Abstract
Background
Aberrant epithelial remodeling and/or abnormalities in mucociliary apparatus in airway epithelium contribute to infection and inflammation. It is uncertain if these changes occur in both large and small airways in non-cystic fibrosis bronchiectasis (non-CF bronchiectasis). In this study, we aim to investigate the histopathology and inflammatory profile in the epithelium of bronchi and bronchioles in bronchiectasis.
Methods
Excised lung tissue sections from 52 patients with non-CF bronchiectasis were stained with specific cellular markers and analyzed by immunohistochemistry and immunofluorescence to assess the epithelial structures, including ciliated cells and goblet cells morphology. Inflammatory cell counts and ciliary proteins expression levels of centrosomal protein 110 (CP110) and dynein heavy chain 5, axonemal (DNAH5) were assessed.
Results
Epithelial hyperplasia is found in both bronchi and bronchioles in all specimens, including hyperplasia and/or hypertrophy of goblet cells. The median cilia length is longer in hyperplastic epithelium [bronchi: 8.16 (7.03–9.14) µm, P<0.0001; bronchioles: 7.46 (6.41–8.48) µm, P<0.0001] as compared to non-hyperplastic epithelium (bronchi: 5.60 µm; bronchioles: 4.89 µm). Hyperplastic epithelium is associated with overexpression of CP110 and decreased intensity of DNAH5 expression in both bronchial and bronchiolar epithelium. Though infiltration of neutrophils is predominant (63.0% in bronchi and 76.7% in bronchioles), eosinophilic infiltration is also present in the mucosa of bronchi (30.8%) and bronchioles (54.8%).
Conclusions
Aberrant epithelial remodeling with impaired mucociliary architecture is present in both large and small airways in patients with refractory non-CF bronchiectasis. Future studies should evaluate the interplay between these individual components in driving chronic inflammation and lung damage in patients.
Keywords: Epithelial remodeling, mucociliary, non-cystic fibrosis, bronchiectasis, pathology
Introduction
Non-cystic fibrosis bronchiectasis (non-CF bronchiectasis) is a suppurative lung disease characterized by dilated and distorted airway architecture and abnormal mucus production; patients present with chronic cough with purulent sputum production and recurrent infective exacerbations and progressive functional decline (1). Non-CF bronchiectasis imposes a significant socioeconomic burden on patients (2). They need longer hospital stays, more frequent outpatient visits, and more extensive medical therapy than control subjects, with a cost of approximately $630 million annually in America (3). Mortality rate ranged 10–16% over an approximate 4-year observation period (3).
The pathogenesis of non-CF bronchiectasis is still under investigation. Currently, the most widely accepted concept for its development is the “vicious cycle hypothesis” (4). It suggests that characteristic airway distortion and abnormal mucus impedes mucociliary clearance (MCC), which in turn promotes bacterial colonization, persistent neutrophilic inflammation and proteases production that perpetuate the process of airway destruction (5-7).
The airway epithelium forms the first line of defense to injurious external stimuli and regulates the immune functions that bridges both innate and adaptive immunity and might be an important regulator of this destructive process. In non-CF bronchiectasis, the epithelium is damaged and has increased susceptibility to injury as compared to normal airway epithelium. Failure of appropriate growth and differentiation of epithelial cells causes persistent mucosal injury (8). Ciliary dyskinesia and mucus over-production weakens clearance of respiratory pathogens from the airway (7). These dysregulated immune responses and repairment contribute to airway structural remodeling, characterized by epithelial hyperplasia, loss of cartilage and fibro-muscular tissues and fibrosis in the bronchi (3,5,9). These pathologic changes might lead to small airways obstruction, chronic inflammation, and progressive lung function impairment (1,9,10).
The histopathological changes of the airway epithelium in the bronchi and bronchioles in non-CF bronchiectasis have not been described. This is an important area to investigate as different lung compartments play different roles and interact to cause lung damage. In recent years, the small airway (<2 mm in diameter) is recognized to be the major site of airflow limitation in chronic inflammatory airway diseases such as asthma and chronic obstructive pulmonary disease (COPD) (11,12). However, small airways haven’t been well studied in non-CF bronchiectasis as thoracic high-resolution computed tomography (HRCT) and spirometric tests cannot accurately assess small airway dysfunction. Thus, we aim to investigate if there is any change in (I) epithelial remodeling (e.g., epithelial hyperplasia and metaplasia); and (II) morphology of goblet and ciliated cells. The results will contribute to a better understanding in pathogenesis of non-CF bronchiectasis.
Methods
Study patients and study design
The study conformed to guidelines of the amended Declaration of Helsinki and has obtained the approval from the Institutional Review Boards of The Third Affiliated Hospitals of Sun Yat-sen University [No. (2017) 2-22]. All patients have given their written consents. We evaluated lung tissues from 52 non-CF bronchiectasis patients aged from 17 to 50 years, who underwent a lobectomy between January 2010 and March 2014 at the First and Third Affiliated Hospitals of Sun Yat-sen University, China. The indications for surgery include: recurrent infection refractory to antibiotics and/or massive hemoptysis that were resistant to medical treatment. The diagnosis of non-CF bronchiectasis was based on thoracic HRCT. Smokers were defined as current cigarette smokers with a smoking history of more than 10 pack years. Lung specimens and clinical information of included patients were collected for histological evaluation and analysis, respectively.
