Abstract
We conducted a randomized controlled trial of a six-session behavioral intervention designed to reduce frequency of condomless sex and numbers of sex partners among recently incarcerated, bisexual Black men. One hundred participants were assigned to the small-group intervention, Men in Life Environments (MILE), and 112 were assigned to the control condition. Among those assigned to MILE, 69% attended at least one session, 88% of whom attended all sessions. At 3-months’ follow-up, large reductions in risk behaviors were reported by both groups. Means for episodes of condomless sex in the previous 3 months declined from 27.7 to 8.0 for the intervention and 25.6 to 6.7 for the control group. Reductions were not greater for the intervention than those of the control group. Regression to the mean, respondent burden, and implementation issues, such as moving from office-based to field-based survey administration at follow-up, may have contributed to the large declines reported by both groups.
Keywords: Behavioral intervention, HIV risk behavior, Bisexual, Black/African American men, Incarceration
Introduction
HIV infection rates among Black men who have sex with both men and women (MSMW) are disproportionately high compared with those of MSMW of all other races and ethnicities [1], with very few HIV prevention intervention trials for Black MSMW [2–4]. Because Black MSMW are less likely to participate in gay communities and less likely to disclose same sex contact than other MSMW, Black MSMW are unlikely to respond to interventions targeting gay men [5–9]. Such a glaring research gap points to the need for Black MSMW-tailored HIV prevention interventions.
Disproportionate incarceration of Black Americans complicates HIV prevention needs [10–14]. HIV disease and HIV risk factors are high among criminal justice-involved populations [10, 15–19] and incarceration is an all-too-frequent experience for Black men who are two to six times more likely to be imprisoned than other men [20, 21]. Incarceration is particularly prevalent among Black men ages 20–44 years, among whom 7.7–9.9% are in jail or prison [20, 21]. An estimated 12–16% of the HIV-infected US population passes through a correctional facility at some point in any given year [22]. Post-incarcerated individuals frequently encounter problems finding sufficient employment, housing, mental health services, and health care—all resources that can support them in avoiding recidivism and risky behaviors [23–26]. Hence, Black MSMW who have been incarcerated may comprise a specific subgroup of at-risk Black Americans for acquiring and transmitting HIV and other sexually transmitted infections (STIs).
In order to address the increased HIV risks associated with sexual risk group, incarceration, and race/ethnicity, we developed and tested the Men in Life Environments (MILE) Intervention for recently incarcerated African American MSMW. MILE was adapted from the Men of African American Legacy Empowering Self (MAALES) Project. It aims to reduce condomless sex, number of sex partners, and sex while intoxicated. In a randomized trial of 386 Black MSMW, MAALES demonstrated efficacy in reducing the frequency of total sex acts, unprotected sex acts with females, and number of female partners at 6 months [2].
Methods
MILE Intervention
The manualized MILE intervention was informed by the Theory of Reasoned Action and Planned Behavior [27, 28], the Critical Thinking and Cultural Affirmation (CTCA) model [29], and the Empowerment Theory [30]. It is comprised of six 2-h small-group sessions held over 3 weeks and facilitated by two African American men. The two sessions focus each sequentially on past, present, and future, encouraging participants to assess how the choices that they have made in the past and the historical and present contexts of their families and communities result in their circumstances in the present, to determine their current HIV and other health risks, and to plan for the future in ways that can lead to healthier lives for themselves and others. Participants received $20 for completing each group session in compensation for the travel and time associated with participation.
MAALES, which has been described elsewhere [2, 4], involves didactic and skill-building components addressing HIV/STI knowledge, sexual negotiation, risk reduction (including proper condom use and reducing riskly sexual behaviors related to substance use), and HIV and STI testing and treatment. Identifying holistic health issues and goal setting, particularly related to smoking and diet, was also addressed and encouraged. Role plays, stress-reduction exercises, games, and examination of contemporary media facilitated engagement with material and group dialogue. MAALES was adapted for MILE to address the needs of recently incarcerated Black MSMW. Adaptations included addressing HIV risks and harm-reduction options in prisons and jails, challenges experienced during reentry, and HIV testing and stigma in custody settings; however, the intervention structure and flow remained the same. Furthermore, MILE did not include the two booster sessions that were part of MAALES. These adaptations were determined based on a thorough review of the MAALES curriculum by our partnering agency’s Director of Education and an advisory board composed of African American MSMW and two other race/ethnicity male staff members with histories of incarceration. Like MILE, MAALES included people regardless of their HIV status, based on community input supporting the need for interventions not limited by HIV status and not requiring HIV testing for entry.
Post-Incarceration Supplemental Services
Both the intervention and control groups were offered post-incarceration supplemental services and condoms at the baseline and follow-up surveys. Our partner agency—Center for Health Justice (CHJ)—provided the supplemental services that included an individual assessment to determine immediate and long-term service needs. Specific services included one-on-one counseling, assistance in accessing emergency shelter, provision of bus tokens, guidance in making appointments for obtaining general relief or social security, clothing vouchers to a local thrift store, hygiene kits, food vouchers, referrals to substance abuse treatment, or aid to HIV-positive clients in accessing case management and treatment education and peer support. Long-term transitional assistance included assisted referrals to educational resources and vocational support.
Study Eligibility, Recruitment, and Randomization
To be eligible, potential subjects had to self-report being incarcerated in the past 12 months; oral, vaginal, or anal sex with at least one man and one woman in the last 12 months; at least one episode of vaginal or anal sex without a condom in the last 3 months; and two or more sex partners in the last 3 months. All subjects were 18 years of age or older and self-identified as Black/African American. Individuals reporting injection drug use in the last 12 months were excluded. Study procedures were approved by the institutional review boards at the University of Southern California and Charles R. Drew University of Medicine and Science.
