Abstract
Purpose
To investigate the prognostic factors of visual and anatomic outcomes in patients with diabetic macular edema (DME) treated with intravitreal injection of dexamethasone implant.
Methods
We retrospectively studied 32 eyes of 31 patients with DME for best-corrected visual acuity (BCVA), central macular thickness, and height and width of both intraretinal fluid (IRF) and subretinal fluid. Logistic regression analysis was used to examine correlations between the baseline characteristics and outcomes at 3 and 6 months.
Results
Baseline predictor of BCVA ≥20 / 40 at month 3 was short height of baseline IRF (p = 0.02), while good baseline BCVA was a predictor for month 6 (p = 0.01). Predictors of improvement in logarithm of minimum angle of resolution BCVA 0.2 at month 3 were the absence of baseline IRF and poor baseline BCVA (p = 0.02 and p = 0.009, respectively), while poor baseline BCVA was the sole predictor at month 6 (p = 0.01). Predictor of central macular thickness ≤300 µm at month 3 was younger age (p = 0.03), while the absence of IRF was the predictor for BCVA improvement at month 6 (p = 0.02). BCVA ≤20 / 100 at month 3 was predicted by poor baseline BCVA (p = 0.01), and increased width of total IRF was the predictor at month 6 (p = 0.02). Predictor of loss of logarithm of minimum angle of resolution BCVA 0.2 at month 6 was increased width of total IRF at baseline (p = 0.04). Additional injection within 6 months was negatively associated with the presence of baseline DME (p = 0.03).
Conclusions
The visual and anatomical outcome of DME treatment with dexamethasone implant can be predicted by baseline visual acuity and IRF morphology.
Keywords: Diabetic retinopathy, Macular edema, Prognosis, Spectral-domain optical coherence tomography
Diabetic macular edema (DME) is the most common cause of vision loss in patients with diabetic retinopathy [1]. DME is thought to occur due to fluid collection induced by breakdown of the blood retinal barrier [2]. Intravitreal dexamethasone implant injection has become a popular treatment as it quells the activity of multiple inflammatory mediators [3]. Treatment outcomes are favorable even for patients refractory to anti-vascular endothelial growth factor (VEGF) treatment [4]. However, intravitreal dexamethasone implant injection does not work for all DME patients based on our clinical experience. Due to the lack of knowledge regarding the prognostic factors after dexamethasone implant treatment, the reason for this failure is unclear [5]. This study sought to determine reasons for the varied outcomes in patients with DME treated with dexamethasone implant. The baseline prognostic factors for visual and anatomical outcomes of DME patients after intravitreal injection of dexamethasone implant were investigated based on detailed measurement of microstructures. In addition, the factors associated with retreatment within 6 months after the first injection were examined.
Materials and Methods
Patients
The medical records of patients examined between April 2014 and August 2016 were retrospectively reviewed. This study followed the tenets of the Declaration of Helsinki and was approved by the institutional review board (KUH1100042). The inclusion criteria were type 1 or type 2 diabetes mellitus, DME treated with one or more intravitreal dexamethasone implants and followed up for at least 6 months, and central macular thickness (CMT) >300 µm based on spectral-domain (SD) optical coherence tomography (OCT). The exclusion criteria were prior vitrectomy, panretinal laser photocoagulation within 3 months, prior laser-treatment of the macula, and ocular diseases other than diabetic retinopathy and cataract. Each patient was followed monthly and received a complete ophthalmic examination including medical and ocular history, treatment history of DME, determination of glycosylated hemoglobin level, and ophthalmologic examination that included best-corrected visual acuity (BCVA), fundus photography, and SD-OCT. Data obtained at baseline and 3 and 6 months were included in this study. Dexamethasone implant containing 0.7 mg dexamethasone (Ozurdex; Allergan, Irvine, CA, USA) was injected intravitreally. Retreatment was performed based on the anatomic criteria such as CRT >175 mm on OCT or evidence of residual edema on OCT, considered intraretinal cysts or regions of retinal thickening.
