We read with great interest the excellent article by Della Torre et al. on the management of acute respiratory distress syndrome (ARDS) in patients with traumatic brain injury (TBI) (1). This thorough review, which deals extensively with the ventilatory management of patients with ARDS and TBI, may benefit from further expansion on the challenging hemodynamic management of these patients.
Like the authors, we believe that maintaining an adequate cerebral perfusion pressure (CPP) and cerebral oxygen delivery, while avoiding hypoxemia and further pulmonary deterioration, are the main targets in the care of this susceptible population. We feel, however, that in order to provide the most optimal hemodynamic resuscitation and avoid the detrimental effects of either hypovolemia or fluid overload, a more advanced hemodynamic monitoring approach has to be implemented. The transpulmonary thermodilution technique, available on the PiCCO® (Pulsion Medical System/GETINGE, Feldkirchen, Germany) or the VolumeView® (Edwards Lifesciences, Irvine, California, USA) monitors, enables the measurement of continuous cardiac output (CO), global end-diastolic volume (GEDV, a volumetric estimate of cardiac preload), extravascular lung water (EVLW, a quantitative measure of lung edema), and pulmonary vascular permeability index (PVPI, a marker of lung microvascular permeability). The monitoring of these cardiorespiratory parameters offers great help in achieving resuscitation targets safely in ARDS complicating TBI.
In patients with TBI, ARDS may result from the cerebral lesion itself, as is often seen in isolated subarachnoid hemorrhage, or from other injuries in case of multiple trauma. The hemodynamic status of these patients may be quite complex and poorly appreciated by clinical signs and basic hemodynamic monitoring. For example, the early phase of subarachnoid hemorrhage may be associated with hypertension and increased CO which may mask the presence of severe hypovolemia, as evidenced by low GEDV values (2). In those patients, transpulmonary thermodilution derived GEDV was reported to identify for optimal fluid status and being associated with a positive impact on the development of delayed cerebral ischemia (3). Furthermore, goal-directed fluid management of patients with subarachnoid hemorrhage has been found to have positive influence especially in patients with higher grade of severity (2).
The clinical scenario in patients with TBI is characterized by a difficult estimation and maintenance of adequate cardiac preload and the risk of fluid overload with potentially aggravating extravasation of fluid into the brain and other tissues. When TBI is associated with ARDS, an aggressive resuscitation strategy, aimed at increasing cerebral perfusion, may be particularly counterproductive due to possible worsening of pulmonary function and oxygenation as a result of increased EVLW (4). Increased EVLW levels have been repeatedly shown to be associated with worse outcome (5,6). Hence EVLW levels, besides quantifying the degree of pulmonary edema and the severity of ARDS, can be used as a safety parameter during fluid resuscitation, especially when pulmonary microvascular permeability is increased (high PVPI) (7).
International recommendations suggest the use of transpulmonary thermodilution or pulmonary artery catheterization in patients with severe shock especially in the presence of ARDS (8). These recommendations also include the measurement of CO to evaluate the response to fluids or inotropes in patients that are not responding to initial therapy (8). However, even in patients that respond to fluids (“fluid-responsive”) fluid management should be titrated carefully when elevated intravascular filling pressures or increased EVLW are present (8).
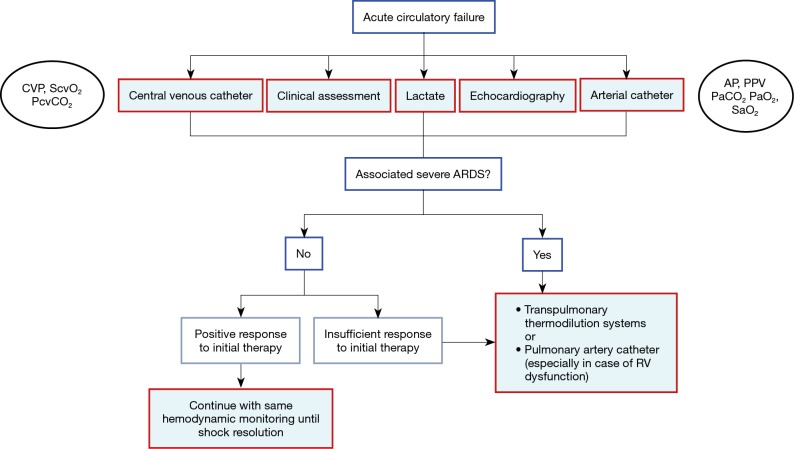
Current clinical practice is represented by a survey from Swiss intensivists, who regarded transpulmonary thermodilution as the most appropriate tool for extended hemodynamic monitoring, and CO and EVLW as most frequently used parameters, in patients with ARDS or septic shock (9). Transpulmonary thermodilution is also useful for the repetitive calibration of continuous CO from the arterial pressure waveform, which also provides important dynamic parameters (i.e., pulse pressure variation, stroke volume variation) for the assessment of fluid responsiveness in ventilated patients. Recently, the “Cardiovascular Dynamic Section” of the European Society of Intensive Care Medicine suggested monitoring by transpulmonary thermodilution or pulmonary artery catheterization (in case of right ventricular dysfunction) in patients with circulatory failure associated with severe ARDS or when response to initial therapy is regarded insufficient (10) (Figure 1). In accordance with this international group of experts, and as justified by the complex cardio-pulmonary pathology, we recommend the use of extended hemodynamic monitoring by the transpulmonary thermodilution technique in patients with ARDS and TBI.