Recruitment was accomplished through outreach by CHJ staff and chain referrals from eligible participants. Within the jail, the study team conducted direct outreach in the unit housing self-identified gay and bisexual men and male-to-female transgendered persons and to a lesser extent, identified potential participants in the general population of Men’s Central Jail. CHJ staff members who regularly work in the unit also provided study information to individuals while providing regular services such as education. These staff provided study information and invited those interested to call following their release. After obtaining verbal consent, study staff collected contact information from those interested for the purpose of following up with them post release. Eligibility screening was conducted post-release via phone. Study staff also recruited subjects from non-custody locales likely to serve Black MSMW, but not focused on reaching sexual minority groups. These included HIV service agencies, job-training centers, drug treatment programs, and agencies serving post-incarcerated populations.
Enrolled subjects were invited to recruit their peers. A $10 incentive was provided to each participant for each successful referral of an eligible person, up to three persons. Overall, 44% of the study participants were referrals from peers; however, in most (64%) of these cases, the referring participant did not seek out or obtain compensation for his referrals.
To reduce the potential for contamination between study arms, participants referred through peers were assigned to the same arm as the peer who referred them. Each time 10 confirmed new eligible subjects had completed the baseline interview, randomization was conducted. Each was re-contacted by phone, and all of those reached were assigned to either the intervention or the control arm, using the next assignment generated through a balanced block randomization program (SAS™ Cary, NC, USA). Those not reached were considered unassigned; contact with them was attempted for up to 3 months following their baseline interview. Individuals who were assigned to the intervention, but who did not attend the first session of the intervention, were permitted to enroll in either of the next two cohorts of intervention sessions held.
Assessments
Interested and eligible individuals completed the consent process and baseline interviews at the study offices located in CHJ. They provided written informed consent using an IRB-approved consent form that outlined study procedures; potential risks, benefits, and compensation; protection related to our Certificate to Confidentiality; and limits to confidentiality. Participants then completed the audio computer-assisted self-interview (ACASI) survey (median completion time: 118 min). Participants later completed two additional self-administered surveys. One survey, which largely focused on post-intervention changes in knowledge and attitudes, was scheduled for 2 weeks after the intervention period. Another was scheduled at 3 months (median completion time: 50 min) following the intervention period to allow for assessment of behavioral changes in the preceding 3 months. Participants received $30 for completing the first survey, $20 for the short second survey, and $40 for the full-length 3-month follow-up survey. Follow-up surveys were conducted either at CHJ or in the field, with field follow-up being used when efforts to complete the survey in the office were unsuccessful or during the last 2 months of data collection when the study space at CHJ was no longer available.
Survey Instrument
The survey instrument was developed as part of the Centers for Disease Control and Prevention initiative that funded this and two other intervention studies of Black MSMW. The instrument drew from previously tested items and scales (see [31] for details). The instrument assessed key background characteristics, including sociodemographics, incarceration history, HIV/STD testing, and diagnoses. It also assessed for hypothesized mediators of the intervention—including HIV risk perception, lack of support/alienation, integrated racial and sexual orientation identity, symptoms of psychological distress, HIV/STD knowledge, condom carrying, and condom-related self-efficacy, outcome expectancies, peer norms, and intentions. However, these were not examined in this preliminary examination of intervention effectiveness. The instrument measured the following behavioral outcomes over the prior 3-month period at baseline and 3-month follow-up:
Number of main and non-main male, female, and transgender sex partners.
Number of episodes of vaginal and anal sex with and without condoms with each partner gender.
Substance use, with items on alcohol use quantity and frequency from the Rapid Alcohol Problem Screen (RAPS) measure [32] and frequency of other substance use.
We focus only on the reported sexual behaviors here.
Intervention Fidelity
To assess fidelity to the curriculum and avoid drift, all sessions were audio-recorded. Initially, all recordings were reviewed by the Project Director (USC) and by the lead curriculum author (John Williams). The reviewers used a detailed form to rate the facilitators on a 4-point quality assurance scale for adherence to each curriculum activity procedure and core element and provided them with feedback after each session. Importantly, the strength of the quality assurance lies in reviewing audio recordings of sessions, providing direct supervision to facilitators, and having a standardized process to ensure all core activities being addressed as intended. Listening to recordings permits supervisors to evaluate not only content, but also process such as pacing, verbal tone, and voice inflection. It also provides the opportunity to assess rapport and to offer strategies for improvement of facilitation. These quality assurance strategies have been shown to be effective [33–35].
The mean per-session scores initially indicated 76% adherence. A comprehensive retraining session on the curriculum then followed. Subsequently, one out of each of the six-session recordings was randomly selected, and scored for adherence. These scores averaged 82%, and all but one session scored at or above the accepted target of 80% adherence [36].
Data Analysis
Descriptive analyses comparing differences between those randomized to each study arm were conducted. Outcome variables of interest were also examined for baseline differences between the intervention and control arms. Chi-square tests were used for categorical variables and t tests for count variables.
