Abstract
Mitochondrial dysfunction is a global term used in the context of “unhealthy” mitochondria. In practical terms, mitochondria are extremely complex and highly adaptive in structure, chemical and enzymatic composition, subcellular distribution and functional interaction with other components of cells. Consequently, altered mitochondrial properties that are used in experimental studies as measures of mitochondrial dysfunction often provide little or no distinction between adaptive and maladaptive changes. This is especially a problem in terms of generation of oxidant species by mitochondria, wherein increased generation of superoxide anion radical or hydrogen peroxide (H2O2) is often considered synonymously with mitochondrial dysfunction. However, these oxidative species are signaling molecules in normal physiology so that a change in production or abundance is not a good criterion for mitochondrial dysfunction. In this review, we consider generation of reactive electrophiles and consequent modification of mitochondrial proteins as a means to define mitochondrial dysfunction. Accumulated evidence indicates that 4-hydroxynonenal (HNE) modification of proteins reflects mitochondrial dysfunction and provides an operational criterion for experimental definition of mitochondrial dysfunction. Improved means to detect and quantify mitochondrial HNE-protein adduct formation could allow its use for environmental health risk assessment. Furthermore, application of improved mass spectrometry-based proteomic methods will lead to further understanding of the critical targets contributing to disease risk.
Keywords: 4-hydroxynonenal, mitochondria, lipid peroxidation, protein adduct, redox, electrophile
INTRODUCTION
In common use, mitochondrial dysfunction represents a generic disease mechanism, i.e., if mitochondria do not function properly, disease will result either directly or due to unmasking of other disease processes. Mitochondria not only provide ATP but are central to metabolism of each of the major energy fuels, biosynthesis of nonessential amino acids, biosynthesis of DNA precursors, signaling of cell death and survival, and biosynthesis of hormones and cell signaling molecules. Consequently, mitochondrial dysfunction can be manifest in many ways and impact both acute and chronic disease processes. Such impact is apparent in Parkinson’s disease and other neurodegenerative diseases, vascular disease, metabolic syndrome and diabetes, cardiomyopathies, and renal failure. Accordingly, environmental influences that cause mitochondrial dysfunction pose a major health burden.
Extensive research has focused on mechanisms of mitochondrial dysfunction, and a general “autocatalytic” scheme explains many characteristics of an insidious mitochondrial dysfunction, which occurs with aging and chronic disease. In this scheme, low-level production of free-radical oxidants cause mtDNA damage, which then causes more mitochondrial dysfunction that results in higher levels of free radical oxidants, etc. The key event in this scheme is oxidative damage to mtDNA, which can result in protein mutation, generation of additional free radicals and altered energy production.
However, alternative models could also explain such an insidious mitochondrial dysfunction. For instance, the function of mitochondria occurs under nonequilibrium conditions where diffusion of O2 and substrates into mitochondria must balance the need for ATP production. If macromolecular aggregates alter the rates of these processes, then compensatory mechanisms must exist to maintain ATP production. Superoxide anion radical (O2⋅−) or hydrogen peroxide (H2O2) produced by the electron transfer chain could provide a signaling molecule to allow such compensation, adjusting diffusional or transport characteristics to maintain the appropriate flow of precursors under the nonequilibrium steady state. Importantly, if increased production of either O2⋅− or H2O2 were used as signaling molecules to maintain ATP production, then increased production of these reactive species could have a positive role in maintaining mitochondrial function. Circumstantial evidence is available for mitochondrial O2⋅− having an activating role for oxidative phosphorylation [Jones, 2006a] and mitochondrial H2O2 providing an extramitochondrial adaptive signal [Go et al., 2010; Imhoff and Hansen, 2009].
Extensive research on extramitochondrial signal transduction shows that reactive cysteine (Cys) residues of specific proteins function as sensors in redox signaling mechanisms. In addition, Cys residues are also critical components of the protein machinery for oxidative phosphorylation, mitochondrial replication, mitochondrial transcription, mitochondrial translation, mitochondrial protein import, and mitochondrial proteolysis. Because thiols are readily oxidized and subject to irreversible loss by reaction with electrophiles, disruption of thiol redox systems provides a mechanism for mitochondrial dysfunction, which can occur in combination with mtDNA damage to contribute to mitochondria-related disease. Unlike damage to mtDNA, oxidized protein thiols are often readily reduced. Thus, the best information relating to the disruption of thiol redox systems in toxicity is derived from studies of protein modifications by reactive electrophiles.
4-Hydroxynonenal (HNE) is an endogenously generated electrophile derived from breakdown of lipid hydroperoxides, which is highly reactive to protein thiols and likely to play a central role in environmentally related mitochondrial dysfunction. A recent review provides a survey of the evidence for a role of HNE in disease [Mattson, 2009]; this review focuses on HNE in mitochondria from the perspective that HNE effects in mitochondria provide a prototype example of and endogenous reactive species which disrupts redox signaling and control. In the present discussion, we provide a brief description of the chemistry and metabolism of HNE with specific consideration of generation of HNE in mitochondria, identification of mitochondrial protein targets of HNE, and assessment of functional consequences of HNE generation. Specific examples of the relevance of this aldehyde to mitochondrial dysfunction are provided in terms of protein thiols and HNE protein adducts in the mitochondria. The review identifies specific needs to distinguish protein modification and mtDNA damage as critical events in environmental toxicities. In addition, the review indicates that application of mass spectrometry-based proteomic methods could improve identification of critical protein targets in mitochondrial dysfunction.
MITOCHONDRIAL FUNCTION, DYSFUNCTION, AND OXIDATIVE STRESS
Highly aerobic tissues rely on oxidative phosphorylation for ATP production and include muscle and nervous tissue, transport epithelia, steroid hormone producing cells, liver and kidneys. The overall process of oxidative phosphorylation is highly controlled and involves integration and balance of oxidation of fatty acids, pyruvate, ketoacids and a range of other intermediary metabolites, with the transport of bicarbonate, inorganic phosphate, ATP, ADP, and regulatory ions such as Ca2+. Mitochondrial dysfunction is most often considered in terms of alterations in electron flux through the mitochondrial electron transport chain (ETC) with increased oxidant production, decreased ATP production or failure of calcium regulation, but also can involve abnormal activation of apoptosis. The most active aerobic tissues are particularly susceptible to mitochondrial dysfunction [Wallace et al., 1997], and mitochondrial dysfunction is a hallmark of doxorubicin-mediated cardiotoxicity, diabetic neuropathy, neurodegenerative diseases, and alcoholic liver disease [Lin and Beal, 2006; Berthiaume and Wallace, 2007; Akude et al., 2009].
