Abstract
Fluoroquinolones are usually well tolerated with a minimum of serious adverse effects; renal toxicity is uncommon. Apart from the renal side effects of ciprofloxacin, we aimed to highlight the renal impact of a ciprofloxacin overdose, and thus conducted a prospective study in the Department of Nephrology at La Rabta Hospital between 2010 and 2015. The cohort database was continually updated until the inclusion of five patients who were subjected to an overdose and who were initially admitted to the medical intensive care unit and then transferred to our department for acute renal failure (ARF) due to ciprofloxacin ingestion requiring urgent hemodialysis. All patients developed ARF after 12–36 h of ingestion. Renal ultrasound was normal in all cases. Twenty-four-hour proteinuria was present but not significant in one case, while microscopic hematuria was present in one case. Treatment consisted of supportive therapy and extrarenal purification by conventional intermittent hemodialysis. Four patients recovered normal renal function within 3 weeks and the remaining patient eventually had chronic kidney failure.
Key Points
| Drug-induced nephrotoxicity is one of the leading causes of acute kidney injury worldwide. Nephrotoxicity of ciprofloxacin is often underestimated. |
| In addition to causing acute kidney injury, chronic drug toxicity can in some cases lead to chronic kidney disease and eventually end-stage renal disease. Thus, the prevention of ciprofloxacin nephrotoxicity should be at the forefront of the approaches employed to counteract drug-induced kidney failure. |
| We have specifically highlighted ciprofloxacin overdose through our cases, with an updated review of the literature. |
Background
Nephrotoxic reactions to ciprofloxacin appear to be unusual but potentially serious. It has previously been reported that fluoroquinolones could cause acute renal failure (ARF) after the ingestion of large quantities, but it is now recognized that therapeutic doses of fluoroquinolones can also cause renal injury. Allergic interstitial nephritis (AIN) is thought to be the most common cause and is attributed to hypersensitivity reaction type III [1], while a ciprofloxacin overdose often causes acute tubular necrosis (ATN; the normal dose range for ciprofloxacin is between 500 and 750 mg/12 h). An improvement in renal function that followed discontinuation of the offending antibiotic supports the presumptive diagnosis of ciprofloxacin-induced ARF; however, as there were few articles on this topic, our study was devoted to emphasizing the renal effects of a ciprofloxacin overdose and to an overview of the literature regarding ciprofloxacin intoxication.
Patients and Methods
This study was conducted in the Department of Nephrology at La Rabta Hospital between 2010 and 2015. The cohort database was continually updated until the inclusion of five patients admitted from the medical intensive care unit to our department for ARF due to ciprofloxacin ingestion requiring urgent hemodialysis (Table 1). The following patient data were collected: age, sex, clinical symptoms and signs, doses of ciprofloxacin, and eventually other ingested drugs, laboratory data, dialysis settings and evolution. ARF was defined as an abrupt or rapid decline in renal filtration function that develops rapidly over a few hours or a few days [2]. Considering the fact that the distribution is non-Gaussian and with a small sample size, only medians were used.
Table 1.
Biological signs of ciprofloxacine intoxication
| Patients | Age (years old) | Medical history | Amount of CP ingested (g) | Concomitant drug intoxication/dose (g) | Circumstances of the ingestion | SGOT/SGPT (UI/l) | Hypovolemia | Eosinophils (cells/mm3) | Creatinine (µmol/l) | CPK/LDH (UI/l) |
| 1 | 27 | – | 10 | Erythromycin/5 | Suicide/depression | 146/120 | Yes | 120 | 328 | 21/125 |
| 2 | 19 | – | 7.5 | _ | _ | 96/58 | – | 90 | 464 | 34/210 |
| 3 | 19 | – | 5 | Diclofenac/4 | Suicide/bipolar syndrome | 47/25 | Yes | 150 | 643 | 52/214 |
| 4 | 20 | Recurrent UTI | 7 | _ | Suicide/schizophrenia | 34/56 | – | 80 | 420 | 36/345 |
| 5 | 31 | HT, diabetes | 30 | _ | Suicide/depression | 326/270 | Yes | 60 | 931 | 128/456 |
CP ciprofloxacine, UTI urinary tract infection, HT hypertension, SGPO serum glutamooxaloacetate transferase, SGPT serum glutamopyruvate transferase, CPK creatinine phosphokinase, LDH lactate dehydrogenase
