Abstract
Background
Circulating tumour cells (CTCs) are a potential surrogate for distant metastasis and are considered a useful clinical prognostic marker for metastatic colorectal cancer (mCRC). This prospective study evaluated the preoperative CTC count as a prognostic factor for pulmonary metastasectomy in mCRC patients.
Methods
Seventy-nine mCRC patients who underwent curative-intent pulmonary metastasectomy were included. Preoperatively, 7.5 mL of peripheral blood from each patient was quantitatively evaluated for CTCs with the CellSearch® system. The clinical significance of CTC count was evaluated according to Kaplan-Meier analyses and log-rank test. Multivariate analyses of the perioperative variables were performed.
Results
The distribution of CTC counts were as follows; 0 in 66 patients (83.5%), 1 in eight patients (10.1%), 2 in three patients (3.8%), and 3 and 6 in one patient (1.3%). The patients with multiple CTCs (CTC count ≥2) had significant shorter disease-free survival (DFS) (P=0.005, median DFS; 19.8 vs. 8.6 months) and overall survival (OS) (P=0.035, median DFS; not reached vs. 37.8 months), respectively. Multivariate analysis showed the patients with multiple CTCs had elevated risk of recurrence [hazard ratio (HR), 3.28; 95% confidence interval (CI), 1.24–8.67; P=0.017].
Conclusions
The detected rate of CTCs was quite low in mCRC patients who underwent pulmonary metastasectomy. The patient with multiple CTCs had shorter DFS in this study. The larger prospective clinical study is needed to establish the meaning of CTC in mCRC candidate for pulmonary metastasectomy.
Keywords: Colorectal cancer, circulating tumour cell (CTC), pulmonary metastasectomy
Introduction
Pulmonary metastasectomy for metastatic colorectal cancer (mCRC) is widely performed, and many studies have shown that it provides a favourable prognosis in selected patients (1-5). Studies have revealed potential prognostic factors, such as the number of metastases (6-8), the site of metastases (7,9), thoracic nodal involvement (5), the disease-free interval (DFI) (6,8) and the preoperative serum level of carcinoembryonic antigen (CEA) (3-8,10,11). However, reliable predictors of long-term survival remain controversial (12,13).
Circulating tumour cells (CTCs) are a potential surrogate for distant metastasis and is a novel and promising biomarker in the diagnosis and therapy of various malignancies (14-19). It has been reported that the CTC count measured using the semi-automated, quantitative CellSearch® CTC detection system (Veridex LLC, Raritan, NJ, USA) was a useful clinical predictive marker for the therapeutic response and survival in mCRC patients (18,19). We hypothesised that the CTC count would also be a useful clinical prognostic factor for mCRC patients undergoing pulmonary metastasectomy. The aim of this prospective study, therefore, was to evaluate the clinical value of the CTC count for mCRC patients undergoing pulmonary metastasectomy.
Methods
Patients
Consecutive mCRC patients who underwent pulmonary metastasectomy for pulmonary metastasis at the Department of Thoracic Surgery, Hyogo College of Medicine Hospital, between November 2007 and March 2012 were enrolled in this prospective study. The study was approved by the Institutional Review Board of Hyogo College of Medicine Hospital (No. 715) and written informed consent for participation was obtained from each patient.
The inclusion criterion was that the patients met our surgical criteria for curative-intent pulmonary metastasectomy, which were as follows: (I) the pulmonary metastasis was technically resectable; (II) the general and functional risks were tolerable; (III) the primary tumour was controlled, and (IV) there was no detectable extra-thoracic lesion (13,20). We did not take into consideration the number and location of pulmonary metastases, provided complete resection was possible. The surgical procedure was chosen by surgeons according to the location and number of pulmonary metastases. Complete resection was defined as the absence of tumour cells at the surgical margin of the resected lung, examined both macroscopically and histologically.
Complete clinical data were collected for all the enrolled patients, including clinical history, a physical examination, and laboratory and radiographic studies. In addition, the serum level of CEA was measured preoperatively using a commercially available electrochemiluminescence immunoassay system (Roche Diagnostics K.K., Tokyo, Japan), following the manufacturer’s instructions. The manufacturer’s suggested cut-off value of CEA that discriminates between non-malignant disease and a malignant tumour was 5 ng/mL. After the operation, the patients who enrolled this study routinely received a radiological examination to evaluate tumour recurrence.