Acute mitochondrial failure occurs due to many natural toxins, which inhibit specific mitochondrial functions. In contrast, exposure to low doses of environmental agents often does not cause acute failure. Under conditions of low exposure, distinction between adaptive mitochondrial responses and mitochondrial dysfunction can be difficult. Changes in generation of oxidants may or may not be reflective of dysfunction. On the other hand, endogenously generated reactive electrophiles, such as conjugated aldehydes, irreversibly modify proteins and are not known to have any beneficial effects. Electrophiles are generated within cells by many processes, such as nitric oxide synthase-mediated nitric oxide production and biotransformation of xenobiotics to reactive intermediates; however, the decomposition of lipid hydroperoxides to yield reactive lipid aldehydes serves as an excellent example of endogenous reactive species generation and could provide a useful index for mitochondrial dysfunction.
REACTIVE SPECIES—4-HYDROXYNONENAL
The mitochondrion is a major site of cellular superoxide and hydrogen peroxide production, with most of this oxidant burden being derived from Complexes I and III of the ETC. Unlike NAD+ and NADP+-coupled electron transfer in which hydride ion transfer allows electrons to be transferred in pairs, electrons are transferred singly within the ETC. When the 1-electron carriers, especially the flavoproteins, hemoproteins, iron–sulfur proteins and ubiquinone/ubisemiquinone/ubiquinol, transfer 1 electron to O2, a free transition metal or a redox-cycling quinone, they produce free radicals. These free radicals are typically very short lived because the unpaired electrons are paired with another unpaired electron (e.g., superoxide dismutase and superoxide-nitric oxide reaction), abstract an electron from a free-radical scavenger such as vitamin E or ubiquinol or are rapidly transferred back into the ETC [Guidot et al., 1995].
Low levels of oxidants are produced due to normal cellular respiration, and higher rates of oxidant production can contribute to cell injury [Goossens et al., 1995; Garcia-Ruiz et al., 1997; Corda et al., 2001]. Although oxidants, including hydrogen peroxide, lipid hydroperoxides and probably superoxide, are produced as signaling molecules and have been extensively studied in extramitochondrial cell signaling pathways, it is unknown whether the constitutive oxidant production by the ETC serves a signaling function. On the other hand, numerous processes and agents are known to increase mitochondrial oxidant production. Some of these produce oxidants by redox cycling, like paraquat and doxorubicin, whereas others stimulate production by altering mitochondrial homeostasis, like ethanol and TNF-α [Bailey and Cunningham, 2002; Berthiaume and Wallace, 2007; Castello et al., 2007; Cocheme and Murphy, 2008]. The oxidants produced include lipid peroxides which are largely metabolized by GSH peroxidases; however, in the presence of a 1-electron donor, lipid peroxides undergo nonenzymatic rearrangement and scission to produce HNE [Esterbauer et al., 1991].
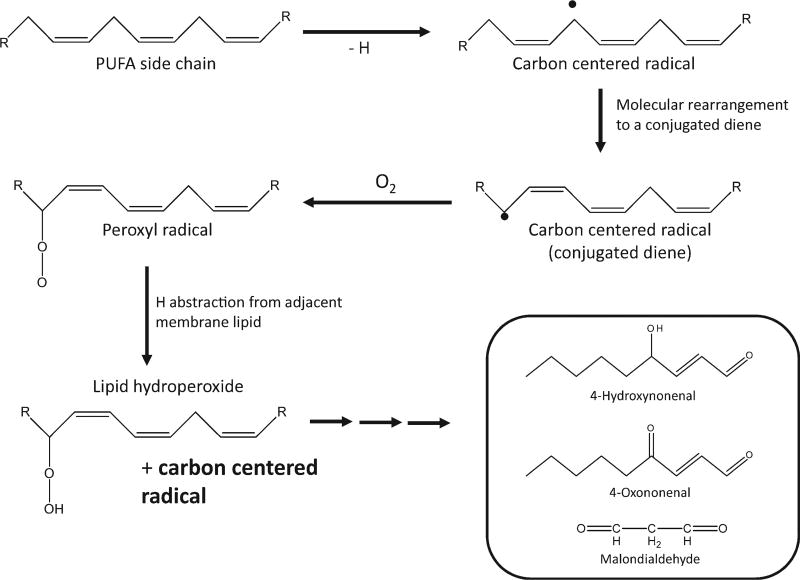
The overall process of lipid peroxidation can be broadly defined as the auto-oxidative deterioration of polyunsaturated fatty acids (PUFA). This process, depicted in Figure 1, is extensively implicated in the etiology of an extensive list of pathologies, some of which include cardiovascular disease, diabetes, and alcoholic liver disease [Wu and Cederbaum, 2003]. The primary products of the free-radical attack of biological membranes are lipid hydroperoxides, which can subsequently decompose to form toxic secondary end products. Lipid aldehydes, like malondialdehyde and HNE, are considered secondary end products of lipid peroxidation [Esterbauer et al., 1991]. It is perhaps important to note that there is little evidence for free-radical chain reactions occurring under usual biologic conditions, especially in mitochondria. An abundance of chemical targets and scavengers effectively limit propagation reactions so that secondary end products largely reflect rates of initiation.
Fig. 1.
4-HNE is derived from lipid hydroperoxides generated during the process of lipid peroxidation. Lipid peroxidation is initiated by a free-radical-mediated abstraction of a hydrogen from a bisallylic carbon of a PUFA. This forms a carbon centered radical, which rearranges to form a conjugated diene. Reaction of this radical with molecular oxygen results in a peroxyl radical, which, in the presence of an additional PUFA acyl chain, can abstract an additional hydrogen forming a lipid hydroperoxide.
HNE IS GENERATED NONENZYMATICALLY AND AS A CONSEQUENCE OF H2O2-DEPENDENT OXIDATION OF CARDIOLIPIN CATALYZED BY CYTOCHROME C
HNE is a common product of peroxidation of linoleic, arachadonic, and linolenic acid acyl chains [Esterbauer et al., 1991]. A characterization of the lipid composition of rat liver subcellular membranes found, compared with the plasma membrane, that the inner mitochondrial membrane (IMM) has a high concentration of these unsaturated fatty acid acyl chains. For instance, Colbeau et al. [1971] found that greater than 50% of the fatty acyl chains in the IMM were unsaturated. Approximately 16 and 19% of the acyl chains were found to be linoleic acid (18:2) and arachadonic acid (20:4), respectively. This unsaturated nature of the IMM renders it susceptible to attack by free radicals resulting in HNE production.
Mitochondria are also rich in cardiolipin (CL), which contributes greatly to the unsaturated nature of the IMM. CL, making up ~20–25% of the IMM, is a mitochondrial phospholipid that contains three glycerol backbones and four acyl chains. The acyl chain composition of CL is very diverse between species and tissues; however, humans and rodents contain primarily linoleic acid (18:2) side chains. For example, the relative abundance of this fatty acid in mouse CL ranges from ~60% in skeletal muscle to greater than 80% in the heart and liver [Houtkooper and Vaz, 2008].
