Abstract
Background
The approaches to thoracoscopic thymectomy in myasthenia gravis (MG) are debatable. We developed a novel approach via subxiphoid and subcostal arch, with a significantly shorter duration of operation and hospital stay, less estimated blood loss, and lower postoperative pain.
Methods
From December 2012 to December 2014, 77 myasthenia gravis patients with or without thymoma underwent thoracoscopic extended thymectomy at our hospital. Among them, 41 patients were operated via the subxiphoid and subcostal arch approach and the other 36 via the conventional unilateral approach. The patient outcomes were retrospectively reviewed and evaluated.
Results
The thoracoscopic extended thymectomy was performed safely via the subxiphoid and subcostal arch approach. In this approach, no drainage tube was inserted after operation except in the first two patients. Two of the 41 patients were switched to trans-sternal approach due to the tight adhesion between the thymoma and the left innominate vein. No major complications occurred. Compared with the unilateral approach, the duration of the procedure via subxiphoid and subcostal arch was significantly shorter, with less estimated blood loss, shorter hospital-stay and lower postoperative pain (P<0.001). The cosmetic scores were comparable between the two groups (P=0.369).
Conclusions
The novel subxiphoid and subcostal arch approach is technically feasible and safe. It is an acceptable alternative to conventional thoracoscopic extended thymectomy.
Keywords: Subxiphoid approach, thoracoscopic surgery, thymectomy, myasthenia gravis
Introduction
Thoracoscopic thymectomy has been used as an effective therapy for the treatment of myasthenia gravis (MG) worldwide (1-7). It is recommended that all the thymus including encapsulated and extracapsular tissues, should be removed during open or thoracoscopic surgery resulting in better therapeutic effect in MG patients (8-10). However, owing to the widespread distribution of ectopic thymic tissues in the anterior mediastinum, the surgical approaches are debatable. In addition to the conventional unilateral (right- or left-sided) approach, the bilateral, subxiphoid, transcervical or combination strategies, have been designed and optimized for thoracoscopic thymectomy, especially for extended thymectomy (11-21). All these approaches require additional incisions to expose the areas beyond the scope of conventional unilateral approach. Until now, no “ideal” approach to thoracoscopic extended thymectomy was available, which adequately exposed the whole anterior mediastinum and facilitated the resection of the fat and ectopic thymic tissue without additional incision (15,16,19,20,22).
By carefully analyzing the anatomy of anterior mediastinum, we have developed a new approach via subxiphoid and subcostal arch. The whole anterior mediastinal space was adequately visualized. It is technically feasible to dissect the anterior mediastinal fat pads near the phrenic nerves, the cardiac-diaphragmatic angles, the pulmonary artery window and the thyroid without the fourth incision. The novel subxiphoid and subcostal arch approach facilitated the thoracoscopic thymectomy and mediastinal fat dissection with minimal blood loss, less postoperative pain, fewer complications and better cosmetic effect.
A retrospective study was performed to evaluate the clinical outcomes of thoracoscopic extended thymectomy via the subxiphoid and subcostal arch and the right-sided approaches, for the treatment of patients with MG. This study has received ethical approval from the Fourth Military Medical University Tangdu Hospital Medical Ethics Committee (TDLL-20150074).
Methods
Patients
Thoracoscopic mediastinal surgery via subxiphoid and subcostal arch has been available since December 2013 at the Tangdu hospital, Xi’an, China. Between December 2013 and December 2014, 41 cases of MG with or without thymoma [(Masaoka stage I and II) were treated via the subxiphoid and subcostal arch thoracoscopic extended thymectomy by Drs. Qiang Lu, Jinbo Zhao, Juzheng Wang and Yongan Zhou. A series of 36 MG patients (with or without thymoma, Masaoka stage I and II)] treated via right-sided thoracoscopic thymectomy from December 2012 to December 2013 were selected as the conventional unilateral approach group.
All of the patients undergoing surgery were evaluated by thoracic surgeons, neurologists and anesthetists before operation. All these patients were diagnosed as MG and classified by neurologists according to the criteria established by the MG Foundation of America (MGFA) (23), and were referred for surgical intervention. The patients with thymomas were evaluated according to the following criteria: (I) a well-capsulated tumor was indicated in CT imaging; (II) no evidence of tumor invasion to the trachea, the heart, the aorta and the other big vessels; (III) tumors size not exceeding 5 cm based on CT scan.
Informed consent was obtained from all patients. The patients were informed about the risks and potential benefits of this new approach, as well as the possibility of switch to trans-sternal approach if larger vessels were injured or the new surgical approach was not feasible. This retrospective study was approved by the ethics committee of Tangdu Hospital. All the patients were followed up for at least 12 months.
