Abstract
Background
Aberrant expression of programmed cell death-ligand 1 (PD-L1) and protein 53 (P53) has been observed in various malignancies, and recently, the mechanism of PD-L1 regulation by P53 has been elucidated. We aimed to explore possible correlations between PD-L1 and P53 expression and the prognosis of patients with resected pulmonary lymphoepithelioma-like carcinoma (LELC).
Methods
A total of 67 consecutive patients with primary pulmonary LELC who underwent radical resection from January 2003 to December 2014 were enrolled in our study. Membranous PD-L1 and nuclear P53 expression were detected by immunohistochemical staining (IHC).
Results
Positive expression of PD-L1 in tumor cells (TCs), PD-L1 in tumor-infiltrating lymphocytes (TILs) and P53 was investigated in 44 patients (65.7%), 37 patients (55.2%), and 34 patients (50.7%), respectively. Using univariate and multivariable analysis, both PD-L1 (+) in TCs and P53 (+) were observed to be significantly independent prognostic factors associated with longer disease-free survival (DFS, P=0.037 and 0.039, respectively), along with early stage LELC (P=0.037), but had no association with overall survival (OS) (P>0.05). In the P53 (+) group, the rate of patients with PD-L1 (+) in TCs was significantly higher than in the P53 (−) group (85.3% vs. 45.5%, P=0.001). In addition, among the 45 patients who underwent adjuvant chemotherapy, DFS was significantly longer in patients with either PD-L1 (+) in TCs or P53 (+) (P=0.036 and 0.044, respectively).
Conclusions
PD-L1 and P53 may be potential therapeutic targets for primary pulmonary LELC. PD-L1 (+) in TCs and P53 (+) were reliable predictors for longer DFS and benefits from adjuvant therapy in resected cases. Routine detection of these two indices in lung LELC may be warranted.
Keywords: Lymphoepithelioma-like carcinoma (LELC), programmed cell death-ligand 1 (PD-L1), protein 53 (P53), tumor-infiltrating lymphocytes (TILs), prognosis
Introduction
Primary pulmonary lymphoepithelioma-like carcinoma (LELC) is a subtype of lung cancer related to the EB virus. In limited literature reports, patients with primary pulmonary LELC survive longer than patients with other lung cancer subtypes (1-3). However, almost thirty-one percent of early stage patients experienced recurrence within five years after multimodality treatment, and nearly thirty-two percent of all patients with lung LELC presented with advanced disease at initial visits (1,3-5). Thus, discovery of new therapeutic targets is needed to improve survival and quality of life further for recurrent or metastatic patients. However, it has been reported that lung LELC lacks common driver gene mutations to existing drug targets, such as epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) gene rearrangement, among others (6,7). In addition, survival benefits from monoclonal antibodies against programmed cell death 1 (PD-1) and programmed cell death-ligand 1 (PD-L1) have been reported, and positive or high expression of PD-L1 in tumor cells [PD-L1 (+) in TCs] or tumor-infiltrating lymphocytes [PD-L1 (+) in TILs] have been suggested to be a biomarker for survival prediction and optional immunotherapy. However, although positive or high expression of PD-L1 in TCs has also been detected in primary pulmonary LELC using immunohistochemical staining (IHC), the prognostic significance of PD-L1 expression for this rare population is still controversial (5,6).
In addition, recent reports found that mutated P53 can modulate PD-L1 expression via microRNA34 and that nuclear P53 and membranous PD-L1 expression levels were correlated in non-small cell lung cancer (NSCLC) (8,9). It is well-known that P53 mutations, which are strongly linked with mutation burdens of lung cancer and poor survival, have been detected in greater than half of patients with NSCLC (10). However, to date, a correlation between PD-L1 and P53 expression in primary pulmonary LELC has not been shown. In addition, the prevalence and prognostic significance of P53 mutation status has never been reported.
Therefore, for the first time, we aimed to explore the correlation and prognostic significance of PD-L1 and P53 status using IHC in sixty-seven postoperative pathological stage I-IIIa primary pulmonary LELC patients.
Methods
Study population selection
Clinical, pathological, radiological and follow-up information of all patients diagnosed with lung LELC at Sun Yat-sen University Cancer Center between January 2003 and December 2014 was analyzed with statistical software (IBM SPSS Statistics version 23.0, Chicago, IL, USA) from the Department of Medical Records. Subsequently, consecutive patients with primary pulmonary LELC who received radical resection (R0) at Department of Thoracic Surgery were collected. Patients enrolled in the study needed to meet the following criteria: (I) no preoperative chemotherapy or radiotherapy; (II) had preserved surgically resected paraffin-embedded specimens; (III) no operation-related death; and (IV) no second primary neoplasms. This retrospective study was approved by the board-certified Research Ethics Committee at our center.