Assessment of retinal microstructures observed using SD-OCT
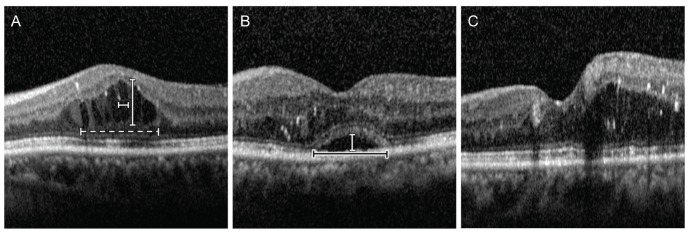
A 9-mm × 6-mm area of the macular region was examined using SD-OCT with a Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany). Volume scans of 25 sections were centered on the fovea, and nine B-scan images of each section were averaged. Using a virtual caliper in the software (Heidelberg Eye Explorer ver. 6.0), the height and width of both subretinal fluid (SRF) and intraretinal fluid (IRF) were measured (Fig. 1A, 1B). When multiple IRFs were present, the nearest IRF from the center was selected. In addition, IRF over 100 µm in width observed only in the outer nuclear layer were considered in quantification because the IRF in the inner retinal layer were usually too small and/or had vague boundaries that limited assessment of their height and width. Total IRF width was measured as the maximal width of IRF clusters within a 3,000-µm circle centered at the fovea. Macular edema was separated into three groups based on SD-OCT: diffuse retinal thickening (DRT), cystoid macular edema (CME), and serous retinal detachment (SRD) [6,7,8,9,10]. The CME pattern was characterized by intraretinal cystoid spaces (Fig. 1A). The SRD pattern was defined as a detachment of the retina with a hyporeflective space between the neurosensory retina and retinal pigment epithelium (Fig. 1B). The DRT pattern was characterized by sponge-like swelling of the macula (Fig. 1C). If DRT was combined with CME or SRD, it was classified as either CME or SRD. If all patterns were combined, the classification was SRD. CMT was automatically calculated using built-in software as the average retinal thickness within a 500-µm radius circle centered on the fovea, based on the volume scan data containing the target circle area. Two graders (HL and KEK) blinded to the data assessed the types of DME and height and width of fluids.
Fig. 1. Patterns of diabetic macular edema observed on optical coherence tomography. (A) Cystoid macular edema type shows prominent intraretinal fluid (IRF) in the outer nuclear layer. The height and width of IRF were measured (vertical and horizontal white bars). Total width of IRF was measured as the maximal width of IRF clusters within a 3,000-µm area (white dashed bar). (B). Serous retinal detachment type shows subretinal hyporeflective space. The height and width of fluid were measured (vertical white bar and horizontal black bar). (C) Diffuse retinal thickening type shows a sponge-like swelling with reduced intraretinal reflectivity.
Statistical analyses