Subxiphoid and subcostal arch thoracoscopic extended thymectomy
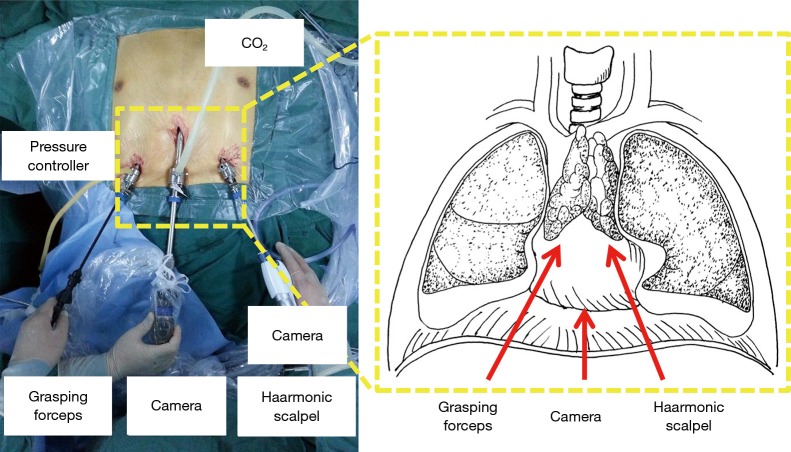
All the patients were held in a supine position on the operating table, with the surgeon standing between the patient’s legs. The first assistant was on the patient’s right side (Figure 1). The patient was anesthetized and ventilated through a single-lumen endotracheal tube. Skin was prepared from the chin to the umbilicus. A 3-cm incision was cut below the xiphoid. The rectus abdominis was split and the retrosternal space was created by finger dissection to introduce a 30-degree oblique, 10 mm thoracoscope. Under the guidance of the operator’s finger, two 5 mm extra pleural thoracic ports were created at the midclavian line intersecting with the bilateral costal arch to introduce a 45-cm longer thoracoscopic grasping forceps on the patient’s right side and a harmonic scalpel (45-cm longer) on the patient’s left side (Figure 2). Under direct thoracoscopic guidance, the anterior mediastinal space was further dissected. A pneumomediastinum was created by insufflation of an 8 cmH2O positive pressure carbon dioxide (CO2), which enabled enlargement of the retrosternal space and facilitated dissection of the thymus (Figure 3). Both the right- and left-sided mediastinal pleura were cut. The whole thymus with or without the thymoma and all the associated fat tissues were excised en-bloc. First, the lower poles of the thymus were lifted with the grasping forceps and the ascending aorta and the innominate veins were exposed. Tracing the course of the innominate veins, all the thymic veins were easily identified. After resecting the thymic veins, the thymus was pulled down by the grasping forceps and the two upper thymic lobes were meticulously dissected. Simultaneously, all the fat pads near the phrenic nerves were dissected with the harmonic scalpel. The pre-pericardial fat and the fat pads near the cardiac-diaphragmatic angles, the aorto-pulmonary window (A-P window) and the lower poles of the thyroid gland were carefully dissected and removed. The superior vena cava, both innominate veins and the aorta were skeletonized. All the specimens were placed in a plastic bag and removed from the mediastinum via the subxiphoid port.
Figure 1.
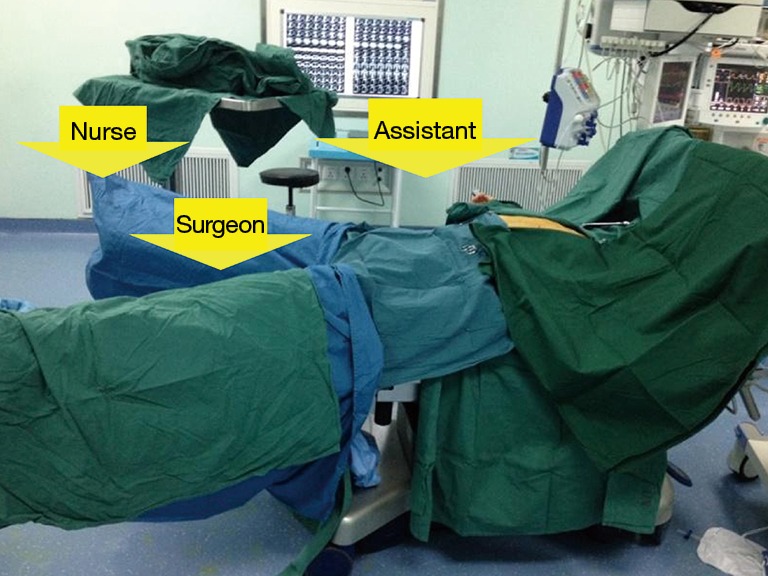
The patient and surgical team in the subxiphoid and subcostal arch thoracoscopic extended thymectomy.
Figure 2.
Thoracoscopic extended thymectomy via subxiphoid and subcostal arch approach: the camera was introduced into the anterior mediastinum via the subxiphoid incision; a 45-cm-long thoracoscopic grasping and a 45-cm-long harmonic scalpel were introduced into the anterior mediastinum through the bilateral costal arch ports.