A longitudinal, nested group-randomized model was conducted to examine differences between study arms while controlling for baseline values and other covariates and while accounting for clustering of subjects within cohorts. The number of condomless sex acts and the number of sex partners in the prior 3 months were analyzed as both a count of episodes and as a dichotomous variable. To reduce skewing, the number of episodes of sex without condoms in the prior 3 months was limited to 100. For outcome analyses, we examine behavioral changes at the 3-month assessments compared to baseline. Utilizing SAS 9.3 Proc Countreg, we attempted to fit the observed distribution for the outcome variables with four possible expected distributions (Poisson, zero-inflated Poisson, negative binomial, and zero-inflated negative binomial). However, the data for both count outcomes at follow-up were highly skewed (with 50% or more of the sample reporting zeros) and did not fit the distributions. Therefore, we limited our statistical approach to dichotomous outcomes (any condomless sex and any sex partners versus none). Proc Glimmix was used to model logistic regressions, incorporating possible data clustering within cohorts as a random effect and an unstructured variance-covariance to remove restrictions and allow for the best possible model fit. All analyses were conducted using SAS (version 9.3; SAS Institute Inc., Cary, NC, USA). In post hoc analysis, we addressed concerns about interview setting effects and by conducting additional analyses without the last 55 participants (who were all interviewed in the field) and by controlling for an indicator of this.
Results
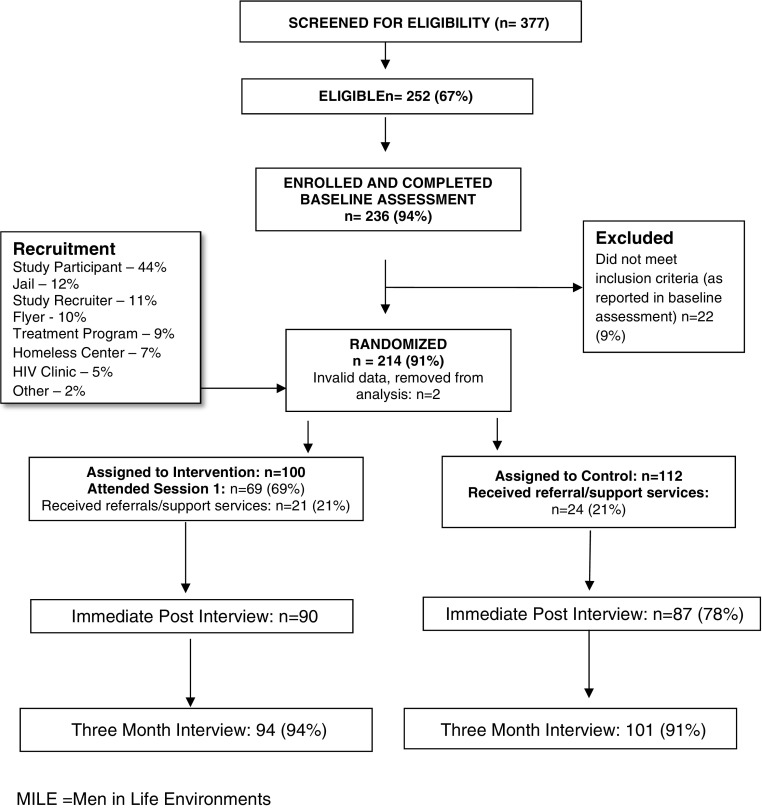
The CONSORT chart (Fig. 1) describes recruitment, enrollment, random assignments, and follow-up for the trial [19]. Outreach and recruitment were conducted from 2011 to 2012. Of the 377 screened, 252 were eligible, and 236 completed baselines. However, 22 of these participants were withdrawn because their baseline assessments indicated that they did not meet the eligibility criteria. This left 214 persons who were assigned to the two study arms across 23 cohorts, including 11 intervention and 12 control cohorts. Due to incomplete data, two people were excluded from the final analysis leaving n = 212, with 100 in the intervention and 112 in the control. Of those assigned to the intervention, 69% participated in at least one MILE session with mean session attendance being 5.
Fig. 1.
CONSORT chart: recruitment, enrollment, and random assignment of participants in the Center for Health Justice (CHJ) MILE Intervention Trial. MILE: Men in Life Environments
Both the intervention and control groups were eligible to receive referrals to additional support services. Nineteen participants (9%) refused the needs assessment to initiate this process. The remainder completed the assessment, but a relatively low percentage accepted services, with just 21% (n = 45) of both arms receiving at least one service. Most of these received the free hygiene kits.
Most study participants were between the ages of 40 and 49 and of lower socioeconomic status; nearly half (49.0% excluding missing) had spent at least 3 years of their adult lives incarcerated. Table 1 shows the comparison between the intervention and control condition on sociodemographics and risk behaviors at baseline. Intervention and control condition participants differed (p < 0.10) in employment, time since last incarceration, and sex with transgender partners.
Table 1.