Blood sampling and CTC counting
Immediately prior to the operation, a 7.5-mL sample of peripheral blood was drawn in a CellSaver tube (Veridex LLC, Raritan, NJ, USA) and submitted for a CTC test. CTCs were evaluated using the semi-automated CellSearch system, following the manufacturer’s protocol as described previously (20-23). In brief, CTCs were immune-magnetically captured using ferroparticles coupled to a monoclonal antibody against the epithelial cell adhesion molecule (EpCAM), and the CTCs-enriched samples were then stained with 4',6-diamidino-2-phenylindole (DAPI) and an anti-cytokeratin antibody conjugated with phycoerythrin (CK-PE). Contaminated white blood cells were excluded by negative selection for CD45. Stained cells were then analyzed on a florescent microscope using the Cell Track Analyzer II (Veridex LLC, Johnson & Johnson, NJ, USA). The criteria for each cell to be defined as a CTC are as follows: round to oval morphology, a visible DAPI-positive nucleus, positive cytokeratin staining in the cytoplasm, and negative staining for CD45. The number of CTCs per 7.5 mL of peripheral blood was presented as the CTC count. The evaluations were performed blind to the clinical characteristics of the patients, and the CTC counts were evaluated after surgery within 72 hours. The results of CTC counts did not affect the indication of pulmonary metastasectomy.
Statistical analysis
Two clinical outcome measures were used for the analysis. Disease-free survival (DFS) was defined as the time from the pulmonary metastasectomy to tumour progression or censorship. Overall survival (OS) was defined as the time from the pulmonary metastasectomy to death or censorship. The survival difference correlation according to CTC count was evaluated using Kaplan-Meier curves. The clinical impact of the CTC count was then evaluated based on multivariate analyses including preoperative variables. Survival curves were compared using the log-rank test. Parameters significant at the 0.05 level for DFS and OS were applied to a first proportional hazards model regression analysis of survival (the Cox model) using forward stepwise selection. Results are expressed as hazard ratios (HRs) with 95% confidence interval (CI).
The statistical analyses were performed with GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA) and EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan). EZR is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
Of total of 79 enrolled patients, complete resection could not achieve in six patients. The patients’ characteristics are summarised in Table 1. There were 47 men and 32 women, with a median age of 68 years (range, 36–85 years). The primary colorectal cancer was located at the rectum in 43 patients (54.4%) and in the colon in the other 36 patients (45.6%). The pathological stage of the primary colorectal cancer was stage I in 4 patients (5.0%), stage II in 16 patients (20.3%), stage IIIA in 21 patients (26.6%), stage IIIB in 15 patients (19.0%), and stage IV in 23 patients (29.1%). The number of pulmonary metastasis was 1 in 50 patients (63.3%), 2 in 18 patients (22.8%), 3 to 5 in four patients (5.1%), and 6 or more in seven patients (8.9%). Pulmonary metastasis was located unilaterally in 58 patients (73.4%) and at bilaterally sites in 21 patients (26.6%). The most common surgical procedure was partial resection, performed for 45 patients (57.0%). Liver metastasectomy was performed for 36 patients (45.6%) prior to pulmonary metastasectomy. The preoperative serum level of CEA was elevated (>5 ng/mL) in 28 patients (35.4%). The CTC count was 0 in 66 patients (83.5%), 1 in eight patients (10.1%), 2 in three patients (3.8%), and 3 and 6 in one patient (1.3%).
