Abstract
Background
A proportion of patients with chronic obstructive pulmonary disease (COPD) may progress rapidly to small cell lung cancer (SCLC). As the forced expiratory volume in 1 second (FEV1)/forced volume vital capacity (FVC) ratio is usually impaired in patients with COPD, and given that the FEV1 and FVC are not necessarily related to lung cancer development, we performed this study to test our hypothesis that the FEV1/FVC ratio predicts survival of patients with limited-stage (LS)-SCLC.
Methods
We assessed 74 patients with LS-SCLC treated with chemoradiotherapy. The patients were divided into two FEV1/FVC ratio groups: <0.74 (n=24) and ≥0.74 (n=50).
Results
The 3-year overall survival (OS) and 3-year progression-free survival (PFS) rates were significantly lower in patients with FEV1/FVC <0.74 than in those with FEV1/FVC ≥0.74 group (35.4% vs. 61.2%, P=0.0033; and 11.7% vs. 51.8%, P=0.0072, respectively). On multivariate analysis, the low FEV1/FVC group was independently associated with OS and PFS [hazard ratio (HR) (95% confidence interval): 2.15 (0.99–4.63), P=0.052; and 2.13 (1.04–4.39), P=0.039, respectively].
Conclusions
Pretreatment FEV1/FVC ratio appears to be a potential prognostic factor for patients with LS-SCLC.
Keywords: Forced expiratory volume, forced volume vital capacity (FVC), small cell lung cancer (SCLC), prognosis
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with lung cancer development (1,2), as is pulmonary impairment, which can be measured by spirometry (3,4). COPD is a risk factor for both small cell lung cancer (SCLC) and the squamous cell carcinoma type of non-small cell lung cancer (NSCLC) (5,6). Chronic inflammation following oxidative stress due to exposure to inhaled toxic and carcinogenic materials (such as smoke or pollutants) comprises the biological aetiology of both lung cancer and COPD, as both diseases share many common pathological pathways (7-10). Hence, COPD in patients diagnosed with lung cancer might be associated with poor clinical outcomes.
Previous studies supported the notion that COPD and the lower limit of normal forced expiratory volume in 1 second (FEV1)/forced volume vital capacity (FVC) ratio, as well as the FEV1 and FVC values themselves, influenced the outcomes of patients with lung cancer (11-14). However, such studies were mainly limited to NSCLC, while those that addressed SCLC are rare. This was likely because patients with the latter disease comprise only 15–20% of those afflicted with lung cancer while patients with NSCLC comprise 80–85%; hence any underlying COPD is likely to be missed or underdiagnosed (15,16). Moreover, the lag time between the occurrence of COPD and diagnosis of lung cancer may be shorter for SCLC than NSCLC owing to the former's rapid progression when the malignancy is related to COPD. For these reasons, detecting COPD before the development of lung cancer is challenging. However, it may be feasible to estimate the prognosis of patients with SCLC through determining the severity of COPD (or, in the absence of a diagnosis, airway obstructive damage) by measuring the FEV1/FVC ratio, which has been used as a standard measure of airway obstruction for over 50 years (17), under the assumption that the FEV1 and FVC are not associated with the extent of cancer progression as are the tumor volume (TV) or neutrophil-to-lymphocyte ratio (NLR) (18).
Hence, we investigated whether pretreatment FEV1/FVC ratio could independently predict treatment outcomes in patients with limited-stage-SCLC (LS-SCLC).
Methods
This study was approved by the Institutional Review Board (AJOU-MED-MDB-17-057). The requirement for informed consent was waived owing to the retrospective nature of the study. Of 84 patients with LS-SCLC who were treated with chemoradiotherapy (CRT) between May 2001 and July 2015, we analyzed 74 after excluding 10 who did not undergo spirometry. The patients had no evident COPD or interstitial lung disease, and did not undergo clinical evaluation for these disorders. All patients had histologically confirmed small cell carcinoma and underwent imaging studies including computed tomography (CT), positron emission tomography-CT or abdominal ultrasonography/bone scintigraphy, and brain magnetic resonance imaging or brain CT. They underwent 2–6 cycles of concurrent, alternating, or sequential etoposide plus cisplatin chemotherapy (CTx) and radiotherapy (RT). RT for concurrent CRT commenced at the beginning of the second CTx cycle. External beam RT to the gross tumour was administered once or twice daily using 54–64 Gy in 27–32 fractions or 45–51 Gy in 30–36 fractions, respectively. Responses were evaluated via chest CT 1 month after treatment. Follow-up was performed every month for the first 3 years and every 6 months thereafter. The median follow-up was 22 months (range, 3–159 months), and endpoints were overall survival (OS) and progression-free survival (PFS). Disease progression was determined according to clinical symptoms and imaging.