Baseline comparison of intervention (n = 100) and control (n = 112) groups on selected sociodemographic and risk-related characteristics, among all randomized participants—The Men in Life Environments (MILE) study of Black MSMa
| Overall n = 212 | Intervention (n = 100) | Control (n = 112) | p value | |
|---|---|---|---|---|
| Age group | 0.39 | |||
| Less than 30 | 18% | 23% | 14% | |
| 30 to 39 | 23% | 23% | 23% | |
| 40 to 49 | 37% | 33% | 40% | |
| 50+ | 22% | 21% | 23% | |
| Level of education | 0.35 | |||
| Less than HS | 25% | 23% | 28% | |
| GED/HS diploma or some college | 67% | 67% | 67% | |
| College graduate | 8% | 10% | 5% | |
| Current employment | 0.06 | |||
| Unemployed/disabled/retired | 86% | 91% | 82% | |
| Employed part/full time | 13% | 9% | 18% | |
| Housing instability (past 12 months)b | 62% | 62% | 63% | 0.83 |
| On probation/parole | 36% | 39% | 34% | 0.45 |
| Cumulative lifetime in prison 3+ years | 45% | 46% | 44% | 0.74 |
| Self-reported HIV status | 0.99 | |||
| HIV positive | 31% | 31% | 30% | |
| HIV negative | 60% | 60% | 61% | |
| HIV other (indeterminate, inconclusive, never tested) | 9% | 9% | 9% | |
| Drug use history | ||||
| Ever alcohol/drug treatment program | 46% | 45% | 47% | 0.74 |
| Drug use past 30 days | 67% | 73% | 64% | 0.17 |
| Length of time since last released from incarceration | 0.07 | |||
| Less than 30 days | 29% | 26% | 33% | |
| 30 to 90 days | 18% | 25% | 12% | |
| 90 to 180 days | 27% | 27% | 27% | |
| 180+ days | 26% | 22% | 29% | |
| Self-identified sexual orientation | 0.47 | |||
| Heterosexual | 7% | 5% | 10% | |
| Gay/homosexual/SGL | 17% | 18% | 15% | |
| Bisexual | 71% | 71% | 71% | |
| Other/none of the above | 5% | 6% | 4% | |
| Exchange sex (selling or buying, past 3 months) | ||||
| With women | 21% | 26% | 18% | 0.16 |
| With men | 30% | 33% | 28% | 0.40 |
| Sex in jail (prior 12 months) | ||||
| With men | 25% | 24% | 27% | 0.62 |
| With women | 2% | 1% | 2% | 0.62 |
| With transgender | 6% | 5% | 7% | 0.51 |
| With any gender | 33% | 30% | 36% | 0.31 |
| Any unprotected sex (prior 3 months) | ||||
| With male partners | 92% | 95% | 89% | 0.13 |
| With female partners | 82% | 81% | 82% | 0.56 |
| With transgender partners | 28% | 22% | 34% | 0.06 |
| With any partners | 100% | 100% | 100% | 1.0 |
| Episodes of unprotected sex (prior 3 months) | Mean (SD) | |||
| With men | 10.4 (14.3) | 11.4 (12.6) | 9.8 (12.6) | 0.56 |
| With women | 16.6 (29.4) | 18.6 (36.6) | 14.8 (21.1) | 0.35 |
| With transgender | 0.4 (3.5) | 0.2 (0.7) | 0.6 (4.8) | 0.40 |
| Mean number of sex partners (vaginal or anal in prior 3 months) | ||||
| With men | 2.8 (2.8) | 2.9 (2.7) | 2.6 (2.8) | 0.50 |
| With women | 2.9 (3.5) | 2.9 (3.3) | 2.8 (3.6) | 0.66 |
| With transgender (among those with transgender partners) | 2.6 (3.6) | 2.6 (3.8) | 2.4 (3.4) | 0.86 |
aChi-square test for dichotomous bivariate analysis. t test for difference in means for count variables
bIn the past 12 months, have you ever spent one night without a regular place to stay? (This may include when you stayed at a shelter, transitional housing facility, or a public or private place like a car or a park)
Table 2 presents baseline and follow-up outcome measures for the intervention and control conditions for the entire sample and with the final 55 participants excluded (referred to as the “limited dataset”). The limited dataset was constructed because we observed a significant decline in reports of any sexual activity among these participants, all of whom were interviewed in field settings. Participants interviewed in the field during the last 2 months of data collection were less likely to report any sexual partner in the prior 3 months (23 vs 77%; p < 0.001), and any condomless sex (11 vs. 59%; p < 0.001). Outcomes considered in bivariate analyses for both groups included “any” condomless sex, mean and median episodes of condomless sex, and mean and median numbers of partners.
Table 2.
Associations of intervention group assignment with condomless sex and number of partner outcomes. The Men in Life Environments study of Black MSMa
| Full sample | Limited sampleb | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |||||
| BL | FU | BL | FU | BL | FU | BL | FU | |
| n = 100 | n = 92 | n = 112 | n = 99 | n = 67 | n = 67 | n = 69 | n = 69 | |
| Any condomless sex (with all genders) | 100% | 48% | 100% | 34% | 100% | 69% | 100% | 49% |
| Any condomless sex with male partners (anal) | 95% | 38% | 89% | 32% | 93% | 54% | 90% | 41% |
| Any condomless sex with female partners (vaginal and anal) | 82% | 19% | 82% | 15% | 82% | 28% | 88% | 22% |
| Number of episodes | ||||||||
| Condomless sex | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| With all partners (all genders) | 27.7 (26.8) | 8.0 (17.4) | 25.6 (26.9) | 6.7 (18.0) | 27.5 (27.8) | 10.6 (19.7) | 27.6 (27.6) | 9.0 (20.1) |
| With male partners (anal) | 11.0 (12.7) | 6.0 (16.8) | 9.7 (14.4) | 4.2 (14.7) | 10.6 (13.6) | 7.7 (19.2) | 10.8 (16.8) | 5.8 (17.4) |
| With female partners (vaginal and anal) | 15.8 (23.1) | 2.0 (5.3) | 14.8 (20.7) | 3.1 (12.8) | 16.1 (22.7) | 2.7 (6.0) | 16.6 (21.4) | 4.0 (15.0) |
| Number of partners | ||||||||
| Total number partners (all genders; anal or vaginal) | 5.8 (5.1) | 1.8 (2.1) | 5.4 (6.0) | 1.5 (3.2) | 5.7 (4.1) | 2.2 (2.0) | 2.7 (7.0) | 1.9 (3.6) |
| Number male partners (anal) | 2.9 (2.7) | 1.1 (1.6) | 2.6 (2.9) | 1.0 (2.4) | 2.7 (2.2) | 1.4 (1.6) | 2.9 (3.4) | 1.3 (2.6) |
| Number female partners (vaginal or anal) | 3.0 (3.3) | 0.7 (1.3) | 2.8 (3.6) | 0.5 (1.3) | 3.0 (3.3) | 0.8 (1.5) | 2.8 (4.1) | 0.6 (1.5) |
| Medians | ||||||||
| Number of episodes | ||||||||
| Condomless sex | Median | Median | Median | Median | Median | Median | Median | Median |
| All partners (all genders) | 18 | 1 | 14.5 | 0 | 15 | 3 | 20 | 0 |
| With male partners (anal) | 8 | 0 | 5 | 0 | 7 | 1 | 5 | 0 |