Immunohistochemistry and staining interpretation
The PD-L1 and P53 expression in tumor tissue were detected by IHC, as described in previous studies (5,11). In our studies, IHC was performed using a rabbit monoclonal anti-PD-L1 antibody (E1L3N™, Cell Signaling Technology, Danvers, MA, USA; dilution 1:200) and rabbit monoclonal antibodies to P53 (1:50, DAKO, Glostrup, Denmark) to determine expression. Two pathologists were blinded to patients’ information, and independently assessed the expression of membranous PD-L1 and nuclear P53 protein. Discordant interpretations were reassessed in a double-head microscope and a final result was agreed.
The tumor sample was considered positive for PD-L1 expression in tumor cells if moderate-to-strong membrane staining was observed in ≥5% of TCs based on previous larger studies (12). However, TILs were considered to have positive PD-L1 expression if ≥1% of TILs exhibited moderate-to-strong membrane staining, as previously described (13,14). P53 expression was considered positive when ≥10% of the TC nuclei showed orange or brown staining, based on two meta-analyses (15,16). In addition, H-score is determined by multiplying each percentage of positive cells by an intensity score (0, absent; 1, weak; 2, moderate; 3, strong).
End-point and statistical analysis
The duration of disease-free survival (DFS) was calculated from the day of radical resection to when radiological progression was initially observed. The overall survival (OS) time was calculated from the day of radical resection to when death was caused by cancer. Those patients who had not reached the above-mentioned end-points or were lost to surveillance by the latest follow-up date (June 1, 2017) were regarded as censored. The Chi-square (χ2) test and Fisher’s exact test were used to analyze differences in categorical variables (including gender, age, smoking history, surgical procedure, tumor location, TNM stage, and adjuvant therapy) between positive PD-L1/P53 expression groups and negative PD-L1/P53 expression groups. In addition, univariate survival analysis and survival curves were conducted by Kaplan-Meier method and the log-rank test was routinely used to test for significant differences. Multivariate survival analysis was conducted with the Cox proportional hazards model. All statistical analyses were performed by IBM SPSS (version 23.0) and two-sided P values <0.05 were defined as significantly different.
Results
Clinical and pathological characteristics in resected primary pulmonary LELC
A total of 67 consecutive patients with resected primary pulmonary LELC, including 36 women and 31 men, were enrolled for detection of nuclear P53 and membranous PD-L1 expression. These patients’ clinicopathological and IHC characteristics are described in Table 1. The mean age at diagnosis was 52.52 (interquartile range, 45.00–59.00) years, and 25.4% of these patients (17/67) have a current or former smoking history with a median smoking index of 400 (range from 9 to 1,350). Based on the eighth edition of the TNM classification for lung cancer, the stage distribution was stage I in 23 patients, stage II in 16 patients and stage IIIa in 28 patients. Forty-five patients had received postoperative adjuvant therapy and the most frequently used regimens were pemetrexed with cisplatin/carboplatin (18/45, 40%) and paclitaxel/docetaxel with cisplatin/carboplatin (22/45, 48.9%).
Table 1. The clinical, pathological and immunohistochemical characteristics of the 67 patients with resected primary pulmonary LELC; and the univariate analysis for disease-free survival.
| Characteristics (n) | No. of cases | Univariate analysis | |
|---|---|---|---|
| 5-year DFS (%) | Pa | ||
| Age (mean ± SD, years) | 52.52±10.01 | ||
| <60 | 54 | 50.5 | 0.69 |
| ≥60 | 13 | 58.6 | |
| Gender | |||
| Female | 36 | 41.2 | 0.13 |
| Male | 31 | 63.4 | |
| Smoking status | |||
| Smokers | 17 | 68.3 | 0.64 |
| Nonsmokers | 50 | 48.4 | |
| Tumor location | |||
| Left lobe | 29 | 48.3 | 0.42 |
| Right lobe | 38 | 54.9 | |
| Tumor size | |||
| ≤3 cm | 26 | 34.8 | 0.3 |
| >3 cm | 41 | 70 | |
| Angiolymphatic invasion | |||
| Yes | 23 | 67.1 | 0.78 |
| No | 34 | 47.4 | |
| Tumor stage | |||
| T1 | 11 | 33.7 | 0.47 |
| T2 | 50 | 58.3 | |
| T3 | 6 | 62.5 | |
| TNM Stage | |||
| I–II | 39 | 62.9 | <0.001 |
| IIIa | 28 | 35.4 | |
| Adjuvant therapy | |||
| Yes | 45 | 51.1 | 0.32 |
| No | 22 | 52.5 | |
| Surgical approach | |||
| Lobectomy | 52 | 49.8 | 0.61 |
| Bilobectomy | 8 | 75 | |
| Pneumonectomy | 7 | 42.9 | |
| PD-L1 expression in TCs | |||
| Positive | 44 | 59.7 | 0.04 |
| Negative | 23 | 39.5 | |
| P53 expression status | |||
| Positive | 34 | 69.2 | 0.01 |
| Negative | 33 | 38.2 | |
| PD-L1 expression in TILs | |||
| Positive | 37 | 59.9 | 0.55 |
| Negative | 30 | 50.6 | |
| PD-L1 in TCs/P53 expression | |||
| PD-L1 (+) and P53 (+) | 29 | 61.9 | 0.01 |
| Either PD-L1 (+) or P53 (+) | 20 | 63 | |
| PD-L1 (−) and P53 (−) | 18 | 26.7 | |
a, the P value less than 0.05 was defined as significantly difference in statistical analysis. PD-L1, programmed cell death ligand 1; P53, human tumor protein 53; TCs, tumor cells; TILs, tumor-infiltrating lymphocytes.