The FEV1 (% of predicted), FVC (% of predicted), and FEV1/FVC (ratio) were collected from pulmonary function test (PFT) results measured by spirometry at diagnosis. FEV1 (% of predicted) and FVC (% of predicted) were defined as the FEV1% and FVC% of the patient divided by the same values obtained from an average healthy person of similar age, sex, and body composition, respectively. The cut-off values of FEV1 (% of predicted), FVC (% of predicted), and FEV1/FVC (ratio) were determined using maximal log-rank tests, which define the maximum absolute values (M) of the standardized two-sample linear rank statistics (|Sµ|) of all possible cutoff points (µ) (19). The cut point, where the standardized statistics take their maximum (M=max|Sµ|), represents the maximum difference between the two groups divided by it (19).
We compared age, sex, Eastern Cooperative Oncology Group performance status, smoking history, CTx cycle, CRT method, RT fractionation, achievement of complete response, and administration of prophylactic cranial irradiation between patients with high vs. low FEV1/FVC ratios. Death, progression, local progression (LP), distant metastasis (DM), TV, and NLR were sequentially compared between the two groups. Similarly, these parameters were compared between patients with high FEV1 (% of predicted) vs. low FEV1 (% of predicted), and between patients with high FVC (% of predicted) vs. low FVC (% of predicted). Pretreatment TV contoured on chest CT before treatment was calculated by the Varian Eclipse Planning System, version 10.0 (Varian Medical Systems, Palo Alto, CA, USA). NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count before treatment. The FEV1 (% of predicted) was plotted as a function of the FVC (% of predicted) for each of the high vs. low FEV1/FVC ratio groups, and their trend lines were drawn respectively.
We analysed all data using the R software version 3.2.3 (R foundation of Statistical Computing, available online: http://www.r-project.org). Maximal log-rank tests were performed using the maxstat package (19). Categorical and continuous variables in both groups were compared using Fisher’s exact test or the χ2 and The Mann-Whitney U tests, respectively. We used the Kaplan-Meier method and log-rank test to evaluate survival differences. Adjusted hazard ratios (AHRs) were calculated via the Cox proportional hazards model for age, sex, and variables that were statistically significant (P value <0.05) on univariate analyses.
Results
Patients’ characteristics are described in Table 1. The median values (ranges) of FEV1 (% of predicted), FVC (% of predicted), FEV1/FVC (ratio), TV (cc), and NLR were 72 [41–137], 81.5 [41–147], 0.68 (0.44–0.9), 86 [6–415], and 2.07 (0.97–9.25), respectively.
Table 1. Comparison between patients with low vs. high FEV1/FVC ratios.