Table 1. Patient characteristics (n=79).
| Characteristics | Patient number |
|---|---|
| Age, median [range] (years) | 68 [36–85] |
| Sex | |
| Male | 47 |
| Female | 32 |
| Site of primary tumour | |
| Colon | 36 |
| Rectum | 43 |
| Stage of primary tumour | |
| I–IIIB | 56 |
| IV | 23 |
| Number of lung metastasis | |
| Solitary | 50 |
| Multiple | 29 |
| Location of lung metastasis | |
| Unilateral | 58 |
| Bilateral | 21 |
| Preoperative serum CEA level (ng/mL) | |
| ≤5 | 51 |
| >5 | 28 |
| Disease free interval (months) | |
| ≤24 | 59 |
| >24 | 20 |
| History of liver metastases | |
| None | 43 |
| Present | 36 |
| Surgical procedure | |
| Wedge resection | 45 |
| Anatomical resection | 34 |
| Complete resection | |
| Achieved | 73 |
| Not achieved | 6 |
| Adjuvant chemotherapy | |
| Yes | 60 |
| No | 19 |
| CTC count | |
| 0 | 66 |
| 1 | 8 |
| 2 | 3 |
| 3 | 1 |
| 6 | 1 |
CEA, carcinoembryonic antigen; CTC, circulating tumour cell.
Clinical outcomes
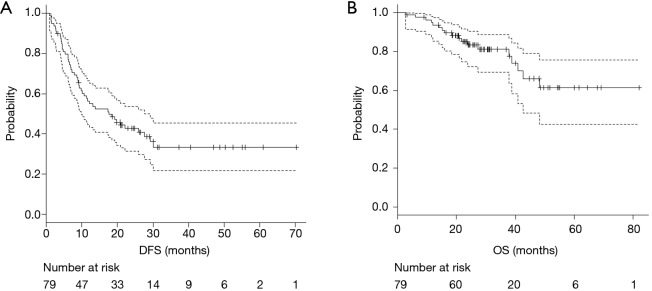
The median follow-up period after pulmonary metastasectomy was 28 months (range, 3–70 months). Of the 79 patients in the analysis, 31 (39.2%) remained free of disease after pulmonary metastasectomy, and 48 patients (60.8%) developed recurrence. Of these 48 patients, 13 died from the disease and 5 from other causes. The median DFS was 17.7 months (95% CI, 9.8–27.6 months), and median OS was not reached (95% CI, 42.6 months–not reached), respectively (Figure 1A,B).
Figure 1.
The survival curves in all patients for DFS (A) and OS (B). The median DFS was 17.7 months (95% CI, 9.8–27.6 months), and median OS was not reached (95% CI, 42.6 months–not reached). DFS, disease-free survival; OS, overall survival.
The clinical outcomes according to CTC count
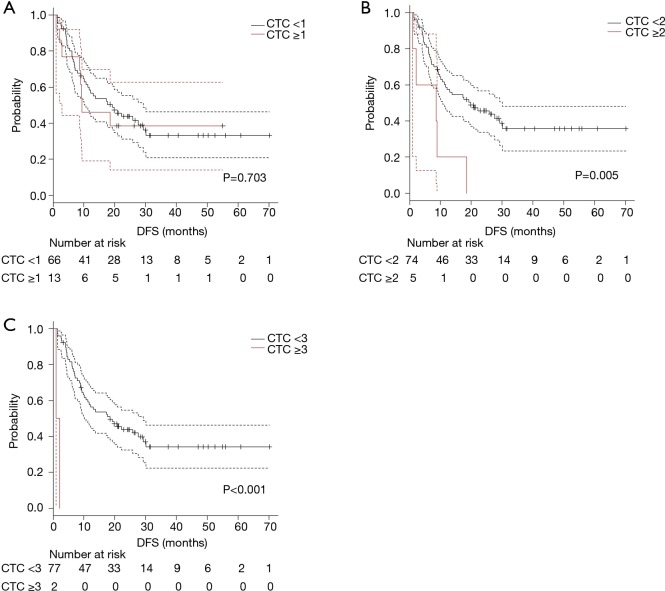
The survival curves were compared according to CTC count. Due to low detection rate of CTC, survival difference was exploratory evaluated using cut-off value of CTC count as 1, 2 and 3.
The survival curves for DFS were shown in Figure 2A (cut-off value as 1; 0 vs. ≥1), Figure 2B (2; <2 vs. ≥2), and Figure 2C (as 3; <3 vs. ≥3). Significant shorter DFS were observed in which using cut-off value as 2 or more. Patients with multiple CTC (CTC count ≥2) had a significant (P=0.005) shorter DFS (median DFS; 19.8 vs. 8.6 months).