| With female partners (vaginal and anal) | 6 | 0 | 7 | 0 | 6 | 0 | 10 | 0 |
| Number of partners | Median | Median | Median | Median | Median | Median | Median | Median |
| Total number partners (all genders; anal or vaginal) | 5 | 1 | 4 | 1 | 5 | 2 | 4 | 1 |
| Number male partners (anal) | 2 | 1 | 2 | 0 | 2 | 1 | 2 | 1 |
| Number female partners (vaginal or anal) | 2 | 0 | 2 | 0 | 2 | 0 | 2 | 0 |
aNumber of episodes was limited to 100+
bLimited sample excludes 55 cases that were known to have been interviewed in field settings at follow-up
Since condomless sex was an inclusion criterion for the study, we expected to observe declines in this variable. However, the decline was quite large, from 100% at baseline to 48 and 34% for the intervention and control groups, respectively. The reported means for episodes of sex without condoms declined from 27.7 to 8.0 for the intervention and 25.6 to 6.7 for the control. Condomless sex with females accounted for the largest proportion of the reported reductions; the intervention and control arms reduced from 15.8 to 2.0 and 14.8 to 3.1, respectively. To a lesser extent, steep drops were seen for number of partners across the intervention and control arms, with the total number of partners, reducing from a mean of 5.8 to 1.8 and 5.4 to 1.5, respectively.
We explored the possibility that the declines resulted from regression to mean (RTM), using an approach outlined, by Hopkins [37] and Trochim [38]. The first step was to calculate the correlation (r) between pre- and post-test responses (0.17). The percentage of RTM was then calculated via the formula Prm = 100 (1−r), where r equals this pre-post correlation. This suggests 83% of the decline from the average pre-test score to the average post-test score can be explained by RTM. The second step was to use data from a previous study [2] to represent a population mean for comparison to our sample means at each time point. This study, known as MAALES, included a more general population of bisexual Black men in Los Angeles. Harawa et al. [2] found a mean number of total condomless sex acts in the past 3 months of 7 at baseline and of 6 at follow-up for the control group, a 14% reduction. Given this difference in estimated population pre- and post-means, we could expect our current study pre- or baseline mean of 27 condomless sex acts to similarly drop by 14% to 23 due to RTM. Moreover, given our pre/post correlation of 0.17, we could also expect to see RTM that covers 83% of the distance from the expected post mean of 23 to the MAALES-estimated population post mean of 6. Given this, we would expect a post-test mean of 9 resulting from RTM alone. The means for the current study post-test are 8 and 6.7 for the intervention and control groups, respectively. This analysis indicates that a considerable portion of the observed declines in risk behavior could be due to RTM.
The final outcomes analysis relied on the dichotomous variables, any condomless sex in the past 3 months versus none and any sex partners versus no sex partners. Results from the final multiple regression outcomes are shown in Table 3. The model controlled for reported baseline values of the dependent variable, current employment, education, time to follow-up interview, interview proctor, and whether the interview was conducted in the final phase of follow-up data collection. No statistically significant differences were found between the intervention and control arms for any condomless sex with male or female partners or across all gender groups. Participants in the intervention arm were found to have higher odds of sex with any partner 2.49 (95% CI 1.09, 5.71). An identical logistic regression is also presented in Table 3 that excludes from the analysis those whose data were collected in the final phase rather than controlling for this phase in the model. However, no significant differences in outcomes were found between interventions and control conditions when excluding those who were surveyed during the final phase of follow-up.
Table 3.
Logistic regression and odds ratios for primary risk behavior outcomes comparing the intervention (MILE) to the control arms at 3-month follow-up, controlling for baselinea
| Dichotomous outcomes | Odds ratios (95% CI) complete caseb | Odds ratios (95% CI) limited sampleb |
|---|---|---|
| Any sex without condoms (prior 3 months) | 1.74 (0.70, 4.36) | 2.22 (0.90, 5.47) |
| Any sex with females without condoms (prior 3 months) | 1.43 (0.48, 4.24) | 2.03 (0.67, 6.29) |
| Any sex with males without condoms (prior 3 months) | 1.26 (0.53, 2.99) | 1.54 (0.65, 3.67) |
| Any sex partner at follow-up (prior 3 months) | 2.49 (1.09, 5.71) | 2.21 (0.86, 5.67) |
| Any female partner at follow-up (prior 3 months) | 1.76 (0.73, 4.23) | 1.42 (0.60, 3.34) |
| Any male partner at follow-up (prior 3 months) | 1.59 (0.61, 4.10) | 1.67 (0.60, 4.49) |
MILE Men in Life Environments
aLogistic regression models controlled for length of time between baseline and follow-up interviews and baseline values for current employment, level of education, the proctor conducting the interview, and whether the interview was conducted in the final phase of follow-up data collection. Models also accounted for clustering within groups
bAnalyses were conducted both as a complete case design as well as excluding cases (n = 55) collected during final phase of follow-up. These cases were known to have been interviewed in field settings at follow-up
Discussion
MILE, which was designed for African American MSMW who had experienced recent incarceration, failed to show intervention impacts, as similar declines in risk behaviors were observed in both the intervention and control groups. Reasons for the large, across-the-board declines in condomless sex and number of sexual partners are not completely clear, but we considered a number of possible explanations including RTM, respondent burden, and interview setting effects.
