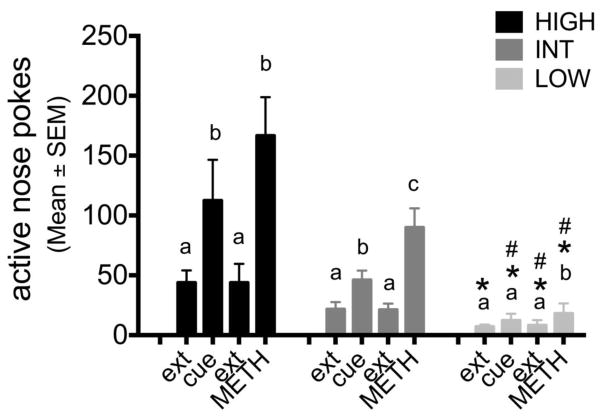
Figure 7.
Cue- and METH-induced reinstatement per category. Different letters above the bars indicate a significant change between days, within each category (a,b p<0.05). *p<0.05: significant difference between HIGH and LOW, #p<0.05: INT vs. LOW
3.3.3 Extinction/reinstatement
Not all animals underwent extinction and reinstatement testing. For those that did, the distribution of the number of individually housed and socially housed females into motivational categories was similar to the distribution of the total population (individually housed HIGH: n=6, social HIGH: n=5, individually housed INT: n=14, social INT: n=7, individually housed LOW: n=2, social LOW: n=11).
Extinction
In the HIGH and INT groups, active nose pokes during the 7 days of extinction differed between days (k=7, n=10, F=29.022, p≤0.001 and k=7. n=20, F=52.0, p≤0.001 resp.) with AP’s decreasing over time. The LOW category showed no change over time (k=7, n=10, F=1.880, p=0.930).
Reinstatement
During reinstatement, there was a significant effect of motivational category on the number of nose pokes to the cue (F=17.015, p≤0.001) with the HIGH and INT groups showing significantly more NP’s in response to the cue that the LOW group. An effect of category was also found for the response to the METH infusion (F=17.892, p≤0.001), with LOW animals showing significantly fewer nose-pokes than the INT and the HIGH groups (Figure 7).
Rats in the HIGH groups reinstated nose-poking in response to the cue and the METH infusion (k=2 n=10, F=54, p=0.007 and k=2 n=10, F=53, p=0.009 resp.) as did the INT group, where both the cue and METH increased active nose-poking compared to prior extinction day (k=2 n=20, F=193, p=0.001 and k=2 n=20, F=201, p≤0.001 resp.). However the INT group responded significantly more to the METH infusions than the cue (k=2 n=20, F=191.5, p=0.001; Figure 7).
Animals in the LOW category showed no significant increase in responding to the cue (k=2 n=10, F=26, p=0.262), but did respond to the METH infusion (k=2 n=10, F=51.5, p=0.014). No difference was found between responding to the cue and the METH infusion.
4.1 Discussion
This is the first study to investigate individual differences in the effects of social housing and OT on the motivation for METH in female rats. We found that social housing reduced the motivation to self-administer METH in female rats and this was mediated primarily by a shift in the proportion of animals with INT motivation to LOW motivation for METH. Interestingly, social housing did not reduce the proportion of animals with HIGH motivation for METH even though OT treatment was effective in decreasing motivation for METH in HIGH and INT rats. The effect of OT was most marked in individually housed females, but all animals with HIGH or INT motivation for METH initially exhibited decreased motivation after OT treatment. Social housing did not reduce the risk for relapse, as individually housed and socially housed females did not differ in cue-induced reinstatement, however individually housed females did exhibit greater METH-induced responding during reinstatement. Thus, we find that social housing and OT interact with individual differences to modulate the motivation for METH.
The protective effects of social housing on METH self-administration found in the current study corroborates our previous data that social housing is protective in females for cocaine self-administration. On a PR schedule, individually housed females showed a greater motivation to self-administer cocaine than did socially-housed females [52]. The finding that social housing did not affect the proportion of animals with HIGH motivation may reflect different qualities of social experience among the pairs. The weight gain (as an index of stress) was the same for HIGH, INT and LOW pairs (data not shown) so there did not seem to be greater stress for the HIGH group, but this does not rule out other more subtle individual differences in social housing pairs.