The Snellen BCVA was converted to a logarithm of the minimal angle of resolution (logMAR) equivalent. To validate the agreement between the two graders, intra-class coefficient (ICC) was calculated. Repeated measures ANOVA was used to compare logMAR BCVAs at baseline, month 3, and month 6. When sphericity violations were detected using Mauchly's test in repeated measures ANOVA, the Greenhouse-Geisser correction was applied to the degrees of freedom used in the F-test because ε was <0.75 in all cases. Mann-Whitney test was conducted to compare the parameters of CME type and SRD type. The parameters of DRT type could not be used in statistical comparison because only one eye was analyzed. Logistic regression analyses were performed to assess the relevant baseline factors associated with logMAR BCVA, CMT at 3 and 6 months, and reinjection before 6 months. Baseline factors analyzed were age, duration of diabetes, history of hypertension, duration of DME, glycosylated hemoglobin level, number of previous bevacizumab injections, history of previous cataract surgery, logMAR BCVA, CMT, presence or absence of each DME type, and height and total width of both IRF and SRF. Changes of 0.1 and 50 µm logMAR BCVA in microstructures were considered as standard units of changes in regression analyses. Individual factors were subjected to univariate logistic regression analysis and were subsequently entered in the multivariate linear regression analysis in a forward conditional manner if p < 0.05 was determined. All statistical analyses were performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). A p-value <0.05 was considered statistically significant for all analyses.
Results
Baseline characteristics
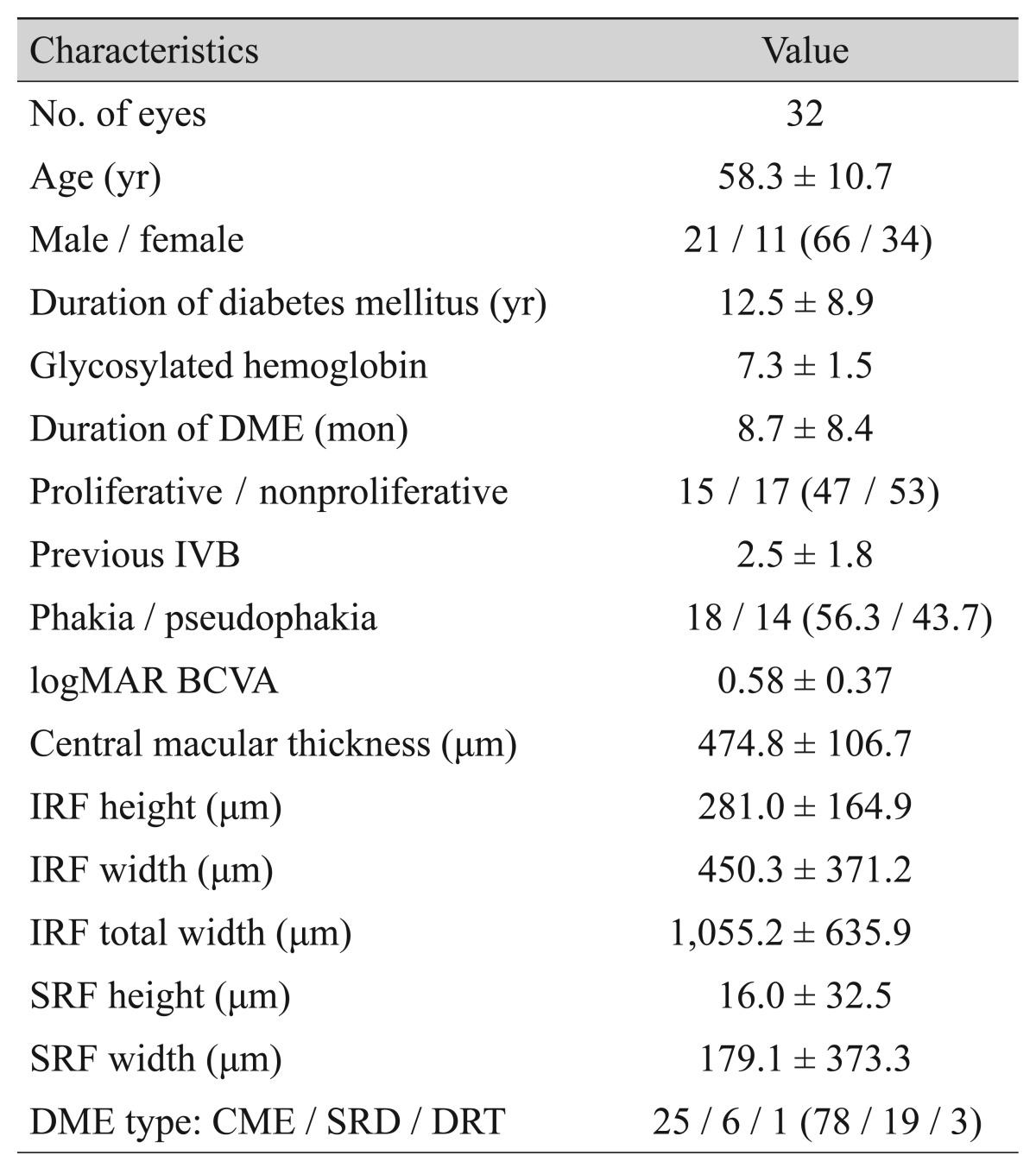
A total of 32 eyes of 31 patients (21 males, 10 females; mean age, 58.3 ± 10.7 years) were included in the present study. Seven eyes were treatment-naïve, and the other 25 eyes had received 2.5 ± 1.8 intravitreal bevacizumab injections before injection of the dexamethasone implant. The mean interval between bevacizumab treatment and dexamethasone implantation was 5.9 ± 6.6 months. Among the 25 eyes with previous bevacizumab injections, 19 were treated with dexamethasone implants within 6 months after the previous bevacizumab injections, and 10 eyes were treated within 3 months. Eighteen eyes were phakic and 14 eyes were pseudophakic. Among the phakic patients, no patient received cataract surgery during the 6 months because the cataracts did not progress significantly. Other detailed baseline characteristics are depicted in Table 1. The determination of DME types did not differ between the two graders. In addition, there was significant agreement in quantifying the fluid characteristics (ICC for IRF height, 0.92; ICC for IRF width, 0.93; ICC for IRF total width, 0.89; ICC for SRF height, 0.95; ICC for SRF width, 0.95; all p < 0.05).
Table 1. Baseline characteristics.
Values are presented as number, mean ± standard deviation, or number (%).
DME = diabetic macular edema; IVB = intravitreal bevacizumab injection; logMAR = logarithm of the minimal angle of resolution; BCVA = best-corrected visual acuity; IRF = intraretinal fluid; SRF = subretinal fluid; CME = cystoid macular edema; SRD = serous retinal detachment; DRT = diffuse retinal thickening.