Correlation of PD-L1 and P53 expression in resected primary pulmonary LELC
Positive PD-L1 expression in TCs [PD-L1 (+) in TCs] was observed in 44 patients (65.7%) with a median H-score of 40 (interquartile range, 0 to 80). When defining high expression as ≥50% of TCs with moderate-to-strong staining at the membrane, the overall proportion of PD-L1 high expression in TCs was 41.8% (28/67). As described in Table 2, clinical and pathological variables showed no significant link with PD-L1 (+) in TCs. However, in the positive P53 expression [P53 (+)] group, the proportion of patients with PD-L1 (+) in TCs was 85.3% (29/34), which was significantly higher than in the P53 (−) group (15/33, 45.5%, P=0.001). Upon further analysis, there was no statistically significant positive correlation between expression levels of P53 and PD-L1 in TCs (r=0.153, P=0.317). In addition, 37 patients (55.2%) with moderate to strong TIL membrane PD-L1 staining (≥5% of all cells) were observed. Similarly, positive PD-L1 expression in TILs [PD-L1 (+) in TILs] also showed no significant relation to any clinical and pathological variables.
Table 2. The clinical, pathological and immunohistochemical characteristics according to PD-L1/P53 expression status.
| Characteristics (n) | PD-L1 expression in TCs | P53 expression | |||||
|---|---|---|---|---|---|---|---|
| Positive (≥5%) (n=44) | Negative (<5%) (n=23) | Pa | Positive (≥10%) (n=34) | Negative (<10%) (n=33) | Pa | ||
| Age (mean ± SD, years) | 51.21±9.60 | 54.11±10.61 | 0.18 | 50.90±9.72 | 54.15±10.21 | 0.2 | |
| Gender | |||||||
| Female | 21 | 15 | 0.2 | 17 | 19 | 0.63 | |
| Male | 23 | 8 | 17 | 14 | |||
| Smoking status | |||||||
| Smokers | 13 | 4 | 0.38 | 10 | 7 | 0.58 | |
| Nonsmokers | 31 | 19 | 24 | 26 | |||
| Tumor location | |||||||
| Left lobe | 18 | 11 | 0.47 | 15 | 14 | 0.86 | |
| Right lobe | 26 | 12 | 19 | 19 | |||
| Tumor stage | |||||||
| T1 | 5 | 6 | 0.17 | 4 | 7 | 0.33 | |
| T2 | 36 | 14 | 28 | 22 | |||
| T3 | 3 | 3 | 2 | 4 | |||
| Nodal stage | |||||||
| N0 | 18 | 6 | 0.49 | 15 | 9 | 0.33 | |
| N1 | 9 | 6 | 6 | 9 | |||
| N2 | 17 | 11 | 13 | 15 | |||
| TNM stage | |||||||
| I | 18 | 5 | 0.21 | 14 | 9 | 0.8 | |
| II | 9 | 7 | 8 | 8 | |||
| IIIa | 17 | 11 | 12 | 16 | |||
| P53 expression | |||||||
| Positive | 29 | 5 | <0.001 | – | – | – | |
| Negative | 15 | 18 | |||||
| PD-L1 expression in TCs | |||||||
| Positive | – | – | – | 29 | 15 | <0.001 | |
| Negative | 5 | 18 | |||||
| PD-L1 expression in TILs | |||||||
| Positive | 24 | 13 | 0.88 | 17 | 20 | 0.38 | |
| Negative | 20 | 10 | 17 | 13 | |||
a, the P value less than 0.05 was defined as significantly difference in statistical analysis. PD-L1, programmed cell death ligand 1; P53, human tumor protein 53; TCs, tumor cells; TILs, tumor-infiltrating lymphocytes.