The functional importance of CL is proposed to arise from its unique ability to interact with proteins and its role in maintaining membrane fluidity and osmotic stability. It has been reported that CL interacts with numerous proteins including all of the complexes of the ETC, adenine nucleotide transporter (ANT) and other mitochondrial transporters. A detailed list of CL-dependent mitochondrial proteins is available [Chicco and Sparagna, 2007]. Because of the interaction of this phospholipid with the complexes of the ETC and high PUFA content, CL is a potential target of oxidants produced by mitochondrial respiration. Using t-butyl hydroperoxide-treated mitochondria from rat heart, Paradies et al. [1998] showed a direct correlation between lipid peroxidation, decreased CL content and decreased cytochrome c oxidase activity. Sen et al. [2006], stimulating lipid peroxidation in rat brain mitochondria with an iron–ascorbate system, also found that increased lipid peroxidation (MDA/TBARS) correlated with a decrease in cardiolipin.
Additionally, CL has been shown to be peroxidized by cytochrome c. Kagan et al. [2005] have shown that, in the presence of hydrogen peroxide, cytochrome c can act as a CL oxygenase. This results in oxidation of CL, subsequent release of cytochrome c and the initiation of apoptosis. Finally, Barth syndrome provides direct evidence that changes in cardiolipin content result in pathology. Barth syndrome is an X-linked disease that is believed to result from a mutation in the TAZ gene, which encodes proteins involved in CL remodeling. This disease manifests as lethal cardioskeletal myopathy and loss of CL content is believed to be an etiological factor [Chicco and Sparagna, 2007].
HNE IS DETOXIFIED BY OXIDATION AND CONJUGATION WITH GSH
Detoxification pathways for HNE have been described in many tissues and cell types such as hepatocytes, hepatic stellate cells, Kupffer cells, thymocytes, synovial fibroblasts, and perfused rat heart and perfused rat kidney [Hartley et al., 1995; Grune et al., 1997; Ullrich et al., 1997; Siems et al., 1998; Srivastava et al., 1998; Reichard et al., 2000; Luckey and Petersen, 2001]. These studies show that both oxidative conversion of HNE to 4-hydroxynonenoic acid via aldehyde dehydrogenase (ALDH) and GST-mediated GSH conjugation are the primary metabolic fates. Compared with these studies that used whole cells to assess HNE metabolism, experiments using isolated mitochondria from both kidney and brain found that the oxidative products predominated while GSH-HNE conjugates were a very minor metabolite [Ullrich et al., 1994; Murphy et al., 2003; Honzatko et al., 2005]. For example, after a 3-min incubation with HNE only 10% of HNE administered can be found in the GSH-HNE pool of mitochondria from rat kidney. Additionally, ~8% of HNE exogenously administered resulted in protein adducts. Ullrich et al. [1994] demonstrated that the majority of the protein adducts were located in the inner membrane space; however, adducts were also detected in the IMM and matrix.
Together, the available data show that both radical initiation events and enzymatic catalysis by cytochrome c in the presence of H2O2 can lead to generation of HNE in mitochondria. The IMM is enriched with PUFAs, which are susceptible to oxidation and generate HNE upon decomposition. Detoxification of HNE in mitochondria occurs primarily via ALDH-mediated oxidation and GSH conjugation, but about 8% of the total HNE reacts with protein. Modification of critical proteins is expected to result in decreased function of the protein so that measurement of such modifications could provide a useful means to define and assess mitochondrial dysfunction.
IMPORTANCE OF PROTEIN THIOLS
The development of an understanding of redox signaling mechanisms and the failure of large-scale, double-blind interventional trials with free-radical scavenging antioxidants to provide significant improvements in health outcomes has led to a revised definition of oxidative stress. The earlier concept of oxidative stress as an imbalance of prooxidants and antioxidants [Sies and Cadenas, 1985] has been replaced by a broader definition [Jones, 2006b], which includes two different types of oxidative stress, one due to free radical mechanisms resulting in macromolecular damage and the other due to nonradical oxidants, which disrupt redox signaling and control mechanisms. In quantitative terms, the latter appears to predominate under most biologic conditions [Jones, 2008].
Protein thiols are the most common and sensitive targets of nonradical oxidants. The human genome encodes proteins with 214,000 distinct Cys residues [Jones, 2008]. These Cys residues exist in different forms, e.g., as disulfides, as free thiols and as thiols stabilized by interaction with Zn2+. The thiols have considerable variability in reactivity due to the characteristics of the protein domain. For instance, Wong and Liebler [2008] compared reactivities of mitochondrial proteins with iodoacetamide and maleimide and found that only 438 cysteine sites in 1,255 cysteinyl peptide adducts (35%) and 362 of the 809 identified protein targets (45%) were adducted by both electrophiles. Consequently, it is readily apparent that different oxidants and electrophiles will be selective in targeting specific proteins.
Mitochondrial proteins are likely to have an enhanced vulnerability to such attack because of the alkaline pH. The ionized (thiolate) forms of Cys are more reactive and thiols are more ionized at higher pH values. Thus an important generalization is that mitochondrial proteins in an alkaline environment are inherently more vulnerable to modification by electrophiles. Despite this vulnerability, Cys residues are critical components of protein machinery for oxidative phosphorylation, mitochondrial replication, mitochondrial transcription, mitochondrial translation, mitochondrial protein import, and mitochondrial proteolysis.
Two thiol antioxidant systems are present in mitochondria and depend, respectively, on GSH and thioredoxin-2 (Trx2). These systems are parallel and nonredundant in their functions [Zhang et al., 2007]. The GSH system involves protein S-glutathionylation/deglutathionylation by glutaredoxin-2 as well as multiple GSH peroxidase and GSH transferase reactions [Go and Jones, 2008]. The Trx2 system depends on thioredoxin reductase-2 and peroxiredoxin-3 and −5 [Arner and Holmgren, 2000; Rhee et al., 2005]. Importantly, although dozens to hundreds of reactive thiols are present in mitochondrial proteins, detailed mechanistic research has only recently begun to organize these into functional redox pathways. The expectation is that delineation of the relevant redox signaling and control pathways will provide the basis to understand critical targets of electrophiles, which cause mitochondrial dysfunction.
IMPACT OF HNE PROTEIN ADDUCTION
As stated by Esterbauer et al. [1991] in their landmark 1991 review on the topic of the chemistry and biochemistry of HNE, “unlike free radicals, aldehydes are rather long lived and can therefore diffuse from the side of their origin (i.e., membranes) and reach and attack targets intracellularly and extracellularly, which are distant from the initial radical event.” Being a strong electrophile, HNE has demonstrated the propensity to modify cellular targets like phospholipids, DNA, and proteins [Marnett et al., 2003; Petersen and Doorn, 2004; Bacot et al., 2007]. Oxidatively modified mitochondrial proteins, HNE-adducts and protein carbonyls, have been observed in vivo. For example, increased mitochondrial HNE-protein adducts were observed in a rat models of chronic ethanol consumption, diabetic cardiomyopathy, and traumatic brain injury [Carbone et al., 2005; Lashin et al., 2006; Opii et al., 2007].