Figure 1.
Simplified algorithm for the choice of hemodynamic monitoring in patients with acute circulatory failure. AP, arterial pressure; ARDS, acute respiratory distress syndrome; CVP, central venous pressure; PaCO2, carbon dioxide pressure in the arterial blood; PaO2, oxygen pressure in the arterial blood; PcvCO2, carbon dioxide pressure in the central venous blood; PPV, pulse pressure variation; RV, right ventricular; SaO2, arterial blood oxygen saturation; ScvO2, central venous blood oxygen saturation. Modified from (10) (with permission).
Acknowledgements
None.
Footnotes
Conflicts of Interest: Drs. Sakka, Tagami, Kirov and Perel are members of the medical advisory board of Pulsion Medical System/GETINGE, Feldkirchen, Germany.
References
- 1.Della Torre V, Badenes R, Corradi F, et al. Acute respiratory distress syndrome in traumatic brain injury: how do we manage it? J Thorac Dis 2017;9:5368-81. 10.21037/jtd.2017.11.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutoh T, Kazumata K, Ajiki M, et al. Goal-directed fluid management by bedside transpulmonary hemodynamic monitoring after subarachnoid hemorrhage. Stroke 2007;38:3218-24. 10.1161/STROKEAHA.107.484634 [DOI] [PubMed] [Google Scholar]
- 3.Tagami T, Kuwamoto K, Watanabe A, et al. Optimal range of global end-diastolic volume for fluid management after aneurysmal subarachnoid hemorrhage: a multicenter prospective cohort study. Crit Care Med 2014;42:1348-56. 10.1097/CCM.0000000000000163 [DOI] [PubMed] [Google Scholar]
- 4.Cecchetti C, Elli M, Stoppa F, et al. Neurogenic pulmonary edema and variations of hemodynamic volumetric parameters in children following head trauma. Minerva Anestesiol 2013;79:1140-6. [PubMed] [Google Scholar]
- 5.Sakka SG, Klein M, Reinhart K, et al. Prognostic value of extravascular lung water in critically ill patients. Chest 2002;122:2080-6. 10.1378/chest.122.6.2080 [DOI] [PubMed] [Google Scholar]
- 6.Tagami T, Nakamura T, Kushimoto S, et al. Early-phase changes of extravascular lung water index as a prognostic indicator in acute respiratory distress syndrome patients. Ann Intensive Care 2014;4:27. 10.1186/s13613-014-0027-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monnet X, Anguel N, Osman D, et al. Assessing pulmonary permeability by transpulmonary thermodilution allows differentiation of hydrostatic pulmonary edema from ALI/ARDS. Intensive Care Med 2007;33:448-53. 10.1007/s00134-006-0498-6 [DOI] [PubMed] [Google Scholar]
- 8.Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 2014;40:1795-815. 10.1007/s00134-014-3525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegenthaler N, Giraud R, Saxer T, et al. Haemodynamic monitoring in the intensive care unit: results from a web-based Swiss survey. Biomed Res Int 2014;2014:129593. 10.1155/2014/129593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teboul JL, Saugel B, Cecconi M, et al. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 2016;42:1350-9. 10.1007/s00134-016-4375-7 [DOI] [PubMed] [Google Scholar]