Abstract
Sir Peter Medawar experimentally demonstrated immunological tolerance through his tissue transplantation experiment in the early and mid-1950s. He made a central contribution to modern biomedicine by showing that genetically distinct cells introduced into a body during its foetal phase could not only be permanently tolerated but also make the host accept any subsequent skin grafts from the original cell donors. However, this discovery had only a limited clinical applicability. None could practise Medawar's method on human foetuses in preparation for their future need for organ or skin transplantation. I analyse this problem by focusing on his management of ‘failures’ during the tissue transplantation experiments. Through statistical, material, theoretical and rhetorical strategies, he managed unsatisfactory findings of his research, including unexpected skin infection, sudden animal death and irregularities in homograft survival times. I argue that these strategies and their inherent ambiguities constituted the course of Medawar's research, enabling him to delineate the temporal dimensions of tolerance and a clinical relevance, which were mutually contradictory. This paper thus illustrates the multiple roles that failures play in scientific research as well as the conflicting outcomes of investigators' efforts to manage them.
There is no calling in life in which mere incompetence is any obstacle to professional advancement: we are brought into the world by incompetent obstetricians and if we survive we are baptized by incompetent clergymen who will as likely as not drop us into the font.
Peter Medawar, Memoir of a thinking radish (1986)
Introduction
In 1953, Peter Brian Medawar (1915–1987) demonstrated immunological tolerance through tissue transplantation experiments.1 Although it had been known that mammals, including humans, could not accept tissues grafted from different individuals, Medawar discovered that they could be induced to accept a foreign body if its cells had been introduced into them in utero. This phenomenon was genetically specific, because the tolerant animals failed to accept grafts from other inbred strains. Subsequently, his research led other scientists to investigate the body's way of distinguishing its ‘self’ from ‘non-self’, which highlighted the biological, rather than chemical, aspects of immunity.2 As this work confirmed Sir Frank Macfarlane Burnet's theoretical prediction in 1949, Medawar and Burnet shared the Nobel Prize in Physiology or Medicine in 1960. However, Medawar's 1953 experiments had little relevance to actual clinical practices.3 No one could use his work as a basis for making a human body tolerate skin or organs from another person.
I offer a historical interpretation of these two dimensions of Medawar's work, which has not attracted much scholarly attention.4 In particular, this paper focuses on his efforts to manage failures in his experiments. I use the term ‘failures’ following Medawar's own expression in his autobiography and laboratory notes.5 From the outset, the main part of his tissue transplantation research seemed to accompany a relatively clear definition of failures because it started from the wartime clinical imperative of treating burn patients. Despite his strategic ambiguity, his primary work aimed at making skin grafts ‘tolerated’ on a foreign body for an extended period. He thus took many cases without this long-term survival as potential failures. In effect, he wrote, ‘For one reason or another some of the experiments failed’, especially as he began his study using foetal mice in 1952.6 In general, I think, there were three types of failures. The first were those from explicit errors by Medawar and his colleagues, Rupert Billingham and Leslie Brent; this included their mistakes during animal surgery, such as loose skin stitches and other problems, which could be easily corrected. The second were the cases for which his team was not responsible, but whose causes, according to their view, could still be identified, at least partly; the graft exchange failures within the same inbred strain due to their residual genetic heterogeneity belonged to this type. Yet more troublesome was the third kind: the issues whose causes seemed to lie beyond the scope of Medawar's understanding or responsibility. This included mysterious deaths of operated animals, as well as the rejection of tissues by mice after receiving cells in utero from the strain out of which the tissues were extirpated. In their laboratory notes, Medawar's group meticulously recorded all these difficulties. It was only after years of struggle that they succeeded in showing that their mice could develop complete tolerance toward foreign skin.
To analyse these efforts, I borrow perspectives from the scholarship on ‘errors’, ‘mistakes’ and ‘failures’. As Giora Hon and other historians have argued, errors and mistakes can play heuristic roles in scientific research.7 The American neurobiologist Stuart Firestein even declared that failure is a driving force of scientific enterprises.8 As Henry Petroski has illustrated, we can learn a lot from historical studies of erroneous judgments in technical projects, because failure is a great teacher.9 Yet, some scholars take a further step by questioning the nature of failure itself.10 From his philosophical scrutiny, Hasok Chang has claimed that scientific ideas that were deemed unsuccessful in the past can be re-evaluated now for what he calls ‘complementary science’.11 Studying twentieth-century biosciences, Hans-Jörg Rheinberger has also shown that scientists do not necessarily fail due to unwanted or unexpected results, because such results, as ‘novelties’, can help researchers construct new epistemic domains.12 As Graeme Gooday has illustrated, failures in technical projects are flexibly interpreted, depending on ‘socio-technical relations of usage’.13
Medawar's research is relevant to these scholars' study of the nature of failures. As I have mentioned, he had a relatively clear definition of failures, which led his team to search for the causes. However, they could not find such causes in all cases, as some of their unsuccessful trials—belonging to the third type—could be neither understood nor controlled.14 Remarkably, however, he rendered many of his failures relevant in a series of measures. Material from spoiled cases could be reused for different purposes, and could also foster new lines of investigation. Yet, the most crucial measure came from his mathematical expertise. Putting his unsuccessful or partially successful cases in a statistical scheme, he accounted for why tolerance was not an ‘all-or-nothing’ phenomenon.15 Strikingly, Medawar and his colleagues simultaneously gave their readers an impression that tolerance was a phenomenon with a clearly delimited boundary equivalent to acquired immunity. Medawar's presentation of tolerance was thus ambiguous, but this ambiguity, integrated within his rhetorical, theoretical and statistical strategies, contributed to making his work convincing despite its limited applicability.
My analysis of this ambiguity draws on the historical scholarship on quantification and statistics, especially on the significance of variation. Theodore Porter depicted how statisticians changed their focal point from the mean as a reified quantity to variations and dynamics in populations.16 Ian Hacking has also analysed longstanding debates on chance and certainty, alongside heterogeneity and regularity, amid the ‘avalanche of numbers’ generated by statistics.17 Similarly, J. Rosser Matthews has illustrated how the concept of ‘errors of technique’ was pitted against the idea of the statistical ‘problems of random sampling’, while Eileen Magnello discussed the differences between vital and mathematical statistics, which correspondingly stressed ‘averages’ and ‘variations’.18 More recently, Tiago Moreira and Paolo Palladino have analysed the ideas of ‘laboratory populations’ and ‘population laboratories’, which were geared to gerontologists' distinct views of averages and variations, respectively.19 In all these works, the key problem has been about how to interpret observations: what ontological significance should we assign to variations in scientific observations? Do they reflect errors in research or actual representations of nature's remarkable versatility? I explore this ‘perennial question’ through Medawar's research.20
I argue that Medawar's statistical, material, theoretical and rhetorical management of failures fostered his strategic ambiguity toward irregularities in his experiments—understood as something between errors in research and nature's variability—which enabled him to delineate the temporal dimensions of tolerance and a clinical relevance, which contradicted each other, reflecting this ambiguity. In my paper, ‘managing failures’ means a broad range of practices for dealing with unsatisfactory outcomes. After experiencing failure, scientists can modify experimental procedures for better results. But they also try to make the best use of such failure in various ways, which may not lead to logically coherent outcomes, as Medawar's work illustrates.
This effort explains Medawar's later philosophical arguments. In 1964, he claimed that most scientific papers, including his own, were ‘fraudulent’, not because they contained any fabricated records, but because they did not show the complex paths of research.21 Published papers seriously distorted the actual process of scientific work, which depended on ‘uncharted by-ways of thoughts’ that were impossible in traditional inductivism. Instead, he defended other philosophies, especially Karl Popper's work, which appeared to represent real scientific endeavours. As Neil Calver has argued, however, Popper was not a ‘guru of falsificationism’ to Medawar, but a science philosopher standing for creativity, imagination and researchers' persistent efforts to address ‘mistakes’.22 As Medawar's research enabled him to understand the inherent instability and complexity of science unrepresented by traditional inductivism, he thought that Popper's work reflected his experience. To Medawar, science was full of pitfalls, but they could be creatively managed in research. Yet, this effort could also make the meaning of the research contradictory.