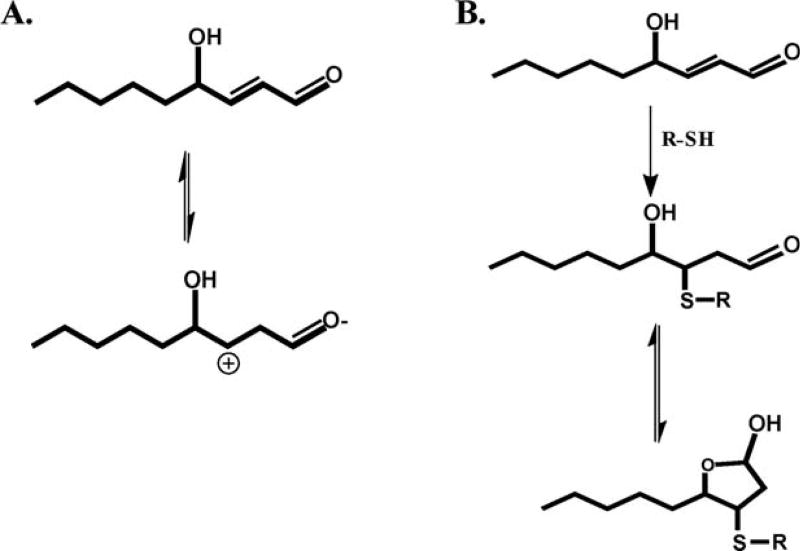
This α,β-unsaturated aldehyde, contains three different functional groups: a hydroxyl group on carbon 4, C—C double bond between carbons 2 and 3, and the aldehyde moiety on carbon 1. Carbon 3 is an electrophilic position given the inductive effect of the carbonyl oxygen (at carbon 1) and the resulting resonance structures (due to rearrangement as shown in Fig. 2A). The electrophilicity and partial positive charge of carbon 3 gives rise to the observed 1,4-Michael addition. Although mentioned in the article of Witz [1989], the influence of the hydroxyl group on reactivity and electrophilicity of carbon 3 is of some question given that 4-hydroxy alkenals are only twofold at most, more reactive than the corresponding alkenal without the hydroxyl group. In other words, the hydroxyl group probably does not significantly polarize the alkene group. In aqueous solution, HNE reacts with the side chains of cysteine, histidine, and lysine with an order of reactivity: Cys >> His > Lys. Based on the rate constants, free thiols have been clearly demonstrated to be the most reactive nucleophile toward HNE (Fig. 2B) [Petersen and Doorn, 2004]. As shown in Figure 2B, HNE can undergo a secondary reaction to cyclize forming a hemiacetal. This cyclization is due increased rotation at the C2—C3 bond and equilibrium favors the formation of this cyclized product [Schaur, 2003; Petersen and Doorn, 2004].
Fig. 2.
4-HNE contains a reactive center located on carbon 3 (A). This reactive center can then form a Michael addition product with a protein thiol (B).
As previously mentioned, mitochondrial thiol residues can be particularly vulnerable to HNE adduction due to the conditions present. For instance, the pKa values of protein thiols are typically in the range of 8–8.5. The pH of the mitochondria is more basic compared with that of the cytosol (~pH 8 vs. ~pH 7.2), which would render mitochondrial thiol residues more reactive [Lin et al., 2002]. Additionally, due to the lipophilic properties of HNE, this aldehyde can accumulate in biological membranes. For example, it has been estimated in nonmitochondrial studies that local concentrations of aldehyde in peroxidizing membranes can reach millimolar concentrations [Benedetti et al., 1984]. Therefore, membrane proteins are excellent targets for modification by lipophilic electrophiles like HNE.
The adenine nucleotide translocator (ANT) is an essential mitochondrial protein affected by lipid peroxidation products. Located in the IMM, this protein is responsible for the translocation of ADP and ATP across the inner membrane. Compromised ANT activity has been observed in aging and other pathologies involving oxidative stress [Yan and Sohal, 1998]. Chen et al. [1995] found that HNE significantly decreases ANT activity and that the drop in activity directly correlates to a loss in protein sulfhydryls. Cytochrome c oxidase is another example of a membrane protein shown to be affected by HNE modification.
Cytochrome c oxidase is the terminal component (complex IV) of the ETC. This protein catalyzes electron transfer from cytochrome c to molecular oxygen, generating a proton gradient required for ATP synthesis. Chen et al. [1998, 2000] have demonstrated that complex IV is a target for reactive aldehydes in vivo. They have also shown that HNE modification results in inhibition of cytochrome c oxidase activity. This protein contains copper centers which are important redox factors involved in electron transfer from cytochrome c to O2. The machinery involved in cytochrome c oxidase biogenesis and copper incorporation utilize redox sensitive Cys residues. These residues are involved in complex redox reactions as well as copper binding and transfer. A copper chaperone involved in this process, Sco1, contains a thioredoxin-like CX3C motif and this sequence has been shown previously to be essential for proper function [Rentzsch et al., 1999]. Cox17p is also involved in copper transfer and this protein employs six conserved redox sensitive Cys residues. All of these redox active Cys residues are potential targets for HNE adduction and it has been documented that defective cytochrome c oxidase biogenesis results in mitochondrial dysfunction frequently involving brain, skeletal muscle, and heart [Horn and Barrientos, 2008].
In addition to the membrane components of oxidative phosphorylation, proteins that function in the TCA cycle have also been demonstrated to be targets of HNE modification. In intact cardiac mitochondria, HNE inhibits NADH-linked respiration by reducing the steady state levels of NADH. Humphries et al. [1998] found that HNE inhibited the activity of α-ketoglutarate dehydrogenase, an enzyme that is integral in the production of NADH, in a concentration dependent manner. Succinate dehydrogenase has also been shown to be adducted by HNE in vivo. This protein is involved in the TCA cycle by catalyzing the oxidation of succinate to fumarate [Dykens and Will, 2008]. Using a rat model of drug-induced diabetes, Lashin et al. [2006] demonstrated HNE modification of succinate dehydrogenase using affinity chromatography and immunoblotting. The site of modification was reported to be the FAD-containing subunit and in vitro assessment of activity showed HNE-mediated inhibition.
In addition to energy supply component, HNE inactivates NADP+-dependent isocitrate dehydrogenase. Mammalian tissues possess two different forms of this protein, cytosolic and mitochondrial. The mitochondrial isoform predominates in the heart and is confined to cardiomyocytes. Both isoforms catalyze the reversible interconversion between isocitrate and α-ketoglutarate [Benderdour et al., 2003]. Along with the proton translocating, nicotinamide nucleotide transhydrogenase, NADP+-dependent isocitrate dehydrogenase is a major system involved in the regeneration of NADPH in the mitochondria [Orrenius et al., 2007]. The activity of this enzyme is decreased compared with controls in spontaneously hypertensive rats. Progression of cardiac disease is typically associated with increased oxidative stress and lipid peroxidation; therefore, Benderdour et al. investigated the in vitro significance of HNE modification of NADP+-dependent isocitrate dehydrogenase. These investigators found that HNE inhibits this protein in a concentration dependent manner and that the mechanism of inactivation might be cysteine specific [Benderdour et al., 2003].