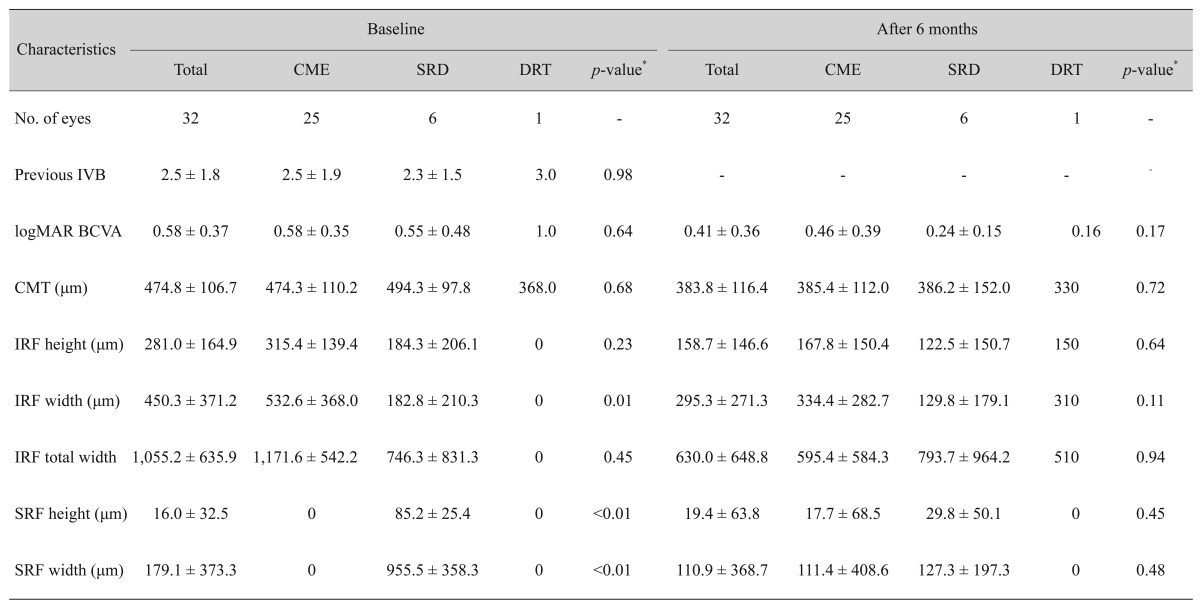
When comparing the number of previous bevacizumab injections and CMT of CME type with SRD type, no significant differences were found (Table 2). The parameters of DRT type could not be compared with other types because only one eye was included in DRT type.
Table 2. Characteristics according to diabetic macular edema type.
All values except the number of eyes are depicted as mean ± standard deviation.
CME = cystoid macular edema; SRD = serous retinal detachment; DRT = diffuse retinal thickening, IVB = intravitreal bevacizumab injection; logMAR = logarithm of the minimal angle of resolution; BCVA = best-corrected visual acuity; CMT = central macular thickness; IRF = intraretinal fluid; SRF = subretinal fluid.
*Mann-Whitney test for the comparison of CME parameters with SRD type. DRT type could not be included in statistical analysis because only 1 eye was included.
Change of BCVA and CMT
The mean baseline logMAR BCVA and CMT were 0.58 ± 0.37 and 474.8 ± 106.7 µm, respectively. The mean logMAR BCVA significantly improved at month 3 and month 6 compared to baseline (0.38 ± 0.34, p = 0.02 and 0.41 ± 0.36, p = 0.02, respectively). BCVA was not significantly different between months 3 and 6 (p = 0.35). Mean CMT at months 3 and 6 was significantly lower than the CMT at baseline (341.4 ± 97.0 µm, p < 0.001 and 388.1 ± 117.0 µm, p = 0.017, respectively). CMT was not statistically significantly different between months 3 and 6 (p = 0.153).
Predictors of good visual outcome
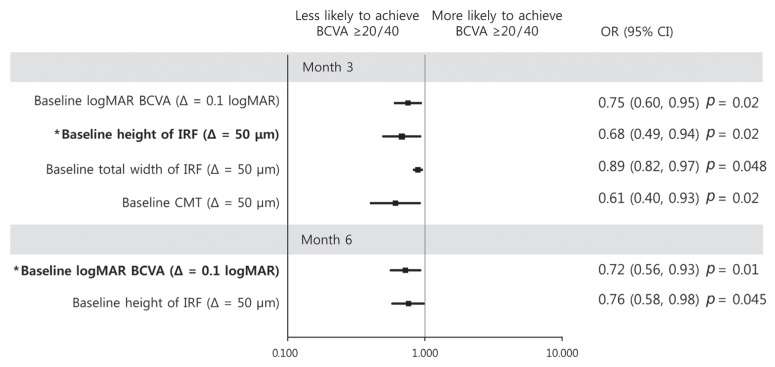
BCVA of 20 / 40 or better at month 3 was associated with good baseline logMAR BCVA (odds ratio [OR], 0.75; p = 0.02), short IRF height (OR, 0.68; p = 0.02), lower total IRF width (OR, 0.89; p = 0.048), and CMT at baseline (OR, 0.61; p = 0.02) in univariate analysis. Multivariate analysis showed that baseline IRF height was negatively associated with a good final BCVA at month 3 (OR, 0.68; p = 0.02) (Fig. 2). Conversely, at month 6, BCVA of 20 / 40 or better was associated with good baseline BCVA (OR, 0.72; p = 0.01) and short IRF height at baseline (OR, 0.76; p = 0.045) in univariate analysis. Multivariate analysis showed that good baseline BCVA was the only prognostic factor of good BCVA at month 6 (OR, 0.73; p = 0.01) (Fig. 2).
Fig. 2. Graphs showing baseline predictors associated with a best-corrected visual acuity (BCVA) ≥20 / 40 at month 3 (upper) and month 6 (lower). With increment of intraretinal fluid (IRF) height at baseline, a patient was less likely to achieve BCVA ≥20 / 40 at month 3. With increment of logarithm of the minimal angle of resolution (logMAR) BCVA at baseline, a patient was less likely to achieve a BCVA ≥20 / 40 at month 6. Error bar represents logarithmic transformations of 95% confidence intervals (CIs). OR = odds ratio; Δ = change; CMT = central macular thickness. *Baseline factors in multivariate logistic regression model.