| Variables | All (n=74) | FEV1/FVC ratio ≥0.74 (n=24) | FEV1/FVC ratio <0.74 (n=50) | P |
|---|---|---|---|---|
| Age, years | 0.68 | |||
| ≤65 | 36 (49%) | 13 (54%) | 23 (46%) | |
| >65 | 38 (51%) | 11 (46%) | 27 (54%) | |
| Sex | 0.02 | |||
| Female | 10 (13%) | 7 (29%) | 3 (6%) | |
| Male | 64 (87%) | 17 (71%) | 47 (94%) | |
| ECOG PS score | 0.37 | |||
| 0 | 20 (27%) | 5 (21%) | 15 (30%) | |
| 1 | 44 (59%) | 17 (71%) | 27 (54%) | |
| 2 | 10 (14%) | 2 (8%) | 8 (16%) | |
| Smoking history | 0.23 | |||
| No | 9 (12%) | 5 (21%) | 4 (8%) | |
| Yes | 65 (88%) | 19 (79%) | 46 (92%) | |
| Chemotherapy cycle | 0.16 | |||
| <4 | 6 (8%) | 4 (17%) | 2 (8%) | |
| ≥4 | 68 (92%) | 20 (83%) | 48 (92%) | |
| Sequential CRT | 0.40 | |||
| No | 61 (82%) | 18 (75%) | 43 (86%) | |
| Yes | 13 (18%) | 6 (25%) | 7 (14%) | |
| RT fractionation | 0.28 | |||
| Once daily | 12 (16%) | 6 (25%) | 6 (12%) | |
| Twice daily | 62 (84%) | 18 (75%) | 44 (88%) | |
| Complete response | 1.00 | |||
| No | 32 (43%) | 10 (42%) | 22 (44%) | |
| Yes | 42 (57%) | 14 (58%) | 28 (56%) | |
| PCI | 1.00 | |||
| No | 40 (54%) | 13 (54%) | 27 (54%) | |
| Yes | 34 (46%) | 11 (46%) | 23 (46%) |
FEV1, pretreatment forced expiratory volume in 1 second; FVC, forced vital capacity; ECOG PS, Eastern Cooperative Oncology Group performance status; CRT, chemoradiotherapy; RT, radiotherapy; PCI, prophylactic cranial irradiation
Treatment outcomes
The 3-year OS (3OS) and 3-year PFS (3PFS) rates for all patients were 44.1% and 24%, respectively. Of 74 patients, 45 died during the follow-up period. Fifty-six patients experienced progression, of whom 35 progressed locally and 21 had DM. Sites of progression included the lung (35 cases), brain [7], bone [4], liver [3], lymph node [3], abdomen [2], adrenal gland [2], and kidney [1].
Variables according to FEV1/FVC
As shown in Table 1, there was no difference in the achievement of complete response and administration of prophylactic cranial irradiation for patients with FEV1/FVC ratios ≥0.74 vs. those with ratios <0.74. The latter group included fewer female patients than the former.
FEV1/FVC and cancer extent
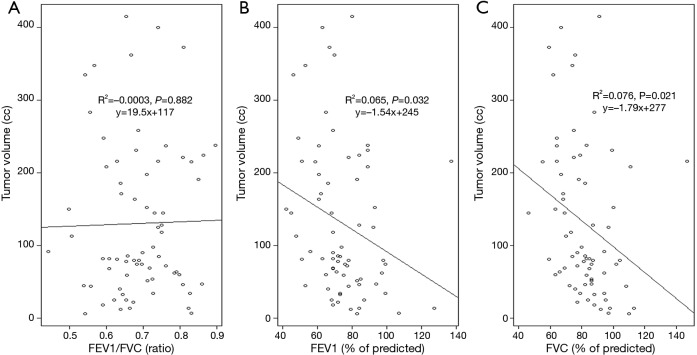
The FEV1/FVC ratio was not associated with TV and NLR, while both the FEV1 (% of predicted) and FVC (% of predicted) were significantly correlated with the NLR (Table 2). Both the FEV1 (% of predicted) and FVC (% of predicted) were inversely correlated with TV, while the FEV1/FVC ratio was not (Figure 1). The FEV1 (% of predicted) and FVC (% of predicted) showed a linear correlation with each other in both the high and low FEV1/FVC ratio groups; the slope for the FEV1/FVC ratio ≥0.74 group was steeper than that for the ratio <0.74 group (Figure 2).
Table 2. Comparison of treatment outcome, tumor volume, and neutrophil-to-lymphocyte ratio between low and high pulmonary function parameters divided by the cutoff values determined via maximal log-rank tests.