Two mathematical styles in Medawar's early research
Starting his scientific education and career in Magdalen College and the Zoology Department at the University of Oxford in 1932, Medawar became interested in mathematical approaches to biological problems. According to his autobiography, Bertrand Russell's The principles of mathematics (1903) was the first book that led him to understand the importance of mathematical methodologies.23 Thereafter, Medawar was exposed to two different styles of mathematical reasoning in biology. Initially, he learned from D'Arcy Thompson's Growth and form (1917), which explained the development of organisms and interspecies relationships through mathematical means. Being sceptical of Darwinian evolution, Thompson was what Magnello called an ‘Aristotelian essentialist’, as his work dealt with strict geometric correlations among species that hardly changed.24 Medawar's correspondence with Thompson illustrates his deep respect toward this senior scholar, for whom he edited Essays on growth and form presented to D'Arcy Wentworth Thompson (1945).25 Medawar's scholarship was also shaped by a group of researchers with an opposite perspective—British evolutionary biologists who contributed to the neo-Darwinian synthesis, including Ronald Fisher, J. B. S. Haldane and Julian Huxley.26 Viewing living organisms as constantly changing entities, they not only championed Darwinian evolutionism but also stressed variations and diversity in natural populations revealed in mathematical analysis. Medawar incorporated their approach as he knew of them through the alumni–faculty network of Oxford's zoology department.27
Medawar's first research project relied on his learning from these scholars, although it is unclear which approach he then adopted. In his experiment in Howard Florey's laboratory in the late 1930s, he mathematically delineated the changing growth rate of chicken embryos' cells under a substance in malt extracts that inhibited animal cells' growth in a culture medium.28 Appropriating Fisher's ‘specific death rate’, Medawar showed that the rate of the cells' growth consistently declined over time, which he interpreted as a sign of ‘senescence’.29 As I have discussed elsewhere, he found that even rapidly growing cells in an embryo underwent ageing, as was revealed by their declining growth rate.30
Medawar reconfirmed this view of growth and ageing in his 1944 paper, which, unlike his earliest work, made clear his Thompsonian approach. Using a schematic drawing of human growth from an anatomy textbook, he delineated a mathematical formula to describe the changing distance of the fork, navel, nipples and chin from the bottom line. Titled ‘The shape of the human being as a function of time’, this paper was indeed an exercise of a Thompsonian methodology. Citing Thompson, he showed how a ‘standard’ human body underwent transformations following a rigorous mathematical rule, which demonstrated that ‘the rate of change of shape of the human being falls off progressively in time’.31 He probably thought that this was not the only possible pattern of growth, but he believed that it was quite close to the standard. Ageing, expressed in terms of lowering growth rate, proceeded following a fixed mathematical pattern.
However, in another work, Medawar chose a different approach. Adopting the ideas of major contributors to the neo-Darwinian synthesis, his evolutionary theory of ageing in 1946 explained the evolutionary emergence of ageing as a consequence of random mutation and natural selection.32 As I have detailed elsewhere, this theory posited that a gene's time of activation determined the amount of selective force exerted on it.33 A gene activated later in life received lesser force of natural selection. Hence, most genes in the final phase of life tended to mutate freely under low selective pressure, and would become responsible for the symptoms of ageing, which would be manifest after organisms came to live in protective environments preventing their early death. Medawar could thus account for a wide variation of timing and rate of ageing among diverse living organisms, depending on their shifting environmental conditions that had no settled pattern.
This theory, inspiring many later students of the evolution of ageing, reflected an increasing concern over Britain's ageing populations that resulted in the creation of its post-war welfare services following the surveys of the Royal Commission on Population.34 The problems of ageing also led to the establishment of the British Society for Research on Ageing, for which Medawar served as an executive committee member.35 With the founders of this society, Medawar shared the anxiety about the increasing number of seniors in Britain and its future impact on the country's economy and medical services.36
But Medawar's gerontological activity then remained an ‘intellectual pastime’.37 Rather, he came to concentrate on the ‘homograft problem’, a major medical issue emerging with the outbreak of the war. Yet, he still had in mind the two different mathematical perspectives, which respectively assumed an unchanging truth or dynamic variability in nature. He adopted these two perspectives in managing failures in his transplantation research.
Failures and numbers in transplantation
The Second World War initiated Medawar's lifelong endeavour in tissue transplantation. After seeing a victim who had lost 60% of his skin due to a third-degree burn after a plane crash in Oxford, he determined to study how to make homografts—tissue grafts from different individuals belonging to the recipient's species—successful for patients who had little remaining skin.38 A great opportunity came when he joined the Burns Unit at the Glasgow Royal Infirmary through a Medical Research Council (MRC) arrangement.39 There, Medawar, together with Leonard Colebrook and Thomas Gibson, investigated burn victims and concluded that homografts could not survive long because of the immune reaction incurred by the activities of antibodies circulating in the entire body rather than local tissues.40 Homograft rejection was thus ‘systemic’ and ‘humoral’ in nature.41 Furthermore, like other immunogenic agents, a second-set graft after the failure of the first-set was broken down more rapidly.42
At Glasgow, Medawar also tried to use tissue culture techniques that he had mastered in Florey's laboratory.43 He cultivated patients' skin in vitro under trypsin in order to increase its amount before transplanting it back to the patients. Yet, this work, which appeared to enable him to solve the problem without using homografts, was not successful because of severe post-surgical contractions that disfigured the operated area.
Thereafter, Medawar, with an MRC grant, started more systematic experiments on homografts in his Oxford laboratory, which continued after he became Mason Professor of Zoology at the University of Birmingham in 1947.44 In general, he pursued three interrelated lines of research using rabbits and mice. First, he tried to find a way to block or mitigate homografts' immunogenic power. Second, he examined the roles of various factors relevant to immunity, including serum, blood cells, vascularization, sites of transplantation and differences among species. Third, he scrutinized the quantitative facets of homograft rejection by measuring the influence of donors and recipients' age and the ‘dosage’ of grafted tissues. He also traced the variations of mitosis in grafts over time and the number of antigens responsible for homograft rejection.
None of these studies was easy, as Medawar often obtained results he did not want. As early as 1943, he found that his use of cellophane for dressing grafted skin brought about substantive necrosis, and the trauma of skin removal made some operated animals inadequate for further use.45 The improper size and thickness of removed skin, as well as mechanical glitches—such as a leaky or faulty tap stopper of tissue incubators—were no less significant.46 But even more problematic were troubles regarding experimental animals. Bacterial infection was quite common in all stages of his work, while fungal infection also occurred.47 Hence, his animals occasionally contracted various diseases, including dysentery and ‘cold’, and some of them died.48 Moreover, perhaps owing to the stressful condition in the cage, some animals attacked others.49
Medawar could reduce the occurrence of these unwanted outcomes by managing the ‘faults’.50 After he failed with a cellophane dressing, he wrote: ‘No cellophane to be used in the future.’51 Indeed, there were a number of alternatives for dressing purposes. Likewise, the size and thickness of the cut became more adequate as he refined his surgical skill, and he fixed mechanical glitches quickly.52 He also tried to improve the condition of experimental animals in cages by providing better feeds and antibiotics.53 Admittedly, this measure was not always effective. Infection and illness could never be completely avoided, as several sociologists have shown through their study of contemporary surgical practices and biological experiments.54 However, he was able to control bacterial infection at least partially with the post-war dissemination of antibiotics through Florey's work that Medawar had assisted in.55 Furthermore, his use of antibiotics spawned a new set of experiments that he called ‘S-test’. While cultivating a rabbit's skin along with serum, lymph nodes and spleen from another animal immunized against it, he added streptomycin in one batch but omitted it in the other to examine the effect of antibiotics in culturing tissues undergoing immune reactions.56 Failed works could be useful in another way: as Leslie Brent has mentioned, Medawar's team had to struggle with their limited laboratory equipment after the war.57 They thus tried to save as much material as they could by using botched experimental subjects for different purposes. For example, as a graft recipient showed a poor prognosis, he wrote that the animal had ‘no further use at present’ and it should ‘serve as homograft donor’.58 He also tried to withdraw blood from an animal, but this job was ‘bungled’ owing to his mistake, which left permanent damage on the animal body. He thus determined to use it for ‘class demonstration purposes’.59
With these efforts, Medawar could successfully perform diverse experiments, but many of them brought about only ambiguous results. Medawar incubated skin patches in Ringer's solution at body temperature for a while and transplanted them to a different individual, finding that few survived there.60 He also discovered that a low-temperature treatment of skin before homografting slightly enhanced its survivability, while turpentine treatment lowered it.61 Yet, this difference did not mean much because all homografts soon broke down. In another set of studies, he examined the effect of cell-free extracts, as well as cell suspensions, which were expected to reduce or delay immune response, if they were injected into the host before homografting. However, no practically or theoretically pertinent outcome was found from this work.62 He also transplanted homografted skin back to its original donor, and surrounded this ‘autograft’—the graft attached to the original donor's body—with six homografts ‘in a symmetrical ring’.63 Through these experiments, Medawar found that a prolonged contact with foreign tissues barely affected the life of autografts, which would survive in any case. At the same time, he co-cultured the donor and the recipient's skin with either the donor's or the recipient's serum. But this work showed little meaningful result, just as his experiment of transplanting homografts from two different individuals, alongside ‘heterografts’ from a different species, failed to show anything remarkable.64
Medawar's effort to count the number of antigens responsible for homograft rejection was equally inconsequential. Using 25 rabbits and the mathematical technique of permutation and combination, he conducted an extensive set of skin transplantations, which prompted him to conclude that there were at least seven antigens involved in rejecting homografts.65 Yet, this conclusion, which might be technically correct, revealed no more about the nature of immunity.