Social housing also caused a significant attenuation of the DA response to a METH challenge in naïve animals, while acute treatment with OT had no effect on METH-induced DA overflow in the NAc. This is to our knowledge the first study to investigate the effect of systemic OT on DA release in the NAc using microdialysis. It is thought that systemic OT mediates its effects on drug related behaviors by interacting with DA in the NAc, where systemic OT attenuates reinstatement-induced neuronal activation [34]. Social housing had a small but significant effect of attenuating METH-induced DA release in the results reported here, but we did not see the expected result of decreased METH-induced dopamine release in the NAc with a single treatment with systemic OT. Effects of a single dose of OT have been seen on neural activity in the NAc, but at a dose significantly higher (1.0 mg/kg) than the 0.3 mg/kg used here [34]. It is also possible that OT has peripheral effects that take repeated exposures to induce behavioral changes, for example peripheral OT may be influencing the vagus nerve to indirectly activate the brain [40]. This is something that requires additional research to determine.
Only a small percent of systemically administered OT is thought to cross the blood brain barrier [53]. Nevertheless, an i.p. injection of OT increased both plasma and brain OT levels significantly within 30 minutes [54]. Furthermore, an OT antagonist delivered to the NAc has been found to prevent the effects of systemic OT to reduce the motivation for METH as well as the effect of systemic OT to attenuate reinstatement of METH seeking [34]. It is, therefore, unlikely that the absence of an OT effect on DA release is due to a lack of increased OT in the brain. Isolation is a stressor for female rats [13,55] and this could have affected DA signaling in the NAc [56,57]. The lack of an acute effect of OT on DA release is consistent with the self-administration data in this study, as we did not see an immediate effect of OT on motivation.
Nevertheless, systemic treatment with OT reduced motivation to self-administer METH in both individually housed and socially housed females as demonstrated by reduced BP. OT was more effective in individually housed animals, and significantly reduced BP during the first week of treatment in contrast to socially housed females where a reduction in motivation was only seen in the second week. These data corroborate previously published results showing that OT reduces motivation for METH [34,37,38] and goes on to show that repeated treatment with a low dose of OT can have a sustained effect in the most vulnerable female rats.
Chronic METH self-administration has been reported to result in dysregulation of the OT system with increased plasma OT and slight decreases in OT receptor immunoreactivity in the NAc of male rats [58]. On the other hand, in male mice OT receptor binding was up-regulated in the amygdala and hypothalamus after chronic METH treatment, but no effect in the NAc [59]. Whether the differences in findings are due to the different modes of METH delivery or species differences is not known. Repeated cocaine administration has been found to decrease OT levels in plasma and several brain regions of male rats [60]. We suggest that those females with higher levels of METH intake are more likely to have dysregulated oxytocinergic systems, and this may explain why it took longer for OT treatment to have an effect in the HIGH motivation females. Future research will investigate whether the dysregulation occurs at the OT receptors, OT distribution or a combination of effects.
Addiction generally starts with recreational use or self-medication which transitions into compulsive drug-seeking and dependence. Fortunately, only a small percentage of people who have used drugs develop dependence [61]. Even people who use regularly for years show different levels of use [62]. Divergent drug self-administration patterns have also been observed in rats. Rats with equal cocaine intake scored differently on addiction-like criteria, with only 17% scoring high on all criteria [63,64]. In our paradigm divergent patterns of ‘drug-use’ also became apparent, even with all animals having equal opportunity to self-administer METH. Limited training before exposure to the PR schedule results in escalation of METH intake, as has been shown for cocaine [18,19]. An additional component of this paradigm is the emergence of individual variation in motivation consistent with the clinical condition of addiction in humans.