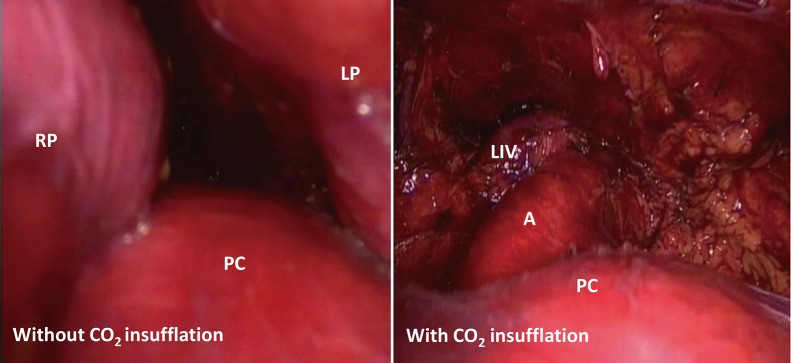
Figure 3.
Insufflation of an 8-cmH2O positive pressure CO2 enlarged the mediastinal space. LP, left pleura; RP, right pleura; PC, pericardium; A, aorta; LIV, left innominate vein.
The surgical field was carefully monitored and the thoracoscope was removed. A drainage tube was inserted into the mediastinum thorough the subxiphoid incision. The air in the chest cavities was evacuated by inflating the lungs. The drainage tube was removed and the skin incisions were sutured.
In the first two cases, chest tubes were placed. Little effusion was drained or accumulated in the chest cavity, which was confirmed by the postoperative chest radiological examination. No drainage tube was placed in the other 39 patients.
Right-sided thoracoscopic thymectomy
The right-sided thoracoscopic approach was similar to the procedures reported previously (1,3,24). The observing port was created at the right axillary line in the sixth intercostal space. The other ports were created along the anterior axillary lines in the appropriate intercostal spaces. The right mediastinal pleura was cut near the sternum. The thymus was grasped to the right side to expose the left upper and lower lobes. After the thymus was removed, the pre-pericardial fat and the right epiphrenic fat pads were dissected. The fat near the right phrenic nerve was removed. The fat pads near the left phrenic nerve and left cardiac-diaphragmatic angle were removed as much as possible. A chest tube was inserted and the skin incisions were sutured.
Postoperative care and evaluation
After surgery, all the patients were managed according to the same protocol. All the patients resumed their preoperative medications for the control of MG. Oxygen saturation and electrocardiographic parameters were monitored in the early postoperative period. A sitting bedside chest X-ray was obtained the first day after operation. The chest tube in the conventional group was removed if the drainage was less than 100 mL in the past 24 h. Analgesia may exacerbate respiratory inhibition and even cause MG crisis. Therefore, intercostals blocks were used in the traditional group and no other postoperative analgesics were used. If the pain was intolerable, analgesics were used as recommended by the anesthetists. Postoperative pain was evaluated using the Visual Analog Pain Scale at 24, 48 h and 7 days after operation. A patient-reported cosmetic score was used to evaluate the cosmetic effect at the time of hospital discharge. The cosmetic evaluation was reported by the patients themselves with a score ranging from 0 to 100 to indicating very dissatisfied to very satisfied status.
All the complications were recorded. Among the patients switched to trans-sternal approach, the reasons were recorded. Pleural effusion was defined as the estimated effusion greater than 500 mL entailing thoracocentesis. Temporary phrenic nerve palsy was defined as postoperative phrenic nerve palsy. However, the function of phrenic nerve was recovered a month after operation.
Statistical analysis
The results were expressed as mean ± standard deviation. The continuous variables were analyzed with an unpaired Student’s t-test or ANOVA, and the categorical variables were analyzed with Fisher’s exact test. The GraphPad Prism software (Version 5.0, GraphPad Software, Inc., CA) was used for all data analysis. All statistical tests were 2-sided. A P value of less than 0.05 was considered significant.
Results
The patient demographics and clinical baseline data are summarized in Table 1. No differences in gender, age, thymoma status or size, MGFA clinical classification or the use of steroids or immunosuppressive drugs between the two groups were observed. No patient developed preoperative MG crisis in this study. All the patients with thymomas showed clear margins according to postoperative pathological examination.