Results
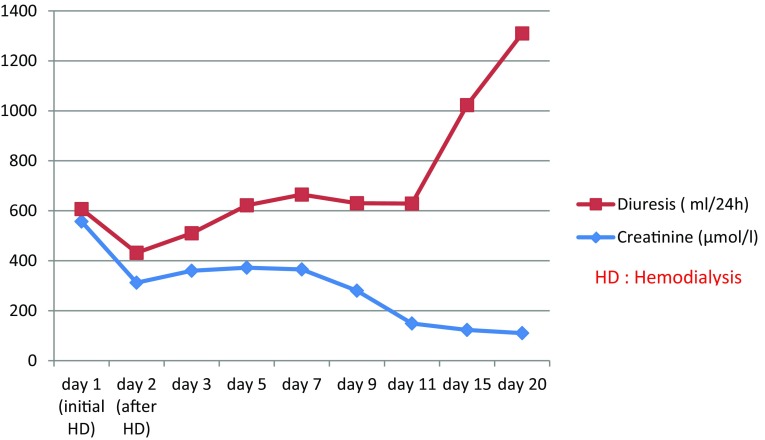
Five female patients aged 22 ± 5 years experienced acute ciprofloxacin intoxication requiring dialysis, four of whom had presented with a psychiatric history of depression and bipolar disorder. One patient had a history of hypertension and diabetes but without documented nephropathy, while another patient had a history of recurrent urinary tract infection. All five patients presented abdominal pain and vomiting after the intake of ciprofloxacin. The median ciprofloxacin dose was 17 ± 5 g. On clinical examination, no skin rash or fever was present. Fatigue, sunken eyes and concentrated urine were noted in three cases in relation to a moderate dehydration state. Neurological examination was normal. Three patients were completely anuric, while the remaining two patients were oliguric; urinalysis had shown hematuria in one patient and proteinuria in the other. Laboratory findings revealed renal failure, with a mean serum creatinine of 557.2 µmol/L (328–931 µmol/L). The median delay between ciprofloxacin intoxication and the occurrence of ARF was 10 ± 6 h. No eosinophilia or urinary leucocytes were noted, and urinary crystal deposits were not identified. The summary of the biological assessment of our patients is shown in Table 1. Proteinuria was negative in four cases and at 0.6 g/24 h in the diabetic patient. Abdominal ultrasonography showed normal-sized kidneys in all cases. Renal biopsy was not performed. The first hemodialysis session was indicated within an average of 36 h of ingestion (24–72 h), while the main indication for dialysis was acidosis in all cases and uremic encephalopathy in one case. The patients had several hemodialysis sessions (an average of 4.6). We noted an improvement of renal function in four patients, with a full recovery in four patients within 3 weeks, on average. Renal function evolution, as well as diuresis, is illustrated in Fig. 1. One patient had incipient renal failure 1 month later, with a serum creatinine of 104 µmol/L (glomerular filtration rate 56 mL/min); the patient then received no further consultations.
Fig. 1.
Mean serum creatinine and 24-h diuresis evolution in our patients
Discussion
ARF due to ciprofloxacin has been described in the literature, mainly in case reports [3–5]. We should distinguish between an overdose of ciprofloxacin and renal side effects related to ciprofloxacin use [6, 7]. At a therapeutic dose, ARF is probably not dose-related and has occurred with doses of ciprofloxacin as low as 200–250 mg twice daily. Several authors emphasized the potential risk factors for this complication, including increased age, low body mass, and co-ingestion of other potentially nephrotoxic medications. In clinical practice, ciprofloxacin-induced ARF has predominantly been the result of an AIN [5]; however, classic symptoms of a hypersensitivity reaction are not always observed. Histologically, we found an infiltrate composed of lymphocytes and plasma cells. Cholestatic liver injury, hypereosinophilia, and rhabdomyolysis have also been reported as side effects of ciprofloxacin [8]. In our series, we only focused on ciprofloxacin overdose, which occurred in young patients, who, for the majority of patients, no medical history was available. In spite of this, these patients developed acute kidney injury. ATN has been the most reported condition related to an overdose of ciprofloxacin [9], and there have been several case reports of ATN secondary to an overdose of ciprofloxacin [9, 10]. Unlike our series, all patients had non-oliguric renal failure, which was completely reversed after discontinuation of the fluoroquinolone [11]. This report shows that the main cause of ARF in our patients was ATN; however, no renal biopsy was performed in our study considering the good evolution in four cases and the presence of underlying nephropathy in the remaining case. Combination therapy with multiple nephrotoxins can result in synergistic nephrotoxicity [12]. In multiple intoxication cases, an ingested dose of ciprofloxacin is lower compared with an overdose of ciprofloxacin only, hence the necessity to consider a kidney complication in less important doses, for multiple drug intake [13, 14]. The absence of other associated causes of ARF, the interval of time between ingestion and ARF, and the good renal outcome are all factors advocating for the diagnosis of drug-induced renal disease.