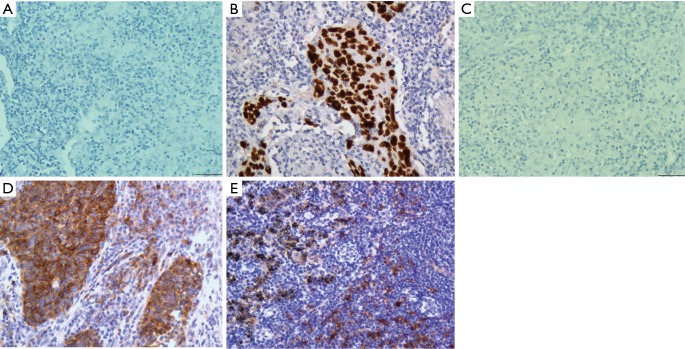
Of all 67 patients, 50.7% (34/67) had P53 (+) expression. Based on the Chi-square test and Fisher’s exact test, no significant difference in clinicopathological characteristics was found between the P53 (+) group and the P53 (−) group, including gender, age, smoking history, tumor location, and TNM stage (Table 2). In addition, there were 29 patients (43.3%) who were both PD-L1 (+) in TCs and P53 (+), and 18 patients (26.9%) who had negative expression of both PD-L1 in TCs and P53. Representative images of PD-L1 and P53 immunostaining results in primary pulmonary LELC are shown in Figure 1.
Figure 1.
Representative images of PD-L1 and P53 expression by IHC (magnification 400×). (A) Negative P53 expression; (B) positive P53 expression; (C) negative PD-L1 expression; (D) positive PD-L1 expression in TCs and TILs; (E) positive PD-L1 expression in lymphocytes distal from TCs. PD-L1, programmed cell death ligand 1; TCs, tumor cells; TILs, tumor-infiltrating lymphocytes.
Prognostic significance of PD-L1 and P53 expression in resected primary pulmonary LELC
In our cohort, the duration of follow-up ranged from 7 to 144 months (median: 33 months). There were 25 recurrences and five tumor-related deaths during the follow-up period. The 1-, 3- and 5-year DFS rates were 88%, 68.9% and 51.9%, respectively and the median DFS time was 68.4 months. However, the median OS time had not yet been reached and the 5-year OS rate was 82.3%.
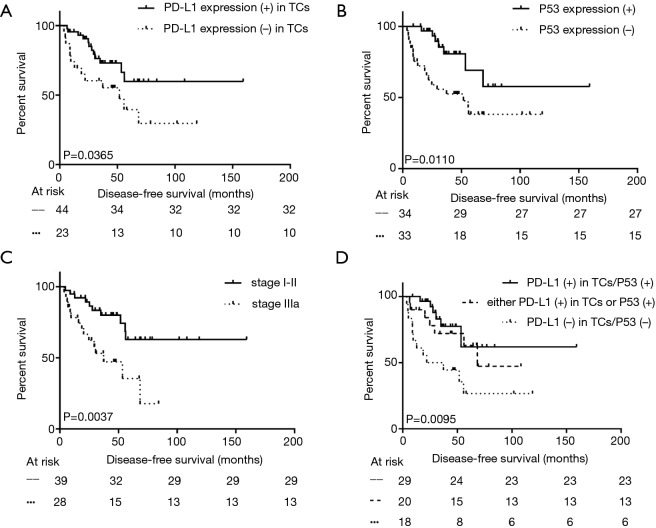
Using Kaplan-Meier univariate analysis as described in Table 1, well-known prognostic predictors including postoperative pathological stage, and presentation of mediastinal lymph nodes metastasis were apparently related to DFS and OS (P<0.050, Figure 2). However, PD-L1 (+) in TCs and P53 (+) were all significantly associated with longer DFS (P=0.037 and 0.011, respectively, Figure 2), but not with OS (P>0.050). In addition, the 29 patients with both PD-L1 (+) in TCs and P53 (+) had longer DFS than the all-negative group (median DFS: not reached vs. 21.9 months, P=0.006, Figure 2). Moreover, this phenomenon was also observed between either the PD-L1 (+) in TCs group or the P53 (+) group and the all-negative group, and the p value was close, but did not reach statistical significance (median DFS: 68.4 vs. 21.9 months, P=0.052). Through analysis with the Cox multivariable survival model, only early postoperative pathological stage, PD-L1 (+) in TCs, and P53 (+) were found to be independent prognostic predictors with longer DFS and are shown in Table 3.
Figure 2.
Kaplan-Meier univariate analysis of disease-free survival among all 67 patients who received surgical therapy according to PD-L1 expression in TCs (A), P53 expression (B), pathological stage (C), and combination of PD-L1 and P53 expression in TCs (D). PD-L1, programmed cell death ligand 1; TCs, tumor cells.