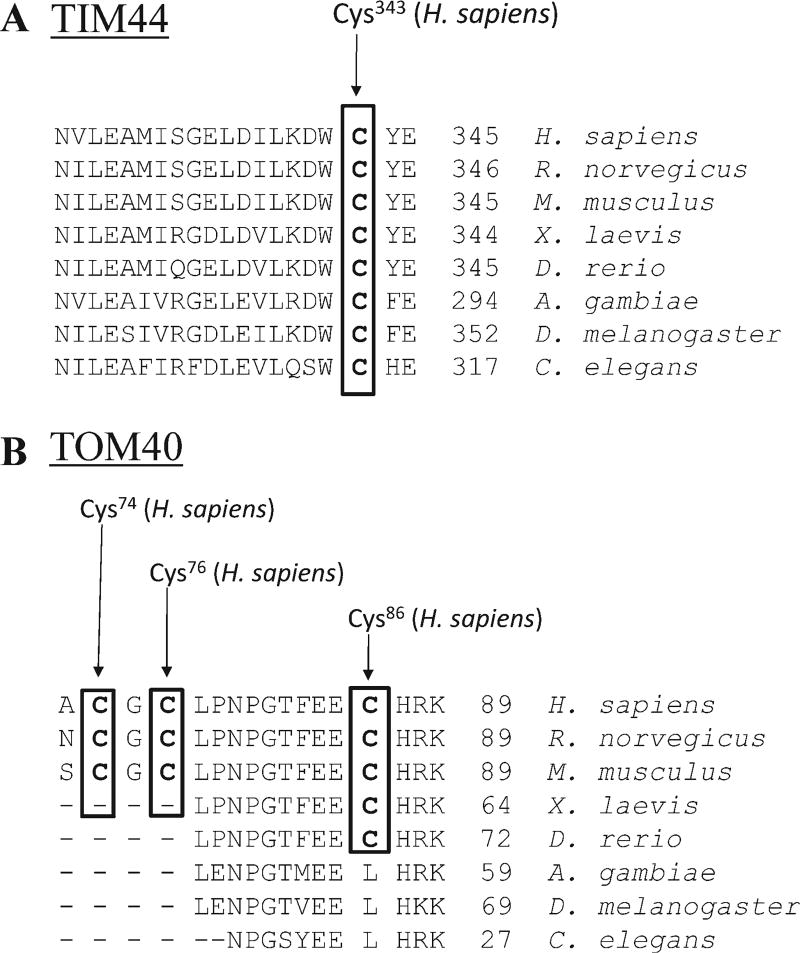
Although mitochondria contain DNA and the machinery for transcription and translation, only about 1% of all mitochondrial proteins are encoded by the mitochondrial genome. Because of this, the majority of the mitochondrial proteome needs to be transported into the organelle. The import of proteins targeted to mitochondria is performed by specific translocases that aid in recognition, translocation, and membrane insertion of precursor proteins [Wiedemann et al., 2004]. Proteins involved in mitochondrial protein import include the translocase of the outer mitochondrial membrane complex (TOM complex) and the translocase of the IMM (TIM23 complex) [Wiedemann et al., 2004]. Both of these processes employ cysteine-rich proteins involved in redox-sensitive functions. Also, many intermembrane space proteins contain conserved cysteine residues and are involved in cofactor and metal binding and the formation of disulfide bonds [Stojanovski et al., 2008]. Interestingly, constituents of these translocases are susceptible to modification by electrophiles. In particular, Wong and Liebler used model electrophiles to identify both protein targets and their sensitive cysteine residues in vitro. Their results revealed two TIM proteins (TIM44 and TIM50) and one TOM protein (TOM40) as adducted by electrophiles [Wong and Liebler, 2008]. It is important to note that the model electrophile used for these studies, BMCC, is a Michael acceptor whose chemistry mimics that of α,β-unsaturated lipid oxidation products, like HNE. Additionally, the identified cysteine residues correspond to conserved residues as evidenced in Figure 3, indicating evolutionally conserved importance.
Fig. 3.
Sequence alignment of TIM44 (A) and TOM40 (B) illustrate conserved cysteine residues across multiple species. The cysteine residues highlighted by bold type and boxed were found by Wong and Liebler [2008] to be modified by the model electrophile BMCC.
Also, biogenesis of iron–sulfur proteins is a mitochondrial process that involves critical cysteine residues. These residues are essential for proper assembly of the scaffold that orients the iron–sulfur center. Although much is known about bacterial and eukaryotic iron–sulfur cluster biogenesis, data regarding specific mammalian mechanisms are still lacking [Lill and Muhlenhoff, 2008]. To summarize, modification of the chaperones and protein import machinery is likely to provide a general mechanism which impairs protein–protein interactions necessary for proper import and folding of matrix or IMM proteins.
Approximately 14% of the mitochondrial proteome is involved in oxidative phosphorylation, whereas 25% of the mitochondrial proteins are predicted to be involved in maintaining and expressing the mitochondrial genome [Khalimonchuk and Winge, 2008]. The mitochondrial ribosomal proteins contain conserved Cys residues and are also encoded in the nuclei, so mitochondrial translation is likely to be sensitive to electrophiles. Proteins involved in replication and repair of mtDNA are encoded by nuclear genes and these proteins must be imported into mitochondria. Therefore, if the import apparatus is impaired, repair of mtDNA damage may be affected resulting in altered expression of mitochondrially encoded genes and mitochondrial dysfunction.
Finally, it is estimated that ~1% of all mammalian genes encode for zinc finger proteins. This class of proteins is involved in DNA–protein and protein–protein interactions. These proteins utilize cysteine and/or histidine residues to complex with zinc atoms. This complex aids in protein folding to facilitate DNA and protein interactions. Zinc finger proteins include those involved in gene transcription, steroid hormone receptors, DNA repair, cell cycle control, and protein degradation [Hartwig, 2001]. Zn-S Cys sites in proteins are reactive and oxidants like GSSG, nitric oxide, and aldehydes can release zinc from proteins [Hao and Maret, 2006]. For example, Carbone et al. [2004] have demonstrated that HNE will modify cysteine residues involved in zinc chelation of alcohol dehydrogenase in vitro. Additionally, Rothfuss et al. [2009] showed that parkin, a RING finger containing protein, protects mtDNA from oxidative damage and supports mitochondrial dysfunctionNA repair. Interestingly, cysteine residues of parkin can be modified by dopamine quinone, a reactive intermediate of dopamine metabolism, resulting in loss of activity and aggregation [LaVoie et al., 2005; Wong et al., 2007]. HNE has a similar reactivity and may therefore exacerbate effects caused by other reactive species.