Histological evaluation
Immunohistochemical (IHC) staining
Intraoperative lung biopsies of bronchiectasis were embedded in paraffin and sectioned at 4 µm with a microtome (Leica, Wetzlar, Germany). IHC staining was performed with a modified horseradish peroxidase (HRP) technique with the Dako Cytomation EnVision+System-HRP (Dako A/S). Sections were processed with Target Retrieval Buffer (Dako A/S). Endogenous peroxidase activity was blocked with 0.3% H2O2. Sections were then stained and incubated at 4 °C overnight. Eosinophils and lymphocytes were stained by hematoxylin and eosin (H&E). Neutrophils were stained with mouse anti-human neutrophil elastase monoclonal antibody (mAb) (Clone NP57; Dako A/S, Glostrup, Denmark). Cilia were stained with rabbit anti-human dynein heavy chain axonemal (DNAH5) polyclonal antibody (pAb) (Abcam, Cambridge, MA). The slides were then incubated with Dako Envision +System-HRP (Dako A/S) at room temperature for 30 minutes followed by applying HRP substrate (diaminobenzidine) for color development. All slides were counterstained with hematoxylin.
Immunofluorescence (IF)
Ciliated cells were also assessed by IF staining with mouse anti-human type IV-β tubulin mAb (Clone, ONS.1A6; Abcam) and rabbit anti-human centrosomal protein 110 (CP110) (Proteintech group, Chicago, USA). Tissue sections were processed with Target Retrieval Buffer (Dako A/S). The slides were then incubated with primary antibodies at 4°C overnight, then incubated with Alexa Fluor 488 or 594 conjugated secondary antibodies (goat-anti mouse or rabbit immunoglobulin G [IgG], H+L; Molecular Probes, Carlsbad, CA) at 1:400 in the dark at room temperature for 1 hour, and mounted with Antifade reagent with 4’, 6-diamidino-2-phenylindole (DAPI; Molecular Probes).
Goblet cells were analyzed by staining with Alcian Blue periodic acid-Schiff (PAS). Sections were immersed in Alcian Blue pH 2.5 staining solution for 30 minutes, followed by periodic acid solution for 5 minutes, Schiff reagent for 15 minutes, and counterstained with hematoxylin.
Normal adjacent lung tissue as healthy controls
As it’s not feasible to obtain open lung biopsy samples from healthy subjects, and the heterogeneity of pathological changes throughout the bronchial tree in patients with bronchiectasis, comparisons were made with the normal lung tissue areas without inflammatory cell infiltration and/or epithelial hyperplasia/remodeling, from the same tissue sections (hereafter referred to as ‘healthy controls’).
Bronchi and bronchioles
Based on the histological criteria for classifying intrapulmonary structures, airways with wider and non-collapsed lumen, lined with pseudostratified columnar epithelium and the presence of hyaline cartilage plates, goblet cells and glands were defined as bronchi. Airways with narrow collapsible lumen, simple columnar ciliated or cuboidal non-ciliated epithelium without cartilage plates, glands or goblet cells (except larger respiratory bronchioles) were defined as bronchioles (13,14).
Evaluation of epithelium
In bronchi, epithelium with >4 layers was considered hyperplastic and severe epithelial hyperplasia as >6 layers (15). In bronchioles, epithelium with ≥2 layers was considered hyperplastic and severe epithelial hyperplasia as >4 layers (14). Squamous metaplasia was determined by the appearance of the stratified squamous epithelium.
Inflammatory cell infiltration evaluation
Five individual fields with infiltration of inflammatory cells were selected for total and differential cells counts. Total cells counts were derived from counting 200 leukocytes (under ×400 magnification). Differential cell counts of the inflammatory cells were expressed as the percentage—number of positive staining cells/200 leukocytes ×100%. Inflammatory cell infiltration is categorized by more than 10%.
Goblet cell evaluation
In bronchi, goblet cell hyperplasia was defined if they were in ≥2 layers, and goblet cell hypertrophy was defined when doubling in the volume (14). Generally, goblet cells disappear in subsequent divisions in bronchioles. Therefore, goblet cell hyperplasia was defined when it appeared in bronchiolar epithelium.
Cilia length evaluation
Cilia length was evaluated on hyperplastic and non-hyperplastic epithelium in bronchi and bronchioles by assessing type IV-β tubulin staining at ×400 magnifications, as previously described (15). Five areas of the cilia staining from the tissue sections or cytospin cells were randomly selected. Cilia length was measured using Image J software. The mean value of cilia length was calculated from 20 measurements for each paraffin section.
Evaluation of CP110 and DNAH5
Staining images were captured at ×400 magnifications with a fluorescence microscope. Protein expression of these cilia related genes were analyzed by Image J, through calculating the value of the positively stained area and the mean fluorescence intensity (MFI) for each marker. Total fluorescence intensity (TFI) measurements were performed through multiplying the positive area by MFI and corrected by subtracting the background autofluorescence (15). The low intensity of DNAH5 positive staining was defined as DNAH5 abnormal expression (16).
Microbiological examination
Patients were routinely received microbiological testing using sputum smear microscopy and sputum culture before operations. After surgery, microorganisms such as fungi and tubercle bacillus will be also detected in the lung specimen. However, as most patients have received long course of antibiotic treatment, microbiological examinations cannot completely explain their etiologies.