Table 3. Prognostic factors for disease-free survival of the 67 patients with resected primary pulmonary LELC according to multivariate analysis.
| Characteristics | Groups | Hazard ratio (95%CI) | Pa |
|---|---|---|---|
| Gender | Female/male | 3.545 (0.906–13.871) | 0.069 |
| Tumor size | ≤3/>3 cm | 0.611 (0.177–2.112) | 0.436 |
| TNM stage | I–II/IIIa | 0.032 (0.001–0.810) | 0.037 |
| PD-L1 expression in TCs | Positive/negative | 7.593 (1.130–51.032) | 0.037 |
| P53 expression status | Positive/negative | 17.054 (1.148–253.374) | 0.039 |
| PD-L1 in TCs/P53 expression | All positive/either positive/all negative | 8.911 (0.377–210.494) | 0.175b |
a, the P value less than 0.05 was defined as significantly difference in statistical analysis; b, the other two was used to compared with all positive group. PD-L1, programmed cell death-ligand 1; P53, human tumor protein 53; TCs, tumor cells; CI, confidence interval.
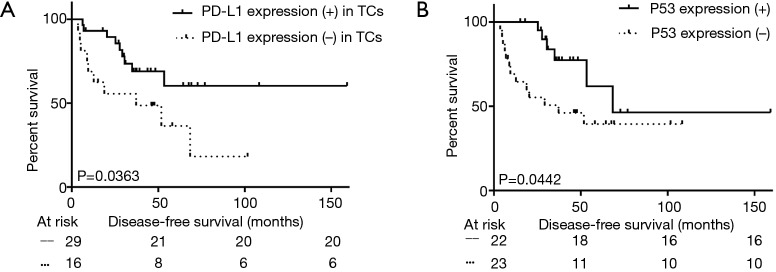
Among the 45 patients with adjuvant chemotherapy, either PD-L1 (+) in TCs or P53 (+) effected the DFS (median DFS: not reached vs. 37.2 months in negative PD-L1 group, P=0.036; not reached vs. 21.2 months in negative P53 group, P=0.044, Figure 3A,B and Table 4). However, these trends were not observed in the group that did not undergo adjuvant therapy (P>0.050).
Figure 3.
Disease-free survival according to (A) PD-L1 expression in TCs or (B) P53 expression among 45 patients who received adjuvant therapy. PD-L1, programmed cell death ligand 1; TCs, tumor cells.
Table 4. The clinical, pathological and immunohistochemical characteristics of the 45 patients with adjuvant chemotherapy.
| Characteristics (n) | PD-L1 expression in TCs | P53 expression | |||||
|---|---|---|---|---|---|---|---|
| Positive (≥5%) (n=29) | Negative (<5%) (n=16) | Pa | Positive (≥10%) (n=22) | Negative (<10%) (n=23) | Pa | ||
| Age (mean ± SD, years) | 51.72±8.61 | 53.43±11.41 | 0.57 | 50.12±8.31 | 54.42±10.50 | 0.13 | |
| Gender | |||||||
| Female | 14 | 11 | 0.19 | 10 | 15 | 0.18 | |
| Male | 15 | 5 | 12 | 8 | |||
| Smoking status | |||||||
| Smokers | 9 | 2 | 0.17 | 7 | 4 | 0.26 | |
| Non-smokers | 20 | 14 | 15 | 19 | |||
| Tumor location | |||||||
| Left lobe | 12 | 9 | 0.16 | 11 | 10 | 0.70 | |
| Right lobe | 17 | 7 | 11 | 13 | |||
| Tumor stage | |||||||
| T1 | 1 | 4 | 0.03 | 1 | 4 | 0.12 | |
| T2 | 26 | 9 | 20 | 15 | |||
| T3 | 2 | 3 | 1 | 4 | |||
| Nodal stage | |||||||
| N0 | 9 | 1 | 0.14 | 8 | 2 | 0.08 | |
| N1 | 7 | 4 | 4 | 7 | |||
| N2 | 13 | 11 | 10 | 14 | |||
| TNM Stage | |||||||
| Ib | 9 | 1 | 0.38 | 8 | 2 | 0.25 | |
| II | 7 | 4 | 5 | 6 | |||
| IIIa | 13 | 11 | 9 | 15 | |||
| Surgical approach | |||||||
| Lobectomy | 23 | 12 | 0.95 | 18 | 17 | 0.37 | |
| Bilobectomy | 3 | 2 | 1 | 4 | |||
| Pneumonectomy | 3 | 2 | 3 | 2 | |||
| Adjuvant regimen | |||||||
| Pemetrexed plus platinum | 15 | 3 | 0.12 | 11 | 7 | 0.22 | |
| Paclitaxel plus platinum | 7 | 5 | 6 | 6 | |||
| Docetaxel plus platinum | 4 | 6 | 2 | 8 | |||
| Gemcitabine plus platinum | 2 | 2 | 2 | 2 | |||
| Vinorelbine plus platinum | 1 | 0 | 1 | 0 | |||
| Disease recurrence status | |||||||
| Yes | 9 | 10 | 0.04 | 6 | 13 | 0.04 | |
| No | 20 | 6 | 16 | 10 | |||
| Alive status | |||||||
| Death | 1 | 2 | 0.24 | 1 | 2 | 0.58 | |
| Alive | 28 | 14 | 21 | 21 | |||
a, the P value less than 0.05 was defined as significantly difference in statistical analysis. PD-L1, programmed cell death ligand 1; P53, human tumor protein 53; TCs, tumor cells.
