Abstract
Background
Due to the non-homogeneity of specimens collected from tuberculosis (TB) suspects, repeated Xpert MTB/RIF (Xpert) may have potential clinical benefits. Incremental cost-effectiveness was analyzed for the second Xpert assay to detect Mycobacterium tuberculosis (Mtb) and rifampicin (RIF) resistance.
Methods
Specimens were collected from 1,063 pulmonary TB (PTB) and 398 extrapulmonary TB (EPTB) suspects, who had two Xpert tests sequentially within one week. The specimens were subjected to smear, culture, Xpert and drug susceptibility testing. Incremental cost-effectiveness of the serial Xpert assays was evaluated.
Results
Among 813 Xpert-positive TB patients, 755 (92.87%) were identified by the first assay whereas the additional 58 (7.13%) were identified by the second assay. The second Xpert assay had higher incremental yield for smear-negative than for smear-positive specimens (12.07% vs. 1.84%, P<0.001), and higher incremental yield for EPTB than for PTB (10.71% vs. 4.65%, P=0.003). About 94.48% (137/145) of the RIF-resistant patients were identified by the first Xpert assay and 5.52% (8/145) were identified by the second Xpert assay. After the first assay, the incremental cost of performing a second Xpert was huge: US$22.82 vs. US$467.72 (P<0.001) and US$35.02 vs. US$291.87 (P<0.001) for PTB and EPTB, respectively. The incremental cost of performing a second Xpert is lower in smear-negative than in smear-positive group in both PTB and EPTB.
Conclusions
One Xpert assay is sufficient for smear-positive cases, and a second Xpert assay is beneficial not only for Mtb detection but also for RIF-resistant diagnosis for smear-negative TB suspects, whereas the incremental cost for the second Xpert is huge.
Keywords: Tuberculosis (TB), diagnostic tests, rifampicin, resistance
Introduction
Tuberculosis (TB) is an ancient infectious disease caused by Mycobacterium tuberculosis (Mtb) that typically affects the lungs (pulmonary TB, PTB) but can affects other sites as well (extrapulmonary TB, EPTB) (1). Despite global efforts, TB is yet to be fully controlled and therefore requires improved methods for rapid identification and early treatment of patients with active disease (2). Xpert MTB/RIF (Xpert) assay (Cepheid, Sunnyvale, CA, USA) is an automated real-time nucleic acid amplification technology for rapid simultaneous detection of Mtb and rifampicin (RIF) resistance within 2 hours (3). The assay has been evaluated for PTB and EPTB diagnosis in abundant studies (4-7), and the results elucidated its excellent accuracy. In 2010 and 2013, the Xpert assay was endorsed by the World Health Organization (WHO) for PTB and EPTB diagnosis, respectively (8,9).
Examination of multiple specimens from same TB patient improves the sensitivity of detection by microscopy and culture (10). Likewise, examination of multiple specimens would improve the detection sensitivity using Xpert assay, although increase the working load and cost as well. Economic evaluations of diagnostic strategies are needed to guide decisions on prioritizing the health care resources in TB control (11). However, little data on the utility of Xpert assay for additional specimen is available. Cost-effectiveness analysis is a measure to evaluate the efficiency of the repeated Xpert assay, and thus facilitate identifying the cost effective route for carrying out the test appropriately. Therefore, the current analysis was conducted to determine the incremental cost-effectiveness of the second Xpert test.
Methods
Ethical approval
The ethical approvals for this study were obtained from the Beijing Chest Hospital Ethics Committee (ethical approval number: BJXK-2015-05). A written informed consent was acquired from each participant.
Patients and sample collection
This prospective study was conducted from March 2015 to April 2017 in Beijing Chest Hospital (Beijing, China), which is the only national referral TB center in China. A total of 1,063 PTB suspects and 398 EPTB suspects who had two Xpert tests sequentially within one week were enrolled. Totally 2,922 Xpert tests were performed on 2,126 sputum, 288 pus, 176 cerebrospinal fluid, 208 pleural fluid, 24 urine and 100 bronchoalveolar lavage fluid specimens.