But there were more meaningful studies. In one experiment, Medawar performed a ‘heterotopic’ grafting by placing skin homografts into the brain or the eye's anterior chamber.66 As far as vascularization did not take place in such locations, skin patches after these operations, especially in the eye's chamber, could survive for some time. This meant that the ‘breakdown of grafts must be “active” and not merely a consequence of partial or total withdrawal of blood supply’, because the host destroyed the graft through its antibodies in the blood.67 He also studied the difference between adult and young rabbits of between two and four weeks of age, when they were used as homograft recipients.68 Although this investigation merely showed that there was no significant difference between them, it would place a stepping stone toward his later experiments on tolerance, which also focused on the significance of age.
Despite these promising works, several problems persisted when it came to tracking the shifting condition of grafted tissues. Medawar had to trace transplanted skin's changing conditions to see if his operation was successful. This work, involving an examination of cellular mitosis and migration as well as the mean diameter of grafts, was less than straightforward. Among theses, measuring cellular migration and the mean diameter was relatively uncomplicated. Medawar wrote: ‘The judgment, whether epithelium has spread or not, is hardly subject to error.’69 However, counting cellular mitosis of homografted skin was a tricky job because it was often difficult to tell whether a cell underwent mitosis or not. Hence, he made a new category, namely ‘dubious mitosis’, alongside ‘definite mitosis’.70 Indeed, as he counted the number of mitosis, such ‘dubious’ cases were frequently found. Furthermore, Medawar had to consider ‘abnormal mitosis’. As his system was ‘ill-adapted to chromosome study’, he observed numerous cases of cell division that looked ‘abnormal’. In fact, he found ‘the long axes of the dividing cells may lie at any angle with regard to the plane of section’, distorting the image of mitosis under his microscope.71 In such instances, ‘the errors of personal judgment become unduly large’.
What did Medawar do to cope with these problems? Strikingly, he did little: ‘no attempt was made to distinguish “normal” and “abnormal” mitotic figures’, because ‘any such discrimination probably introduces as many errors as it can hope to remove’. Likewise, he did not do anything about ‘dubious mitosis’, because it was found mostly in the second-set homografts, which did not exhibit many cellular divisions in any case. Ignoring these problems, he then calculated the ‘mean mitosis’ per diameter which determined the ‘degree of survival’ of homografts.72 He also computed the ‘sampling errors’ out of his mitosis counts to examine how the number of divisions he measured through ‘sampling’ corresponded to the hypothetical average representing all instances of mitosis.73
This work reveals ambiguity in Medawar's approach. As Matthews has shown, the statistical notion of sampling errors assumed heterogeneity in a population that could be shown only partly through sampling, in contrast to the concept of personal errors of technique that postulated a singular truth, which most experimenters might not reach without committing ‘errors’.74 By computing sampling errors and ignoring personal errors, Medawar seemed to stress heterogeneity in his research subject. Yet, this heterogeneity, even if it existed, should not be too great. As sampling errors were ‘a very small fraction of the mean computed from the readings’, the mean value that he posed should be regarded as a singular truth.
This attitude led Medawar to draw a qualitative conclusion out of quantitative research, especially since the mean values of mitosis counts revealed a significant difference between the first- and second-sets.75 He observed a very small number of mitosis in second-set homografts, in contrast to the first-sets, which displayed substantial cellular proliferation. This was a great piece of evidence for the immunological nature of graft rejection. Most cells in second-set homografts could not divide, because the host body stopped their proliferation by recognizing their physical pattern known from the previous encounter. The quantitative study based on the notion of sampling errors was thus phased into the qualitative affirmation of the distinction between first- and second-sets.
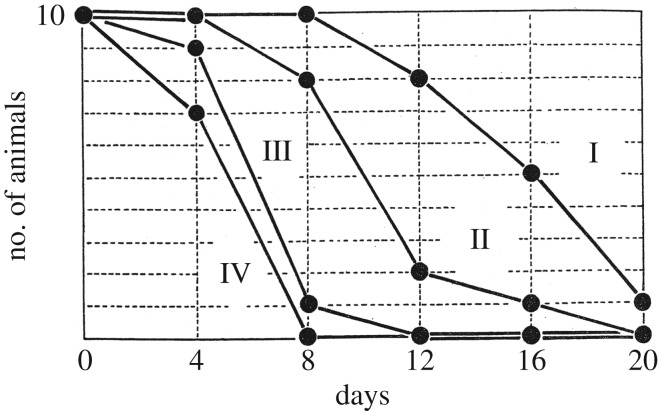
Medawar's analysis of declining numbers of surviving homografts over time also represented how he found a qualitative implication from quantitative studies. As I have reviewed in another paper, Medawar traced the number of surviving homograft patches on rabbits' bodies.76 The second-set grafts were rejected more rapidly than the first-sets, as the difference between groups I and II (first-sets) and groups III and IV (second-sets) illustrated (figure 1). This reconfirmed that the homograft failure was an immunological phenomenon.
Figure 1.
Changing number of rabbits with surviving skin homografts. The horizontal axis is the number of days after transplantation, while the vertical axis is the number of rabbits maintaining homografts, converted to a scale with ten as the maximum. Peter Medawar, ‘The behaviour and fate of skin autografts and skin homografts in rabbits’, J. Anat. 78, 176–199 (1944), at p. 186.
In this experiment, he also examined the relevance of ‘homograft dosage’ as well as the site of operation. In particular, the dosage difference initially seemed important, as large homograft patches (group II) appeared to break down more rapidly than smaller ones (group I). But Medawar's statistical study using probit analysis illustrated that the dosage affected only the length of the ‘latent period which must pass before the homograft reaction becomes effective’.77 After the rejection response started, all homografts broke down at the same rate without regard to their size. The location of homografting also turned out to be less significant. In terms of the speed of rejection, the second-set skin grafted on a new site (group III) did not differ much from that reattached on to the site where the first-set had already been destroyed (group IV). Homograft rejection was thus a ‘systemic’ rather than a ‘local’ reaction.
But this conclusion entailed an uneasy tension. How large was the gap between groups II and III if compared with that between groups I and II? To some, the distance between I and II might look greater than that between II and III. Viewing the graphs in this way, it was possible to say that there was no clear-cut qualitative distinction between the first- and second-set homografts, which seemed to exist in a continuous spectrum. There was another problem: to what extent could Medawar justify his argument that the location of graft was unimportant by pointing to the minimal distance between groups III and IV? Was it not large enough to prove that the site did matter? Medawar indeed exercised his discretion in drawing qualitative conclusions from the numbers he acquired, but his paper did not articulate its potential problems.
Managing failure for tolerance
According to a standard historical account, there was a great turning point for Medawar in 1948. At a scientific conference he met Hugh Donald, head of the Animal Breeding and Genetics Research Organisation in the Agricultural Research Council.78 After hearing Donald's concerns over distinguishing monozygotic from fraternal twins among his cattle, Medawar visited his experimental farm and showed that skin transplantation was useful in making this distinction: monozygotic twins accepted each other's skin, while fraternal twins did not. Yet, he was surprised by some fraternal twins that did accept each other's tissues.79 Initially puzzled by this discovery, Medawar soon found an answer in other scholars' publications. Above all, he came across Burnet and Fenner's Production of antibodies (1949), which predicted that extraneous entities introduced into an animal during its early life would be indefinitely ‘tolerated’ because ‘the process by which self-pattern becomes recognizable takes place during the embryonic … stages’.80 This idea was backed up by a piece of evidence provided by Ray Owen, who showed that the blood type of freemartin cattle—cows that were genetically female but had some masculine characteristics—was the same as that of their fraternal twins because of their shared blood circulation during foetal life, which caused their freemartinism as well as tolerance toward their twin's cells.81 Indeed, Medawar's animals that accepted their fraternal twin's skin were also freemartins. After this discovery, he started his systematic experiments to demonstrate tolerance using inbred mice, which culminated in his landmark discovery at University College London (where he began to teach from 1951).