Women appear to be more sensitive to the addictive effects of psychostimulants [1,65], which has been duplicated in preclinical studies [13,66–70]. We have previously shown that when rats have a choice between self-administering cocaine or a palatable food pellet more females than males will prefer cocaine, thus resulting in a higher group average for cocaine intake. When looking at just cocaine preferring animals, however, males and females had similar behavior (Perry et al., 2013). Thus, we propose that sex differences in apparent vulnerability for high motivation for addiction-like behavior is driven, at least in part, by the proportion of individuals within a group with greater vulnerability. This suggests that sex differences in drug-taking are based on a population sex difference [71,72]. Whether there are also underlying sex differences in the mechanisms mediating addiction remains to be determined.
Summarizing, social housing of female rats protected against escalation of BP and increased METH use, although it did not affect the risk for reinstatement of drug seeking. Individually housed females showed greater BP than socially housed females. When examining individual differences, the number of individually housed and socially housed females that showed HIGH levels of motivation was similar, while a higher percentage of socially housed females fell into the LOW category. Thus, the group difference was mostly driven by increases in the proportion of LOW females in the socially housed group compared to the individually housed females.
Social housing did not enhance the effects of OT to reduce the motivation to self-administer METH. This may be related to the fact that many showed lower levels of motivation already, which could not be further reduced by OT. These results do not necessarily rule out an interaction between social environment and OT. In the current experiments animals were either individually housed or socially housed throughout self-administration. It is possible that in animals exposed to drugs in isolation, social housing or environmental enrichment in combination with OT would enhance the efficacy of the social environment in reducing reinstatement.
Long-term self-administration on a schedule where rats have to increase their effort for each infusion results in a natural divergence of animals with low and high motivation for drugs of abuse. Social housing of females protects against the risk of enhanced motivation to self- administer drugs. Understanding how a positive social environment can reduce the susceptibility to drug abuse and examining the differences between high and low risk subpopulations, may provide important insight in the underlying neurobiology of addiction-vulnerability.
Acknowledgments
We gratefully acknowledge support from the National Institute on Drug Abuse R01DA012677, R21-DA-032747 and R01DA039952.
Footnotes
Conflict of Interest: the authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- 2.Reichel CM, Chan CH, Ghee SM, See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl) 2012;223:371–80. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendler KS, Myers J, Prescott CA. Sex differences in the relationship between social support and risk for major depression: a longitudinal study of opposite-sex twin pairs. Am J Psychiatry. 2005;162:250–6. doi: 10.1176/appi.ajp.162.2.250. [DOI] [PubMed] [Google Scholar]
- 4.Glazer SS, Galanter M, Megwinoff O, Dermatis H, Keller DS. The role of therapeutic alliance in network therapy: a family and peer support-based treatment for cocaine abuse. Subst Abus. 2003;24:93–100. doi: 10.1080/08897070309511537. [DOI] [PubMed] [Google Scholar]
- 5.Amagasa S, Fukushima N, Kikuchi H, Oka K, Takamiya T, Odagiri Y, et al. Types of social participation and psychological distress in Japanese older adults: A five-year cohort study. PLoS ONE. 2017;12:e0175392. doi: 10.1371/journal.pone.0175392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green KM, Doherty EE, Reisinger HS, Chilcoat HD, Ensminger M. Social integration in young adulthood and the subsequent onset of substance use and disorders among a community population of urban African Americans. Addiction. 2010;105:484–93. doi: 10.1111/j.1360-0443.2009.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havassy BE, Wasserman DA, Hall SM. Social relationships and abstinence from cocaine in an American treatment sample. Addiction. 1995;90:699–710. doi: 10.1046/j.1360-0443.1995.90569911.x. [DOI] [PubMed] [Google Scholar]
- 8.Dobkin PL, De CM, Paraherakis A, Gill K. The role of functional social support in treatment retention and outcomes among outpatient adult substance abusers. Addiction. 2002;97:347–56. doi: 10.1046/j.1360-0443.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–17. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raz S, Berger BD. Social isolation increases morphine intake: behavioral and psychopharmacological aspects. Behav Pharmacol. 2010;21:39–46. doi: 10.1097/FBP.0b013e32833470bd. [DOI] [PubMed] [Google Scholar]