Table 1. Patient demographics and clinical baseline characteristics.
| Clinical characters | The subxiphoid and subcostal arch “Three ports” approach (n=41) | The unilateral approach (n=36) | P |
|---|---|---|---|
| Gender (male/female) | 16/25 | 13/23 | 0.818 |
| Age (year) | 36.3±8.2 | 38.5±9.1 | 0.268 |
| Thymoma (yes/no) | 10/31 | 12/24 | 0.453 |
| Steroids/immunosuppressive drugs use before operation (yes/no) | 2/39 | 2/34 | 1.000 |
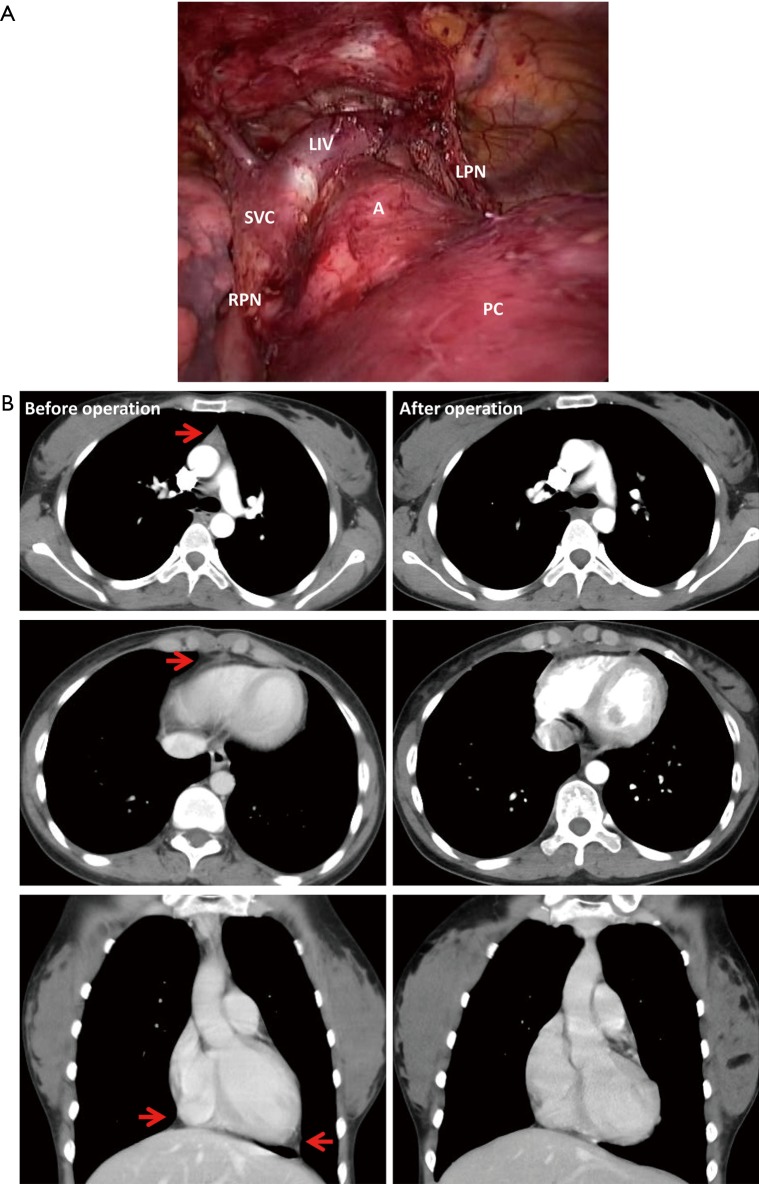
The thymus and all the fat tissues near the phrenic nerves, the pre-pericardia fat and the cardiac-diaphragmatic angles, the A-P window and the lower poles of the thyroid gland could be totally resected via the subxiphoid and subcostal arch approach (Figures 4,5). Compared with the patients treated by the conventional unilateral approach, the patients treated by the subxiphoid and subcostal arch approach with features of significantly shorter operation period, less estimated blood loss and shorter hospital stay after operation (P<0.001). In the group treated via conventional unilateral approach, the chest tubes were removed after 3.6±1.2 days and the total drainage amounted to 523.1±68.5 mL. In the group treated via subxiphoid and subcostal arch approach no drainage tubes were inserted postoperatively. All the detailed informations are summarized in Table 2.
Figure 4.
Comparison before and after subxiphoid and subcostal arch thoracoscopic extended thymectomy: (A) the whole thymus and the fat tissues in the anterior mediastinum were totally excised; (B) the hyperplastic thymus, the pre-pericardial fat and the fat pads near the bilateral cardiac-diaphragmatic angles (the arrows) were totally excised. LIV, left innominate vein; SVC, super vena cava; RPN, right phrenic nerve; LPN, left phrenic nerve; PC, pericardium; A, aorta.