Although this account is largely correct, it does not completely illuminate Medawar's complex path of research. Most of all, the idea that an intervention into embryos and foetuses would bring about significant changes did not stem only from Burnet's and Owen's work, since it also came from Medawar's own longstanding interest in the concatenation between growth and ageing. Medawar took note of Burnet's and Owen's studies because they struck a chord with Medawar's view that embryogenesis, as revealed in his tissue culture, was a key period that accompanied both rapid growth and ageing.82 Medawar later found that an animal's ability to develop tolerance also underwent rapid decline during its growth in embryonic and foetal stages. The idea that ageing and growth simultaneously occurred can also be found in his 1946 paper on the evolution of ageing, which postulated that senile changes took place right after birth.83
Medawar devised a new line of immunological research out of this 1946 paper, which proposed that tissue exchanges between old and young organisms would reveal significant information about the ageing processes. In 1951, Medawar's team began a systematic grafting study among animals of different ages.84 The goals of his research expanded because he also investigated the relevance of age in homografting as well as the mechanisms of senescence. Indeed, he had already investigated whether young rabbits differed from old in responding to homografts in 1945. He was also aware of James Murphy's and others' earlier work on embryos which temporarily accepted foreign grafts.85 In his own experiments in 1951 and 1952, using rabbits and mice, Medawar inquired if age differences between the graft and the host affected homografts and autografts.86 Simultaneously, he used a deep-freezer: he could temporarily stop the ageing of skin grafts by storing them at −70 °C for a while. Then, these ‘young’ tissues would be thawed and grafted to a host.87 What would happen to these ‘young’ homografts attached to an older host? Would they survive any longer?
There was another project that also addressed age and development. In 1947, Medawar's team showed that a piece of darker skin would gradually colour its neighbours with a lighter hue.88 Conspicuously, this experiment brought forth the significance of development, because Medawar explained it through cytoplasmic inheritance theories, which—according to historian Jan Sapp—were deeply associated with embryologists' view that development and heredity were integrated.89 In fact, Medawar's postulation of darker skin cells' cytoplasmic hereditary entity and its migration into its surrounding cells was based on embryologists' long-lasting speculation on the roles of cytoplasmic inheritance in cellular differentiation during foetal growth. Rupert Billingham later claimed that his and Medawar's study of cytoplasmic inheritance would contribute to explaining ‘embryonic differentiation’.90 It is probably no coincidence that Burnet, who focused on early life as the period of the formation of immune ‘self’, also incorporated cytoplasmic inheritance in his theory of antibody formation.91
The relationship between pregnancy and homografting pertained to this research. If embryonic and foetal life was important in forming immunity, pregnancy must be investigated, too. To Medawar, foetuses were similar to homografts, because both were genetically distinct from their host. As early as 1948, he thus tried to find a way to a successful homografting by investigating the mother–foetus relations, because foetuses were not normally subject to mothers' immune reaction, except for the case of the Rh disease, known to him through the problems of the wartime transfusion service which highlighted the risk of an Rh+ foetus in an Rh– mother's uterus.92 At that time, Medawar started investigating the influence of pregnancy on homografts' survival, alongside the influence of maternal hormones, including ‘cortisone or something like it’, which seemed to be secreted by the mother's body to reduce or stop its immunological reaction toward the foetus.93
In all these experimental projects, Medawar, Billingham and Brent experienced numerous problems, which they could partly manage. During their experiment on ageing, they improved their method of storage in a freezer, after finding ‘a faculty method’.94 They also managed some petty issues such as ‘confusion of labelling’ and overcame the problems of their coordination, especially when Brent—then a postgraduate student—made a mistake.95 Medawar's team could also find the right amount of cortisone for controlling the homograft response after their initially disappointing trials performed with inadequate amounts.96
But Medawar's experiments were still not entirely satisfactory. In particular, the sex of mice was a troublesome factor because he thought that female mice could become pregnant by males in the same cage and thereby—according to him—might show aberrant responses to homografts. He also thought that female mice could be killed or harmed by male mice.97 Hence, he tried to segregate animals according to their sex, but his effort occasionally failed, as he erred in confirming the sex of mice.
More problematic was what I have called the third type of failure, namely the disappointing attempts that could not be explained. For instance, a recipient animal, during an experiment on ageing, ‘died 2hrs AFTER OPERATION’ for no good reason.98 In another investigation, Medawar was not able to explain why grafts on a mouse grew so slowly. He suspected that ‘there is definitely something wrong with this animal’, but could not tell what was actually ‘wrong’.99 He also thought that operations that ‘were clumsily and incompetently done’ were the cause of the ‘excessive drying’ of the part on which extraneous tissues were grafted,100 but he could not point to the specific steps in the procedure in which he made a mistake.101 Likewise, he found that a graft's condition became ‘hopeless’ during an experiment on pigment spreading, but this ‘cannot be due to faulty technique’, since everything appeared properly done.102 Indeed, this issue was very annoying because he then transplanted autografts, which should usually be successful.
Medawar continued to face obstacles well into his main tolerance research using young mice. Based on his longstanding studies of growth and ageing, as well as Burnet's and Owen's investigations, he started homografting on newborn mice in July 1952 and in September he adopted even younger hosts, foetal mice.103 In these works, he consistently implemented his research scheme—inoculating young inbred hosts with cell suspensions from a different inbred strain and examining whether the host, after completing growth, could accept skin from the donor strain—but he found that ‘some of the experiments failed’.104 His laboratory notes clearly express his attitude toward these failures, which ‘must be prevented in future’.105
Indeed, Medawar appeared able to identify the causes of some failures, such as a ‘blunt’ needle, improper cell suspensions including ‘bony matter’, stitches that became ‘adrift’ after operation and an erroneous use of cortisone.106 In such cases, Medawar and his colleagues could easily correct the problems. They could also use the lymph nodes from mice that failed to develop tolerance for a different experiment—on the ‘passive transfer’ of immunity—in order to save materials.107
However, some of their problems could not be brought under complete control. In particular, foetus manipulation was not easy. In fact, Medawar and his colleagues had to ‘see’—and identify with his fingers—foetuses through the mother's body wall exposed by a mid-ventral incision.108 Therefore, they occasionally erred in counting the number of foetuses. The process of foetal injection was equally problematic. He sometimes killed his mice due to an overly ‘thorough’ injection of cell suspensions.109 He also injected cells into ‘the head region’ of foetal mice, which thereafter perished.110 But even more problematic was the third type. For example, Medawar could not account for some experimental animals' sudden death and stillbirth. Other mice were excessively sensitive to anaesthesia, yet he could not explain the reason.111 He also wrote that a ‘young mouse has disappeared without a trace’ and could not find it again, although he suspected that it was lost during cage changes.112
The irregularities within Medawar's inbred mice were equally annoying, even though he thought that he partially knew the causes. Since 1945, he had maintained several inbred mouse strains that he acquired from the Jackson Laboratory.113 Although all inbred mice in one strain should exchange skin without immune reaction, he found contrary evidence among some: definite immune reaction was found during intra-strain skin grafting with varying survival times. In fact, this finding was alarming because different inbred mice had to provide their skin after the original donor from the same strain was killed having given its organs for creating cell suspension. If the later skin donors were different from the original cell donor, it was hard to test whether the recipient developed tolerance.