- 11.Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology (Berl) 2016;233:3201–10. doi: 10.1007/s00213-016-4368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozarth MA, Murray A, Wise RA. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol Biochem Behav. 1989;33:903–7. doi: 10.1016/0091-3057(89)90490-5. [DOI] [PubMed] [Google Scholar]
- 13.Westenbroek C, Perry AN, Becker JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvia CP, Jaber M, King GR, Ellinwood EH, Caron MG. Cocaine and amphetamine elicit differential effects in rats with a unilateral injection of dopamine transporter antisense oligodeoxynucleotides. Neuroscience. 1997;76:737–47. doi: 10.1016/s0306-4522(96)00399-5. [DOI] [PubMed] [Google Scholar]
- 15.Taheri S, Xun Z, See RE, Joseph JE, Reichel CM. Cocaine and methamphetamine induce opposing changes in BOLD signal response in rats. Brain Res. 2016;1642:497–504. doi: 10.1016/j.brainres.2016.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell J, Sever PS. The biochemical pharmacology of abused drugs. I. Amphetamines, cocaine, and LSD. Clin Pharmacol Ther. 1974;16:625–38. doi: 10.1002/cpt1974164625. [DOI] [PubMed] [Google Scholar]
- 17.Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology (Berl) 2011;214:557–66. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oleson EB, Roberts DCS. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts DCS, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–24. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- 21.O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–31. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 22.Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 23.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–76. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 25.Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl) 2010;208:211–22. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauvet C, Lardeux V, Goldberg SR, Jaber M, Solinas M. Environmental enrichment reduces cocaine seeking and reinstatement induced by cues and stress but not by cocaine. Neuropsychopharmacology. 2009;34:2767–78. doi: 10.1038/npp.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofford RS, Darna M, Wilmouth CE, Dwoskin LP, Bardo MT. Environmental enrichment reduces methamphetamine cue-induced reinstatement but does not alter methamphetamine reward or VMAT2 function. Behav Brain Res. 2014;270:151–8. doi: 10.1016/j.bbr.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–94. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 29.Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–98. doi: 10.1016/S0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- 30.Wei D, Lee D, Cox CD, Karsten CA, Peñagarikano O, Geschwind DH, et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci U S A. 2015;112:14084–9. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–84. doi: 10.1016/S0018-506X(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 32.Neumann I. Brain oxytocin: a key regulator of emotional and social behaviors in both females and males. J Neurroendocrin. 2008;20:858–65. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso C, Ellenbogen MA, Serravalle L, Linnen A-M. Stress-induced negative mood moderates the relation between oxytocin administration and trust: evidence for the tend-and-befriend response to stress? Psychoneuroendocrinology. 2013;38:2800–4. doi: 10.1016/j.psyneuen.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G. Oxytocin acts in nucleus accumbens to attenuate methamphetamine seeking and demand. Biol Psychiatry. 2017;81:949–58. doi: 10.1016/j.biopsych.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferland CL, Reichel CM, McGinty JF. Effects of oxytocin on methamphetamine-seeking exacerbated by predator odor pre-exposure in rats. Psychopharmacology (Berl) 2016;233:1015–24. doi: 10.1007/s00213-015-4184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baracz SJ, Everett NA, McGregor IS, Cornish JL. Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats. Addict Biol. 2016;21:316–25. doi: 10.1111/adb.12198. [DOI] [PubMed] [Google Scholar]
- 37.Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS. Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats. Neuropharmacology. 2010;58:38–43. doi: 10.1016/j.neuropharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 38.Cox BM, Young AB, See RE, Reichel CM. Sex differences in methamphetamine seeking in rats: impact of oxytocin. Psychoneuroendocrinology. 2013;38:2343–53. doi: 10.1016/j.psyneuen.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leong K-C, Zhou L, Ghee SM, See RE, Reichel CM. Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats. Exp Clin Psychopharmacol. 2016;24:55–64. doi: 10.1037/pha0000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumais KM, Kulkarni PP, Ferris CF, Veenema AH. Sex differences in neural activation following different routes of oxytocin administration in awake adult rats. Psychoneuroendocrinology. 2017;81:52–62. doi: 10.1016/j.psyneuen.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Baracz SJ, Cornish JL. Oxytocin modulates dopamine-mediated reward in the rat subthalamic nucleus. Horm Behav. 2013;63:370–5. doi: 10.1016/j.yhbeh.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Romero-Fernandez W, Borroto-Escuela DO, Agnati LF, Fuxe K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol Psychiatry. 2013;18:849–50. doi: 10.1038/mp.2012.103. [DOI] [PubMed] [Google Scholar]