Smear microbiology
Direct smear was prepared and stained with auramine, and then examined by light-emitting diode (LED) microscopy. The smear was read and interpreted in accordance with WHO guidelines (12).
Xpert MTB/RIF
The assay was performed following the manufacturer instructions. For pus specimens, 1 mL of pus was mixed with 2 mL of Xpert sample reagent, vortexed for at least 10 s and incubated at room temperature for 10 min. The mixture was again vortexed for 10 seconds and incubated at room temperature for 5 min. For other specimens, 1 mL was mixed with 2 mL of Xpert sample reagent. After vortexed for several seconds, the reaction mixture was incubated at room temperature for 15 min to inactivate the living bacteria. Then, a total of 2 mL processed mixture was transferred into the Xpert cartridge and loaded onto the GeneXpert instrument. The automatic detection procedure was run afterwards.
Mycobacterial culture and susceptibility testing by mycobacteria growth indicator tube (MGIT) 960 system
The MGIT 960 system is based on fluorescence detection of mycobacteria growth in a tube containing modified Middlebrook 7H9 medium together with fluorescence quenching-based oxygen sensor (13). The specimens were decontaminated with N-acetyl-L-cysteine-sodium hydroxide (BBL MycoPrep; Becton Dickinson, Sparks, MD) for 20 min, then neutralized with sterile saline phosphate buffer (PBS; pH 6.8) to a final volume of 45 mL, and the centrifuged at 3,000 ×g for 15 min at 4 °C. The pellet was resuspended in 1.5 mL of PBS. Each MGIT960 tube was inoculated with 0.5 mL of the resulting specimen, incubated at 37 °C in an automated MGIT960 apparatus (Becton Dickinson) for a maximum of 42 days. The MGIT960 outcomes were recorded according to the manufacturer’s instruction. The standard drug susceptibility testing (DST) with RIF was carried out for the positive cultures using the MGIT960 IR kit (Becton Dickinson) according to the manufacturer’s instructions.
Cost parameters
Since its endorsement by WHO in 2010, over 23 million Xpert tests have been procured by 130 countries (14). Market price is around US$ 50,000 for the four-cartridge module plus computer extension and US$ 65 per cartridge. Nevertheless, it is provided at negotiated prices to some low- and middle-income countries with high TB burden at US$17,000 for the equipment and US$9.98 for each cartridge (15). The cost for one Xpert test was US$13.2 in this study, including equipment US$2.84 (at a price of US$17,000 per four-module instrument), building space US$0.02, maintenance US$0.18, staff US$0.11, cartridge US$9.98 (at negotiated price), and consumables US$0.07.
Cost-effectiveness analysis
Cost-effectiveness was calculated by determining the cost for each TB cases detected. An average cost-effectiveness ratio was estimated by dividing the total cost of an intervention by its measure of effectiveness. An incremental cost-effectiveness ratio considers change from the first to the second Xpert test; in this instance, the additional cost per additional TB case identified from examining progressively more specimens per patient.
Statistical analysis
The incremental yields of the first and second Xpert assay were compared using χ2 test. The Student’s t-test was performed to assess statistical significance between the costs of different groups. Statistical analyses were performed with SPSS (version 19.0). Differences were considered statistically significant at P<0.05.
Results
Detection of Mtb
Totally 645 PTB suspects produced Xpert-positive outcomes. Among them, 615 (95.35%) could be identified by the first Xpert assay and 30 (4.65%) additional cases were identified by a second assay. Meanwhile, 168 EPTB suspects had Xpert-positive outcomes, and 150 (89.29%) of them were identified by the first Xpert assay, while 18 (10.71%) more cases were identified by the second assay (Table 1). Overall, the second Xpert assay had higher incremental yield for EPTB samples than for PTB samples [10.71% (18/168) vs. 4.65% (30/645), χ2=8.820, P=0.003].