Statistical analysis
All data were analyzed using the SPSS statistical software V18.0 (SPSS Inc., Chicago, USA). Continuous variables were expressed using mean ± standard deviation if normally distributed or median (interquartile range) if not. Categorical variables were expressed by percentage. Differences in continuous variables were compared by using Student’s t-test or non-parametric test such as Wilcoxon signed rank test, and in categorical variables were compared by using the Chi-squared test or Fisher exact test. Correlation analysis was performed using Spearman r. In all analyses, differences were considered significant if P<0.05.
Results
Patient characteristics
The demographics and clinical characteristics of patients were summarized in Table 1. Their mean age was 49.06 years old. There were significantly more male smokers (21.15%) than female smokers (5.77%, P=0.017). Pseudomonas aeruginosa (5.77%), Haemophilus influenzae (3.85%) and Haemophilus parainfluenzae (1.92%) showed positive in bronchial wash culture in 11.54% patients. Aspergillus species (15.38%) and mycobacterium tuberculosis (MTB, 11.53%) were the main pathogens identified in pathologic analysis in lung biopsy specimens. Lymphocytic inflammation was more prevalent among females as compared to males (62.5% vs. 85.7%, P=0.046).
Table 1. Clinical characteristics and microbiological results.
| Characteristics | All patients (n=52) | Male (n=24) | Female (n=28) | P§ |
|---|---|---|---|---|
| Age, year | 49.06±12.49 | 52.25±12.7 | 46.32±11.85 | 0.088 |
| Smoker | 14 (26.93) | 11 (48.53) | 3 (10.71) | 0.017 |
| Positive sputum cultures | 6 (11.54) | 3 (12.50) | 3 (10.71) | NA |
| Pseudomonas aeruginosa | 3 (5.77) | 1 (4.17) | 2 (7.14) | NA |
| Haemophilus influenzae | 2 (3.85) | 2 (8.33) | 0 | NA |
| Haemophilus parainfluenzae | 1 (1.92) | 0 | 1 (3.57) | NA |
| Infection* | 16 (30.77) | 6 (25.00) | 10 (35.71) | NA |
| Aspergillus species | 8 (15.38) | 3 (12.50) | 5 (17.86) | NA |
| Mycobacterium tuberculosis | 6 (11.53) | 2 (8.33) | 4 (14.28) | NA |
| Bacterial infection | 2 (3.85) | 1 (4.16) | 1 (3.57) | NA |
| Inflammatory cell infiltration** | ||||
| Eosinophilic | 32 (61.53) | 15 (62.50) | 17 (60.71) | NA |
| Neutrophilic | 41 (78.84) | 22 (91.67) | 19 (67.85) | 0.142 |
| Lymphocytic | 39 (75.00) | 15 (62.50) | 24 (85.71) | 0.046 |
Data are presented as number (%) and mean ± standard deviation. §, the statistical analysis was performed using chi-square tests; *, infection is evidenced by histopathological examination with specific stain in the excised lung tissue; as for TB detection, patients were diagnosed through the specific pathologic change of tuberculosis in patients’ biopsy samples, such as caseous necrosis; **, inflammatory cell infiltration is categorized by more than 10%.
Histopathological examinations
We were able to identify airway epithelium in 51 out of 52 patient specimens – bronchi only, bronchioles only and both bronchi and bronchiole structures were seen in 7, 24 and 20 specimens respectively. Table 2 summarizes the histological differences between the bronchi and bronchioles.
Table 2. Differences in histology in bronchi and bronchioles.
| Histological change | Bronchi (n=27)* | Bronchioles (n=44)* | P |
|---|---|---|---|
| Epithelial hyperplasia | 25 (92.6) | 43 (97.7) | 0.141 |
| Mild-moderate# | 13 (48.2) | 29 (65.9) | 0.139 |
| Severe## | 12 (44.5) | 14 (31.8) | 0.284 |
| Goblet cell hyperplasia | 20 (74.1) | 35 (79.6) | 0.592 |
| Goblet cell hypertrophy | 17 (63.0) | 10 (22.7) | 0.001 |
| Goblet cell hyperplasia and hypertrophy | 15 (55.6) | 9 (20.5) | 0.004 |
| Squamous metaplasia | 2 (7.4) | 1 (2.3) | 0.141 |
| Cilia length, µm | 8.16 (7.03–9.14) | 7.46 (6.41–8.48) | <0.0001** |
Data are presented as number (%), and median (interquartile range). #, mild-moderate epithelial hyperplasia is defined as the presence of 5–6 cell layers in bronchi and 3–4 cell layers in bronchioles; ##, Severe epithelial hyperplasia is defined as >6 cell layers in bronchi and > 4 cell layers in bronchioles; *, Bronchi only, bronchioles only and both bronchi and bronchiole structures were seen in 7, 24 and 20 specimens respectively; **, Compared to median lengths of bronchi and bronchioles in non-diseased control regions i.e., 5.60 (4.99–6.61) µm and 4.89 (4.50–5.99) µm respectively.