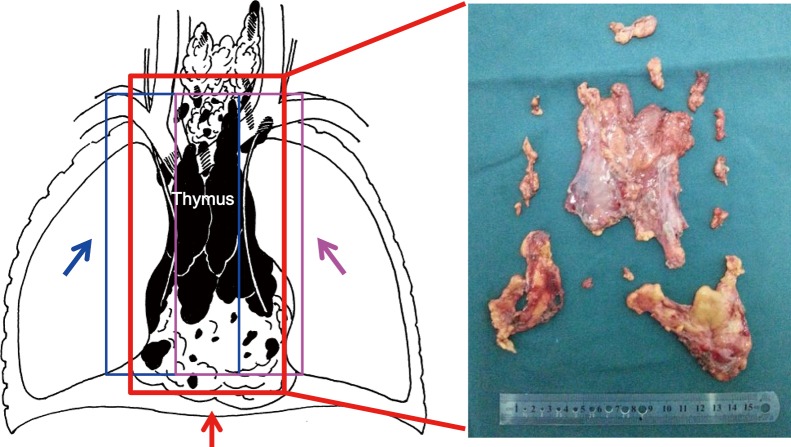
Figure 5.
Excision of thymus and fat tissues via different approaches: The fat tissues near the right side (the blue line) were removed via the right-sided approach (the blue arrow) and the fat tissues near the left side (the purple lines) were removed via the left-sided approach (the purple arrow). However, almost all the fat tissues in the anterior mediastinum (the red lines) were excised in subxiphoid and subcostal arch thoracoscopic extended thymectomy (the red arrow).
Table 2. The preoperative results in two groups.
| Clinical characters | The subxiphoid and subcostal arch “Three ports” approach (n=41) | The unilateral approach (n=36) | P |
|---|---|---|---|
| Operation times (min) | 95.3±25.5 | 120.0±24.6 | <0.001 |
| Blood lose (mL) | 25.5±10.6 | 55.1±10.4 | <0.001 |
| Total drainage (mL) | 0* | 523.1±68.5 | – |
| Times of drainage removal (days after operation) | 0* | 3.6±1.2 | – |
| Hospital stay after operation (day) | 3.6±1.3 | 7.4±2.3 | <0.001 |
| Conversion to transsternal approach | |||
| Larger vessels adhesion (n) | 2 | 2 | 1.000 |
| Larger vessels injury (n) | 0 | 2 | 0.215 |
| Pleural effusion (n) | 1 | 0 | 0.468 |
| Temporary phrenic nerve palsy (n) | 0 | 1 | 0.468 |
| Postoperative pain [1–10] | |||
| 24 h after operation | 4.1±1.2 | 7.5±0.8 | <0.001 |
| 48 h after operation | 3.2±1.4 | 5.6±1.3 | <0.001 |
| 7 days after operation | 2.8±0.7 | 4.5±1.5 | <0.001 |
| Cosmetic score [1–100] | 90.5±12.3 | 87.6±15.8 | 0.369 |
*, the first two patients and the two patients with sternotomy were excluded.
The mortality was comparable in the two groups. In the group treated via subxiphoid and subcostal arch approach two patients were switched to trans-sternal approach due to tight adhesion between the thymoma and the left innominate vein. In these two patients, all the adhesions were well visualized and no accidental big vessel injury occurred. However, in the conventional unilateral approach group, four conversions occurred due to close adhesion of lesions with the left innominate vein (two cases) and hemorrhage caused by accidental injury to the left innominate vein (two cases). In the subxiphoid and subcostal arch approach group, two patients suffered from pleural effusion and pneumothorax. They were treated by thoracentesis and no thoracostomy was needed. No myasthenia crisis occurred in both groups. All the patients were followed up for at least 12 months. The complications in the two groups are summarized in Table 2.
Compared with the patients treated via conventional unilateral approach group, the patients subjected to subxiphoid and subcostal arch thoracoscopic thymectomy reported significantly less postoperative pain at 24, 48 h and 7 days (P<0.001). There were no significant differences in the patients-reported cosmetic evaluation between the two groups (P=0.369). The postoperative pain and cosmetic results are summarized in Table 2.
Discussion
Generally, thymectomy is indicated for patients diagnosed with myasthenia gravis (10). However, the appropriate surgical approaches remain arguable. Considering the wide distribution of ectopic thymic tissues in the anterior mediastinum, most thoracic surgeons recommend to remove as much mediastinal fat tissues as possible during thymectomy to avoid leaving ectopic thymic tissues, regardless via the thoracoscopic or open approaches (5,6,10,14,22,24) (Figure 5). In the open surgery, the trans-sternal approach is recognized as the standard procedure for extended thymectomy. However, different approaches exist for thoracoscopic thymectomy, which includes unilateral (right- or left-sided), bilateral, subxiphoid, transcervical or a combination approach (12,13,15-18,22,25,26). Until now, no consensus is available regarding the standard thoracoscopic approach for thoracoscopic thymectomy, especially extended thymectomy. The surgeons usually choose the thoracoscopic surgical approaches based on their training experiences and preference.