| Variables | FEV1/FVC ratio | FEV1 (% of predicted) | FVC (% of predicted) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≥0.74 | <0.74 | P | ≥76 | <76 | P | ≥91 | <91 | P | |||
| N=74 | 24 | 50 | 31 | 43 | 18 | 56 | |||||
| Death | 0.01 | 0.04 | 0.01 | ||||||||
| No | 15 (63%) | 14 (28%) | 17 (55%) | 12 (28%) | 12 (67%) | 17 (30%) | |||||
| Yes | 9 (38%) | 36 (72%) | 14 (45%) | 31 (72%) | 6 (33%) | 39 (70%) | |||||
| Progression | 0.01 | 0.10 | 0.94 | ||||||||
| No | 11 (46%) | 7 (14%) | 11 (35%) | 7 (16%) | 5 (28%) | 13 (23%) | |||||
| Yes | 13 (54%) | 43 (86%) | 20 (65%) | 36 (84%) | 13 (72%) | 43 (77%) | |||||
| Local progression | 0.36 | 0.39 | 0.03 | ||||||||
| No | 15 (63%) | 24 (48%) | 14 (45%) | 25 (58%) | 5 (28%) | 34 (61%) | |||||
| Yes | 9 (38%) | 26 (52%) | 17 (55%) | 18 (42%) | 13 (72%) | 22 (39%) | |||||
| Distant progression | 0.20 | 0.01 | 0.01 | ||||||||
| No | 20 (83%) | 33 (66%) | 28 (90%) | 25 (58%) | 18 (100%) | 35 (63%) | |||||
| Yes | 4 (17%) | 17 (34%) | 3 (10%) | 18 (42%) | 0 (0%) | 21 (38%) | |||||
| N=70 | 22 | 48 | 29 | 41 | 18 | 52 | |||||
| Tumor volume (cc), mean ± SD | 145±111 | 123±103 | 0.42 | 109±98 | 146±108 | 0.14 | 101±109 | 141±103 | 0.17 | ||
| N=69 | 20 | 49 | 28 | 51 | 17 | 52 | |||||
| Neutrophil to lymphocyte ratio, mean ± SD | 2.6±1.9 | 2.5±1.3 | 0.86 | 2±0.9 | 2.9±1.6 | <0.01 | 1.9±0.9 | 2.7±1.6 | 0.01 | ||
FEV1, pretreatment forced expiratory volume in 1 second; FVC, forced vital capacity; SD, standard deviation.
Figure 1.
Simple linear regressions and trend lines for tumor volume according to (A) the FEV1/FVC ratio, (B) FEV1 (% of predicted), and (C) FVC (% of predicted). FVC, forced volume vital capacity; FEV1, forced expiratory volume in 1 second.
Figure 2.
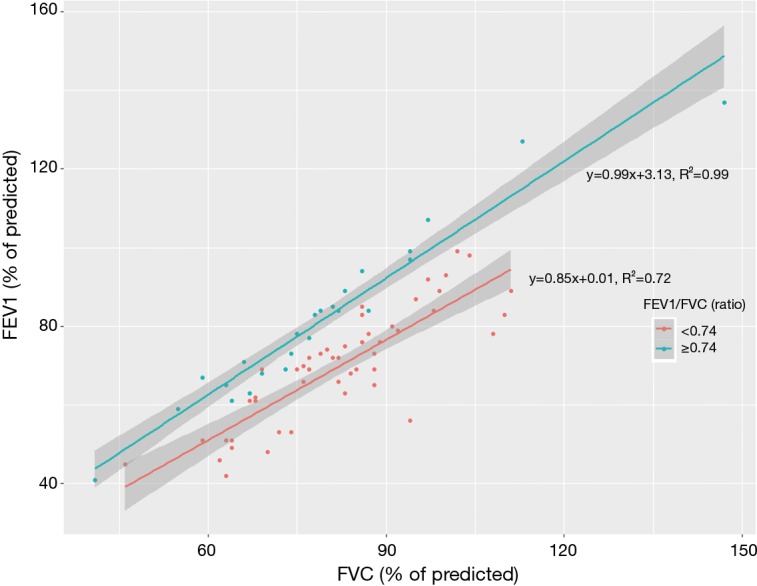
Scatter plot and trend lines between FVC (% of predicted) and FEV1 (% of predicted) in patients with FEV1/FVC ratios <0.74 vs. those with FEV1/FVC ratios ≥0.74. FVC, forced volume vital capacity; FEV1, forced expiratory volume in 1 second.