Predictors of improved visual acuity
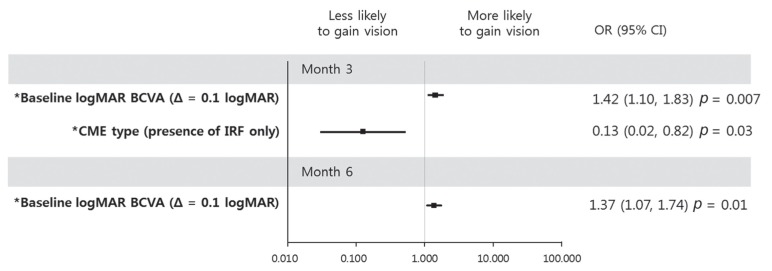
Improvement of logMAR BCVA ≥0.2 at month 3 correlated with poor baseline logMAR BCVA and absence of IRF at baseline in both univariate and multivariate analyses (OR, 1.42; p = 0.007 and OR, 0.13; p = 0.03, respectively, in univariate analysis. OR, 1.60; p = 0.009 and OR, 0.04; p = 0.02, respectively, in multivariate analysis) (Fig. 3). At month 6, improvement of BCVA was only associated with poor baseline BCVA (OR, 1.37; p = 0.01) in univariate analysis (Fig. 3).
Fig. 3. Graphs showing baseline predictors associated with an improvement of logarithm of minimum angle of resolution (logMAR) best-corrected visual acuity (BCVA) 0.2 at month 3 (upper) and month 6 (lower). At month 3, a patient with increased logMAR BCVA at baseline was more likely to gain vision, while the presence of intraretinal fluid (IRF) only at baseline was less likely to result in increased vision. For increment of logMAR BCVA at baseline, a patient was more likely to gain vision at month 6. Error bar represents logarithmic transformation of 95% confidence interval (CI). OR = odds ratio; Δ = change; CME = cystoid macular edema. *Baseline factors in multivariate logistic regression model.
Predictors of resolution of macular edema
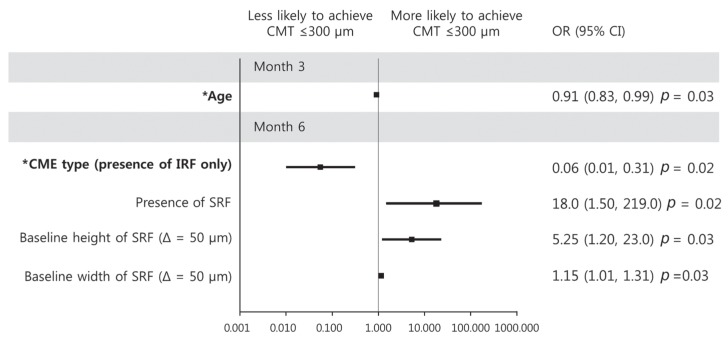
CMT ≤300 µm at month 3 was predicted only by younger age (OR, 0.91; p = 0.03) (Fig. 4). At month 6, CMT ≤300 µm was predicted by absence of CME (OR, 0.06; p = 0.02), presence of SRD type (OR, 18.0; p = 0.02), greater SRF height (OR, 5.25; p = 0.03) and greater SRF width (OR, 1.15; p = 0.03) at baseline in univariate analysis. The absence of IRF at baseline was the only factor persisting in multivariate analysis (OR, 0.06; p = 0.02) (Fig. 4).
Fig. 4. Graphs showing baseline predictor variables associated with central macular thickness (CMT) ≤300 µm at 3 and 6 months. With increment of age, a patient was less likely to achieve CMT reduction at month 3. With the presence of intraretinal fluid (IRF) only at baseline, a patient was less likely to achieve CMT reduction at month 6. Error bar represents logarithmic transformation of 95% confidence interval (CI). OR = odds ratio; SRF = subretinal fluid; Δ = change. *Baseline factors in multivariate logistic regression model.