To conclude, mitochondrial HNE-protein adducts have been observed in multiple disease states. Proteins found to be adducted were those involved in the ETC, cellular respiration, and TCA cycle. Examples of these proteins include the ANT, cytochrome c oxidase, and succinate dehydrogenase. Additionally, proteins involved in mitochondrial protein import are susceptible to modification by reactive electrophiles and alteration of this import process is likely to have deleterious effects on mitochondrial function. Finally, zinc containing proteins are involved in DNA binding and protein–protein interactions. Reactive cysteine residues are involved in chelation of these metal ions and modification of these residues has been demonstrated. This modification would in turn lead to altered DNA binding and/or protein misfolding.
PERSPECTIVES
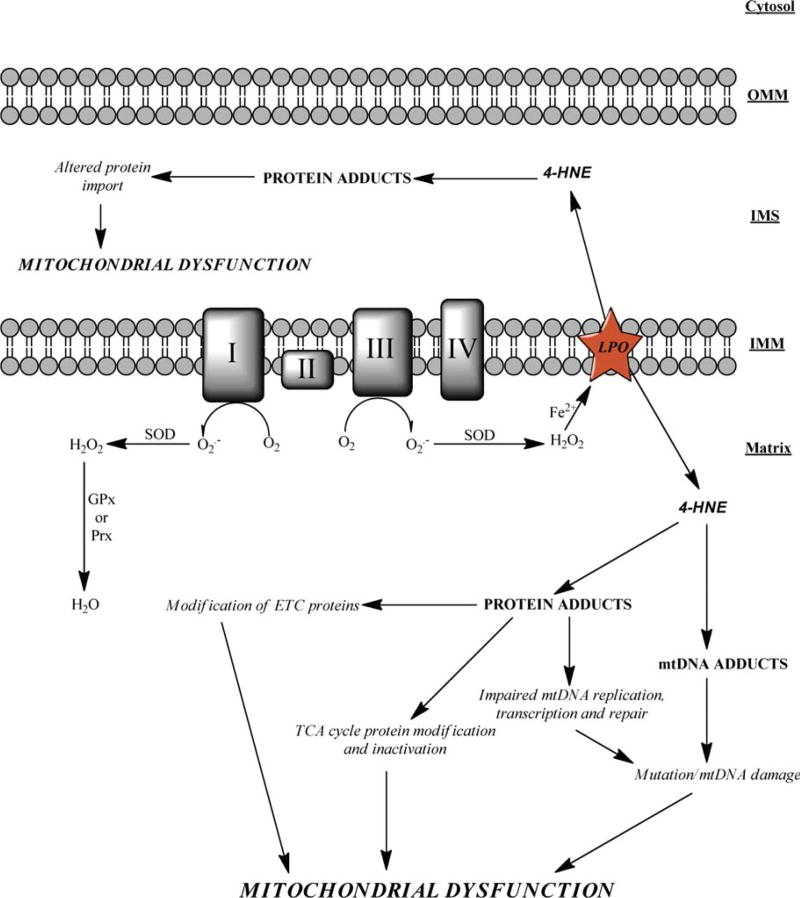
Mitochondrial dysfunction is a hallmark of many disease states like alcoholic liver disease and neurodegenerative diseases. A potential etiological factor of this dysfunction is the production of reactive electrophiles, which include species like nitric oxide, xenobiotics, and lipid peroxidation products. HNE was chosen as a prototypic endogenously generated electrophile to address how reactive species can affect mitochondrial function. The mitochondrion is primed for attack by reactive aldehydes because of its capacity for oxidant production, the abundance of PUFAs in the IMM, and the alkaline pH of the matrix. An increase in mitochondrial protein adduction by HNE has been observed in various disease states and proteins found to be adducted include those clearly required for mitochondrial energy supply. Furthermore, components of the mitochondrial protein import system are modified by electrophiles, demonstrating that agents such as HNE are likely to cause an insidious mitochondrial dysfunction (Figure 4).
Fig. 4.
A general schematic representation demonstrating how 4-HNE can cause mitochondrial dysfunction is shown. ROS produced within the mitochondrion can result in lipid peroxidation and 4-HNE. This aldehyde can diffuse into the matrix and/or the IMS resulting in protein modification. It is this protein modification that causes altered protein activity resulting in mitochondrial dysfunction. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Not addressed in this review, HNE can form adducts on DNA bases resulting in exocyclic adducts, such as etheno adducts, and this is a subject worthy of additional study relevant to mitochondrial dysfunction. These etheno adducts result in base mispairing and are mutagenic in bacterial and mammalian cells [Chung et al., 1996; Marnett et al., 2003]. While HNE reacts less readily with DNA when compared with protein reactivity, exposure of single-stranded phage DNA to HNE resulted in inhibition of DNA replication [Singh et al., 2005]. Additionally, adducts of the N2 group of guanine are found to be highly inhibitory to DNA synthesis by replicative DNA polymerases [Wolfle et al., 2006]. HNE-DNA adducts have been pointed to as etiological factors for human cancers that show a mutation for p53 at codon 249 and exocyclic HNE-DNA adducts have been proposed as oxidative stress markers in colon carcinogenesis [Poli et al., 2008]. While the aforementioned examples of DNA adducts refer to nuclear DNA, it is very plausible that mtDNA can accumulate HNE modified bases, which can lead to mutation. Factors that favor adduction of mtDNA by HNE are mitochondrial ROS generation, high concentration of PUFAs in the IMM, lack of histones, and proximity of mtDNA to the ETC and membrane.
Additional proteomic investigations are needed to identify mitochondrial proteins that are modified by HNE in diseases like alcoholic liver disease, diabetes, and neurodegenerative diseases. All of these pathologies have implicated both HNE and mitochondrial dysfunction as etiological factors. Two-dimensional electrophoresis techniques coupled with immunodetection of adducts and mass spectrometry can be utilized to achieve this objective. Two-dimensional liquid chromatography coupled to MS/MS proteomic approaches can also be employed to assess specific cysteine modifications. These approaches include redox DIGE and ICAT procedures [Hurd et al., 2007; Fu et al., 2008]. Redox–Western blots, which allow sensitive detection of specific modification of known proteins, can be used to detect modified proteins [Chen et al., 2008]. Such a targeted approach, as opposed to a global survey, can be utilized to investigate if HNE adducts are present on specific proteins involved in mtDNA replication, transcription, translation, and mitochondrial protein import.