Epithelium hyperplasia/squamous metaplasia
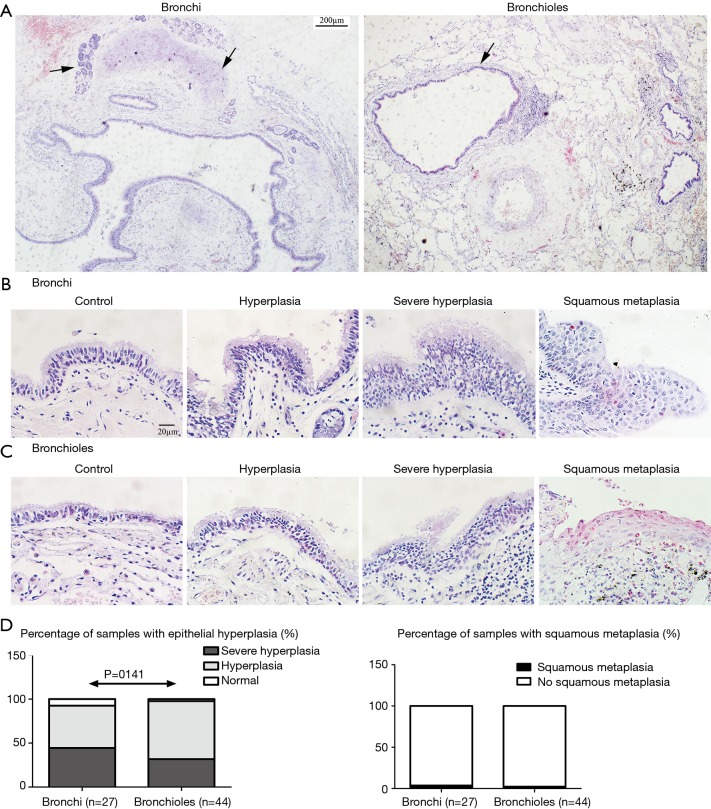
In bronchiectatic airways (Figure 1A) compared to the non-diseased areas, there was an increase in epithelial hyperplasia in both the bronchi (Figure 1B) and in bronchioles (Figure 1C). There was no difference in the prevalence or severity of epithelial hyperplasia between the bronchi and bronchioles (92.6% vs. 97.7%, P=0.141, Figure 1D). Squamous metaplasia was noted in 2 specimens (1 bronchi and 1 bronchiole specimen, Figure 1B,C,D).
Figure 1.
Epithelium of bronchi and bronchioles. Bronchi has large lumen, pseudostratified columnar epithelium and hyaline cartilage plates with glands in the wall, which were showed by “↑”. Bronchioles simple cuboidal epithelium without cartilage and glands in the wall, and goblet cell disappear. “↑” showed an enlarged bronchioles (as compared to the adjacent pulmonary vein) (A, ×40 magnification; scale bar =200 µm). The common epithelium, epithelial hyperplasia, severe hyperplasia and squamous metaplasia in bronchi (B, ×400 magnification; scale bar =20 µm) and in bronchioles (C, ×400 magnification; scale bar =20 µm). Comparison of epithelial hyperplasia and squamous metaplasia (D, Fisher exact test) in bronchi and bronchioles.
Goblet cell hyperplasia and hypertrophy
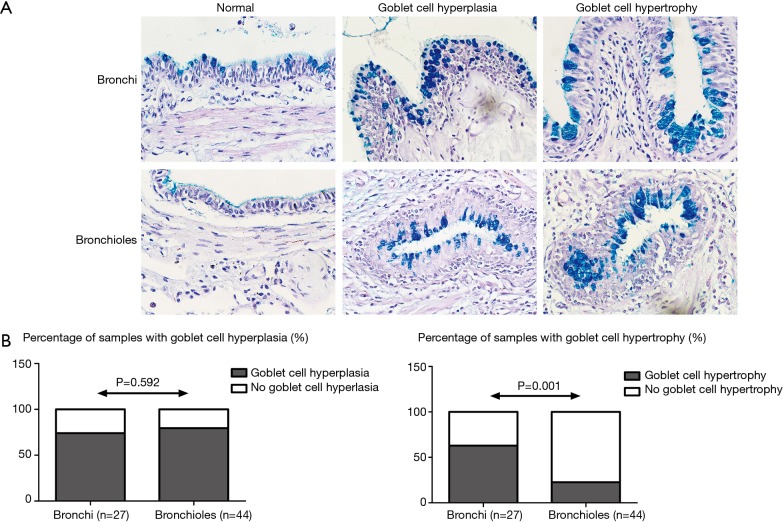
The prevalence of goblet cell hyperplasia (Figure 2) was similar in both bronchi and bronchioles (74.1% vs. 79.6%, P=0.592). The prevalence of goblet cell hypertrophy (Figure 2) was significantly higher in bronchi compared to bronchioles (63.0% vs. 22.7%, P=0.001). The prevalence of both goblet cell hyperplasia and hypertrophy was higher in bronchi than in bronchioles (55.6% vs. 20.5%, P=0.004). Two specimens did not contain goblet cells (identified by PAS staining).
Figure 2.
Goblet cell hyperplasia and hypertrophy. The goblet cell hyperplasia and hypertrophy in bronchi and bronchioles (A, ×400 magnification; scale bar =20 µm). Comparison (B, Fisher exact test) of goblet cell hyperplasia and hypertrophy in bronchi and bronchioles.