Figure 2.
The survival curves for DFS were shown according to several cut-off values [(A) 1; (B) 2; (C) 3]. Significant difference in DFS were observed in (B) (P=0.005) and (C) (P<0.001), respectively. DFS, disease-free survival; CTC, circulating tumour cell.
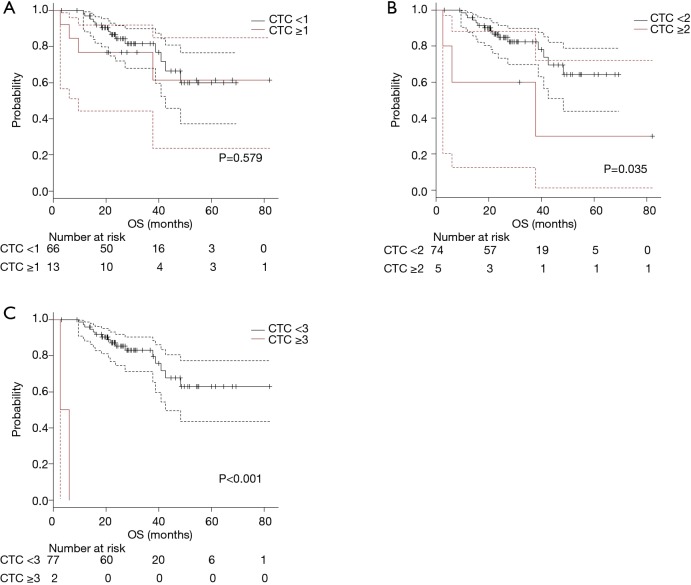
The survival curves for OS were shown in Figure 3A (cut-off value as 1; 0 vs. ≥1), Figure 3B (2; <2 vs. ≥2), and Figure 3C (as 3; <3 vs. ≥3). Significant shorter OS were observed in which using cut-off value as 2 or more. Patients with multiple CTC (CTC count ≥2) had a significant (P=0.035) shorter DFS (median DFS; not reached vs. 37.8 months).
Figure 3.
The survival curves for OS were shown according to several cut-off values [(A) 1; (B) 2; (C) 3]. Significant difference in OS were observed in (B) (P=0.035) and (C) (P<0.001), respectively. OS, overall survival.
Based on these exploratory analyses, we divided enrolled patients into two groups according CTC count as 2 (CTC count ≥2 vs. <2).
Survival analysis among preoperative variables
Survival analysis was also performed involving other preoperative clinical variables including sex, site of primary tumour, stage of primary tumour, number of lung metastasis, number of pulmonary metastases, location of pulmonary metastasis, preoperative serum CEA level, DFI, and history of liver. Univariate analyses (Table 2) revealed stage of primary tumour, number of pulmonary metastasis (multiple), location of pulmonary metastasis (bilateral), preoperative CEA level (≥5 ng/mL, DFI ≤24 months) and history of liver metastasis (present) had significant shorter DFS. It also revealed preoperative CEA level, DFI and history of liver metastasis had significant shorter OS.
Table 2. Univariate analysis for DFS and OS among perioperative variables.
| Variables | Number of Patients | P value (DFS) | P value (OS) |
|---|---|---|---|
| Sex | 0.86 | 0.510 | |
| Male | 47 | ||
| Female | 32 | ||
| Site of primary tumour | 0.14 | 0.370 | |
| Colon | 36 | ||
| Rectum | 43 | ||
| Stage of primary tumour | <0.01 | 0.850 | |
| I–III | 56 | ||
| IV | 23 | ||
| Number of lung metastasis | <0.01 | 0.110 | |
| Solitary | 49 | ||
| Multiple | 30 | ||
| Location of lung metastasis | <0.001 | 0.120 | |
| Unilateral | 58 | ||
| Bilateral | 21 | ||
| Preoperative serum CEA level (ng/mL) | <0.001 | <0.001 | |
| ≤5 | 51 | ||
| >5 | 28 | ||
| Disease-free interval (months) | 0.01 | 0.025 | |
| ≤24 | 59 | ||
| >24 | 20 | ||
| History of liver metastasis | <0.01 | 0.046 | |
| None | 43 | ||
| Present | 36 |
CEA, carcinoembryonic antigen; DFS, disease-free survival; CTC, circulating tumour cell.