A number of other studies cite the need to measure for RTM in HIV risk intervention trials and behavioral social science studies [39, 40]. Due to the substantial decline in means observed for both groups and the entry criteria that selected for individuals reporting very high-risk recent behaviors, we explored the possibility that the decline indicated the phenomenon of regression toward the mean in our study sample. This RTM analysis indicated it would be expected to observe nearly as considerable a decline at follow-up as was observed in the current study due to RTM. However, this assumes that the MAALES study population provides reasonable estimates for a population data comparison and does not take into account other possible reasons for the low correlation between baseline and follow-up responses.
One such reason involves participant burden. The baseline survey lasted nearly 2 h on average, with the outcome-related questions placed as part of a very detailed set of sexual risk behavior and partner-related questions in the second third of the survey. The anticipation of a long interview at follow-up may have led participants to select “No” on contingency questions related to sexual risk in order to reduce the time needed to complete the survey.
Interview setting may also have influenced responses. All participants completed the baseline survey in the study office, where they were provided with snacks, had relatively few interruptions or distractions, and were monitored by survey proctors. Some follow-up surveys, including all of the final 55, were conducted in field settings. These settings included the participants’ homes, the interviewers’ cars, or public settings. Participants known to have been interviewed in these settings were significantly more likely to report no sexual activity than other participants. Some anecdotal reports further indicate that the intervention participants were more willing than control participants to put focus and effort into completing the follow-up surveys. Furthermore, the intervention group was significantly more likely to report any sex partner during the 3-month follow-up period in the complete dataset, but not in the limited dataset. The higher likelihood of reporting any sexual partnership among intervention participants likely explains why odds ratios for sex risk outcomes were elevated (though non-significant) for the intervention arm.
Finally, we also point out that regardless of group assignment, all participants received some HIV prevention encouragement, including free condoms and being enrolled in a setting wherein HIV prevention messages and information were displayed and available. It is possible that these activities contributed to short-term across-the-board risk reduction and/or increased participants’ perceived desirability of providing low-risk responses at follow-up.
The MILE trial differed from the efficacious MAALES trial on which it was based. The MAALES study was not limited to men with two or more recent partners or to those reporting recent unprotected sex. The intervention also was designed for and tested in Black MSMW regardless of their incarceration history. Included in the MAALES but not the MILE intervention were booster sessions held at 9 and 18 weeks’ post-completion of the core intervention. In terms of methodological differences, the MAALES trial involved a much larger group of randomized study participants (n = 386) and included follow-up assessments at both 3 and 6 months, with statistically significant intervention-associated declines in risk behaviors observed only at 6 months. Median completion time for the baseline MAALES survey was just 88 min, compared to 118 for MILE. The difference may have motivated more MILE participants to want to shorten the duration of the follow-up survey.
Despite the equivocal findings in the MILE trial, we point to some important successes. The study was able to locate, enroll, and engage a significant number of eligible men from a stigmatized population over a short time period. In addition, an excellent retention rate was achieved, despite high rates of housing instability and recidivism. Furthermore, although the percentage of the intervention group that initiated the MILE intervention sessions was suboptimal (69%), the high attendance rates of those who did and positive participant feedback speak to its value and relevance to attendees. Participants reported being drawn to a holistically targeted intervention that addressed them as Black men rather than men defined by a particular sexual orientation.
Recent developments in pre-exposure prophylaxis (PrEP) [41, 42], research demonstrating the effectiveness of treatment as prevention [43], and the success of HIV testing and rapid entry into HIV care [44, 45] have led to a sharp turn away from purely behavioral interventions toward more biomedical strategies [46]. However, there remains a need for behavioral approaches to ensure that high-risk populations, such as recently incarcerated Black MSMW, have the knowledge, skills, and self-empowerment they need to effectively access biomedical interventions [46–48]. A new set of trials, focused on promoting access and adherence to newer biomedical prevention strategies, is needed to address large racial and social disparities in HIV. Trials such as MILE may be used to inform approaches to recruiting and following high-risk, under-resourced, and frequently ignored groups for purposes of improving utilization of HIV testing and treatment, as well as pre-exposure prophylaxis. We argue that a combination of approaches is needed at both the individual and populations levels, particularly for highly marginalized populations who frequently experience pressing health threats—signaling the importance of intervention benefits that extend beyond reducing HIV incidence and uncontrolled viremia. Nevertheless, implementation issues in testing these approaches must be carefully attended to, and efforts to assess for intervention benefits should avoid substantial assessment burdens for study participants and include objective assessments wherever possible.
Compliance with Ethical Standards
Study procedures were approved by the institutional review boards at University of Southern California and the Charles R. Drew University of Medicine and Science. Interested and eligible individuals completed the consent process and baseline interviews at the study offices located in CHJ. They provided written informed consent using an IRB-approved consent form that outlined study procedures; potential risks, benefits, and compensation; protection related to our Certificate to Confidentiality; and limits to confidentiality. Those initially contacted in jail provided verbal consent for collection of their locator information in jails for potential study enrollment post-release, using IRB approved forms and processes for this purpose.