Ciliary impairment
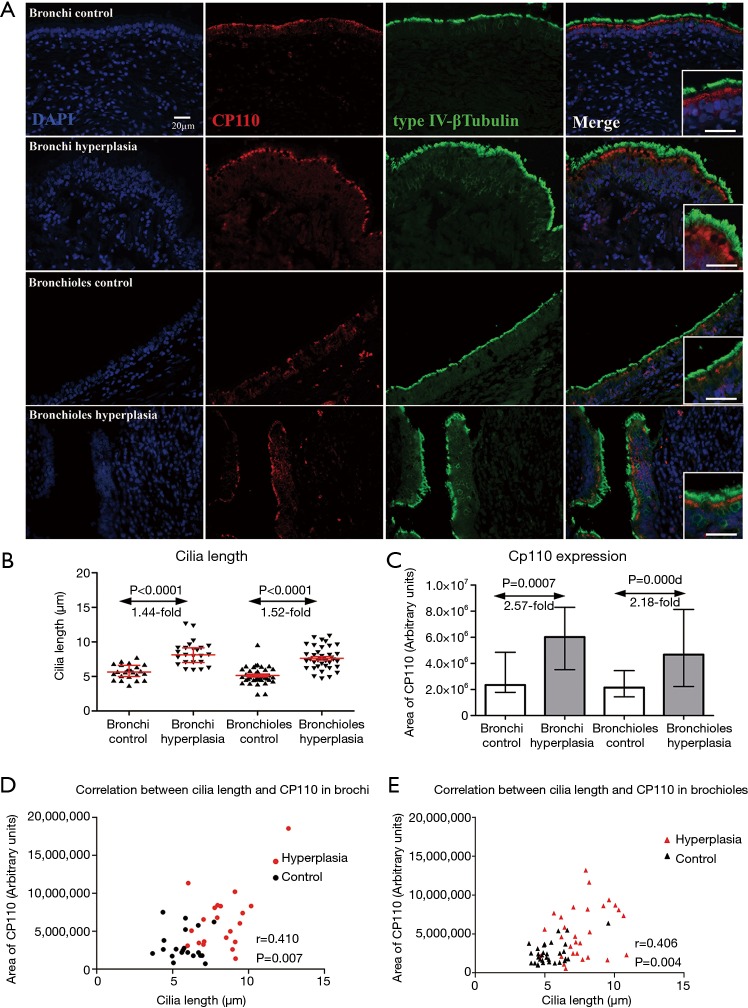
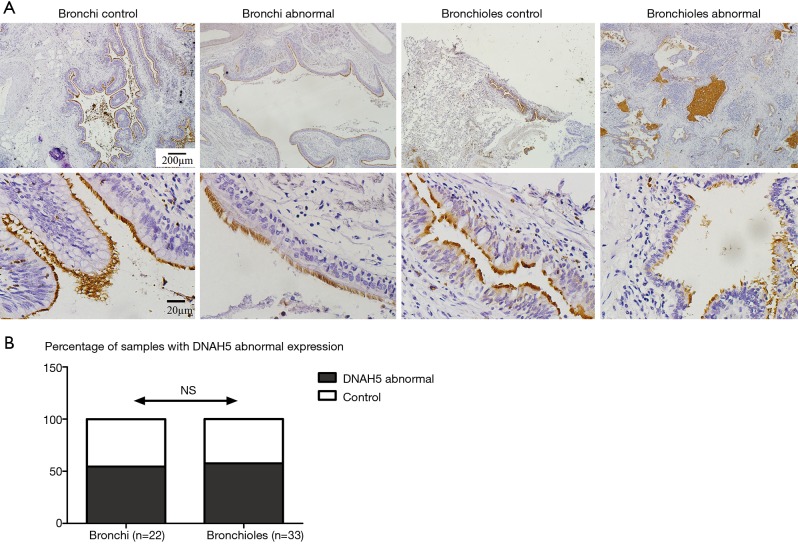
The median cilia length was significantly longer in both bronchi [8.16 (7.03–9.14) µm, P<0.0001] and bronchioles [7.46 (6.41–8.48) µm, P<0.0001] in bronchiectasis airways with epithelial hyperplasia, compared to healthy controls [5.60 (4.99–6.61) and 4.89 (4.50–5.99) µm respectively] (Figure 3A,B). There was a higher expression of CP110 in both bronchi (2.57-fold, P=0.0007) and bronchioles (2.18-fold, P=0.0005) with airway hyperplasia, compared to healthy controls (Figure 3C). There was a positive correlation between cilia length and CP110 expression in both bronchi (r=0.410, P=0.007) and in bronchioles (r=0.406, P=0.004) (Figure 3D,E). Decreased intensity of DNAH5 (by IHC staining) was found in 12 (54.4%) bronchial and 19 (57.6%) bronchiolar specimens (Figure 4).
Figure 3.
Cilia impairment. CP110 (red) were double stained with cilia (βIV-tubulin, green) in non-hyperplastic and hyperplastic epithelium in bronchi and bronchioles respectively (A, ×400 amplifications; scale bar =20 µm). Statistical analysis was performed in non-hyperplastic and hyperplastic epithelium in bronchi and bronchioles in cilia length in paraffin section (B, Mann-Whitney two-tailed U test; Median values with 25th and 75th percentiles are indicated by horizontal red lines). CP110 were evaluated by TFI (C, Mann-Whitney two-tailed U test; Median values with 25th and 75th percentiles). The correlations between TFI of CP110 and cilia length (one-to-one) were analyzed respectively (D and E).
Figure 4.
Abnormal DNAH5 expression pattern. DNAH5 was evaluated by IHC staining (A, upper: ×40 amplifications; scale bar =200 µm; down: ×400 amplifications; scale bar =20 µm). Statistical analysis was performed in non-hyperplastic and hyperplastic epithelium in bronchi and bronchioles in DNAH5 expression (B, Fisher exact test).