Multivariate analysis in all variables
In mCRC patient who underwent pulmonary metastasectomy (Table 3), the multivariate analysis included all variables found to be significant in the univariate analyses and showed that multiple CTCs (i.e., ≥2 cells/7.5 mL) was a significant prognostic factor for DFS (HR, 3.28; 95% CI, 1.24–8.67; P=0.017) but not for OS (HR, 2.51; 95% CI, 0.69–9.09; P=0.176).
Table 3. Multivariate analyses for DFS and OS.
| Variables | Hazard ratio | P value |
|---|---|---|
| DFS | ||
| CTC count (≥2) | 3.28 (1.24–8.67) | 0.017 |
| CEA (>5 ng/mL) | 4.12 (2.25–7.57) | <0.001 |
| Number of lung metastases (multiple) | 2.04 (1.12–3.71) | 0.037 |
| History of liver metastasis (present) | 1.32 (0.69–2.53) | 0.403 |
| Disease-free interval (≤24 months) | 2.45 (1.10–5.45) | 0.028 |
| OS | ||
| CTC count (≥2) | 2.51 (0.69–9.09) | 0.176 |
| CEA (>5 ng/mL) | 5.74 (1.63–20.15) | 0.006 |
| Disease-free interval (≤24 months) | 5.09 (0.61–42.45) | 0.133 |
CTC, circulating tumour cell; CEA, carcinoembryonic antigen; DFS, disease-free survival; OS, overall survival.
Discussion
Colorectal cancer tends to undergo haematogenous metastasis, with the most frequent sites of metastases being the liver and lungs (24). Because the efficacy of liver metastasectomy is well known and contributes to a favourable prognosis (25,26), it has been recommended when complete resection is possible (13). In pulmonary metastases, pulmonary metastasectomy has been shown to provide a favourable prognosis (1-5) and has also been widely performed as a standard therapy (13). Our study also showed a favourable prognosis with this procedure, and it suggested that our patient selection of this study was appropriate.
Cohen and co-workers reported that the detection rate of CTCs in mCRC patients was 48% (18). Conversely, the detection rate of CTC in non-metastatic CRC has been reported as 0–10.5% (27,28). Although only mCRC patients were enrolled in this study, the detection rate of CTCs was 16.5%, which was much lower than in previous reports (18). Indeed, multiple CTCs (i.e., ≥2 cells/7.5 mL) was observed in only five patients (4.7%). These results suggest that most of the patients could be considered as having a “local disease”, and that our surgical criteria for pulmonary metastasectomy for mCRC were justified.
We found a significant correlation between CTC count and DFS, which may inform surgical criteria for pulmonary metastasectomy. Curative-intent surgery for distant metastases should be performed for mCRC patients without other metastatic sites. In general, the presence of distant metastasis in mCRC patients is determined based on conventional radiological examinations such as computed tomography or 18F-fluorodeoxy-glucose positron emission tomography. However, these conventional modalities cannot detect micro-metastases. The findings of this study suggest that the detection of multiple CTCs (i.e., ≥2 cells/7.5 mL) suggests the presence of a micro-metastasis. Bork and co-workers reported that the detection of CTCs in peripheral blood was significantly correlated with short progression-free survival in non-mCRC patients (28). CTC count could be a useful surrogate marker for micro-metastasis in mCRC patients undergoing pulmonary metastasectomy.
Several reports have shown CTC count to be a significant prognostic factor in the OS of colorectal cancer patients with or without distant metastasis (18,28). In the present study, we were unable to lead the CTC count as being a significant prognostic factor for OS. We speculate several reasons for this. First, the therapeutic response to chemotherapy can greatly influence the OS in mCRC patients. The development of chemotherapy, including Bevacizumab, has contributed to improve OS. Longer OS can be achieved in patients with good response to chemotherapy even if a tumour recurrence is observed after pulmonary metastasectomy. Second, patient selection may have influenced these results. Briefly, all of the enrolled patients were well selected through our surgical criteria, and a favourable prognosis may therefore be expected in most of the patients. Finally, the number of enrolled patients may not have been enough to establish the CTC count as a prognostic factor of OS. Indeed, only 16.4% of the patients died from colorectal cancer in the period of observation of this study.