The reported thoracoscopic approaches for thymectomy are associated with several limitations. In the unilateral approach, it is difficult to expose the contralateral side and to remove all the mediastinal fat tissues (12,15-17,25-27) (Figure 5). Although the bilateral approach provided adequate exposure of the anterior mediastinum, a higher number of incisions was needed, which may increase operative trauma and postoperative pain (11,17,26,28). Hsu (24,25), Zielinski (18,29,30) and Suda (19,20) reported thoracoscopic thymectomy partially or only via subxiphoid incisions. Hsu CP created a 6-cm semi-curved subxiphoid incision and used the Kent retractor to lift the sternum. Two ports were created in the sixth intercostal space along the bilateral anterior axillary lines (24,25). Zielinski performed the transcervical, subxiphoid and unilateral incisions for maximal thoracoscopic thymectomy by using two surgeon teams (18,22,29,30) and believe that there are several issues of a minimally invasive thymectomy for MG and thymomas necessitating further clarifications (31). Suda reported single-port thymectomy using the subxiphoid incision to avoid intercostal nerve damage in the field of thoracic surgery (20,21,32). They found that the instruments may interfere with each other. Therefore, they further developed a dual-port thymectomy (19). Although all these approaches provided acceptable view of both sides, there were a few relative shortcomings. First, the subxiphoid incision was huge and a retractor was needed to lift the sternum and enlarge the anterior mediastinal space, leading to potentially greater trauma. Second, the two ports in the intercostal space may injure the intercostal nerves, which may induce postoperative pain. Third, the methods used by Suda were minimally invasive. However, it was difficult to totally remove the fat near the cardiac-diaphragmatic angles using single-port or dual-port subxiphoid approach.
Compared with the aforementioned approaches, a few advantages regarding the novel subxiphoid and subcostal arch intervention were as follows. First, it enabled excellent visualization of the whole anterior mediastinum, facilitating the removal of fat tissues (Figure 5) and minimizing the chance of accidental surgical injury such as accidental vessel laceration or contralateral phrenic nerve injury.
Second, the double lumen endotracheal tube ventilation was not mandatory. In the conventional thoracoscopy, single-lung ventilation was needed. Therefore, patients with severe lung diseases or poor lung function intolerant to single-lung ventilation and the young patients unable to accommodate the smallest double-lumen tube were excluded. However, the subxiphoid and subcostal arch intervention adequately exposed the surgical field under single-lumen tube ventilation, which may provide an acceptable alternative. In this study, except for the first two, all patients treated via the subxiphoid and subcostal arch approach were ventilated with single-lumen endotracheal tubes, without the need for double-lumen endotracheal intubation.
Third, the procedure induced less postoperative pain. Postoperative pain was mainly caused by the chest tube induced the intercostal nerve compression or injury. In the traditional approach group, at least three intercostal nerves would be involved during the surgery. In this study, the threshold to remove the chest tube was 100 mL, which was a high threshold and might cause the longer chest tube duration and drive the increased pain and the increased length of stay in the unilateral approach group. In the subxiphoid approach reported by Hsu CP, two intercostal nerves might be injured (24,25). However, in the new approach via subxiphoid and subcostal arch, no intercostal nerve was compressed or injured. Therefore, the postoperative pain score was far less compared with the conventional approaches.
In addition, no mediastinal drainage tubes were inserted using the new approach, in contrast to the others, since very little effusion drained or accumulated in the chest cavity in the first two patients (for these two patients, the drainage was less than 100 mL in the first 24 h after operation). We speculated several potential reasons. First, in the subxiphoid and subcostal arch approach group, the thymus and the fat tissues were adequately visualized, which might reduce the incidental injury. Second, the lung tissues were flexible and inflated immediately after resection of the bilateral mediastinal pleura and the fat tissues, which reduced the space in the anterior mediastinum and further reduced the effusion production and accumulation. Although our results proved that no drainage tube was safe, the patients should be closely monitored during the early postoperative stages. However, the shortcomings of this technique were as follows. First, it was hard to remove a big tumor through this approach. The space in the anterior mediastinum was limited even though a positive pressure CO2 was used to expand the space. So far, according to our experience, the largest tumor resected through this approach was 11 cm in longer diameter. Second, this approach could not be applied in patients with former anterior mediastinal operation owning to the adhesions in the anterior mediastinum. Third, the pericardium and the heart might be irritated in this approach. Therefore, patients with poor heart function require comprehensive monitoring during adopting this novel approach.
The study limitations are related to small size and short follow-up. No definitive conclusion was possible regarding the long-term therapeutic effect of this new approach. Further, in this retrospective study, a historical and sequential but not simultaneous population was included as a control group, which may provide greater surgical experience for the surgeons. A perspective trial to confirm the advantages and the long-term efficacy of subxiphoid and subcostal arch thoracoscopic thymectomy is under way (NCT02317224).
Conclusions
Thoracoscopic extended thymectomy via subxiphoid and subcostal arch might represent a safe and feasible intervention for MG patients. The approach could provide adequate exposure to the anterior mediastinum and might reduce postoperative pain.