- 44.Baracz SJ, Cornish JL. The neurocircuitry involved in oxytocin modulation of methamphetamine addiction. Front Neuroendocrinol. 2016;43:1–18. doi: 10.1016/j.yfrne.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Bisagno V, Cadet JL. Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin. Behav Pharmacol. 2014;25:445–57. doi: 10.1097/FBP.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur J Neurosci. 2007;26:1026–35. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- 47.Owesson-White C, Belle AM, Herr NR, Peele JL, Gowrishankar P, Carelli RM, et al. Cue-Evoked Dopamine Release Rapidly Modulates D2 Neurons in the Nucleus Accumbens During Motivated Behavior. J Neurosci. 2016;36:6011–21. doi: 10.1523/JNEUROSCI.0393-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–86. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baracz S. Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus. Behav Brain Res. 2011;228:185–93. doi: 10.1016/j.bbr.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 50.Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22–8. doi: 10.1016/j.drugalcdep.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Meth. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 52.Westenbroek C, Ter Horst GJ, Roos MH, Kuipers SD, Trentani A, den Boer JA. Gender-specific effects of social housing in rats after chronic mild stress exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:21–30. doi: 10.1016/s0278-5846(02)00310-x. [DOI] [PubMed] [Google Scholar]
- 53.Mens WB, Witter A, van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–9. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- 54.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–93. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 55.LeFevre J, McClintock MK. Isolation accelerates reproductive senescence and alters its predictors in female rats. Horm Behav. 1991;25:258–72. doi: 10.1016/0018-506x(91)90055-m. [DOI] [PubMed] [Google Scholar]
- 56.Rougé-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–7. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 57.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–8. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 58.Baracz SJ, Parker LM, Suraev AS, Everett NA, Goodchild AK, McGregor IS, et al. Chronic Methamphetamine Self-Administration Dysregulates Oxytocin Plasma Levels and Oxytocin Receptor Fibre Density in the Nucleus Accumbens Core and Subthalamic Nucleus of the Rat. J Neuroendocrinol. 2016:28. doi: 10.1111/jne.12337. [DOI] [PubMed] [Google Scholar]
- 59.Zanos P, Wright SR, Georgiou P, Yoo JH, Ledent C, Hourani SM, et al. Chronic methamphetamine treatment induces oxytocin receptor up-regulation in the amygdala and hypothalamus via an adenosine A2A receptor-independent mechanism. Pharmacol Biochem Behav. 2014;119:72–9. doi: 10.1016/j.pbb.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Sarnyai Z, Szabó G, Kovács GL, Telegdy G. Opposite actions of oxytocin and vasopressin in the development of cocaine-induced behavioral sensitization in mice. Pharmacol Biochem Behav. 1992;43:491–4. doi: 10.1016/0091-3057(92)90182-f. [DOI] [PubMed] [Google Scholar]
- 61.Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26:479–88. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 62.Hser Y-I, Huang D, Brecht M-L, Li L, Evans E. Contrasting trajectories of heroin, cocaine, and methamphetamine use. J Addict Dis. 2008;27:13–21. doi: 10.1080/10550880802122554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–8. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 64.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 65.Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SRB. Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gend Med. 2010;7:402–13. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–37. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 67.Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Current Topics in Behavioral Neurosciences. 2011;8:73–96. doi: 10.1007/7854_2010_93. [DOI] [PubMed] [Google Scholar]
- 68.Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS ONE. 2013;8:e79465. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perry AN, Westenbroek C, Jagannathan L, Becker JB. The Roles of Dopamine and α1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology. 2015;40:2696–704. doi: 10.1038/npp.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37:2605–14. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–63. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Becker JB, McClellan M, Reed BG. Sociocultural context for sex differences in addiction. Addict Biol. 2016;21:1052–9. doi: 10.1111/adb.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]