There were several limitations to this study. The most important was the sensitivity of detection and identification of CTCs using the CellSearch system. This system is the only commercially available method to detect and identify CTCs; however, its detection rate has been reported as less than 50% in CRC patient with distant metastases (18). The CTC-chip, a microfluidic platform for isolating CTCs, has been reported as being a more sensitive detection system for CTCs (29). Recently, Chikaishi and co-workers reported that CTC-chip coated with an anti-EpCAM antibody could provide greater sensitivity in the detection of CTCs with EpCAM expression (30). Another major limitation of this prospective study was that it was a single-institution study with a small number of subjects. The results of this study suggest that the evaluation of CTC count should be performed for a larger number of patients. Finally, since this study was an exploratory study, the prospective clinical study with large number is needed to evaluate the clinical impact of CTC in mCRC.
In conclusion, the detected rate of CTCs was quite low in mCRC patients who underwent pulmonary metastasectomy. The patient with multiple CTCs had shorter DFS in this study. The larger prospective clinical study is needed to establish the meaning of CTC in mCRC candidate for pulmonary metastasectomy.
Acknowledgements
Prof. Jiro Fujimoto, Prof. Naohiro Tomita, Dr. Hidenori Yanagi contributed us to patient recruitment in this study. We are deeply graceful to them. We also thank Miss. Risa Murata for helpful assistance for preparation of the manuscript.
Funding: This work was supported by the Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Young Scientists (grant No. KAKENHI B 22791326), and Grant-in-Aid for Graduate Students, Hyogo College of Medicine.
Ethical Statement: This prospective study was approved by the Institutional Review Board of Hyogo College of Medicine Hospital (No. 715). Informed consent was obtained for all patients before surgery.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hornbech K, Ravn J, Steinbrüchel DA. Outcome after pulmonary metastasectomy: analysis of 5 years consecutive surgical resections 2002-2006. J Thorac Oncol 2011;6:1733-40. 10.1097/JTO.0b013e3182287da2 [DOI] [PubMed] [Google Scholar]
- 2.Suemitsu R, Takeo S, Kusumoto E, et al. Results of a pulmonary metastasectomy in patients with colorectal cancer. Surg Today 2011;41:54-9. 10.1007/s00595-009-4244-x [DOI] [PubMed] [Google Scholar]
- 3.Pfannschmidt J, Muley T, Hoffmann H, et al. Prognostic factors and survival after complete resection of pulmonary metastases from colorectal carcinoma: experiences in 167 patients. J Thorac Cardiovasc Surg 2003;126:732-9. 10.1016/S0022-5223(03)00587-7 [DOI] [PubMed] [Google Scholar]
- 4.Maeda R, Isowa N, Onuma H, et al. Pulmonary resection for metastases from colorectal carcinoma. Interact Cardiovasc Thorac Surg 2009;9:640-4. 10.1510/icvts.2009.202598 [DOI] [PubMed] [Google Scholar]
- 5.Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg 2002;124:1007-13. 10.1067/mtc.2002.125165 [DOI] [PubMed] [Google Scholar]
- 6.Lee WS, Yun SH, Chun HK, et al. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. Int J Colorectal Dis 2007;22:699-704. 10.1007/s00384-006-0218-2 [DOI] [PubMed] [Google Scholar]
- 7.Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238-44. 10.1016/j.athoracsur.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 8.Rena O, Casadio C, Viano F, et al. Pulmonary resection for metastases from colorectal cancer: factors influencing prognosis. Twenty-year experience. Eur J Cardiothorac Surg 2002;21:906-12. 10.1016/S1010-7940(02)00088-X [DOI] [PubMed] [Google Scholar]
- 9.Chen F, Hanaoka N, Sato K, et al. Prognostic factors of pulmonary metastasectomy for colorectal carcinomas. World J Surg 2009;33:505-11. 10.1007/s00268-008-9875-3 [DOI] [PubMed] [Google Scholar]