Acknowledgements
We would appreciate the colleagues in the Department of Anesthesiology, Operation Room and the Department of Neurology for their cooperation in this study, Dr. Yi Wan in the Department of Health Statistics, School of Public Health, Fourth Military Medical University as a consultant for this paper, and Ms Fang Zhao for preparing the illustrations.
Funding: This work was supported by the National Nature Science Foundation of China (No. 81001041), and Science and Technology Innovation Development Foundation of Tangdu Hospital, Fourth Military Medical University.
Ethical Statement: This study has received ethical approval from the Fourth Military Medical University Tangdu Hospital Medical Ethics Committee (TDLL-20150074). Informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tomulescu V, Sgarbura O, Stanescu C, et al. Ten-year results of thoracoscopic unilateral extended thymectomy performed in nonthymomatous myasthenia gravis. Ann Surg 2011;254:761-5; discussion 765-6. 10.1097/SLA.0b013e31823686f6 [DOI] [PubMed] [Google Scholar]
- 2.Tomulescu V, Ion V, Kosa A, et al. Thoracoscopic thymectomy mid-term results. Ann Thorac Surg 2006;82:1003-7. 10.1016/j.athoracsur.2006.04.092 [DOI] [PubMed] [Google Scholar]
- 3.Meyer DM, Herbert MA, Sobhani NC, et al. Comparative clinical outcomes of thymectomy for myasthenia gravis performed by extended transsternal and minimally invasive approaches. Ann Thorac Surg 2009;87:385-90; discussion 390-1. 10.1016/j.athoracsur.2008.11.040 [DOI] [PubMed] [Google Scholar]
- 4.Wagner AJ, Cortes RA, Strober J, et al. Long-term follow-up after thymectomy for myasthenia gravis: thoracoscopic vs open. J Pediatr Surg 2006;41:50-4; discussion 54. 10.1016/j.jpedsurg.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 5.Nakagiri T, Inoue M, Shintani Y, et al. Improved procedures and comparative results for video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc 2015;29:2859-65. 10.1007/s00464-014-3964-1 [DOI] [PubMed] [Google Scholar]
- 6.Pompeo E, Tacconi F, Massa R, et al. Long-term outcome of thoracoscopic extended thymectomy for nonthymomatous myasthenia gravis. Eur J Cardiothorac Surg 2009;36:164-9. 10.1016/j.ejcts.2009.02.021 [DOI] [PubMed] [Google Scholar]
- 7.Bachmann K, Burkhardt D, Schreiter I, et al. Long-term outcome and quality of life after open and thoracoscopic thymectomy for myasthenia gravis: analysis of 131 patients. Surg Endosc 2008;22:2470-7. 10.1007/s00464-008-9794-2 [DOI] [PubMed] [Google Scholar]
- 8.Mineo TC, Ambrogi V. Outcomes after thymectomy in class I myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:1319-24. 10.1016/j.jtcvs.2012.12.053 [DOI] [PubMed] [Google Scholar]
- 9.Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg 2012;143:601-6. 10.1016/j.jtcvs.2011.04.050 [DOI] [PubMed] [Google Scholar]
- 10.Sonett JR, Jaretzki A., 3rd Thymectomy for nonthymomatous myasthenia gravis: a critical analysis. Ann N Y Acad Sci 2008;1132:315-28. 10.1196/annals.1405.004 [DOI] [PubMed] [Google Scholar]
- 11.Caronia FP, Fiorelli A, Santini M, et al. Uniportal bilateral video-assisted thoracoscopic extended thymectomy for myasthenia gravis: A case report. J Thorac Cardiovasc Surg 2015;150:e1-3. 10.1016/j.jtcvs.2015.03.063 [DOI] [PubMed] [Google Scholar]
- 12.Shigemura N, Shiono H, Inoue M, et al. Inclusion of the transcervical approach in video-assisted thoracoscopic extended thymectomy (VATET) for myasthenia gravis: a prospective trial. Surg Endosc 2006;20:1614-8. 10.1007/s00464-005-0614-7 [DOI] [PubMed] [Google Scholar]
- 13.Komanapalli CB, Cohen JI, Sukumar MS. Extended transcervical video-assisted thymectomy. Thorac Surg Clin 2010;20:235-43. 10.1016/j.thorsurg.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 14.Shrager JB. Extended transcervical thymectomy: the ultimate minimally invasive approach. Ann Thorac Surg 2010;89:S2128-34. 10.1016/j.athoracsur.2010.02.099 [DOI] [PubMed] [Google Scholar]
- 15.Yu L, Ma S, Jing Y, et al. Combined unilateral-thoracoscopic and mediastinoscopic thymectomy. Ann Thorac Surg 2010;90:2068-70. 10.1016/j.athoracsur.2010.02.042 [DOI] [PubMed] [Google Scholar]