Table 1. Mtb detection outcomes by Xpert MTB/RIF with two specimens examined.
| Category | Suspects | Cases | Cases identified on 1st Xpert assay (%) | Additional cases identified on 2nd Xpert assay (%) |
|---|---|---|---|---|
| PTB | ||||
| Smear-positive | 464 | 430 | 422 (98.14) | 8 (1.86) |
| Smear-negative | 599 | 215 | 193 (89.77) | 22 (10.23) |
| Total | 1,063 | 645 | 615 (95.35) | 30 (4.65) |
| EPTB | ||||
| Smear-positive | 97 | 60 | 59 (98.33) | 1 (1.67) |
| Smear-negative | 301 | 108 | 91 (84.26) | 17 (15.74) |
| Total | 398 | 168 | 150 (89.29) | 18 (10.71) |
| Total | 1,461 | 813 | 765 (94.10) | 48 (5.90) |
PTB, pulmonary tuberculosis; EPTB, extrapulmonary tuberculosis.
The suspects were further stratified into smear-positive and smear-negative groups, and the results showed that in smear-negative group, the second Xpert assay had apparent incremental yield for both PTB and EPTB patients (Table 1). Overall, the second Xpert assay had higher incremental yield for smear-negative specimens than for the smear-positive specimens [12.07% (39/323) vs. 1.84% (9/490), χ2=36.727, P<0.001].
Detection of RIF resistance
One hundred and twenty-six PTB and 19 EPTB cases produced RIF resistance outcomes by 2 Xpert assays. Among the 145 RIF-resistant patients, 137 (94.48%) were identified by the first Xpert assay and 8 (5.52%) additional cases were identified by a second Xpert assay. Compared with the phenotypic DST, the first Xpert test detected 92.57% (137/148) of the RIF-resistant patients, and the second Xpert test gained 5.41% increment, so the total detection rate was 97.97% (145/148). Furthermore, 2 Xpert tests could correctly detect all of the RIF susceptible patients (Table 2).
Table 2. Rifampicin resistance detection outcomes by Xpert MTB/RIF with two specimens examined.
| Category | Cases identified on 1st Xpert assay, n (%) | Cases identified on 2nd Xpert assay, n (%) |
|---|---|---|
| Rifampicin-resistant cases | 137 (94.48%) | 8 (5.52) |
| Rifampicin-susceptible cases | 390 (100%) | 0 (0) |
Cost-effectiveness
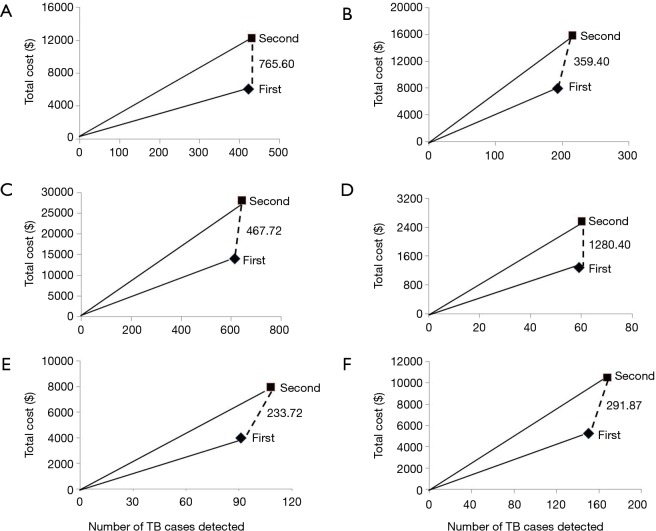
There was a big difference regarding average cost (per TB case diagnosed) between 1st and 2nd Xpert assays: US$22.82 vs. US$43.51 (P<0.001) and US$35.02 vs. US$62.54 (P<0.001) for PTB and EPTB diagnosis, respectively. Compared with the first Xpert MTB/RIF assay, the incremental cost of performing a second test was huge: US$22.82 vs. US$467.72 (P<0.001) and US$35.02 vs. US$291.87 (P<0.001) for PTB and EPTB, respectively. Furthermore, the incremental cost of performing a second Xpert is lower in smear-negative group than in smear-positive group for both PTB and EPTB patients (Table 3, Figure 1).