Finally, proteins are the machinery that carry out all of the biological processes necessary for survival. As reported in many published manuscripts, HNE-protein adducts typically alter the function of the target proteins. Therefore, metabolomic profiling and fingerprinting of both systemic and compartmental changes will provide considerable information concerning function. In particular, changes in metabolite profiles may indicate that a particular metabolic pathway is affected by a disease or drug treatment, and discrimination of adaptive and maladaptive responses will ultimately clarify the key events of environmental toxicities related to mitochondrial dysfunction. In addition to HNE effects on proteins, novel HNE metabolite biomarker discovery could also be conducted via these techniques. General HNE metabolites can be detected in the urine and bile of laboratory animals [Alary et al., 2003]; however, specific, nonprotein biomarkers of mitochondrial HNE production are lacking. Because of the abundance of certain thiol-containing cofactors in mitochondria, like coenzyme A and dihydrolipoate, detection of HNE adducts of these species could potentially signal mitochondrial dysfunction and oxidative stress and provide clinical biomarkers to predict disease onset and/or outcome.
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com).
References
- Akude E, Zherebitskaya E, Roy Chowdhury SK, Girling K, Fernyhough P. 4-Hydroxy-2-nonenal induces mitochondrial dysfunction and aberrant axonal outgrowth in adult sensory neurons that mimics features of diabetic neuropathy. Neurotox Res. 2009;17:28–38. doi: 10.1007/s12640-009-9074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alary J, Gueraud F, Cravedi JP. Fate of 4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol Aspects Med. 2003;24:177–187. doi: 10.1016/s0098-2997(03)00012-8. [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Bacot S, Bernoud-Hubac N, Chantegrel B, Deshayes C, Doutheau A, Ponsin G, Lagarde M, Guichardant M. Evidence for in situ ethanolamine phospholipid adducts with hydroxy-alkenals. J Lipid Res. 2007;48:816–825. doi: 10.1194/jlr.M600340-JLR200. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Benderdour M, Charron G, DeBlois D, Comte B, Des Rosiers C. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: An event that precedes hypertrophy development. J Biol Chem. 2003;278:45154–45159. doi: 10.1074/jbc.M306285200. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Comporti M, Fulceri R, Esterbauer H. Cytotoxic aldehydes originating from the peroxidation of liver microsomal lipids. Identification of 4,5-dihydroxydecenal. Biochim Biophys Acta. 1984;792:172–181. doi: 10.1016/0005-2760(84)90219-4. [DOI] [PubMed] [Google Scholar]
- Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23:15–25. doi: 10.1007/s10565-006-0140-y. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Petersen DR. 4-Hydroxynonenal regulates 26S proteasomal degradation of alcohol dehydrogenase. Free Radic Biol Med. 2004;37:1430–1439. doi: 10.1016/j.freeradbiomed.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem Res Toxicol. 2005;18:1324–1331. doi: 10.1021/tx050078z. [DOI] [PubMed] [Google Scholar]
- Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–14193. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Bertrand H, Yu BP. Inhibition of adenine nucleotide translocator by lipid peroxidation products. Free Radic Biol Med. 1995;19:583–590. doi: 10.1016/0891-5849(95)00066-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Schenker S, Frosto TA, Henderson GI. Inhibition of cytochrome c oxidase activity by 4-hydroxynonenal (HNE). Role of HNE adduct formation with the enzyme subunits. Biochim Biophys Acta. 1998;1380:336–344. doi: 10.1016/s0304-4165(98)00002-6. [DOI] [PubMed] [Google Scholar]
- Chen J, Petersen DR, Schenker S, Henderson GI. Formation of malondialdehyde adducts in livers of rats exposed to ethanol: Role in ethanol-mediated inhibition of cytochrome c oxidase. Alcohol Clin Exp Res. 2000;24:544–552. [PubMed] [Google Scholar]
- Chen Y, Go YM, Pohl J, Reed M, Cai J, Jones DP. Increased mitochondrial thioredoxin 2 potentiates N-ethylmaleimide-induced cytotoxicity. Chem Res Toxicol. 2008;21:1205–1210. doi: 10.1021/tx800012p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- Colbeau A, Nachbaur J, Vignais PM. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971;249:462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Will Y, editors. Drug-Induced Mitochondrial Dysfunction. Hoboken: John Wiley; 2008. p. 616. [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fu C, Hu J, Liu T, Ago T, Sadoshima J, Li H. Quantitative analysis of redox-sensitive proteome with DIGE and ICAT. J Proteome Res. 2008;7:3789–3802. doi: 10.1021/pr800233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Park H, Koval M, Orr M, Reed M, Liang Y, Smith D, Pohl J, Jones DP. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic Biol Med. 2010;48:275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens V, Grooten J, de Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grune T, Siems WG, Petras T. Identification of metabolic pathways of the lipid peroxidation product 4-hydroxynonenal in in situ perfused rat kidney. J Lipid Res. 1997;38:1660–1665. [PubMed] [Google Scholar]
- Guidot DM, Repine JE, Kitlowski AD, Flores SC, Nelson SK, Wright RM, McCord JM. Mitochondrial respiration scavenges extramitochondrial superoxide anion via a nonenzymatic mechanism. J Clin Invest. 1995;96:1131–1136. doi: 10.1172/JCI118100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, Maret W. Aldehydes release zinc from proteins. A pathway from oxidative stress/lipid peroxidation to cellular functions of zinc. FEBS J. 2006;273:4300–4310. doi: 10.1111/j.1742-4658.2006.05428.x. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Ruth JA, Petersen DR. The hepatocellular metabolism of 4-hydroxynonenal by alcohol dehydrogenase, aldehyde dehydrogenase, and glutathione S-transferase. Arch Biochem Biophys. 1995;316:197–205. doi: 10.1006/abbi.1995.1028. [DOI] [PubMed] [Google Scholar]
- Hartwig A. Zinc finger proteins as potential targets for toxic metal ions: Differential effects on structure and function. Antioxid Redox Signal. 2001;3:625–634. doi: 10.1089/15230860152542970. [DOI] [PubMed] [Google Scholar]
- Honzatko A, Brichac J, Murphy TC, Reberg A, Kubatova A, Smoliakova IP, Picklo MJ., Sr Enantioselective metabolism of trans-4-hydroxy-2-nonenal by brain mitochondria. Free Radic Biol Med. 2005;39:913–924. doi: 10.1016/j.freeradbiomed.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Horn D, Barrientos A. Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life. 2008;60:421–429. doi: 10.1002/iub.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–2506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries KM, Yoo Y, Szweda LI. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry. 1998;37:552–557. doi: 10.1021/bi971958i. [DOI] [PubMed] [Google Scholar]
- Hurd TR, Prime TA, Harbour ME, Lilley KS, Murphy MP. Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis: Implications for mitochondrial redox signaling. J Biol Chem. 2007;282:22040–22051. doi: 10.1074/jbc.M703591200. [DOI] [PubMed] [Google Scholar]