Predictors of poor visual outcome
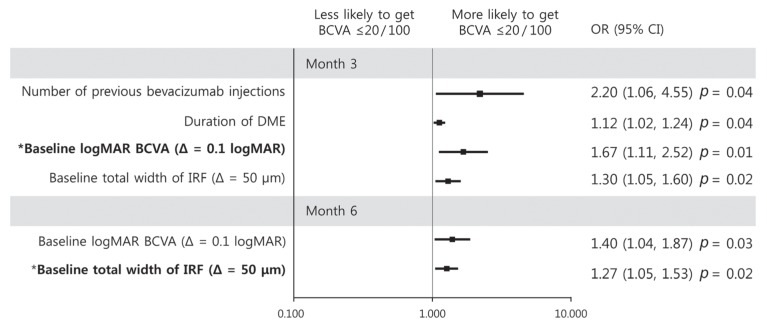
At month 3, BCVA of 20 / 100 or worse was more likely in patients who received more anti-VEGF injections and long duration of DME before dexamethasone implant injection (OR, 2.20; p = 0.04 and OR, 1.12; p = 0.04, respectively), poor baseline BCVA (OR, 1.67; p = 0.01), and increased total IRF width (OR, 1.30; p = 0.02). In multivariate analysis, only poor baseline BCVA persisted (OR, 1.67; p = 0.01) (Fig. 5). At month 6, univariate analysis showed that poor BCVA was associated with poor baseline BCVA (OR, 1.40; p = 0.03) and increased total IRF width (OR, 1.27; p = 0.02). In multivariate analysis, only increased total IRF width at baseline correlated with poor visual outcome (OR, 1.27; p = 0.02) (Fig. 5).
Fig. 5. Graphs showing baseline predictors associated with a best-corrected visual acuity (BCVA) ≤20 / 100 at month 3 (upper) and month 6 (lower). With increment of logarithm of the minimal angle of resolution (logMAR) BCVA at baseline, a patient was more likely to achieve a BCVA ≤20 / 100 at month 3. With increment of total width of intraretinal fluid (IRF) at baseline, a patient was more likely to achieve BCVA ≤20 / 100 at month 6. Error bar represents logarithmic transformation of 95% confidence interval (CI). OR = odds ratio; DME = diabetic macular edema; Δ = change. *Baseline factors in multivariate logistic regression model.
Predictors of loss of visual acuity
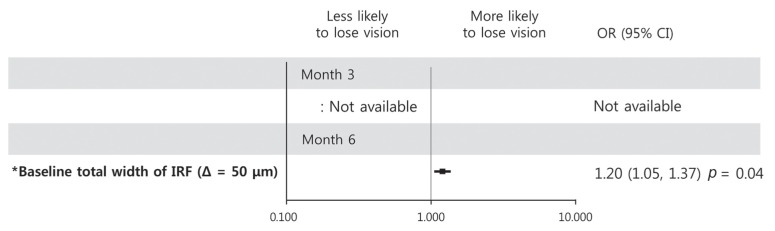
At month 3, statistical analysis could not be performed because only one patient experienced vision loss of logMAR ≥0.2. Regarding loss of BCVA at month 6, only increased total IRF width at baseline was significantly associated in univariate regression analysis (OR, 1.20; p = 0.04) (Fig. 6).
Fig. 6. Graphs showing baseline predictors associated with a loss of logarithm of minimum angle of resolution best-corrected visual acuity 0.2 at month 3 (upper) and month 6 (lower). At month 3, statistical analysis could not be performed because only one patient experienced vision loss of logarithm of minimum angle of resolution ≥0.2. With increment of total width of intraretinal fluid (IRF) at baseline, a patient was more likely to lose vision at month 6. Error bar represents logarithmic transformation of 95% confidence interval (CI). OR = odds ratio; Δ = change. *Baseline factors in multivariate logistic regression model.
Predictors of reinjection within 6 months
Eleven eyes (6 CME, 4 SRD, and 1 DRT) required an additional injection of dexamethasone implant within 6 months. Baseline CME type was associated with reduced reinjection within 6 months (OR, 0.13; p = 0.03). Other baseline factors showed no association with reinjection.
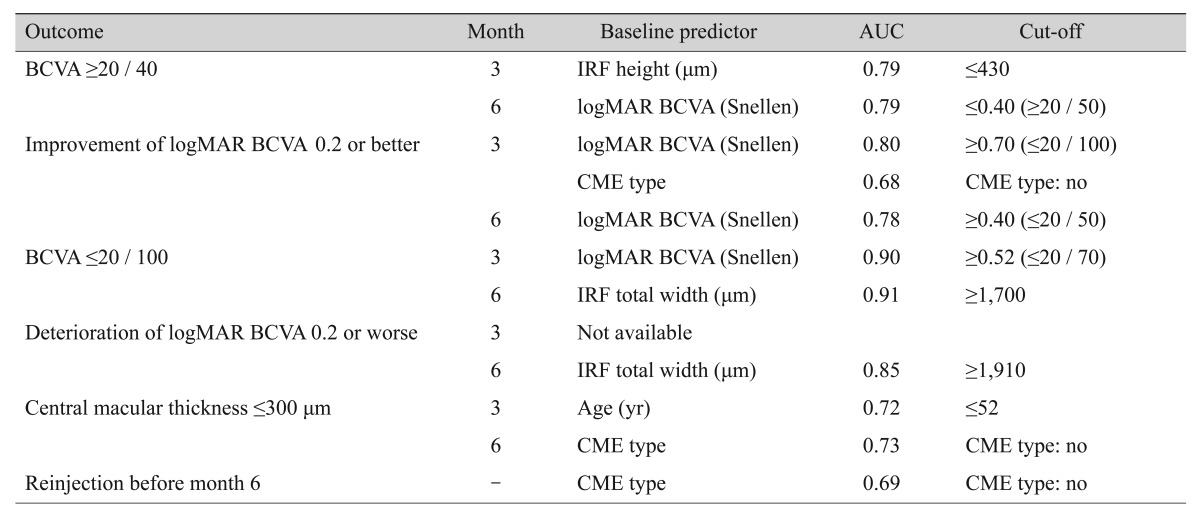
Area under the curve and cut-off values of predictors
Area under the curve and cut-off values of prognostic factors from each multivariate logistic regression model were calculated and are presented in Table 3. All factors showed statistically significant area under the curve values for outcome prediction.