FEV1/FVC and survival
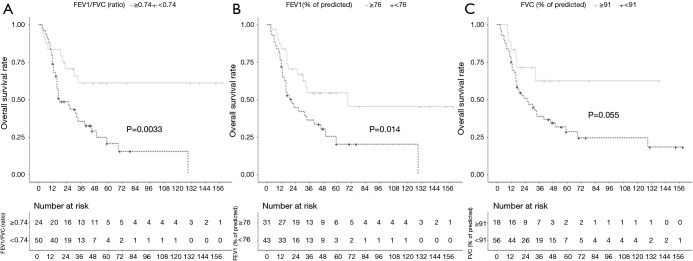
The 50 patients with FEV1/FVC ratios <0.74 experienced more deaths and progression than the 24 patients with FEV1/FVC ratios ≥0.74 (Table 2). Moreover, the 43 patients with FEV1 (% of predicted) values <76 as well as the 56 patients with FVC (% of predicted) values <91 experienced more deaths and DM rates than the 31 patients with FEV1 (% of predicted) values ≥76 and the 18 patients with FVC (% of predicted) values ≥91, respectively. The 3OS and 3PFS rates were significantly lower in patients with FEV1/FVC ratios <0.74 than in those with FEV1/FVC ratios ≥0.74 (35.4% vs. 61.2%, respectively, P=0.0033; and 11.7% vs. 51.8%, respectively, P=0.0072). As shown in Figure 3, the OS rate was lower in the FEV1/FVC ratio <0.74 group than in the FEV1/FVC ratio ≥0.74 group within 1 year of diagnosis, but the survival curves intersected at 1 year and continued to separate afterwards. On the other hand, patients with FEV1 (% of predicted) <76 and those with FVC (% of predicted) <91 showed lower OS rates than those with FEV1 (% of predicted) ≥76 and those with FVC (% of predicted) ≥91, respectively, at all points on the survival curves (FEV1: 36.4% vs. 54.4%, respectively, P=0.014; FVC: 38.9% vs. 62.5%, respectively, P=0.055). On multivariate analysis, the low FEV1/FVC ratio group was independently associated with OS and PFS (Table 3).
Figure 3.
Kaplan-Meier plots showing overall survival according to (A) the FEV1/FVC ratio, (B) FEV1 (% of predicted), and (C) FVC (% of predicted). FVC, forced volume vital capacity; FEV1, forced expiratory volume in 1 second.
Table 3. Univariate and multivariate analyses of patients with limited-stage small cell lung cancer.
| Variables | Overall survival | Progression-free survival | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | AHR (95% CI) | P | HR (95% CI) | P | AHR (95% CI) | P | ||
| Age >65 years | 1.63 (0.9–2.94) | 0.105 | 1.55 (0.85–2.82) | 0.150 | 1.58 (0.93–2.68) | 0.094 | 1.6 (0.92–2.75) | 0.093 | |
| Male sex | 3.6 (1.1–11.8) | 0.034 | 2.93 (0.85–10.1) | 0.089 | 1.84 (0.79–4.3) | 0.160 | 1.01 (0.38–2.64) | 0.99 | |
| ECOG PS score 2 | 1.48 (0.68–3.2) | 0.319 | – | – | 1.46 (0.69–3.11) | 0.322 | – | – | |
| Smoking history | 2.45 (0.75–7.96) | 0.136 | – | – | 1.09 (0.46–2.41) | 0.838 | – | – | |
| Chemotherapy cycle ≤3 | 1.54 (0.6–3.93) | 0.37 | – | – | 1.64 (0.65–4.12) | 0.838 | – | – | |
| Sequential CRT | 1.15 (0.55–2.4) | 0.714 | – | – | 1.27 (0.67–2.41) | 0.463 | – | – | |
| Twice daily RT fractionation | 1.04 (0.46–2.33) | 0.923 | – | – | 0.93 (0.47–1.85) | 0.845 | – | – | |
| FEV1/FVC ratio <0.74 | 2.92 (1.39–6.14) | 0.005 | 2.15 (0.99–4.63) | 0.052 | 2.3 (1.23–4.31) | 0.009 | 2.13 (1.04–4.39) | 0.039 | |
| FEV1 (% of predicted) <76 | 2.19 (1.16–4.14) | 0.016 | 1.75 (0.91–3.35) | 0.094 | 1.9 (1.09–3.3) | 0.024 | 1.56 (0.89–2.76) | 0.122 | |
| FVC (% of predicted) <91 | 2.26 (0.96–5.35) | 0.063 | – | – | 1.4 (0.75–2.6) | 0.292 | – | – | |
HR, hazard ratio; CI, confidence interval; AHR, adjusted HR; ECOG PS, Eastern Cooperative Oncology Group performance status; CRT, chemoradiotherapy; RT, radiotherapy; FEV1, pretreatment forced expiratory volume in 1 second; FVC, forced vital capacity.