- Imhoff BR, Hansen JM. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chem Biol Interact. 2006a;163:38–53. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006b;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- Khalimonchuk O, Winge DR. Function and redox state of mitochondrial localized cysteine-rich proteins important in the assembly of cytochrome c oxidase. Biochim Biophys Acta. 2008;1783:618–628. doi: 10.1016/j.bbamcr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashin OM, Szweda PA, Szweda LI, Romani AM. Decreased complex II respiration and HNE-modified SDH subunit in diabetic heart. Free Radic Biol Med. 2006;40:886–896. doi: 10.1016/j.freeradbiomed.2005.10.040. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- Lill R, Muhlenhoff U. Maturation of iron-sulfur proteins in eukaryotes: Mechanisms, connected processes, and diseases. Annu Rev Biochem. 2008;77:669–700. doi: 10.1146/annurev.biochem.76.052705.162653. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lin TK, Hughes G, Muratovska A, Blaikie FH, Brookes PS, Darley-Usmar V, Smith RA, Murphy MP. Specific modification of mitochondrial protein thiols in response to oxidative stress: A proteomics approach. J Biol Chem. 2002;277:17048–17056. doi: 10.1074/jbc.M110797200. [DOI] [PubMed] [Google Scholar]
- Luckey SW, Petersen DR. Metabolism of 4-hydroxynonenal by rat Kupffer cells. Arch Biochem Biophys. 2001;389:77–83. doi: 10.1006/abbi.2001.2307. [DOI] [PubMed] [Google Scholar]
- Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA, protein. J Clin Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TC, Amarnath V, Picklo MJ., Sr Mitochondrial oxidation of 4-hydroxy-2-nonenal in rat cerebral cortex. J Neurochem. 2003;84:1313–1321. doi: 10.1046/j.1471-4159.2003.01628.x. [DOI] [PubMed] [Google Scholar]
- Opii WO, Nukala VN, Sultana R, Pandya JD, Day KM, Merchant ML, Klein JB, Sullivan PG, Butterfield DA. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J Neurotrauma. 2007;24:772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: Implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Peroxidative damage to cardiac mitochondria: Cytochrome oxidase and cardiolipin alterations. FEBS Lett. 1998;424:155–158. doi: 10.1016/s0014-5793(98)00161-6. [DOI] [PubMed] [Google Scholar]
- Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-hydroxynonenal: A membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- Reichard JF, Vasiliou V, Petersen DR. Characterization of 4-hydroxy-2-nonenal metabolism in stellate cell lines derived from normal and cirrhotic rat liver. Biochim Biophys Acta. 2000;1487:222–232. doi: 10.1016/s1388-1981(00)00095-0. [DOI] [PubMed] [Google Scholar]
- Rentzsch A, Krummeck-Weiss G, Hofer A, Bartuschka A, Ostermann K, Rodel G. Mitochondrial copper metabolism in yeast: Mutational analysis of Sco1p involved in the biogenesis of cytochrome c oxidase. Curr Genet. 1999;35:103–108. doi: 10.1007/s002940050438. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chae HZ, Kim K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Rothfuss O, Fischer H, Hasegawa T, Maisel M, Leitner P, Miesel F, Sharma M, Bornemann A, Berg D, Gasser T, Patenage N. Parkin protects mitochondrial genome integrity and supports mitochondrial DNA repair. Hum Mol Genet. 2009;18:3832–3850. doi: 10.1093/hmg/ddp327. [DOI] [PubMed] [Google Scholar]
- Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Sen T, Sen N, Tripathi G, Chatterjee U, Chakrabarti S. Lipid peroxidation associated cardiolipin loss and membrane depolarization in rat brain mitochondria. Neurochem Int. 2006;49:20–27. doi: 10.1016/j.neuint.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Siems WG, Pimenov AM, Esterbauer H, Grune T. Metabolism of 4-hydroxynonenal, a cytotoxic lipid peroxidation product, in thymocytes as an effective secondary antioxidative defense mechanism. J Biochem. 1998;123:534–539. doi: 10.1093/oxfordjournals.jbchem.a021969. [DOI] [PubMed] [Google Scholar]
- Sies H, Cadenas E. Oxidative stress: Damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- Singh SP, Chen T, Chen L, Mei N, McLain E, Samokyszyn V, Thaden JJ, Moore MM, Zimniak P. Mutagenic effects of 4-hydroxynonenal triacetate, a chemically protected form of the lipid peroxidation product 4-hydroxynonenal, as assayed in L5178Y/ Tk +/− mouse lymphoma cells. J Pharmacol Exp Ther. 2005;313:855–861. doi: 10.1124/jpet.104.080754. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Chandra A, Wang LF, Seifert WE, Jr, DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovski D, Muller JM, Milenkovic D, Guiard B, Pfanner N, Chacinska A. The MIA system for protein import into the mitochondrial intermembrane space. Biochim Biophys Acta. 2008;1783:610–617. doi: 10.1016/j.bbamcr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Grune T, Henke W, Esterbauer H, Siems WG. Identification of metabolic pathways of the lipid peroxidation product 4-hydroxynonenal by mitochondria isolated from rat kidney cortex. FEBS Lett. 1994;352:84–86. doi: 10.1016/0014-5793(94)00922-8. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Huser H, Ehrlich W, Grune T. Intracellular metabolism of 4-hydroxynonenal in primary cultures of rabbit synovial fibroblasts. Free Radic Biol Med. 1997;22:1153–1157. doi: 10.1016/s0891-5849(96)00496-0. [DOI] [PubMed] [Google Scholar]
- Wallace KB, Eells JT, Madeira VM, Cortopassi G, Jones DP. Mitochondria-mediated cell injury. Symposium overview. Fundam Appl Toxicol. 1997;38:23–37. doi: 10.1006/faat.1997.2320. [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Frazier AE, Pfanner N. The protein import machinery of mitochondria. J Biol Chem. 2004;279:14473–14476. doi: 10.1074/jbc.R400003200. [DOI] [PubMed] [Google Scholar]
- Witz G. Biological interactions of alpha,beta-unsaturated aldehydes. Free Radic Biol Med. 1989;7:333–349. doi: 10.1016/0891-5849(89)90137-8. [DOI] [PubMed] [Google Scholar]
- Wolfle WT, Johnson RE, Minko IG, Lloyd RS, Prakash S, Prakash L. Replication past a trans-4-hydroxynonenal minor-groove adduct by the sequential action of human DNA polymerases iota and kappa. Mol Cell Biol. 2006;26:381–386. doi: 10.1128/MCB.26.1.381-386.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HL, Liebler DC. Mitochondrial protein targets of thiol-reactive electrophiles. Chem Res Toxicol. 21:796–804. doi: 10.1021/tx700433m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ES, Tan JM, Wang C, Zhang Z, Tay SP, Zaiden N, Ko HS, Dawson VL, Dawson TM, Lim KL. Relative sensitivity of parkin and other cysteine-containing enzymes to stress-induced solubility alterations. J Biol Chem. 2007;282:12310–12318. doi: 10.1074/jbc.M609466200. [DOI] [PubMed] [Google Scholar]
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27:277–284. [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Sohal RS. Mitochondrial adenine nucleotide translocase is modified oxidatively during aging. Proc Natl Acad Sci USA. 1998;95:12896–12901. doi: 10.1073/pnas.95.22.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Go YM, Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]