Table 3. Stratified analysis of Mtb detection and cost-effectiveness.
| Category | Order of tests | Mtb detection | Costs (US $) | ||||
|---|---|---|---|---|---|---|---|
| Specimens examined | Cases detected | Incremental cases | Average cost for per case | Cost for per incremental case | |||
| Pulmonary tuberculosis | |||||||
| Smear-positive | 1st | 464 | 422 | – | 14.51 | – | |
| 2nd | 464 | 430 | 8 | 28.49 | 765.60 | ||
| Smear-negative | 1st | 599 | 193 | – | 40.97 | – | |
| 2nd | 599 | 215 | 22 | 73.55 | 359.40 | ||
| Total | 1st | 1,063 | 615 | – | 22.82 | – | |
| 2nd | 1,063 | 645 | 30 | 43.51 | 467.72 | ||
| Extrapulmonary tuberculosis | |||||||
| Smear-positive | 1st | 97 | 59 | – | 21.70 | – | |
| 2nd | 97 | 60 | 1 | 42.68 | 1,280.40 | ||
| Smear-negative | 1st | 301 | 91 | – | 43.66 | – | |
| 2nd | 301 | 108 | 17 | 73.58 | 233.72 | ||
| Total | 1st | 398 | 150 | – | 35.02 | – | |
| 2nd | 398 | 168 | 18 | 62.54 | 291.87 | ||
Figure 1.
Cost-effectiveness of sequential Xpert MTB/RIF assays. The dashed lines denote the incremental cost-effectiveness ratios of increasing the number of Xpert MTB/RIF assays that are examined per patient, the figures adjacent to these lines indicate the extra cost of detecting each additional TB case. The steeper the slope is, the less cost-effective the intervention is. (A) Smear-positive pulmonary tuberculosis; (B) smear-negative pulmonary tuberculosis; (C) total pulmonary tuberculosis; (D) smear-positive extrapulmonary tuberculosis; (E) smear-negative extrapulmonary tuberculosis; (F) total extrapulmonary tuberculosis.
Discussion
Accurate, rapid detection of TB and TB drug resistance is critical for improving patient care and decreasing TB transmission. The development of Xpert technique was a landmark event. A Cochrane systematic review (6) found that Xpert has a pooled sensitivity of 88% and pooled specificity of 98% compared with the gold standard of culture. It provides fast results, is easy to use and has a low biohazard risk which facilitates its implementation in rural settings. Cost-effectiveness evaluation for TB diagnostics often provides important information to the policymakers. Data on cost and cost-effectiveness of Xpert in diverse settings had been reported. Xpert was more sensitive, comparably specific, and more cost-effective than smear microscopy in intermediate and low burden areas (16,17). Xpert was superior over microscopic determination of drug susceptibility (MODS) in high TB/HIV prevalence setting (18). Whereas, Vassall et al. (19) reported that Xpert was cost-neutral and did not improve the cost-effectiveness of TB diagnosis in South Africa.
Budgetary constraint is a major consideration influencing the choice of diagnostics in developing countries. This is the first study to assess the incremental cost-effectiveness of the second Xpert assay for detection of TB. Our results showed that the first Xpert assay detected 98.16% (481/490) of the Xpert-positive patients among the smear-positive TB patient group. Nevertheless, the cost-effectiveness analysis showed that the incremental cost of performing a second Xpert was very high for the smear-positive TB patients. As the incremental yield from a second Xpert was relatively small, so one Xpert assay was sufficient for smear-positive patients. Albeit the benefit of performing Xpert assays for those patients was to find RIF resistance, especially in high multidrug-resistant (MDR)-TB burden area.
The global priorities for TB care and control are to improve case detection and to detect cases earlier, including cases of smear-negative disease, and to enhance the capacity to diagnose MDR-TB. Our results showed that the second Xpert assay had an incremental yield of 12.07% (39/323) for smear-negative TB patients. Boehme et al. (5) reported that among patients with smear-negative/culture-positive PTB, the addition of a second Xpert test increased sensitivity by 12.6%, which is similar to our results. Dorman et al. (20) showed that the new generation of Xpert-Xpert MTB/RIF Ultra (Ultra) could increase sensitivity by 17% compared with Xpert for TB detection with smear-negative-culture-positive sputum, while the cost of it will be similar with Xpert. Theoretically, Ultra would be more cost-effective comparing with 2 Xpert tests for smear-negative PTB diagnosis. Cowan et al. (21) reported that the two Xpert tests strategy was more expensive but still cost-effective compared with 3 smears. Due to the unavailable of Ultra in most of countries nowadays, our results suggested that two Xpert tests can benefit the poor diagnosis of smear-negative TB. Notable, for smear-negative (and economically sustainable) TB suspects, a second Xpert assay is not only valuable for Mtb detection but also for RIF resistance diagnosis.