Table 3. AUC and cut-off values of predictors in multivariate regression model.
AUC = area under the curve; BCVA = best-corrected visual acuity; IRF = intraretinal fluid; logMAR = logarithm of the minimal angle of resolution; CME = cystoid macular edema.
Discussion
Studies on DME using anti-VEGF treatment have shown that microstructural changes at baseline might predict the response to treatment. However, there is little information regarding the prognostic factors after treatment with dexamethasone implant [5].
In this study, baseline IRF showed temporary negative effect on visual and anatomical outcomes. Tall IRF at baseline was associated with good BCVA at months 3 and 6 in univariate analysis; however, the IRF did not persist in the multivariate analysis at month 6. Similarly, the negative effect of IRF on BCVA improvement was no longer present after 3 months. Because bipolar cells convey the signal from the photoreceptors, their excessive stretching by IRF could induce mechanical damage of bipolar cells, leading to visual impairment [11]. Because the pathogenesis of IRF formation is related with various inflammatory cytokines as well as VEGF, sole treatment with anti-VEGF might be less effective in CME type [12]. Conversely, the ability of dexamethasone to suppress various inflammatory signals appears to resolve the negative effect of IRF in a short period of time. However, for patients with poor BCVA and those with significant loss of BCVA, the negative effect of IRF was evident until 6 months, unlike in patients with good visual prognosis. Interestingly, the associated property of IRF was not height, as shown in good visual prognosis mentioned above, but the increased total width of IRF ≥1,700 µm. Although the reasons why the width was associated with visual loss and the negative effect was longer than height are unclear, the horizontal stretch appears more harmful to the retina, including bipolar cells, than the vertical stretch. Another interesting finding was the possible different roles of IRF and SRF in different treatment regimens. For ranibizumab treatment, SRD type showed worse visual prognosis than CME or DRT types [8]. Conversely, a recent report regarding dexamethasone implant documented that the SRD and diffuse DME types showed significant visual improvement and CMT reduction [5]. Although direct comparison between previous studies and ours is difficult, the quantified properties of IRF morphology might be useful to predict the visual outcome after treatment with dexamethasone implant.
Other pretreatment factors were also associated with visual prognosis. Good baseline BCVA was correlated with good visual outcome; however, the amount of improvement was higher in patients with poor baseline BCVA. This inverse correlation of BCVA improvement with baseline BCVA represents a ceiling effect, as previously described [13]. In addition, the increased number of previous anti-VEGF injections and long duration of DME were associated with poor BCVA of 20 / 100 or less at month 3. However, these negative effects were not observed at month 6. These results indicate that the dexamethasone implant could gradually compensate for the negative effects from the sustained pathological condition of diabetes mellitus by changing the inflammatory environment of DME.
Lens status (phakic or pseudophakic) was not associated with visual outcome in our study. No patient developed significant cataract requiring cataract surgery during treatment. However, the relatively short follow-up period in this study might be the reason for lack of cataract progression.
Conversely, SRF was associated only with the anatomical outcome of reduced CMT, not with visual outcome. The SRD type with increased SRF height and width at baseline was correlated with CMT ≤300 µm at month 6. Previous studies with anti-VEGF treatment showed no difference in CMT reduction among DME types [8,14]. However, a recent study on dexamethasone implant showed similar results to our study, with a positive correlation between the presence of SRF at baseline and CMT reduction [5]. The favorable anatomical outcome in SRD type with dexamethasone implant treatment might be due to the specific trait of SRF pathogenesis. SRF is supposedly the exudation due to hyperpermeability or retinal pigment epithelium (RPE) dysfunction [15,16]. Conversely, steroids stabilize the existing retinal vasculature and maintain tight junction integrity at the blood retinal barrier by promoting tight junction protein expression and protecting against oxidative stress-induced disruption of tight junction proteins in retinal pigment epithelium cells [3]. Therefore, dexamethasone implants might reduce CMT more effectively in the environment of SRF formation than IRF formation. Interestingly, younger age, especially under 52 years, was the sole factor associated with good anatomical outcome at month 3 but not at month 6. Although the reason is unclear, young age has showed favorable response in functional and anatomical outcomes after 1 year of ranibizumab treatment [16]. Based on temporary favorable effects until 3 months in our study, the dexamethasone implant treatment could compensate for the effect of old age on anatomical outcome as treatment continues.