To cope with this problem, Medawar pointed to several possible factors, which would cause not just graft breakdown but also ‘variation’ in the survival time. Initially, these factors included ‘residual heterozygosity in the inbred lines’, ‘small differences of graft dosage’, ‘differences of physiological state in the grafted skin’ and ‘differences in physiological state of the recipients’ including the influence of their ‘sex’.114 Later, he added another factor, namely ‘mutation’ during inbreeding.115 However, for reasons unknown, he came to disregard all of these factors except ‘residual heterozygosity’—the existence of some genetic heterogeneity within inbred strains. In truth, his transplantation test revealed that the aaUU strain (C-line) was slightly heterogeneous, and he decided to refrain from using this strain, especially for ‘Exp. 73’, detailed in his 1953 paper.116 However, it remained unknown if other factors were really irrelevant. Furthermore, some mice from the A-line, deemed reliable for its ‘known and charted inbreeding’, also displayed some adverse reactions against grafts from the same strain.117
Grafting between different strains was more straightforward, but was still irregular in many aspects. As this was homografting, there was no reason to expect that it would succeed. Yet, its survival time varied considerably, perhaps due to genetic heterogeneity and errors in technique. This variation thus led Medawar to perform some statistical analysis based on his measurement of ‘median survival time’ (MST), determined by a mathematical transformation of the ‘time-frequency distribution of the moments of graft death’ into a curve tracing the ‘percentage mortality’ over time.118 In this new curve, MST was defined as ‘the time at which this curve passes through the ordinate corresponding to 50% graft mortality’ (figure 2).
Figure 2.
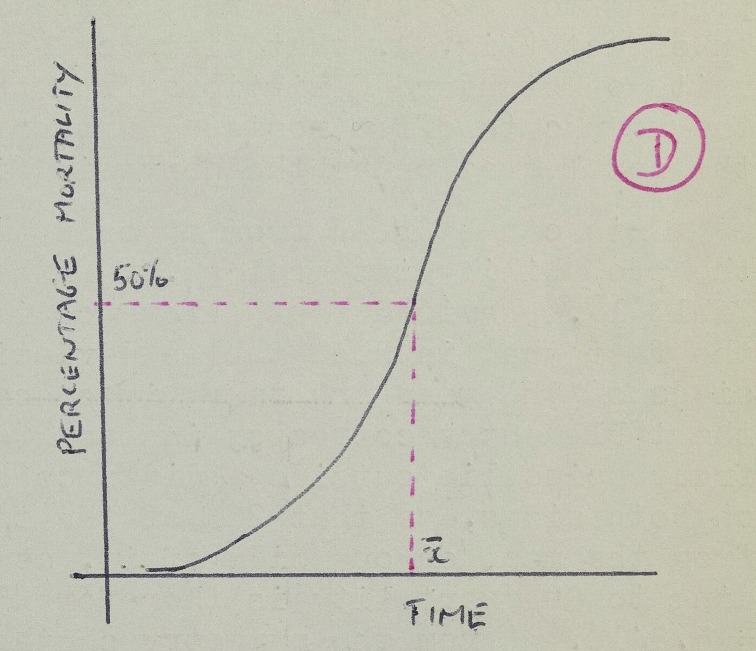
The percentage mortality of homografts over time. ‘Homograft survival times: B series’, Medawar's laboratory notes stored in Box 43, Folder C155, Peter Brian Medawar Papers, Wellcome Library, London. (Online version in colour.)
However, things were not entirely certain. The ‘time-frequency distribution’, which was supposed to be normal, ‘cannot of course be exactly normal: some skew-ness is to be expected, since there is no theoretical limit to time which a homograft may survive’.119 The timing of graft survival was also problematic, because host cells' proliferation may be mistaken as that of grafted skin.120 Moreover, some degenerating grafts had a small number of die-hard cells, whose long life hampered Medawar's attempt to distinguish dead from living grafts.121 If only 10% of a graft's cells were surviving, was it then alive or dead?
Medawar did not publish many of these complications in his 1953 Nature paper. Above all, this paper mentioned only one successful case, which he called ‘Exp. 73’, among a total of 15 sets of experiments on foetal mice conducted before the publication. Whereas all these experiments were recorded in his laboratory notes like a natural history, namely with a full chronological detail of trials and errors, his published account presented his work as a smooth progression toward success with no chronological references.122 Admittedly, he did mention the cases that did not exhibit tolerance in ‘Exp. 73’. Among the six foetal CBA mice inoculated with A-line cells, only two displayed lifetime tolerance toward A-line skin, while another tolerant mouse exhibited ‘a long-drawn-out “spontaneous” involution’ of its graft.123 Among the other three, one was not even born, and the remaining two rapidly rejected new tissues from the A-strain. The problem was that this was not the only issue. His paper did not even mention that he inadvertently picked up a pregnant female mouse for a second-set graft, although he wanted to choose a male that could not become pregnant. Because he suspected that pregnancy influenced homograft survival, this was a mistake that he acknowledged only in his laboratory notes.124 Similarly, he came to use a pregnant female mouse as a recipient of the CBA lymph nodes immunized against A-line in examining the restoration of immunity, which would demonstrate that tolerance was ‘due to a failure of the host's immunological response’.125 Yet, its A-line skin stayed for a longer period than he expected before its breakdown, probably because of the host's pregnancy.126 But a greater disparity was found in the actual success rate. Whereas Medawar's 1953 paper described his production of two tolerant mice out of six, the rate recorded in his laboratory notes was far lower (table 1).
Table 1.
A tabulation of Medawar's experiments for inducing tolerance by inoculating foetal mice with cells from mice of different inbred strains, from September 1952 to May 1953. The data came from Medawar's laboratory notes stored in Box 43, Folder C156, Peter Brian Medawar Papers, Wellcome Library, London.
| Cases | Injected | Failed | Survival for 12–14 days | Survival for 15–30 days | Survival for 31 days or longer |
|---|---|---|---|---|---|
| EMB-85 | 3 | 1 | 1 | 1 | |
| EMB-68 | 4 | 3 | 1 | ||
| EMB-79 | 6 | 5 | 1 | ||
| EMB-103 | 4 | 2 | 1 | 1 | |
| EMB-102 | 7 | 6 | 1 | ||
| EMB-100 | 6 | 5 | 1 | ||
| EMB-94 | 3 | 1 | 2 | ||
| EMB-87 | 4 | 3 | 1 | ||
| EMB-77 | 5 | 3 | 2 | ||
| EMB-74 | 5 | 3 | 2 | ||
| EMB-73 | 6 | 3 | 3 | ||
| EMB-69 | 5 | 5 | |||
| EMB-64 | 5 | 4 | 1 | ||
| EMB-41 | 7 | 6 | 1 | ||
| EMB-180 | 7 | 6 | 1 | ||
| Total | 77 | 56 | 4 | 11 | 6 |
| Rate of success (all) | 27.3% | ||||
| Rate of success (graft survival for 15 days or longer) | 22.1% | ||||
| Rate of success (graft survival for 31 days or longer) | 7.8% | ||||
Among the 77 mice inoculated in utero, those that tolerated homografts for more than 15 days numbered 17 (22.1%), among which only six tolerated for 31 days or longer (7.8%). His laboratory notes clearly expressed his feeling toward some of these unsuccessful attempts: it was ‘depressing’, especially when inoculated mice could not even have a chance to exhibit tolerance owing to their untimely death.127 Admittedly, some will say that Medawar had no obligation to publish all these failed works. I agree, but the absence of his reference to the failures made his narrative in the paper awkward. After discussing ‘Exp. 73’, he suddenly mentioned the result of his inoculations into newborn animals, in which ‘only nine mice [out of 96] showed an increase of tolerance’.128 The word ‘only’ would make sense if he had already shown the full records of his foetal inoculations, whose success rate was supposed to be higher. According to my own count, even this success rate for inoculations into newborn mice (9.4%) was exaggerated, because it probably included rather questionable cases in which grafts survived just for a few additional days plus MST (table 2).129 The mice that tolerated foreign grafts for 15 days or longer were merely four (4.4%), and only one among them showed lifetime tolerance (1.1%).
Table 2.