Inflammatory cell infiltration
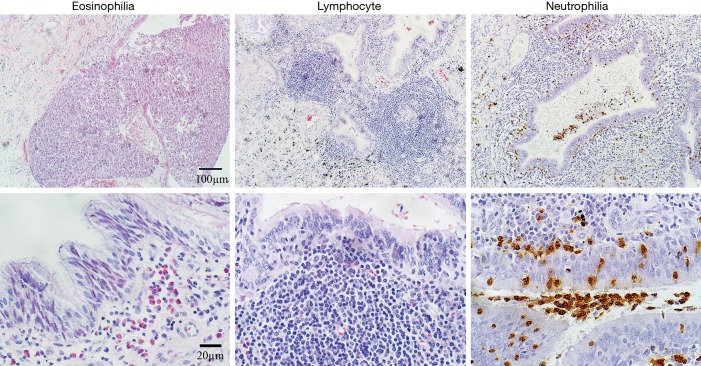
In 51 samples, we found three different patterns of inflammatory cell infiltration (Figure 5). The patterns of inflammatory cell infiltration were summarized in Table 3. There was more severe eosinophilic infiltration in the bronchioles (31.0%) compared to the bronchi (3.8%, P=0.008). Neutrophils were the predominant cell type in both bronchi (62.9%) and bronchioles (76.8%). Besides, we noted lymphocytic infiltration in over half of the bronchi and bronchiole specimens (70.4% and 59.1%, respectively). Furthermore, mixed neutrophilic and eosinophilic inflammation was seen in 6 bronchial (22.2%) and 18 bronchiolar (42.9%) specimens. A small group (<20%) exhibited mixed neutrophilic/eosinophilic/lymphocytic airway infiltration.
Figure 5.
Inflammatory cell infiltration. Inflammatory cell infiltration pattern (A, lower powder view: ×100 amplifications; scale bar =100 µm; B, ×400 amplifications; scale bar =20 µm).
Table 3. Patterns of inflammatory cell infiltration.
| Inflammatory infiltration | Bronchi (n=27), n (%) | Bronchioles (n=44), n (%) | P |
|---|---|---|---|
| Eosinophilica | |||
| None | 18 (69.2) | 19 (45.2) | NA |
| 10–30% | 7 (26.9) | 10 (23.8) | 0.770 |
| >30% | 1 (3.8) | 13 (31.0) | 0.008 |
| Neutrophilicb | |||
| None | 10 (37.0) | 10 (23.3) | NA |
| 10–30% | 11 (40.7) | 23 (53.5) | 0.253 |
| >30% | 6 (22.2) | 10 (23.3) | 0.515 |
| Lymphocytic | |||
| None | 8 (29.6) | 18 (40.9) | NA |
| 10–30% | 13 (48.2) | 21 (47.7) | 0.595 |
| >30% | 6 (22.2) | 5 (11.4) | 0.268 |
| Eosinophilic & neutrophilic | |||
| None | 21 (77.8) | 24 (57.1) | NA |
| 10–30% | 5 (18.5) | 13 (31.0) | 0.266 |
| >30% | 1 (3.7) | 5 (11.9) | 0.382 |
| All three | 5 (18.5) | 5 (11.4) | 0.489 |
a, two samples don’t have HE staining (but one of them had bronchi stained); b, one sample doesn’t have neutrophil staining for bronchioles.
Discussion
In the present study, we found a complex histopathological condition with infection in bronchi and bronchioles of patients with non-CF bronchiectasis. We demonstrated that impaired ciliated cells with lengthened cilia structures on the hyperplasic epithelium in both bronchi and bronchioles of the patients. These histological findings can be of important in understanding the pathogenesis causing acute and chronic airway infection and inflammation, clinical symptoms of coughing and expectoration, and give more evidences to the current “vicious cycle hypothesis”.
MCC is characterized by the upward movement of mucus by ciliary motion that requires a balance between the volume and composition of the mucus, and normal ciliary beat frequency (17,18). In our study, we showed hyperplasia and/or hypertrophy of the goblet cells in both the large and small airways epithelium, which contributed directly to the over-secretion of the mucus and impairment of MCC in the patients. Mucus production, secretion and clearance play a critical role in maintenance of airway health because it maintains hydration in the airway and traps particulates, bacteria, and viruses (18,19). Furthermore, airway goblet cells are proinflammatory effector cells which are able to secrete pro-inflammatory cytokines, chemokines, and growth factors. These inflammatory mediators released from goblet cells may act in an autocrine and paracrine manner to enhance inflammation in diseases such as asthma and leading to airway goblet cell hyperplasia (20,21). Our findings therefore present pathological evidences to the current “vicious cycle hypothesis” in the development of bronchiectasis.
Although different types of epithelial cells contribute to the MCC function, ciliated cells play a central role in maintaining MCC function and preventing chronic airway diseases (18,22). Among the tissue specimens, significantly lengthened cilia are present on the hyperplasic epithelium both in bronchi and bronchioles. As we described previously (15), these lengthened cilia are accompanied with impairment of MCC function, which is a potential cause of chronic mucosal inflammation or infection in chronic airway diseases (e.g., rhinosinusitis). In patients with non-CF bronchiectasis, this abnormal-cilia structure will contribute to impaired MCC, making airways susceptible to chronic infection and microbial colonization, favoring a continuous inflammatory response that persists even after infection has been controlled (3,5,7).