Discussion
Our results revealed that the FEV1/FVC ratio was independently associated with survival in patients with LS-SCLC. Patients with FEV1/FVC ratios ≥0.74 experienced delayed or no progression, as well as long-term survival, while those with FEV1/FVC ratios <0.74 had rapid treatment failure and early deaths. These results supported our hypothesis that coexisting airway obstructive damage in patients with SCLC plays a role in poor treatment outcomes.
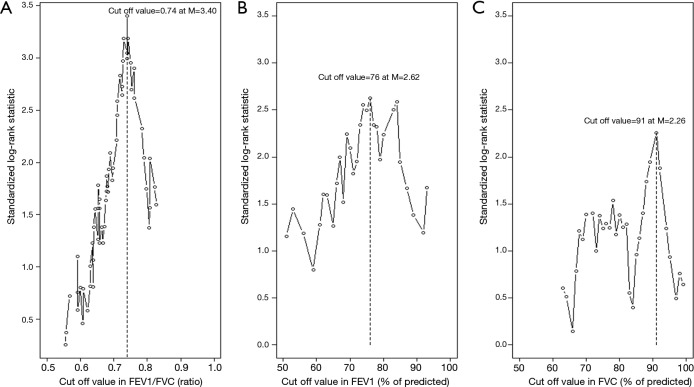
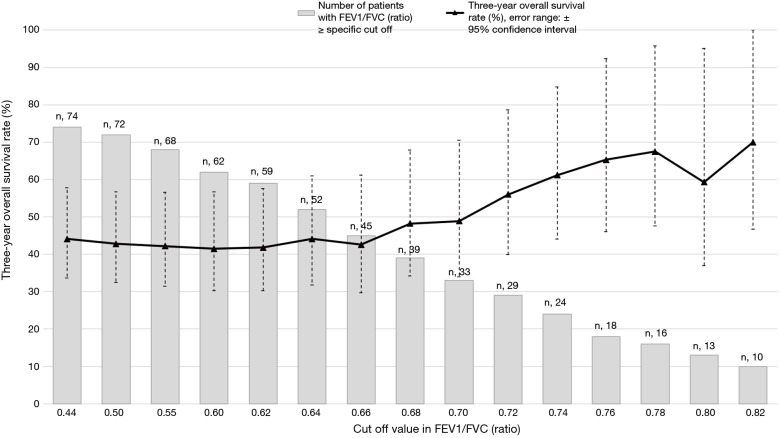
In this study, the optimal cut off values for the FEV1/FVC ratio, FEV1 (% of predicted), and FVC (% of predicted) were estimated using the log-rank statistics maximums (as shown in Figure S1; M =3.40, 2.62, and 2.26, respectively). The standardized log-rank statistics (|Sµ|) of 3 pulmonary function parameters continuously increased commensurate with the potential cutoff points. In particular, the continuous increase in OS rates that corresponded to the increased proportion of patients with high FEV1/FVC ratios (Figure S2) may explain the abrupt escalation of the |Sµ| of the FEV1/FVC ratio until attaining the cutoff value of 0.74. These additional data show that the significant differences in the cutoff values between the two groups were not incidental. Seventy-four patients were too small a population to justify using the median value as a cutoff between the two groups. Moreover, it would have been inappropriate to use standard receiver operating characteristic curves to determine the optimal cutoff value because the occurrences of multiple events over 2 or 3 years required time-dependent analysis. To overcome these vulnerabilities, we used the maximally selected log-rank test for defining the cutoff values.
Figure S1.
Standardized log-rank statistics of all possible cutoff values, indicating the optimal cutoff value as determined by the maximum standardized log-rank statistic [M] for (A) the FEV1/FVC ratio, (B) FEV1 (% of predicted), and (C) FVC (% of predicted). FVC, forced volume vital capacity; FEV1, forced expiratory volume in 1 second.
Figure S2.
The 3-year overall survival rate in patients with FEV1/FVC ratio equal to or above the indicated cutoff value. FVC, forced volume vital capacity; FEV1, forced expiratory volume in 1 second.