A tabulation of Medawar's experiments for inducing tolerance by inoculating newborn mice with cells from mice of different inbred strains, from July 1952 to May 1953. The data came from Medawar's laboratory notes stored in Box 43, Folder C156, Peter Brian Medawar Papers, Wellcome Library, London.
| Cases | Injected | Failed | Survival for 12–14 days | Survival for 15–30 days | Survival for 31 days or longer |
|---|---|---|---|---|---|
| EMB-16 | 5 | 5 | |||
| EMB-14 | 6 | 6 | |||
| EMB-12 | 4 | 4 | |||
| EMB-24 | 7 | 7 | |||
| EMB-31 | 6 | 6 | |||
| EMB-57 | 7 | 5 | 2 | ||
| EMB-58 | 2 | 1 | 1 | ||
| EMB-86 | 9 | 9 | |||
| EMB-28 | 6 | 5 | 1 | ||
| EMB-17 | 6 | 4 | 2 | ||
| EMB-62 | 7 | 6 | 1 | ||
| EMB-8 | 8 | 8 | |||
| EMB-7 | 6 | 6 | |||
| EMB-19 | 7 | 7 | |||
| NB-10 | 4 | 3 | 1 | ||
| Total | 90 | 82 | 4 | 3 | 1 |
| Rate of success (all) | 8.9% | ||||
| Rate of success (graft survival for 15 days or longer) | 4.4% | ||||
| Rate of success (graft survival for 31 days or longer) | 1.1% | ||||
The low success rate could raise a question on Medawar's claim with regard to ‘Burnet and Fenner's … theory’ on the prenatal formation of the immunological ‘self’. Could Medawar's work really be the decisive evidence supporting Burnet's theory if only a small fraction of inoculated mice developed permanent tolerance toward the donor strain?130 Perhaps these fully tolerant mice might be a result of mere chance, if he were to remain consistent with his statement that ‘there is no theoretical limit to time which a homograft may survive’.131 Equally troublesome were his temporarily tolerant mice. Did they ever support Burnet's theory? Maybe they could reflect Medawar's technical mistakes or the genetic heterogeneity of his mice, but their widely varying survival times also indicated that he could not identify—let alone control—the cause of the problems. In effect, he worried about this for a long time, at least until 1986: ‘survival times varied, of course, however much one tried to standardize the conditions under which transplantations were carried out’.132
Medawar's response to these problems was not to find out all their causes. Such efforts might be too time-consuming and unproductive. Perhaps his team could also consider any ‘hidden variable’ behind the low success rate, but ‘Medawar's group, which was so creative in other respects, did not at the time suspect that there might be’ such variables.133
Instead, the Medawar team managed the problems by being strategically ambiguous. This ambiguity came from the two mathematical traditions he had incorporated since the 1940s. If Fisher and Haldane told Medawar that mathematics was useful for addressing diversity and variability in nature, Thompson taught him that there was an unequivocal truth delineated rigorously in mathematical terms. Indeed, referring to the non-tolerant mice of ‘Exp. 73’, Medawar said that ‘this was because they were imperfectly injected’.134 Although Medawar did not mention all other problems—including the heterogeneity of inbred mice, mutation, mistakes in inoculations, hosts' physiological condition, incorrect counting and the ambiguity in measuring graft survival—this statement assumed the possibility of an ideal experiment for ‘perfect’ tolerance. However, he also wrote that ‘the conferment of tolerance is not of an all-or-nothing character; every degree is represented, down to that which gives the test-grafts only a few days of grace beyond the median survival time’. In this statement, he translated errors into the inherent variability of tolerance as a natural phenomenon which could be quantitatively represented around MST. As a result, it became hard to distinguish usual homografts that were destroyed after 13 days of operation from some of his ‘successful’ grafts on mice inoculated with A-line cells right after birth. If both grafts stayed on their hosts' body just for 11 days of MST plus two additional days, why, then, did the former represent a normal breakdown but the latter tolerance?
Reflecting this ambiguity, Medawar proposed a theoretical scheme subsuming irregularities in an apparent order. As I have mentioned, he noted that the rate of failure for inoculations into newborn animals was higher than that for foetal operations. In effect, the graft survival times of most newborn inoculated mice were close to MST—indicating that tolerance failed to develop—while even later inoculation triggered more clear-cut immune reactions in mice, with homograft survival times shorter than MST.135 Then he could say that ‘the pattern of the host's response to foreign tissue cells is turned completely upside down’ from tolerance to immunity during growth. In this scheme, he might assign positive numbers to tolerance as the survival time of grafts on tolerant mice was longer than MST, but negative numbers should be given to immunity as most homografts broke down before reaching MST. Between these two poles, a short span of time after birth was called the ‘null period’, because newborn mice—whose grafts' duration of survival was almost equal to MST—usually showed neither tolerance nor immunity.136 To him, this trend pointed to a steady decline, as his later publication further confirmed. In 1956, Medawar's team showed that very young foetuses, before their eighteenth day after conception, had a greater chance (58%) of developing tolerance than older foetuses (13%).137 In other words, they found failed cases less often with the younger foetuses. Citing two American scientists, Jack Cannon and William Longmire, who discovered a similar phenomenon, Medawar thus claimed that there was ‘the progressive decay, with [hosts’] increasing age, of the power of an antigenic stimulus to confer tolerance'.138 This conclusion was consistent with his earlier view that growth accompanied ageing.
But Medawar's theory was not entirely consistent with his rhetorical presentation of his finding as a possible clinical innovation. His theory, reflecting Medawar's interpretation of the quantitative pattern of failures during the growth of host mice, could potentially facilitate a breakthrough in clinical transplantation, but these failures contributing to his theory made its realization questionable. He indeed claimed that ‘actively acquired tolerance’ was ‘the exact inverse of “actively acquired immunity”’, based on his view that an animal's growth accompanied a decline of its power to develop tolerance, which decreased the rate of success in Medawar's experiments and ultimately brought forth immunity.139 If actively acquired immunity was useful, then actively acquired tolerance could also be useful. Yet, these two phenomena were different. While most forms of ‘actively acquired immunity’ were relatively easy to trigger with vaccination, ‘actively acquired tolerance’ was far more difficult, as his repeated failures demonstrated. In a sense, this problem echoed the ambiguity between failure and nature's variability, a longstanding problem in his research. If his theory represented the dynamics of the transformation from tolerance to immunity, the lack of its clinical prospect reflected the other side of this dynamic—those mice that failed to develop tolerance even after receiving foreign cells during their early life.
In retrospect, there was a major reason why Medawar's work had no clinical relevance. Later, Medawar's team discovered that the causes of this continuing trouble would be found not just in ‘technical errors’ and other irregularities but the graft-versus-host disease, an illness caused by the graft's leucocytes attacking the host.140 Unfortunately, they did not know anything about this disease in the period 1951 to 1956 when they were actively investigating tolerance using young mice.
At the time, Medawar declared that he found a ‘solution’ to a problem that he had long struggled with since the war.141 Although he wrote that it was just a ‘“laboratory” solution’ at the time, he felt ‘certain that the clinical homograft problem is soluble’, and some readers would also feel that his work could bring about an actual clinical application comparable to vaccination.142 These readers, including surgeons, were encouraged by Medawar's work, especially his reference to acquired immunity, which was a convincing rhetoric. Yet, they ultimately had to search for different kinds of tolerance induced by distinct factors, including radiation and immunosuppressants.143
This problem reflected the contradictory roles of failures in Medawar's work. Being likened to acquired immunity, tolerance became a phenomenon with a definite category, which could be violated through failures. Still, these failures did not just violate but also constituted the boundary of tolerance, which formed a long continuum toward its opposite pole, immunity. It was through his failures that Medawar built his theory, placing both tolerance and immunity in a quantitative and temporal continuum within which he forged his claim for a clinical relevance. Curiously, the relevance was contradicted by this very continuum, comprising his interpretation of failures.
Conclusion
Medawar's research has been known as an exemplary scientific achievement with its multiple contributions, including the creation of practical and epistemic bridges between laboratories and clinics in biomedicine.144 I have illuminated his work from a different perspective. Despite its pivotal role in biomedicine, Medawar's work had no clinical relevance. No doctor undertook the risky task of inoculating foreign cells into human foetuses in preparation for their future transplantation.145 But why was it so risky? An obvious reason was found in the low success rate of his experiments. His publications reflected only a small portion of his laboratory research, during which he tried not only to control but also to appropriate various failures.
This paper illustrates how he managed these problems. He certainly learned from his failures, and also utilized materials resulting from unsatisfactory cases for other purposes. But, in some instances, he could not understand why his experiments failed. Nevertheless, he incorporated these failures under a theoretical, rhetorical and statistical scheme. Utilizing two different mathematical approaches, Medawar placed his failures between a deviation from the normal practice and a part of nature's diversity and variability. With this ambiguity, he crafted a theory that integrated his failures in a quantitative–temporal scheme, which enabled him to propose a clinical utility in a rhetorical framework that was contradicted by the basis of the theory—the failures.
This contradiction highlights a new dimension of failures in science. It has been well known that failures can help scientists be creative, but their multiple roles in research programmes have not been well understood. Medawar was undoubtedly a great scientist, and the failures during his research played heuristic roles in his success. Simultaneously, they were also limiting, as his successful research had little clinical implication owing to the manifold failures constituting his experiments.