We should also mention the presence of additional contributing factors to the onset of drug-induced renal failure in some of our patients, i.e. medical history of diabetes and recurrent urinary tract infection in two patients, as well as prior clinical hypovolemia found in three cases. Resolution of the ARF has usually occurred within 1–8 weeks of discontinuation (Table 2). In the literature, some case reports have also documented crystal-induced acute kidney injury with standard doses of ciprofloxacin during 1–8 days of therapy [4, 5]. ARF results from the crystallization of ciprofloxacin with magnesium and proteins, leading to intrarenal obstruction and inflammatory changes in the tubular walls [15]. This was not the case in our patients; notably, no urinary crystal deposits were identified. Rarely, ciprofloxacin use has also been reported to cause granulomatous interstitial nephritis [16]. The treatment of AIN includes hydration and transient dialysis, but treatment with corticosteroids is controversial [17, 18]. Quinolones are known to be partially removed by hemodialysis [19, 20]; however, in our series, the indication of hemodialysis was not for drug epuration but rather for the management of ARF complications such as acidosis and uremic encephalopathy.
Table 2.
Some case reports from literature
| References | Age (years old)/gender | Circumstances of ingestion | Therapeutic dose | Peak of Serum creatinine (µmol/l) | Type of ARF | Follow-up duration | Final creatinine levels (µmol/l) | Evolution |
|---|---|---|---|---|---|---|---|---|
| [12] | 70/F; 44/F | UTI; RTI | Yes | 193; 195 | AIN; AIN | 1 month; 3 months | 109; 116 | CRRF |
| [10] | 15/M | Overdose | No | 548 | ATN | NA | NA | IRF |
| [13] | 29/F | Overdose | No | 320 | AIN | 1 week | NA | CRRF |
| [19] | 31/F | RTI | Yes | 700 | AIN | 2 months | 225 | IRF |
| [9] | 65/M | Cellulitis | Yes | – | ATN | 3 months | – | RBC |
| [11] | 80/M; 81/M | RTI in both cases | Yes | 3.5(mg/dl); 8 (mg/dl) | AIN + vasculitis | 2 weeks; 3 weeks | 2.7 mg/dl; 3 mg/dl | IRF |
| [20] | 90/F | Proteus sp. In culture of tracheal aspirate | Yes | 3.8 (mg/dl) | CICN | 10 days | 1.2 | RBC |
| [21] | 16/F | UTI | Yes | 900 | CICN | 3 months | 70 | IRF |
NA not available, ARF acute renal failure, ATN acute tubular necrosis, AIN allergic interstitial necrosis, RTI respiratory tract infection, UTI urinary tract infection, CICN ciprofloxacin induced crystal nephropathy, CRRF complete recovery of renal function, IRF improvement of renal function, RBC return to baseline creatinine
Conclusion
In our study, we focused on the nephrotoxicity of ciprofloxacin after an overdose. Renal prognosis was generally good in the absence of additional contributing factors or concomitant drug intoxication. Definitive diagnosis requires performance of renal biopsy, although this is not always feasible, while the use of hemodialysis is not systematic, except for uremic complications of ARF.
Funding
No financial support was received for the conduct of this study or the preparation of this article.
Conflict of interest
Meriam Hajji, Hela Jebali, Aymen Mrad, Yassine Blel, Nozha Brahmi, Rania Kheder, Soumaya Beji, Lilia Ben Fatma, Wided Smaoui, Madiha Krid, Fethi Ben Hmida, Lamia Rais, and Mohammed Karim Zouaghi declare that they have no conflicts of interest.