Co-existing airway obstructive damage was evaluated using the FEV1/FVC ratio because none of the patients with LS-SCLC who were treated with CRT had a history of COPD diagnosis or clinical evaluation. Although 0.74 is a greater value than 0.70, which is the absolute FEV1/FVC ratio cutoff for an abnormal spirometry test (18), we used 0.74 as it decreased the chances of including patients who may have been predisposed to airway obstructive damage. Moreover, patients with FEV1/FVC ratios of 0.70–0.74 carried the possibility of having co-existing COPD given the fact that the proportion of COPD in patients with NSCLC (50%) is higher than that in the general population (17%) (20,21). Another reason for using 0.74 as the cutoff was the steady increase in the 3OS rate in patients with FEV1/FVC ratios between 0.68 and 0.78, indicating a possible association between the severity of the obstructive airways damage and prognosis (Figure S2).
To demonstrate an independent association between the FEV1/FFV ratio and survival, we showed that this ratio was not associated with cancer-related factors such as TV or NLR, while both the FEV1 (% of predicted) and FVC (% of predicted) separately tended to be related to these factors (22). The change in FEV1 (% of predicted) as a function of FEV (% of predicted) was linear, indicating that a centrally located large typical SCLC may cause a simultaneous decrease in both the FEV1 (% of predicted) and FVC (% of predicted) in a manner where their ratio remains constant and therefore non-indicative of cancer extent. Hence, the FEV1/FVC ratio may only reflect co-existing obstructive damage of airway (including that associated with COPD) because FEV1 (% of predicted) and FVC (% of predicted) values are directly correlated with the severity of such airway obstruction.
Moreover, the FEV1/FVC ratio reflected progression-related OS without being a significant predictor of LP or DM, while the FEV1 (% of predicted) and FVC (% of predicted) predicted DM-related OS without showing significant differences in progression. In contrast to DMs, LP was associated with high FEV1 (% of predicted) and FVC (% of predicted). The Kaplan-Meier survival curves in the low vs. high FEV1/FVC ratio groups intersected at an early time point, while no such intersections occurred in the high vs. low FEV1 (% of predicted) and FVC (% of predicted) groups. This may indicate that, in patients with a high FEV1/FVC ratio, progression was suppressed by normal airflow rather than low tumor burden; however, in patients with high FEV1 (% of predicted) and FVC (% of predicted), progression was suppressed owing to low cancer-related inflammation and resultant low systemic failure. The linear trend line of the FEV1 (% of predicted) and FVC (% of predicted) scatterplots had slopes of 0.99 (R2=0.99) in patients with FEV1/FVC ratios ≥0.74 and 0.85 (R2=0.72) in those with FEV1/FVC ratios <0.74. This meant that normalized FEV1 as a function of normalized FVC was nearly identical in patients with high FEV1/FVC ratios, confirming normal airflow.
Biologically, reactive oxygen species accumulation and inflammation driven by endogenous and exogenous factors are precursors for COPD and lung cancer via their modulation of cellular and DNA damage, respectively (9). COPD and lung cancer development share genetic and epigenetic changes that are related to oxidant or toxic stresses, alterations to cell cycle regulation, changes in certain cytokine levels, inflammation present in the microenvironment, and increased proteinase levels produced by immune and stromal cells (10). Therefore, these factors are likely to result in poor treatment outcomes despite CRT in patients diagnosed with both COPD and LS-SCLC. According to our data, patients with cancer controlled by CRT who had high FEV1/FVC ratios achieved long-term survival. Our results were also consistent with past studies that showed that pre-existing COPD predicted poor long-term cancer-specific survival in patients with NSCLC (11,12). Taken together, predisposing airway obstructive damage appears to increase the probability of long-term progression and mortality in patients with LS-SCLC.
Our study had several limitations. First, PFT results were based on values measured pre-bronchodilation; therefore, patients with reversible obstruction may have been misclassified into the low FEV1/FVC ratio group (23). Second, our study was retrospective and included a small sample size; therefore, our outcomes should be validated in prospective studies that investigate the correlation between FEV1/FVC ratios and treatment outcomes in patients with LS-SCLC. Despite of these limitations, ours was the first study to investigate the association between the FEV1/FVC ratio in patients with LS-SCLC and survival. Our results implied that active management of airway obstructive damage found by spirometry at diagnosis (i.e., smoking cessation, education, and medication) might improve long-term treatment outcomes of LS-SCLC patients with low FEV1/FVC ratios. Moreover, early diagnosis and treatment of COPD through active spirometry in normal individuals who are frequently exposed to smoke or air pollution may potentially prevent SCLC. Additionally, determining the pretreatment FEV1/FVC ratio might help identify patients who are eligible for aggressive treatments that include CTx cycle prolongation and RT dose escalation,
In conclusion, the FEV1/FVC ratio may be a potential prognostic factor for patients with LS-SCLC. Managing co-existing airway obstructive damage may contribute to improving survival of such patients with CRT.