- 16.Yeh CM, Chen HC, Chou CM, et al. Hybrid combination of small subxiphoid incision and thoracoscopic thymectomy for juvenile myasthenia gravis. J Pediatr Surg 2011;46:780-3. 10.1016/j.jpedsurg.2010.11.044 [DOI] [PubMed] [Google Scholar]
- 17.Caronia F, Fiorelli A, Monte AL. Bilateral thoracoscopic thymectomy using a novel positioning system. Asian Cardiovasc Thorac Ann 2014;22:1135-7. 10.1177/0218492313508563 [DOI] [PubMed] [Google Scholar]
- 18.Zielinski M, Czajkowski W, Gwozdz P, et al. Resection of thymomas with use of the new minimally-invasive technique of extended thymectomy performed through the subxiphoid-right video-thoracoscopic approach with double elevation of the sternum. Eur J Cardiothorac Surg 2013;44:e113-9; discussion e119. [DOI] [PubMed]
- 19.Suda T, Ashikari S, Tochii D, et al. Dual-port thymectomy using subxiphoid approach. Gen Thorac Cardiovasc Surg 2014;62:570-2. 10.1007/s11748-013-0337-y [DOI] [PubMed] [Google Scholar]
- 20.Suda T, Sugimura H, Tochii D, et al. Single-port thymectomy through an infrasternal approach. Ann Thorac Surg 2012;93:334-6. 10.1016/j.athoracsur.2011.08.047 [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Hachimaru A, Tochii D, et al. Video-assisted thoracoscopic thymectomy versus subxiphoid single-port thymectomy: initial results†. Eur J Cardiothorac Surg 2016;49 Suppl 1:i54-8. [DOI] [PubMed] [Google Scholar]
- 22.Zielinski M, Hauer L, Kuzdzal J, et al. Technique of the transcervical-subxiphoid-videothoracoscopic maximal thymectomy. J Minim Access Surg 2007;3:168-72. 10.4103/0972-9941.38911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaretzki A, 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Ann Thorac Surg 2000;70:327-34. 10.1016/S0003-4975(00)01595-2 [DOI] [PubMed] [Google Scholar]
- 24.Hsu CP, Chuang CY, Hsu NY, et al. Comparison between the right side and subxiphoid bilateral approaches in performing video-assisted thoracoscopic extended thymectomy for myasthenia gravis. Surg Endosc 2004;18:821-4. 10.1007/s00464-003-9146-1 [DOI] [PubMed] [Google Scholar]
- 25.Hsu CP, Chuang CY, Hsu NY, et al. Subxiphoid approach for video-assisted thoracoscopic extended thymectomy in treating myasthenia gravis. Interact Cardiovasc Thorac Surg 2002;1:4-8. 10.1016/S1569-9293(02)00003-8 [DOI] [PubMed] [Google Scholar]
- 26.Nesher N, Pevni D, Aviram G, et al. Video-assisted thymectomy with contralateral surveillance camera: a means to minimize the risk of contralateral phrenic nerve injury. Innovations (Phila) 2012;7:266-9. 10.1097/IMI.0b013e3182742a53 [DOI] [PubMed] [Google Scholar]
- 27.Lee CY, Kim DJ, Lee JG, et al. Bilateral video-assisted thoracoscopic thymectomy has a surgical extent similar to that of transsternal extended thymectomy with more favorable early surgical outcomes for myasthenia gravis patients. Surg Endosc 2011;25:849-54. 10.1007/s00464-010-1280-y [DOI] [PubMed] [Google Scholar]
- 28.Shiono H, Kadota Y, Hayashi A, et al. Comparison of outcomes after extended thymectomy for myasthenia gravis: bilateral thoracoscopic approach versus sternotomy. Surg Laparosc Endosc Percutan Tech 2009;19:424-7. 10.1097/SLE.0b013e3181c48242 [DOI] [PubMed] [Google Scholar]
- 29.Zielinski M, Hauer L, Hauer J, et al. Comparison of complete remission rates after 5 year follow-up of three different techniques of thymectomy for myasthenia gravis. Eur J Cardiothorac Surg 2010;37:1137-43. 10.1016/j.ejcts.2009.11.029 [DOI] [PubMed] [Google Scholar]
- 30.Zielinski M, Kuzdzal J, Szlubowski A, et al. Transcervical-subxiphoid-videothoracoscopic "maximal" thymectomy--operative technique and early results. Ann Thorac Surg 2004;78:404-9; discussion 409-10. 10.1016/j.athoracsur.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 31.Zielinski M. Definitions and standard indications of minimally-invasive techniques in thymic surgery. J Vis Surg 2017;3:99. 10.21037/jovs.2017.06.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suda T. Subxiphoid uniportal video-assisted thoracoscopic surgery procedure. Thorac Surg Clin 2017;27:381-6. 10.1016/j.thorsurg.2017.06.006 [DOI] [PubMed] [Google Scholar]