The structural damage to the airways is responsible for progressive small airways obstruction and dilation. Gilley and colleagues reported that conditional deletion of motile cilia in the lung of the adult mouse results in slower cilia beating frequency and the development of bronchiectasis (23). They found that airway dilation in bronchiectasis was due to morphological changes of airway epithelial cells with cellular hyperplasia and proliferation of club cells, and led to loss of cilia and MCC impairment. In bronchiectasis, the mucus itself is often abnormal and more complex. Tracheobronchial clearance in bronchiectasis has been shown to be slower than in normal control subjects independent of the presence of infection (3,5,7). Over time, retained sputum can cause mucous plugs and airway obstruction, obliteration, and damage resulting in more advanced bronchiectasis (6,7). These structural abnormalities in small airways allow for mucus stasis, which favors continued chronic infection and the persisted vicious cycle in patient with bronchiectasis (8,24).
CP110 is a key marker in ciliogenesis process (25,26). Our previous study demonstrated that the increased expression of CP110 in mucosa from patients with chronic rhino sinusitis (CRS) was associated to the poor ciliation (15). DNAH5 encode for dynein proteins of ciliated airway, and DNAH5 mutations is a common cause of primary ciliary dyskinesia with outer dynein arm defects (16). In this study, we assessed the length of cilia in bronchi and bronchioles and found significantly longer cilia with abnormal expression of ciliogenesis markers (e.g., CP110 and DNAH5) in hyperplasic epithelium as compared to normal lung tissue. The longer and messy cilia with outer dynein mutations may cause MCC dysfunction, and decrease the efficacy of pathogens elimination (22). As such, the continual local microbial invasion and inflammatory damage were contribution to permanent lung tissue alteration and airway structural remodeling (6,7). To our knowledge, this is the first study reporting a positive correlation between cilia length and CP110 in bronchiectasis.
An ongoing cycle of airway tracts infection and inflammation may be established following a combination of defection in host defense and bacterial infection (4,7,10). In consistent with previous studies (27-29), inflammatory cells infiltration around airways of non-CF bronchiectasis, including eosinophils, neutrophils and lymphocytes, could be found in both bronchi and bronchioles. As the MCC function is compromised, repeating pathogens infection or colonization led to inflammation, which caused persistent infiltration of different inflammatory cells. Interestingly, the high percentage of eosinophils in respiratory epithelium were observed in patients with bronchiectasis in our study. In fact, most of these patients didn’t have asthma according to their clinical records. Thus, the fungal infection might be a potential cause to recruit eosinophils infiltration into airways (30), because Aspergillus species infection was found to be the most common pathogen in these excised lung tissue sections from patients with non-CF bronchiectasis.
There are several limitations in this study. This is a retrospective study with a relatively small sample size. As a retrospective study, differences in demographic and clinical characteristics should be matched and compared with the healthy controls, which is not available due to the ethics. There is no standard reference of the qualitative and quantitative measures on goblet and ciliary cells in both bronchi and bronchioles, we could therefore only use the control measures obtained from the same specimens in a relative non-diseased area. In this study, the mean age of patients was 49.06 years old, which was younger than the average age in other large-sample reports. However, some studies on surgical treatment of bronchiectasis reported that the median age of these patients was less than 50 years old (31,32). This may be explained by that older patients are more likely accompanied by comorbidities and therefore be less eligible for surgery.
In conclusion, our data demonstrates the different pathologic patterns in bronchi and bronchioles and the impairments of cilia architecture in patients with refractory non-CF bronchiectasis. These findings are of significant importance to our understanding of the “viscous cycle hypothesis” in the development of non-CF bronchiectasis.
Acknowledgements
We thank Dr. Yan Yan and Dr. Li Chunwei for technologic support. We thank all patients for providing their clinical information and specimens.
Funding: This study was supported by the National Natural Science Foundation of China (Grant no. 81470219).
Ethical Statement: The study conformed to guidelines of the amended Declaration of Helsinki and has obtained the approval from the Institutional Review Boards of The Third Affiliated Hospitals of Sun Yat-sen University [No. (2017) 2-22]. All patients have given their written consents.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65:i1-58. 10.1136/thx.2010.136119 [DOI] [PubMed] [Google Scholar]
- 2.Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest 2010;138:944-9. 10.1378/chest.10-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McShane PJ, Naureckas ET, Tino G, et al. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013;188:647-56. 10.1164/rccm.201303-0411CI [DOI] [PubMed] [Google Scholar]
- 4.Boyton RJ, Altmann DM. Bronchiectasis: current concepts in pathogenesis, immunology, and microbiology. Annu Rev Pathol 2016;11:523-54. 10.1146/annurev-pathol-012615-044344 [DOI] [PubMed] [Google Scholar]
- 5.Whitters D, Stockley R. Immunity and bacterial colonisation in bronchiectasis. Thorax 2012;67:1006-13. 10.1136/thoraxjnl-2011-200206 [DOI] [PubMed] [Google Scholar]