- 10.Higashiyama M, Kodama K, Higaki N, et al. Surgery for pulmonary metastases from colorectal cancer: the importance of prethoracotomy serum carcinoembryonic antigen as an indicator of prognosis. Jpn J Thorac Cardiovasc Surg 2003;51:289-96. 10.1007/BF02719380 [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto T, Tsubota N, Iwanaga K, et al. Pulmonary resection for metastases from colorectal cancer. Chest 2001;119:1069-72. 10.1378/chest.119.4.1069 [DOI] [PubMed] [Google Scholar]
- 12.Treasure T. Pulmonary metastasectomy for colorectal cancer: weak evidence and no randomized trials. Eur J Cardiothorac Surg 2008;33:300-2. 10.1016/j.ejcts.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012;17:1-29. 10.1007/s10147-011-0315-2 [DOI] [PubMed] [Google Scholar]
- 14.Liu MC, Shields PG, Warren RD, et al. Circulating tumour cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 2009;27:5153-9. 10.1200/JCO.2008.20.6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller MC, Doyle GV, Terstappen LW. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol 2010;2010:617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cristofanilli M, Hayes DF, Budd GT, et al. Circulating tumour cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23:1420-30. 10.1200/JCO.2005.08.140 [DOI] [PubMed] [Google Scholar]
- 17.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. Erratum in: Clin Cancer Res 2009;15:1506. 10.1158/1078-0432.CCR-08-0872 [DOI] [PubMed] [Google Scholar]
- 18.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumour cells to tumour response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. 10.1200/JCO.2007.15.8923 [DOI] [PubMed] [Google Scholar]
- 19.Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumour cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20:1223-9. 10.1093/annonc/mdn786 [DOI] [PubMed] [Google Scholar]
- 20.Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumours in the lung. J Thorac Cardiovasc Surg 1965;49:357-63. [PubMed] [Google Scholar]
- 21.Tanaka F, Yoneda K, Kondo N, et al. Circulating tumour cells as a diagnostic maerker in primary lung cancer. Clin Cancer Res 2009;15:6980-6. 10.1158/1078-0432.CCR-09-1095 [DOI] [PubMed] [Google Scholar]
- 22.Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumour cells in patients with small-cell lung cancer. J Thorac Oncol 2012;7:512-9. 10.1097/JTO.0b013e31823f125d [DOI] [PubMed] [Google Scholar]
- 23.Okumura Y, Tanaka F, Yoneda K, et al. Circulating tumour cells in pulmonary venous blood of primary lung cancer patients. Ann Thorac Surg 2009;87:1669-75. 10.1016/j.athoracsur.2009.03.073 [DOI] [PubMed] [Google Scholar]
- 24.Goya T, Miyazawa N, Kondo H, et al. Surgical resection of pulmonary metastases from colorectal cancer. 10-year follow-up. Cancer 1989;64:1418-21. [DOI] [PubMed] [Google Scholar]
- 25.Murata S, Moriya Y, Akasu T, et al. Resection of both hepatic and pulmonary metastases in patients with colorectal carcinoma. Cancer 1998;83:1086-93. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Kawamura M, Ishihara T. Surgical treatment for both pulmonary and hepatic metastases from colorectal cancer. J Thorac Cardiovasc Surg 1999;118:1090-6. 10.1016/S0022-5223(99)70106-6 [DOI] [PubMed] [Google Scholar]
- 27.Thorsteinsson M, Söletormos G, Jess P. Low number of detectable circulating tumor cells in non-metastatic colon cancer. Anticancer Res 2011;31:613-7. [PubMed] [Google Scholar]
- 28.Bork U, Rahbari NN, Schölch S, et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br J Cancer 2015;112:1306-13. 10.1038/bjc.2015.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9. 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chikaishi Y, Yoneda K, Ohnaga T, et al. EpCAM-independent capture of circulating tumour cells with a 'universal CTC-chip'. Oncol Rep 2017;37:77-82. 10.3892/or.2016.5235 [DOI] [PubMed] [Google Scholar]