Acknowledgements
None.
Ethical Statement: This study was approved by the Institutional Review Board (AJOU-MED-MDB-17-057); the requirement for informed consent was waived owing to the retrospective nature of the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Purdue MP, Gold L, Jarvholm B, et al. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax 2007;62:51-6. 10.1136/thx.2006.064196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. 10.1183/09031936.00144208 [DOI] [PubMed] [Google Scholar]
- 3.Eberly LE, Ockene J, Sherwin R, et al. Pulmonary function as a predictor of lung cancer mortality in continuing cigarette smokers and in quitters. Int J Epidemiol 2003;32:592-9. 10.1093/ije/dyg177 [DOI] [PubMed] [Google Scholar]
- 4.Wasswa-Kintu S, Gan W, Man S, et al. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax 2005;60:570-5. 10.1136/thx.2004.037135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papi A, Casoni G, Caramori G, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax 2004;59:679-81. 10.1136/thx.2003.018291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang R, Wei Y, Hung RJ, et al. Associated Links Among Smoking, Chronic Obstructive Pulmonary Disease, and Small Cell Lung Cancer: A Pooled Analysis in the International Lung Cancer Consortium. EBioMedicine 2015;2:1677-85. 10.1016/j.ebiom.2015.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev 2008;11:1-15. 10.1080/10937400701436460 [DOI] [PubMed] [Google Scholar]
- 8.Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. Int J Environ Res Public Health 2009;6:445-62. 10.3390/ijerph6020445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durham A, Adcock I. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121-7. 10.1016/j.lungcan.2015.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton AM. Mechanistic links between COPD and lung cancer. Nat Rev Cancer 2013;13:233-45. 10.1038/nrc3477 [DOI] [PubMed] [Google Scholar]
- 11.Sekine Y, Suzuki H, Yamada Y, et al. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg 2013;61:124-30. [DOI] [PubMed] [Google Scholar]
- 12.Zhai R, Yu X, Shafer A, et al. THe impact of coexisting copd on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014;145:346-53. 10.1378/chest.13-1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai J, Liu M, Jiang G, et al. The Impact of Chronic Obstructive Pulmonary Disease on Lung Cancer Survival: A Metaanalysis. Arch Cancer Res 2016;4:1 10.21767/2254-6081.100052 [DOI] [Google Scholar]
- 14.Kiri VA, Soriano J, Visick G, et al. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J 2010;19:57-61. 10.4104/pcrj.2009.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrón Collar D, Pazos Guerra M, Rodriguez P, et al. COPD is commonly underdiagnosed in patients with lung cancer: results from the RECOIL study (retrospective study of COPD infradiagnosis in lung cancer). Int J Chron Obstruct Pulmon Dis 2017;12:1033-8. 10.2147/COPD.S123426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riaz SP, Lüchtenborg M, Coupland VH, et al. Trends in incidence of small cell lung cancer and all lung cancer. Lung Cancer 2012;75:280-4. 10.1016/j.lungcan.2011.08.004 [DOI] [PubMed] [Google Scholar]
- 17.Johns DP, Walters JAE, Walters EH. Diagnosis and early detection of COPD using spirometry. J Thorac Dis 2014;6:1557-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol 2017;53:128-49. [DOI] [PubMed] [Google Scholar]
- 19.Hothorn T, Lausen B. Maximally selected rank statistics in R. R News 2002;2:3-5. [Google Scholar]
- 20.Lee SJ, Lee J, Park YS, et al. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol 2014;9:812-7. 10.1097/JTO.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 21.Kim DS, Kim YS, Jung KS, et al. Prevalence of Chronic Obstructive Pulmonary Disease in Korea. American Journal of Respiratory and Critical Care Medicine 2005;172:842-7. 10.1164/rccm.200502-259OC [DOI] [PubMed] [Google Scholar]
- 22.Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 23.Johannessen A, Lehmann S, Omenaas ER, et al. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med 2006;173:1316-25. 10.1164/rccm.200601-023OC [DOI] [PubMed] [Google Scholar]