Discussion
PD-1 is usually expressed in the cell membranes of activated lymphocytes, which primarily serve as host immune cells responding to harmful invasive microorganisms and TCs. However, when the specific PD-L1 or 2, which is expressed in many malignant solid tumors, interacts with PD-1, the activated lymphocytes, especially CD8+ T cells, are negatively regulated, leading to exhaustion or apoptosis of infiltrating lymphocytes and subsequent immune escape. In recent research, positive or over-expression of PD-L1 has been observed by IHC in NSCLC. In other words, the development and progression of lung cancer might not be affected by tumor-stimulated immune interactions, because binding between PD-L1 in TCs and PD-1 receptor in TILs could induce immune tolerance. However, novel PD-L1/PD-1 monoclonal antibodies could blockade either of the two binding sites, thereby restoring function of exhausted lymphocytes, which could generate a durable antitumor immune response. Although detection of PD-L1 by IHC is not currently required in order to proceed with PD-L1/PD-1 monoclonal antibodies therapy, routine testing may provide potential prognostic value.
With regard to primary pulmonary LELC, a lack of common driver gene mutations has been reported by many scholars, but 63.3–75.8% of the EB virus-associated primary pulmonary carcinoma showed PD-L1 (+) in TCs, which was a higher proportion than in lung squamous cell carcinoma (SCC, 21–56.2%), lung adenocarcinoma (ADC, 18.6–49.0%) and non-selective NSCLC (24.8–57.5%), A similar proportion of samples with PD-L1 (+) in TCs (44/67, 65.7%) was found in our cohort (4-7,9,17,18). However, the prognostic implication of positive or high PD-L1 expression in TCs for this rare population is still controversial. In a report by Jiang et al., the PD-L1 (+) in TCs group was associated with longer DFS, which was consistent with our result (P=0.037) (5). In contrast, no prognostic value of this biomarker for LELC of lung was found in Chang and colleagues’ retrospective analysis, and Fang et al. had observed that high PD-L1 expression in TCs (H score ≥30) was correlated with poor DFS in resectable LELC of lung (4,6). Although there is no agreement on the prognostic significance of PD-L1, these published reports had confirmed that patients with primary pulmonary LELC are a special community with a higher incidence of PD-L1 (+) in TCs and that they might be candidates for immune therapy with PD-L1/PD-1 monoclonal antibodies.
In recent years, up-regulated PD-L1 expression in TILs has been detected in many malignant solid tumors, and its potential predictive value for anti-PD-1/PD-L1 immune therapy and survival outcomes has also been reported. However, the scant reports from Yang et al. and Cha et al. revealed no evidence supporting the application of PD-L1 expression in TILs as a prognostic factor for NSCLC (9,19). Similarly, we found that the DFS and OS were not significantly different between the positive and negative PD-L1 expression in TILs groups. In addition, correlation between PD-L1 expression in TCs and TILs has been shown in SCC and ADC, but our study did not find this correlation in primary pulmonary LELC (9,19).