References
- 1.Centers for Disease Control and Prevention. HIV surveillance report. 2015;27. Published November 2016.
- 2.Harawa NT, Williams JK, McCuller WJ, Ramamurthi HC, Lee M, Shapiro MF, Norris KC, Cunningham WE. Efficacy of a culturally congruent HIV risk-reduction intervention for behaviorally bisexual black men: results of a randomized trial. AIDS. 2013;27(12):1979–1988. doi: 10.1097/QAD.0b013e3283617500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maulsby C, Millett G, Lindsey K, Kelley R, Johnson K, Montoya D, Holtgrave D. A systematic review of HIV interventions for black men who have sex with men (MSM) BMC Public Health. 2013;13(1):625. doi: 10.1186/1471-2458-13-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams JK, Glover DA, Wyatt GE, Kisler K, Liu H, Zhang M. A sexual risk and stress reduction intervention designed for HIV-positive bisexual African American men with childhood sexual abuse histories. Am J Public Health. 2013;103(8):1476–1484. doi: 10.2105/AJPH.2012.301121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mays VM, Cochran SD, Zamudio A. HIV prevention research: are we meeting the needs of African American men who have sex with men? J Black Psychol. 2004;30(1):78–105. doi: 10.1177/0095798403260265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millett G, Malebranche D, Mason B, Spikes P. Focusing “down low”: bisexual black men, HIV risk and heterosexual transmission. J Natl Med Assoc. 2005;97(7 Suppl):52S–59S. [PMC free article] [PubMed] [Google Scholar]
- 7.Myrick R. In the life: culture-specific HIV communication programs designed for African American men who have sex with men. J Sex Res. 1999;36(2):159–170. doi: 10.1080/00224499909551981. [DOI] [Google Scholar]
- 8.Williams JK, Wyatt GE, Resell J, Peterson J, Asuan-O’Brien A. Psychosocial issues among gay- and non-gay-identifying HIV-seropositive African American and Latino MSM. Cultur Divers Ethnic Minor Psychol. 2004;10(3):268–286. doi: 10.1037/1099-9809.10.3.268. [DOI] [PubMed] [Google Scholar]
- 9.Williams JK, Ramamurthi HC, Manago C, Harawa NT. Learning from successful interventions: a culturally congruent HIV risk-reduction intervention for African American men who have sex with men and women. Am J Public Health. 2009;99(6):1008–1012. doi: 10.2105/AJPH.2008.140558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer RA, Magnus M, Kuo I, Wang L, Liu TY, KH M. The high prevalence of incarceration history among Black men who have sex with men in the United States: associations and implications. Am J Public Health. 2014;104(3):448–454. doi: 10.2105/AJPH.2013.301786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iguchi MY, Bell J, Ramchand RN, Fain T. How criminal system racial disparities may translate into health disparities. J Health Care Poor Underserved. 2005;16(4 Suppl B):48–56. doi: 10.1353/hpu.2005.0081. [DOI] [PubMed] [Google Scholar]
- 12.Isaac NE, Sanchez RL. Emergency department response to battered women in Massachusetts. Ann Emerg Med. 1994;23(4):855–858. doi: 10.1016/S0196-0644(94)70325-6. [DOI] [PubMed] [Google Scholar]
- 13.Jones KT, Gray P, Whiteside O, Wang T, Bost D, Dunbar E, Foust E, Johnson WD. Evaluation of an HIV prevention intervention adapted for black men who have sex with men. Am J Public Health. 2007;98(6):1043–1050. doi: 10.2105/AJPH.2007.120337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy ME, Wilton L, Phillips G, 2nd, et al. Understanding structural barriers to accessing HIV testing and prevention services among black men who have sex with men (BMSM) in the United States. AIDS Behav. 2014;18(5):972–996. doi: 10.1007/s10461-014-0719-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harawa N, Adimora A. Incarceration, African Americans and HIV: advancing a research agenda. J Natl Med Assoc. 2008;100(1):57–62. doi: 10.1016/S0027-9684(15)31175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maruschak L, Berzofsky M, Unangst J. Medical problems of state and federal prisoners and jail inmates 2011–12. U.S. Department of Justice; 2015.
- 17.Hammett TM. HIV/AIDS and other infectious diseases among correctional inmates: transmission, burden, and an appropriate response. Am J Public Health. 2006;96(6):974–978. doi: 10.2105/AJPH.2005.066993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinbaum CM, Sabin KM, Santibanez SS. Hepatitis B, hepatitis C, and HIV in correctional populations: a review of epidemiology and prevention. AIDS. 2005;19(Suppl 3):S41–S46. doi: 10.1097/01.aids.0000192069.95819.aa. [DOI] [PubMed] [Google Scholar]
- 19.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabol W, Minton T, Harrison P. Prison and jail inmates at midyear 2006. U.S. Department of Justice, Bureau of Justice Statistics Bulletin. 2007;NCJ 217675.