MDR-TB is an increasing concern globally and directly threatens disease control efforts in many countries. Only 30,000 of nearly 500,000 new cases of multidrug-resistant TB are detected and reported every year, hence misdiagnosis causes thousands of deaths, nosocomial and community transmission, and amplification of drug resistance (22,23). In this study, 5.52% additional RIF resistant cases were identified by the second Xpert assays. Although Xpert had excellent repeatability for RIF resistance detection, our assay demonstrated a second Xpert assay has the benefit to detect more RIF resistant cases.
The incremental cost-effectiveness of the second Xpert is dependent on a number of different setting-specific factors. First, the cost for per TB case detection will decrease with the increase of TB prevalence in the setting (21). Second, higher proportion of TB cases among the suspect population improves the cost-effectiveness of Xpert. Third, the decision analytic modeling demonstrated that when transmission effects are excluded, the cost-effectiveness of Xpert increases as the MDR-TB prevalence increases (24). Fourth, the cost for per Xpert-positive case was higher in sites with lower volumes of testing. Previous study in South Africa suggested that low testing volume and a high number of sites involved could increase Xpert testing cost by 50% or more (25). Other factors are likely to influence the cost-effectiveness as well, such as the proportion of those co-infected with HIV. These findings may help to inform the decision-makers about the appropriateness of a second Xpert deployment in different settings.
The main drawback of Xpert is its cost. As the goal of TB control is to correctly identify as many cases as possible for effective treatment, a cost-effective but simple, easy and rapid diagnostic method that could be readily and widely adopted is need. Our results showed that after the first Xpert assay, the incremental cost of performing a second test is huge. In low-income countries, resourcing for TB services is extremely constrained. In order to end the global TB epidemic, it is the responsibility of the manufacturers, governments and non-profit organizations to lower the price of Xpert assay to make it affordable in low-income countries.
Conclusions
According to our assay, one Xpert assay is sufficient for smear-positive cases, and a second Xpert assay is beneficial not only for Mtb detection but also for RIF-resistant diagnosis for smear-negative TB suspects, whereas the incremental cost for the second Xpert test is huge.
Acknowledgements
Funding: This work was supported by the research funding from National Science and Technology Major Project (2017ZX10201301-004-002, 2017ZX09304009-004), Natural Science Fund of China (81703632), Beijing Natural Science Foundation (7172050), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201824).
Ethical Statement: The ethical approvals for this study were obtained from the Beijing Chest Hospital Ethics Committee (ethical approval number: BJXK-2015-05). A written informed consent was acquired from each participant.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Health Organization. Global tuberculosis report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 2.Raviglione M, Marais B, Floyd K, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet 2012;379:1902-13. 10.1016/S0140-6736(12)60727-2 [DOI] [PubMed] [Google Scholar]
- 3.Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005-15. 10.1056/NEJMoa0907847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theron G, Zijenah L, Chanda D, et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383:424-35. 10.1016/S0140-6736(13)62073-5 [DOI] [PubMed] [Google Scholar]
- 5.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377:1495-505. 10.1016/S0140-6736(11)60438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steingart KR, Schiller I, Horne DJ, et al. Xpert ® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014;1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014;44:435-46. 10.1183/09031936.00007814 [DOI] [PubMed] [Google Scholar]
- 8.World Health Oragnization. WHO endorses new rapid tuberculosis test. Available online: http://www.who.int/mediacentre/news/releases/2010/tb_test_20101208/en/. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 9.World Health Organization. Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children Policy update. Available online: http://www.who.int/tb/publications/xpert-mtb-rif-assay-diagnosis-policy-update/en/. Geneva, Switzerland: WHO, 2013. [PubMed]
- 10.Bonnet M, Ramsay A, Gagnidze L, et al. Reducing the number of sputum samples examined and thresholds for positivity: an opportunity to optimise smear microscopy. Int J Tuberc Lung Dis 2007;11:953-8. [PubMed] [Google Scholar]
- 11.van Hoorn R, Jaramillo E, Collins D, et al. The Effects of Psycho-Emotional and Socio-Economic Support for Tuberculosis Patients on Treatment Adherence and Treatment Outcomes - A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0154095. 10.1371/journal.pone.0154095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Union against Tuberculosis and Lung Disease. Technical guide: sputum examination for tuberculosis by direct microscopy in low income countries, 5th ed. Paris, France: International Union against Tuberculosis and Lung Disease, 2000.