Acknowledgements
I wish to express my deep gratitude toward Hasok Chang and the anonymous referees for their highly valuable comments on earlier versions of this paper. I also thank Leslie Brent for sharing his memory of Medawar and his team during the 1940s and the 1950s. A preliminary form of this paper was presented at the History of Science Society Annual Meeting at Atlanta in 2016. I am very grateful to William Summers, who gave me his insightful comments on this presentation. In addition, I thank Er Xue Min for her work on the scanned files of Medawar's laboratory notes, which made them printable and thus eased my analysis of his experiments. My project was supported by the Academic Research Fund Tier-1 from the Ministry of Education in Singapore (RG 74/14).
NOTES
Rupert E. Billingham, Leslie Brent and Peter Medawar, ‘“Actively acquired tolerance” of foreign cells’, Nature 172, 603–606 (1953).
Warwick Anderson and Ian Mackay, Intolerant bodies: a short history of autoimmunity (Johns Hopkins University Press, Baltimore, 2014), esp. pp. 80–81; Thomas Pradeu, The limits of the self (Oxford University Press, 2012), pp. 53–64; Arthur Silverstein, A history of immunology (Academic Press, London, 2009), 2nd edn, pp. 165–166, 347–365; Alfred Tauber, Immune self: theory or metaphor? (Cambridge University Press, 1994), pp. 81–123; William C. Summers, Félix d'Herelle and the origins of molecular biology (Yale University Press, New Haven, 1999), pp. 47–53, 73–76, 107–112.
David Hamilton, A history of organ transplantation (University of Pittsburgh Press, 2012), pp. 240–241.
Although scientists have written much about Medawar, few historians have paid enough attention to his work, except for short references within a general historical account of immunology. For recent historical scholarship on Medawar, see Neil Calver, ‘Sir Peter Medawar: science, creativity and the popularization of Karl Popper’, Notes Rec. R. Soc. 67, 301–314 (2013); Neil Calver and Miles Parker, ‘The logic of scientific unity? Medawar, the Royal Society and the Rothschild controversy 1971–72’, Notes Rec. R. Soc. 70, 83–100 (2016). See also my paper: Hyung Wook Park, ‘“The shape of the human being as a function of time”: time, transplantation and tolerance in Peter Brian Medawar's research, 1937–1956’, Endeavour 34, 112–121 (2010).
Ben Marsden investigated failures in engineering by tracing the views of historical actors themselves. In historical research, I think that this approach is useful. See Ben Marsden, ‘Blowing hot and cold: reports and retorts on the status of the air engine as success or failure, 1830–1855’, Hist. Sci. 36, 373–420 (1998).
Peter Brian Medawar, Memoir of a thinking radish: an autobiography (Oxford University Press, 1986), p. 132.
Giora Hon, ‘Towards a typology of experimental errors: an epistemological view’, Stud. Hist. Phil. Sci. 20, 469–504 (1989); Antonio Cadeddu, ‘The heuristic function of “error” in the scientific methodology of Louis Pasteur’, Hist. Phil. Life Sci. 22, 3–28 (2000); Douglas Alchin, ‘Error types’, Perspec. Sci. 9, 38–59 (2001); Kathryn Olesko, ‘The meaning of precision: the exact sensibility in early nineteenth-century Germany’, in The values of precision (ed. M. Norton Wise), pp. 103–134 (Princeton University Press, 1995); Gerald Geison, The private science of Louis Pasteur (Princeton University Press, 1995), pp. 110–142; Jutta Schickore, ‘“Through thousands of errors we reach the truth”—but how? On the epistemic roles of error in scientific practice’, Stud. Hist. Phil. Sci. 36, 539–556 (2005); Jerome Ravetz, Scientific knowledge and its social problems (Clarendon Press, Oxford, 1971), pp. 94–101; Sara Delamont and Paul Atkinson, ‘Doctoring uncertainty: mastering craft knowledge’, Soc. Stud. Sci. 31, 87–107 (2001); Giora Hon, Jutta Schickore and Friedrich Steinle (eds), Going amiss in experimental research (Springer, Dordrecht, 2009). In a similar vein, Cyrus Mody has shown multiple dimensions of ‘contamination’ in engineering research, including its constructive roles. See Cyrus Mody, ‘A little dirt never hurt anyone: knowledge-making and contamination in materials science’, Soc. Stud. Sci. 31, 7–36 (2001).
Stuart Firestein, Failure: why science is so successful (Oxford University Press, 2016).
Henry Petroski, To engineer is human: the role of failure in successful design (St Martin's, New York, 1985); Design paradigms: case histories of error and judgment in engineering (Cambridge University Press, 1994). For case studies in the history of technology, see also Timothy Stoneman, ‘A bold new vision: the VOA radio ring plan and global broadcasting in the early Cold War’, Technol. Cult. 50, 316–344 (2009); Kenneth Lipartito, ‘Picturephone and the information age: the social meaning of failure’, Technol. Cult. 40, 50–81 (2003).
Although Stuart Firestein also tried to define and classify many different kinds of failures, he concludes that this may not be a very productive job. According to him, failures are too elusive, confusing and ubiquitous to be subject to a clear definition and classification. See Firestein, op. cit. (note 8), pp. 7–24.
Hasok Chang, ‘Who cares about the history of science?’, Notes Rec. R. Soc. 71, 91–107 (2017). See also Hasok Chang, Inventing temperature: measurement and scientific progress (Oxford University Press, 2004).
Hans-Jörg Rheinberger, Toward a history of epistemic things: synthesizing proteins in the test tube (Stanford University Press, 1997). See also Hans-Jörg Rheinberger, ‘Experimental reorientations’, in Going amiss in experimental research (ed. Giora Hon, Jutta Schickore and Friedrich Steinle), pp. 75–90 (Springer, Dordrecht, 2009).
Graeme Gooday, ‘Re-writing the “book of blots”: critical reflections on histories of technological “failure”’, Hist. Technol. 14, 265–291 (1998).
My previous paper has shown that biomedical scientists, like surgeons and technicians, can fail without knowing what made their work go amiss. This is in part due to ‘irregularities of the body’, including those of experimental and clinical subjects as well as the hands of investigators. See Hyung Wook Park, ‘Constructing failure: Leonard Hayflick, biomedicine and the problems with tissue culture’, Ann. Sci. 73, 303–327 (2016), at pp. 305–306; John Senior, ‘Metrological awakenings: rationalising the body electric in nineteenth-century medicine’, in The road to medical statistics (ed. Eileen Magnello and Anne Hardy), pp. 77–93 (Rodopi, Amsterdam, 2002), at p. 86.
Billingham et al., op. cit. (note 1), p. 604.
Theodore M. Porter, The rise of statistical thinking (Princeton University Press, 1986).
Ian Hacking, The taming of chance (Cambridge University Press, 1990).
J. Rosser Matthews, ‘Almroth Wright, vaccine therapy and British biometrics: disciplinary expertise versus statistical objectivity’, in The road to medical statistics (ed. Eileen Magnello and Anne Hardy), pp. 125–147 (Rodopi, Amsterdam, 2002); Eileen Magnello, ‘The introduction of mathematical statistics into medical research: the roles of Karl Pearson, Major Greenwood and Austin Bradford Hill’, in The road to medical statistics (ed. Eileen Magnello and Anne Hardy), pp. 95–123 (Rodopi, Amsterdam, 2002). Related questions are addressed in Gérard Jorland, Annick Opinel and George Weisz (eds), Body counts: medical quantification in historical and sociological perspective (McGill-Queen's University Press, Montreal, 2005).
Tiago Moreira and Paolo Palladino, ‘“Population laboratories” or “laboratory populations”? Making sense of the Baltimore longitudinal study of aging, 1965–1987’, Stud. Hist. Phil. Biol. Biomed. Sci. 42, 317–327 (2011).
Matthews, op. cit. (note 18), p. 144.
Peter Medawar, ‘My life in science (1) Sir Peter Medawar’, 25 April 1966, p. 6, in Box 3, Folder A40, Peter Brian Medawar Papers, Wellcome Library, London (hereafter PBM); ‘Is the scientific paper a fraud?’ in Experiment: a series of scientific case histories first broadcast in the BBC Third Programme (ed. David Edge), pp. 7–12 (British Broadcasting Corporation, London, 1964).
Calver, op. cit. (note 4); Calver and Parker, op. cit. (note 4).
Medawar, op. cit. (note 6), p. 64.
Magnello, op. cit. (note 18), p. 97. See D'Arcy Wentworth Thompson, On growth and form (Cambridge University Press, 1942), pp. 1092–1095.