References
- 1.Hootkins R, Fenves AZ, Stephens MK. Acute renal failure secondary to oral ciprofloxacin therapy: a presentation of three cases and a review of the literature. Clin Nephrol. 1989;32:75–78. [PubMed] [Google Scholar]
- 2.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114(1):5–14. doi: 10.1172/JCI200422353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804–817. doi: 10.1046/j.1523-1755.2001.060002804.x. [DOI] [PubMed] [Google Scholar]
- 4.Thorsteinsson SB, Bergan T, Oddsdottir S, et al. Crystalluria and ciprofloxacin, influence of urinary pH and hydration. Chemotherapy. 1986;32:408. doi: 10.1159/000238444. [DOI] [PubMed] [Google Scholar]
- 5.Chopra N, Fine PL, Price B, Atlas I. Bilateral hydronephrosis from ciprofloxacin induced crystalluria and stone formation. J Urol. 2000;164:438. doi: 10.1016/S0022-5347(05)67379-X. [DOI] [PubMed] [Google Scholar]
- 6.Ball P, Tillotson G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Saf. 1995;13:343. doi: 10.2165/00002018-199513060-00004. [DOI] [PubMed] [Google Scholar]
- 7.Schluter G. Ciprofloxacin: toxicologic evaluation of additional safety data. Am J Med. 1989;87:37S. doi: 10.1016/0002-9343(89)90018-1. [DOI] [PubMed] [Google Scholar]
- 8.Dichiara AJ, Atkinson M, Goodman Z, Sherman KE. Ciprofloxacin-induced acute cholestatic liver injury and associated renal failure. Case report and review. Minerva Gastroenterol Dietol. 2008;54(3):307–315. [PubMed] [Google Scholar]
- 9.Dharnidharka VR, Nadeau K, Cannon CL, et al. Ciprofloxacin overdose: acute renal failure with prominent apoptotic changes. Am J Kidney Dis. 1998;31:710–712. doi: 10.1053/ajkd.1998.v31.pm9531191. [DOI] [PubMed] [Google Scholar]
- 10.George MJ, Dew RB, 3rd, Daly JS. Acute renal failure after an overdose of ciprofloxacin. Arch Intern Med. 1991;151(3):620. doi: 10.1001/archinte.1991.00400030146039. [DOI] [PubMed] [Google Scholar]
- 11.Allon M, Lopez E, Min K. Acute renal failure due to ciprofloxacin. Arch Intern Med. 1990;150:2187–2189. doi: 10.1001/archinte.1990.00390210141030. [DOI] [PubMed] [Google Scholar]
- 12.Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Curr Opin Crit Care. 2005;11(6):555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- 13.Connor JP, Curry JM, Selby TL, Perlmutter AD. Acute renal failure secondary to ciprofloxacin use. J Urol. 1994;151(4):975–976. doi: 10.1016/S0022-5347(17)35139-X. [DOI] [PubMed] [Google Scholar]
- 14.Lo WK, Rolston KV, Rubenstein EB, et al. Ciprofloxacin induced nephrotoxicity in patients with cancer. Arch Intern Med. 1993;153:1258–1262. doi: 10.1001/archinte.1993.00410100082012. [DOI] [PubMed] [Google Scholar]
- 15.Fogazzi GB, Garigali G, Brambilla C, Daudon M. Ciprofloxacin crystalluria. Nephrol Dial Transplant. 2006;21:2982. doi: 10.1093/ndt/gfl320. [DOI] [PubMed] [Google Scholar]
- 16.Goli R, Mukku KK, Raju SB, Uppin MS. Acute ciprofloxacin-induced crystal nephropathy with granulomatous interstitial nephritis. Indian J Nephrol. 2017;27(3):231–233. doi: 10.4103/0971-4065.200522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shih DJ, Korbet SM, Rydel JJ, et al. Renal vasculitis associated with ciprofloxacin. Am J Kidney Dis. 1995;26:516–519. doi: 10.1016/0272-6386(95)90500-6. [DOI] [PubMed] [Google Scholar]
- 18.Singlas E, Taburet AM, Landru I, Albin H. Ryckelinck JP Pharmacokinetics of ciprofloxacin tablets in renal failure: influence of haemodialysis. Eur J Clin Pharmacol. 1987;31:389–393. doi: 10.1007/BF00606636. [DOI] [PubMed] [Google Scholar]
- 19.Thalhammer F, Kletzmayr J, ElMenyawi I, Kovarik J, Rosenkranz AR, Traunmuller F, et al. Ofloxacin clearance during hemodialysis: a comparison of polysulfone and cellulose acetate hemodialyzers. Am J Kidney Dis. 1998;32:642–645. doi: 10.1016/S0272-6386(98)70029-0. [DOI] [PubMed] [Google Scholar]
- 20.Famularo G, De Simone C. Nephrotoxicity and purpura associated with levofloxacin. Ann Pharmacother. 2002;36(9):1380–1382. doi: 10.1345/aph.1A474. [DOI] [PubMed] [Google Scholar]
- 21.Sedlacek M, Suriawinata AA, Schoolwerth A, Remillard BD. Ciprofloxacin crystal nephropathy: a ‘new’ cause of acute renal failure. Nephrol Dial Transplant. 2006;21:2339–2340. doi: 10.1093/ndt/gfl160. [DOI] [PubMed] [Google Scholar]