Reinjection within 6 months was lower in the CME type (presence of only IRF) than other types. Because IRF negatively affected visual outcome, lower rate of reinjection in CME type initially appeared contradictory. Other types of DME (DRT and SRD types) are thought to have increased vascular hyperpermeability, while liquefaction necrosis of the Muller cells and adjacent neural cells due to persistent edema or ischemia leads to cystoid cavity formation [17,18,19,20]. The presence of SRF and diffuse DME was reported to increase the reinjection of dexamethasone implant [5]. Therefore, CME type could represent functional damage with relatively low recurrence of DME than other types. Due to the small number of eyes in each DME type, further studies are needed to clarify the factors for early reinjection.
The limitations of this study were its retrospective nature and the relatively small size of the study cohorts. The short follow-up period was only 6 months. In addition, we could not find any effect of DRT type because only one patient was DRT type. Further long-term studies with more participants are needed. However, our study has the advantage of detailed quantification of pathomorphological structures and suggesting the cut-off values of predictors.
In conclusion, increased height and total width of baseline IRF were the major morphological factors for poor visual outcome of DME after intravitreal dexamethasone implant injection. Additionally, the baseline CME type was associated with poor anatomical outcome and low reinjection rate. Therefore, more active treatment might be needed for patients with increased baseline IRF.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91:1464–1474. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 2.Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54:1–32. doi: 10.1016/j.survophthal.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Dugel PU, Bandello F, Loewenstein A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol. 2015;9:1321–1335. doi: 10.2147/OPTH.S79948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhioua I, Semoun O, Lalloum F, Souied EH. Intravitreal dexamethasone implant in patients with ranibizumab persistent diabetic macular edema. Retina. 2015;35:1429–1435. doi: 10.1097/IAE.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 5.Moon BG, Lee JY, Yu HG, et al. Efficacy and safety of a dexamethasone implant in patients with diabetic macular edema at tertiary centers in Korea. J Ophthalmol. 2016;2016:9810270. doi: 10.1155/2016/9810270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–693. doi: 10.1016/s0002-9394(99)00033-1. [DOI] [PubMed] [Google Scholar]
- 7.Kim BY, Smith SD, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006;142:405–412. doi: 10.1016/j.ajo.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Seo KH, Yu SY, Kim M, Kwak HW. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina. 2016;36:588–595. doi: 10.1097/IAE.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 9.Mori Y, Murakami T, Suzuma K, et al. Relation between macular morphology and treatment frequency during twelve months with ranibizumab for diabetic macular edema. PLoS One. 2017;12:e0175809. doi: 10.1371/journal.pone.0175809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giocanti-Auregan A, Hrarat L, Qu LM, et al. Functional and anatomical outcomes in patients with serous retinal detachment in diabetic macular edema treated with ranibizumab. Invest Ophthalmol Vis Sci. 2017;58:797–800. doi: 10.1167/iovs.16-20855. [DOI] [PubMed] [Google Scholar]
- 11.Pelosini L, Hull CC, Boyce JF, et al. Optical coherence tomography may be used to predict visual acuity in patients with macular edema. Invest Ophthalmol Vis Sci. 2011;52:2741–2748. doi: 10.1167/iovs.09-4493. [DOI] [PubMed] [Google Scholar]
- 12.Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008;145:854–861. doi: 10.1016/j.ajo.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Shah SU, Harless A, Bleau L, Maturi RK. Prospective randomized subject-masked study of intravitreal bevacizumab monotherapy versus dexamethasone implant monotherapy in the treatment of persistent diabetic macular edema. Retina. 2016;36:1986–1996. doi: 10.1097/IAE.0000000000001038. [DOI] [PubMed] [Google Scholar]
- 14.Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology. 2015;122:1395–1401. doi: 10.1016/j.ophtha.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 15.Ferris FL, 3rd, Patz A. Macular edema: a complication of diabetic retinopathy. Surv Ophthalmol. 1984;28(Suppl):452–461. doi: 10.1016/0039-6257(84)90227-3. [DOI] [PubMed] [Google Scholar]
- 16.Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130:1153–1161. doi: 10.1001/archophthalmol.2012.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanoff M, Fine BS, Brucker AJ, Eagle RC., Jr Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28(Suppl):505–511. doi: 10.1016/0039-6257(84)90233-9. [DOI] [PubMed] [Google Scholar]
- 18.Bringmann A, Reichenbach A, Wiedemann P. Pathomechanisms of cystoid macular edema. Ophthalmic Res. 2004;36:241–249. doi: 10.1159/000081203. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF. Retinal vascular cystoid macular edema: review and new theory. Retina. 2016;36:1823–1842. doi: 10.1097/IAE.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, et al. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016;2016:2156273. doi: 10.1155/2016/2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]