- 13.Harausz E, Lusiba JK, Nsereko M, et al. Comparison of MGIT and Myco/F lytic liquid-based blood culture systems for recovery of Mycobacterium tuberculosis from pleural fluid. J Clin Microbiol 2015;53:1391-4. 10.1128/JCM.02742-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert H, Nathavitharana RR, Isaacs C, et al. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J 2016;48:516-25. 10.1183/13993003.00543-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diagnostics FFI. Negotiated prices for Xpert MTB/RIF and FIND country list. Available online: http://www.finddiagnostics.org/about/what_we_do/successes/findnegotiated-prices/xpert_mtb_rif.html. 2013.
- 16.You JH, Lui G, Kam KM, Lee NL. Cost-effectiveness analysis of the Xpert MTB/RIF assay for rapid diagnosis of suspected tuberculosis in an intermediate burden area. J Infect 2015;70:409-14. 10.1016/j.jinf.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 17.Cowan JF, Chandler AS, Kracen E, et al. Clinical Impact and Cost-effectiveness of Xpert MTB/RIF Testing in Hospitalized Patients With Presumptive Pulmonary Tuberculosis in the United States. Clin Infect Dis 2017;64:482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wikman-Jorgensen PE, Llenas-Garcia J, Perez-Porcuna TM, et al. Microscopic observation drug-susceptibility assay vs. Xpert((R)) MTB/RIF for the diagnosis of tuberculosis in a rural African setting: a cost-utility analysis. Trop Med Int Health 2017;22:734-43. 10.1111/tmi.12879 [DOI] [PubMed] [Google Scholar]
- 19.Vassall A, Siapka M, Foster N, et al. Cost-effectiveness of Xpert MTB/RIF for tuberculosis diagnosis in South Africa: a real-world cost analysis and economic evaluation. Lancet Glob Health 2017;5:e710-9. 10.1016/S2214-109X(17)30205-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorman SE, Schumacher SG, Alland D, et al. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018;18:76-84. 10.1016/S1473-3099(17)30691-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowan JF, Chandler AS, Kracen E, et al. Clinical Impact and Cost-effectiveness of Xpert MTB/RIF Testing in Hospitalized Patients With Presumptive Pulmonary Tuberculosis in the United States. Clin Infect Dis 2017;64:482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang KC, Yew WW. Management of difficult multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis: update 2012. Respirology 2013;18:8-21. 10.1111/j.1440-1843.2012.02257.x [DOI] [PubMed] [Google Scholar]
- 23.Shin SS, Keshavjee S, Gelmanova IY, et al. Development of extensively drug-resistant tuberculosis during multidrug-resistant tuberculosis treatment. Am J Respir Crit Care Med 2010;182:426-32. 10.1164/rccm.200911-1768OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vassall A, van Kampen S, Sohn H, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med 2011;8:e1001120. 10.1371/journal.pmed.1001120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schnippel K, Meyer-Rath G, Long L, et al. Scaling up Xpert MTB/RIF technology: the costs of laboratory- vs. clinic-based roll-out in South Africa. Trop Med Int Health 2012;17:1142-51. 10.1111/j.1365-3156.2012.03028.x [DOI] [PMC free article] [PubMed] [Google Scholar]