Mutant P53 sites are often detected by gene sequencing or IHC in NSCLC (up to 50%) (10). Similarly, P53 mutations were shown to be the most common mutation in primary pulmonary LELC by Dr. Hong SD from our center. This result was chosen as a poster board display during the 2016 Annual Meeting of the American Society of Clinical Oncology (ASCO), but this finding was inconsistent with a study by Chang et al. (20). In this study, P53 status was evaluated using IHC, since the expressed mutant P53 protein, which had been shown to have a longer half-life than wild-type P53 protein, could accumulate at the nuclear membrane and be detected by IHC. In a recent report on the mechanism, Cortez MA and his colleagues elucidated that P53 can regulate PD-L1 expression via microRNA34. There was also a concerning correlation between PD-L1 expression and P53 mutation status (8). We also found that the P53 (+) population had a higher incidence of patients with PD-L1 (+) in TCs (85.3% vs. 45.5%, P=0.001), but found no positive correlation between expression levels of P53 and PD-L1 in TCs. This result differs from findings in non-selected NSCLC reported by Cortez et al. and Cha et al. (8,9). More peculiar was the finding that P53 (+) in primary pulmonary LELC showed a favorable DFS in our analysis. This tendency was also observed by Chang et al. in three primary pulmonary LELC patients with P53 gene mutations, which is completely opposite from results in other subtypes of NSCLC (15,16,20). To our knowledge, this is the first report concerning the prognostic significance of P53 expression in LELC of the lung. In addition, based on the results of Cancer Genome Atlas exome data analysis, there is a concerning link between P53 status and mutation burden in tumors (21). In other words, the evaluation of P53 status could be used as a surrogate biomarker for mutation burden (9,21). At the same time, other research has suggested that higher non-synonymous mutation burdens could improve clinical benefits and PFS after antitumor therapy (22). Similar to our results, the 29 patients with both PD-L1 (+) in TCs and P53 (+) had a prolonged DFS compared to the all-negative group (P=0.006). Moreover, this difference was also observed between the P53 (+) only group and the all-negative group; however, the P value only had a strong tendency towards statistical significance (P=0.061).
The value of postoperative adjuvant therapy for primary pulmonary LELC is another unsettled issue. Similar to a report from Lin et al., adjuvant chemotherapy after radical dissection did not improve DFS and OS in our study (23). However, in other larger cohort, postoperative adjuvant chemotherapy could prolong OS of patients with stage IIIa cancer (2). In addition, among the 45 patients who underwent adjuvant chemotherapy, the PD-L1 (+) in TCs and P53 (+) groups had more favorable DFS than the negative group, which was first observed in LELC of the lung. In prior literature, the PD-L1 (+) in TCs or P53 statuses, which could be detected by IHC or sequencing, had not been shown to be a predictor factor for adjuvant chemotherapy (9,17,24).
Taken together, the following limitations are present in our study. First, this cohort was limited by the scant number of cases, and the fact that the majority of these reported cases came from southern China, such as the Guangdong province. Second, the prognostic significance of PD-L1 and P53 status might be affected by different cut-off values. In our analysis, moderate to strong membranous staining observed in more than 5% of all TCs was defined as PD-L1 (+) in TCs and 5% was also the threshold value in many prospective clinical studies (12,25,26). Similarly, the cut-off for PD-L1 (+) in TILs was defined as moderate to strong staining in more than 1% of infiltrating lymphocytes, based on larger cohort analyses (13,14). Finally, the P53 status was detected by IHC in this study, rather than by P53 gene sequencing, and the individual was considered to be positive when stained nuclei were found in more than 10% of all TCs based on two larger meta-analyses (15,16). Like with ovarian carcinoma, colorectal cancer, and glioblastoma multiforme, etc. (27-30), the optimal thresholds of P53 positivity in LELC of the lung is still debated and further research studies are warranted. We calculated different cutoff values in our cohort including 1%, 5%, 10%, and 50%, and found that 10% is the most appropriate one to be of prognostic value.
In conclusion, PD-L1 and P53 may be potential therapeutic targets for primary pulmonary LELC. Positive PD-L1 in TCs and P53 expression were two credible biomarker predictors for longer non-recurrence survival and survival benefits from adjuvant therapy in resected cases. Routine detection of the above two indices in LELC of the lung may be recommended.
Acknowledgements
First, we thank the department of follow-up for recording recurrence and death data in detail. Second, we are supposed to express our gratitude for the support and suggestion in this study from staffs at department of pathology.
Funding: This study was funded by National Key Research and Development Program, China (2016YFC0905400).