- 21.Brewer RA, Magnus M, Kuo I, Wang L, Liu TY, Mayer KH. Exploring the relationship between incarceration and HIV among black men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2014;65(2):218–225. doi: 10.1097/01.qai.0000434953.65620.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releases from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4(11):e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bracken N, Hilliard C, McCuller WJ, Harawa NT. Facilitators of HIV medical care engagement among former prisoners. AIDS Educ Prev. 2015;27(6):566–583. doi: 10.1521/aeap.2015.27.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harzke AJ, Ross MW, Scott DP. Predictors of post-release primary care utilization among HIV-positive prison inmates: a pilot study. AIDS Care. 2006;18(4):290–301. doi: 10.1080/09540120500161892. [DOI] [PubMed] [Google Scholar]
- 25.Wohl DA, Scheyett A, Golin CE, White B, Matuszewski J, Bowling M, Smith P, Duffin F, Rosen D, Kaplan A, Earp JA. Intensive case management before and after prison release is no more effective than comprehensive pre-release discharge planning in linking HIV-infected prisoners to care: a randomized trial. AIDS Behav. 2011;15(2):356–364. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersilia J. When prisoners come home: parole and prisoner reentry. New York, New York: Oxford University Press; 2003. [Google Scholar]
- 27.Ajzen I. From intentions to actions: a theory of planned behavior. In: Kuhl J, Bechman J, editors. Action control from cognition to behaviour. New York, NY: Springer-Verlag; 1985. [Google Scholar]
- 28.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. Special Issue: Theories of cognitive self-regulation. 1991;50(2):179–211.
- 29.Manago C. A critical thinking and cultural affirmation (CTCA) approach to HIV prevention and risk reduction, consciousness, and practice for African American males at HIV sexual risk. Los Angeles: The AmASSI Center; 1996. [Google Scholar]
- 30.Freire P. Pedagogy of the oppressed. New York, NY: Continuum; 1983. [Google Scholar]
- 31.Joseph HA, Pan Y, Mendoza M, Harawa NT, Lauby J, Hosek SG, et al. HIV acquisition and transmission potential among African-American men who have sex with men and women in three U.S. cities. Arch Sex Behav. 2018;47(1):183–194. doi: 10.1007/s10508-017-1052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherpitel CJ. Screening for alcohol problems in the emergency room: a rapid alcohol problems screen. Drug Alcohol Depend. 1995;40(2):133–137. doi: 10.1016/0376-8716(95)01199-4. [DOI] [PubMed] [Google Scholar]
- 33.Liese BS. New developments, advances, and possibilities in cognitive therapy supervision. Clin Superv. 1998;17(1):115–124. doi: 10.1300/J001v17n01_10. [DOI] [Google Scholar]
- 34.NIMH Multisite HIV/STD Prevention Trial for African American Couples Group Supervision of facilitators in a multisite study: goals, process, and outcomes. J Acquir Immune Defic Syndr. 2008;49(Suppl 1):S59–S67. doi: 10.1097/QAI.0b013e3181844807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perris C. Cognitive psychotherapy and training supervisors. J Cogn Psychother. 1994;8(2):83–102. [Google Scholar]
- 36.Durlak JA, DuPre EP. Implementation matters: a review of research on the influence of implementation on program outcomes and the factors affecting implementation. Am J Community Psychol. 2008;41(3–4):327–350. doi: 10.1007/s10464-008-9165-0. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins W. Regression to the mean. In: A new view of statistics. Available at: http://www.sportsci.org/resource/stats/. Accessed Feb 2016.
- 38.Trochim M. Regression to the mean. In: The research methods knowledge base, 2nd ed. Available at: http://www.socialresearchmethods.net/kb/. Accessed Nov 2015.
- 39.Hughes JP, Haley DF, Frew PM, Golin CE, Adimora AA, Kuo I, Justman J, Soto-Torres L, Wang J, Hodder S. Regression to the mean and changes in risk behavior following study enrollment in a cohort of U.S. women at risk for HIV. Ann Epidemiol. 2015;25(6):439–444. doi: 10.1016/j.annepidem.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu R, Chen L. The need to control for regression to the mean in social psychology studies. Front Psychol. 2014;5:1574. doi: 10.3389/fpsyg.2014.01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapía M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernández T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallás EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV, iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okwundu CI, Uthman OA, Okoromah CA. Antiretroviral pre-exposure prophylaxis (PrEP) for preventing HIV in high-risk individuals. Cochrane Database Syst Rev. 2012;7:CD007189. doi: 10.1002/14651858.CD007189.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hull MW, Montaner J. Antiretroviral therapy: a key component of a comprehensive HIV prevention strategy. Curr HIV/AIDS Rep. 2011;8(2):85–93. doi: 10.1007/s11904-011-0076-6. [DOI] [PubMed] [Google Scholar]
- 44.Schwarcz S, Hsu LC, Scheer S. Disparities and trends in viral suppression during a transition to a “Test and Treat” approach to the HIV epidemic, San Francisco, 2008–2012. J Acquir Immune Defic Syndr. 2015;70(5):529–537. doi: 10.1097/QAI.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen SW, Sansom SL, Brooks JT, Marks G, Begier EM, Buchacz K, DiNenno EA, Mermin JH, Kilmarx PH. A mathematical model of comprehensive test-and-treat services and HIV incidence among men who have sex with men in the United States. PLoS One. 2012;7(2):e29098. doi: 10.1371/journal.pone.0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5(1):143–167. doi: 10.1146/annurev.clinpsy.032408.153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koblin BA, Andrasik M, Austin J. Preparing for the unexpected: the pivotal role of social and behavioral sciences in trials of biomedical HIV prevention interventions. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S183–S186. doi: 10.1097/QAI.0b013e31829a3a4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lippman SA, Koester KA, Amico KR, Lama JR, Martinez Fernandes N, Gonzales P, Grinsztejn B, Liu A, Buchbinder S, Koblin BA. Client and provider perspectives on new HIV prevention tools for MSM in the Americas. PLoS One. 2015;10(3):e0121044. doi: 10.1371/journal.pone.0121044. [DOI] [PMC free article] [PubMed] [Google Scholar]