- 6.Cole PJ. Inflammation: a two-edged sword--the model of bronchiectasis. Eur J Respir Dis Suppl 1986;147:6-15. [PubMed] [Google Scholar]
- 7.Chalmers JD, Hill AT. Mechanisms of immune dysfunction and bacterial persistence in non-cystic fibrosis bronchiectasis. Mol Immunol 2013;55:27-34. 10.1016/j.molimm.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 8.Gohy ST, Hupin C, Pilette C, et al. Chronic inflammatory airway diseases: the central role of the epithelium revisited. Clin Exp Allergy 2016;46:529-42. 10.1111/cea.12712 [DOI] [PubMed] [Google Scholar]
- 9.King P. Pathogenesis of bronchiectasis. Paediatr Respir Rev 2011;12:104-10. 10.1016/j.prrv.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 10.Wilson R, Dowling RB, Jackson AD. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur Respir J 1996;9:1523-30. 10.1183/09031936.96.09071523 [DOI] [PubMed] [Google Scholar]
- 11.Doberer D, Trejo BH, Wenzel SE. Should lung biopsies be performed in patients with severe asthma? Eur Respir Rev 2015;24:525-39. 10.1183/16000617.0045-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contoli M, Bousquet J, Fabbri LM, et al. The small airways and distal lung compartment in asthma and COPD: a time for reappraisal. Allergy 2010;65:141-51. 10.1111/j.1398-9995.2009.02242.x [DOI] [PubMed] [Google Scholar]
- 13.Park S, and Anderson P. Pathology Education Instructional Resource (PEIR). Available online: http://peir.path.uab.edu/wiki/Main_Page
- 14.Shimizu T, Takahashi Y, Kawaguchi S, et al. Hypertrophic and metaplastic changes of goblet cells in rat nasal epithelium induced by endotoxin. Am J Respir Crit Care Med 1996;153:1412-8. 10.1164/ajrccm.153.4.8616574 [DOI] [PubMed] [Google Scholar]
- 15.Li YY, Li CW, Chao SS, et al. Impairment of cilia architecture and ciliogenesis in hyperplastic nasal epithelium from nasal polyps. J Allergy Clin Immunol 2014;134:1282-92. 10.1016/j.jaci.2014.07.038 [DOI] [PubMed] [Google Scholar]
- 16.Olbrich H, Horváth J, Fekete A, et al. Axonemal localization of the dynein component DNAH5 is not altered in secondary ciliary dyskinesia. Pediatr Res 2006;59:418-22. 10.1203/01.pdr.0000200809.21364.e2 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Di YP. Effects of second hand smoke on airway secretion and mucociliary clearance. Front Physiol 2012;3:342. 10.3389/fphys.2012.00342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sears PR, Yin WN, Ostrowski LE. Continuous mucociliary transport by primary human airway epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 2015;309:L99-108. 10.1152/ajplung.00024.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sibila O, Suarez-Cuartin G, Rodrigo-Troyano A, et al. Secreted mucins and airway bacterial colonization in non-CF bronchiectasis. Respirology 2015;20:1082-8. 10.1111/resp.12595 [DOI] [PubMed] [Google Scholar]
- 20.Tanabe T, Shimokawaji T, Kanoh S, et al. Secretory phospholipases A2 are secreted from ciliated cells and increase mucin and eicosanoid secretion from goblet cells. Chest 2015;147:1599-609. 10.1378/chest.14-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanabe T, Rubin BK. Airway goblet cells secrete pro-inflammatory cytokines, chemokines, and growth factors. Chest 2016;149:714-20. 10.1378/chest.15-0947 [DOI] [PubMed] [Google Scholar]
- 22.Boon M, Wallmeier J, Ma L, et al. MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nat Commun 2014;5:4418. 10.1038/ncomms5418 [DOI] [PubMed] [Google Scholar]
- 23.Gilley SK, Stenbit AE, Pasek RC, et al. Deletion of airway cilia results in non-inflammatory bronchiectasis and hyperreactive airways. Am J Physiol Lung Cell Mol Physiol 2014;306:L162-9. 10.1152/ajplung.00095.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilley AE, Walters MS, Shaykhiev R, et al. Cilia dysfunction in lung disease. Annu Rev Physiol 2015;77:379-406. 10.1146/annurev-physiol-021014-071931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walentek P, Quigley IK, Sun DI, et al. Ciliary transcription factors and miRNAs precisely regulate Cp110 levels required for ciliary adhesions and ciliogenesis. Elife 2016;5:e17557. 10.7554/eLife.17557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yadav SP, Sharma NK, Liu C, et al. Centrosomal protein CP110 controls maturation of the mother centriole during cilia biogenesis. Development 2016;143:1491-501. 10.1242/dev.130120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regamey N, Tsartsali L, Hilliard TN, et al. Distinct patterns of inflammation in the airway lumen and bronchial mucosa of children with cystic fibrosis. Thorax 2012;67:164-70. 10.1136/thoraxjnl-2011-200585 [DOI] [PubMed] [Google Scholar]
- 28.Amalakuhan B, Maselli DJ, Martinez-Garcia MA. Update in Bronchiectasis 2014. Am J Respir Crit Care Med 2015;192:1155-61. 10.1164/rccm.201505-0926UP [DOI] [PubMed] [Google Scholar]
- 29.Gaga M, Bentley AM, Humbert M, et al. Increases in CD4+ T lymphocytes, macrophages, neutrophils and interleukin 8 positive cells in the airways of patients with bronchiectasis. Thorax 1998;53: 685-91. 10.1136/thx.53.8.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Dea EM, Amarsaikhan N, Li H, et al. Eosinophils are recruited in response to chitin exposure and enhance Th2-mediated immune pathology in Aspergillus fumigatus infection. Infect Immun 2014;82:3199-205. 10.1128/IAI.01990-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin YX, Zhang Y, Duan L, et al. Surgical treatment of bronchiectasis: a retrospective observational study of 260 patients. Int J Surg 2014;12:1050-4. 10.1016/j.ijsu.2014.08.398 [DOI] [PubMed] [Google Scholar]
- 32.Rademacher J, Ringshausen FC, Suhling H, et al. Lung transplantation for non-cystic fibrosis bronchiectasis. Respir Med 2016;115:60-5. 10.1016/j.rmed.2016.04.007 [DOI] [PubMed] [Google Scholar]