Ethical Statement: This retrospective study was approved by the board-certified Research Ethics Committee at our center (No. B2017-50).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.He J, Shen J, Pan H, et al. Pulmonary lymphoepithelioma-like carcinoma: a Surveillance, Epidemiology, and End Results database analysis. J Thorac Dis 2015;7:2330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-58. 10.1002/cncr.27452 [DOI] [PubMed] [Google Scholar]
- 3.Yu X, Wen Y, Qin R, et al. Prognosis and distribution of lymph nodes metastases in resectable primary pulmonary lymphoepithelioma-like carcinoma: A large cohort from a single center. Thorac Cancer 2018;9:360-7. 10.1111/1759-7714.12586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang W, Hong S, Chen N, et al. PD-L1 is remarkably over-expressed in EBV-associated pulmonary lymphoepithelioma-like carcinoma and related to poor disease-free survival. Oncotarget 2015;6:33019-32. 10.18632/oncotarget.5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang L, Wang L, Li PF, et al. Positive expression of programmed death ligand-1 correlates with superior outcomes and might be a therapeutic target in primary pulmonary lymphoepithelioma-like carcinoma. Onco Targets Ther 2015;8:1451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang YL, Yang CY, Lin MW, et al. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer 2015;88:254-9. 10.1016/j.lungcan.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Lin Y, Cai Q, et al. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis 2015;7:1556-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortez MA, Ivan C, Valdecanas D, et al. PDL1 Regulation by p53 via miR-34. J Natl Cancer Inst 2015;108. pii: djv303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cha YJ, Kim HR, Lee CY, et al. Clinicopathological and prognostic significance of programmed cell death ligand-1 expression in lung adenocarcinoma and its relationship with p53 status. Lung Cancer 2016;97:73-80. 10.1016/j.lungcan.2016.05.001 [DOI] [PubMed] [Google Scholar]
- 10.Salgia R, Skarin AT. Molecular abnormalities in lung cancer. J Clin Oncol 1998;16:1207-17. 10.1200/JCO.1998.16.3.1207 [DOI] [PubMed] [Google Scholar]
- 11.Lei B, Liu S, Qi W, et al. PBK/TOPK expression in non-small-cell lung cancer: its correlation and prognostic significance with Ki67 and p53 expression. Histopathology 2013;63:696-703. [DOI] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao T, Li C, Wu Y, et al. Prognostic value of PD-L1 expression in tumor infiltrating immune cells in cancers: A meta-analysis. PLoS One 2017;12:e0176822. 10.1371/journal.pone.0176822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J 2001;18:705-19. 10.1183/09031936.01.00062201 [DOI] [PubMed] [Google Scholar]
- 16.Mitsudomi T, Hamajima N, Ogawa M, et al. Prognostic significance of p53 alterations in patients with non-small cell lung cancer: a meta-analysis. Clin Cancer Res 2000;6:4055-63. [PubMed] [Google Scholar]
- 17.Schmidt LH, Kummel A, Gorlich D, et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One 2015;10:e0136023. 10.1371/journal.pone.0136023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 2015;41:450-6. 10.1016/j.ejso.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 19.Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer 2016;57:91-103. 10.1016/j.ejca.2015.12.033 [DOI] [PubMed] [Google Scholar]
- 20.Chang YL, Wu CT, Shih JY, et al. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci 2011;102:282-7. 10.1111/j.1349-7006.2010.01768.x [DOI] [PubMed] [Google Scholar]
- 21.Shim HS, Kenudson M, Zheng Z, et al. Unique Genetic and Survival Characteristics of Invasive Mucinous Adenocarcinoma of the Lung. J Thorac Oncol 2015;10:1156-62. 10.1097/JTO.0000000000000579 [DOI] [PubMed] [Google Scholar]
- 22.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124-8. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Z, Situ D, Chang X, et al. Surgical treatment for primary pulmonary lymphoepithelioma-like carcinoma. Interact Cardiovasc Thorac Surg 2016;23:41-6. 10.1093/icvts/ivw064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiller JH, Adak S, Feins RH, et al. Lack of prognostic significance of p53 and K-ras mutations in primary resected non-small-cell lung cancer on E4592: a Laboratory Ancillary Study on an Eastern Cooperative Oncology Group Prospective Randomized Trial of Postoperative Adjuvant Therapy. J Clin Oncol 2001;19:448-57. 10.1200/JCO.2001.19.2.448 [DOI] [PubMed] [Google Scholar]
- 25.Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. 10.1016/j.ejca.2014.01.018 [DOI] [PubMed] [Google Scholar]
- 26.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. 10.1038/labinvest.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yemelyanova A, Vang R, Kshirsagar M, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol 2011;24:1248-53. 10.1038/modpathol.2011.85 [DOI] [PubMed] [Google Scholar]
- 28.Lee SH, Kim H, Kim WY, et al. Genetic alteration and immunohistochemical staining patterns of ovarian high-grade serous adenocarcinoma with special emphasis on p53 immnnostaining pattern. Pathol Int 2013;63:252-9. 10.1111/pin.12060 [DOI] [PubMed] [Google Scholar]
- 29.Lopez I, L PO, Tucci P, et al. Different mutation profiles associated to P53 accumulation in colorectal cancer. Gene 2012;499:81-7. 10.1016/j.gene.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 30.Lotfi M, Afsharnezhad S, Raziee HR, et al. Immunohistochemical assessment of MGMT expression and p53 mutation in glioblastoma multiforme. Tumori 2011;97:104-8. 10.1177/030089161109700118 [DOI] [PubMed] [Google Scholar]