See the Medawar–Thompson correspondence in Box 2, Folder A24, PBM. Evelyn Fox Keller argues that Thompson failed to persuade biologists except Medawar. See Evelyn Fox Keller, Making sense of life: explaining biological development with models, metaphors and machines (Harvard University Press, Cambridge, MA, 2002), pp. 61, 68–76.
See the Medawar–Fisher correspondence in Ronald A. Fisher Papers (hereafter RAF), Rare Books and Special Collections, University of Adelaide Library, Adelaide, Australia. Haldane commented on Medawar's papers. See Haldane to Medawar, undated, Box 17, Folder C23, PBM; Medawar to Haldane, 30 June 1946, Box 45, Haldane/5/2/3/85, Special Collections, University College London, London. Furthermore, Medawar's tutor, John Z. Young, was a student of Gavin de Beer, a close colleague of Julian Huxley. On the Oxford zoologists around Medawar, see Steven James Waisbren, ‘The importance of morphology in the evolutionary synthesis as demonstrated by the contributions of the Oxford group: Goodrich, Huxley and de Beer’, J. Hist. Biol. 21, 291–330 (1988). It is unclear in which year Medawar began his interaction with these scholars, but throughout the 1940s he cited and corresponded with both Thompson and the evolutionary biologists without expressing any sense of contradiction between their distinct viewpoints.
Peter Medawar, ‘Oxford zoology’, Biology, Autumn Term, 1–4 (1944).
Peter Medawar, ‘A factor inhibiting the growth of mesenchyme’, Q. J. Exp. Physiol. 27, 147–160 (1937). Medawar later wrote, ‘I still don't know even approximately what the chemical nature of … inhibitory factor was’. See Medawar, op. cit. (note 6), p. 68. It is unclear when he started and ended this research during the late 1930s.
Peter Medawar, ‘The growth, growth energy and ageing of the chicken's heart’, Proc. R. Soc. Lond. B Bio. 129, 332–355 (1940), at p. 344. The details of Medawar's mathematical approach used in this paper can be found in Hyung Wook Park, ‘Refiguring old age: shaping scientific research on senescence, 1900–1960’, PhD thesis, University of Minnesota (2009), pp. 133–137.
Park, op. cit. (note 4), pp. 112–114.
Peter Medawar, ‘The shape of the human being as a function of time’, Proc. R. Soc. Lond. B Bio. 132, 133–141 (1944), at p. 133.
Peter Medawar, ‘Old age and natural death’, Mod. Quart. 2, 30–49 (1946). On the influence of the Modern Synthesis, see Medawar's handwritten manuscripts in Box 17, Folder C23, C24, C26, PBM.
Hyung Wook Park, Old age, new science: gerontologists and their biosocial visions (University of Pittsburgh Press, 2016), pp. 183–190.
George Williams, ‘Pleiotropy, natural selection and the evolution of death’, Evolution 11, 398–411 (1957); Michael R. Rose, Evolutionary biology of aging (Oxford University Press, New York, 1991); S. C. Stearns, M. Ackermann, M. Doebeli and M. Kaiser, ‘Experimental evolution of aging, growth and reproduction in fruit flies’, Proc. Natl Acad. Sci. USA 97, 3309–3313 (2000). On the British concerns on the ageing populations in the mid twentieth century and the new policies reflecting these concerns, see Pat Thane, Old age in English history (Oxford University Press, 2000), pp. 344–351, 364–371. Medawar mentioned the Royal Commission and the social problems of ageing during his 1951 inaugural lecture at University College London. See Peter Medawar, An unsolved problem of biology: an inaugural lecture delivered at University College London, 6 December 1951 (Lewis, London, 1952), p. 4.
Vladimir Korenchevsky, ‘British Society for Research on Ageing, circular letter no 25’, 31 January 1955, Box 4, Folder C15, Dame Honor Bridget Fell Papers, Wellcome Library, London; Park, op. cit. (note 33), pp. 172–179, 189–192.
Medawar, op. cit. (note 34), pp. 3–4.
Medawar, op. cit. (note 6), p. 84.
Medawar to Leonard Colebrook, 6 March 1967, p. 10, Box 5, Folder C8, Leonard Colebrook Papers, Wellcome Library, London. ‘Homografts’ and ‘allografts’ are now used interchangeably, but in Medawar's time, most transplantation researchers used the former to indicate grafts whose genetic constitution was homologous with, but not the same as, that of the recipient. The homology reflects that the donor and the recipient belonged to the same species.
Peter Medawar, ‘Tolerance and tissue transplantation’, 1968, Box 18, Folder C34, PBM. He went to Glasgow through the recommendation of Frank Green of MRC, who was quite ‘impressed’ by Medawar's experimental studies for overcoming the homograft problems, one of which had been published in the Bulletin of War Medicine. See Medawar, op. cit. (note 6), p. 80.
But Medawar was not the first scientist who discovered the immunological nature of graft rejection. See Silverstein, op. cit. (note 2), pp. 234–237. See also Thomas Schlich, The origins of organ transplantation: surgery and laboratory science, 1880–1930 (University of Rochester Press, 2010).
Thomas Gibson and Peter Brian Medawar, ‘The fact of skin homografts in man’, J. Anat. 77, 299–310 (1943), at p. 299. Medawar, then, was choosing a side in the longstanding debate between humoral and cellular immunity, which started in the nineteenth century. He believed that the immunity against homografts was humoral because it was mediated by antibodies in the bodily ‘humor’. In contrast, he believed, lymphocytes were fixed in their local tissues and played little role in the homograft rejection. See Hamilton, op. cit. (note 3), p. 182. On the debate in historical perspectives, see Arthur Silverstein, ‘Cellular versus humoral immunology: a century-long dispute’, Nat. Immunol. 4, 425–428 (2003).
When a tissue or organ is grafted twice from a single individual, or a different individual belonging to the same inbred strain, they are called the first- and second-set grafts, respectively.
‘Tolerance and tissue transplantation’, Box 18, Folder C35, PBM.
See, for example, ‘Medical Research Council: form of application for a research grant application by P. B. Medawar’, 2 January 1943, FD 1/6959 (Medawar's skin grafting 1942–44), The National Archives, Kew, Richmond, Surrey, UK (hereafter TNA).
‘2nd Dressing’, 29 April 1943; ‘Foreign serum: controls’, 12 April 1943, Box 12, Folder C2, Pt3, PBM.
‘Pinch autografts: trial’, 23 March 1943; ‘Pinch autografts: trial (2)’, 2 April 1943, Box 12, Folder C2, Pt3. ‘Opn, anaerobiosis test (+ iodoacetate) (3 days) AERO: S-23’, 24 May 1946, Box 13, Folder C4, PBM.
‘4th dressing’, 6 May 1943, Box 12, Folder C2, Pt3; ‘Summary tables: detection of antibodies in vitro, etc. 15’, Box 12, Folder C3, Pt1, PBM.
‘Opn, J-16: Submaxillary gland donor (DJ): 552’, 28 June 1946; ‘Opn, D-pair2: Homografts (reverse in vivo) (DD): 490’, 23 January 1946; ‘Opn, blood-series 4: White-cell recipient (RL)’, 27 March 1945, Box 12, Folder C3, Pt2, PBM.
‘Opn, S-pair 3: homograft donor (DS): 396’, 16 March 1945, Box 12, Folder C3, Pt2, PBM.
‘Pinch autografts: trial’, 23 March 1943, Box 12, Folder C2, Pt3, PBM.
‘2nd Dressing’, 29 April 1943, Box 12, Folder C2, Pt3, PBM.
On the thickness, Medawar wrote, ‘the skin incision must not go down to the body wall, but must as far as possible be immediately subcutaneous and over the level of the subcutaneous veins running in the plane of the skin’. On the size, he wrote that he must ‘use smaller pinches in a smaller area’. He did not write down the specific size in his lab notes. See ‘Pinch autografts: trial’, 23 March 1943; ‘Pinch autografts: trial (2)’, 2 April 1943, Box 12, Folder C2, Pt3, PBM.
‘Homograft (high dosage): 106’, 20 December 1943, Box 12, Folder C2, Pt3, PBM. But it is unclear how Medawar managed the problem of stressed animals, which probably came from the small size of their cage.
Kathleen Jordan and Michael Lynch, ‘The mainstreaming of a molecular biological tool: a case study of a new technique’, in Technology in working order: studies of work, interaction and technology (ed. Graham Button), pp. 162–178 (Routledge, London, 1993), at pp. 170–174; Pearl Katz, ‘Ritual in the operating room’, Ethnology 20, 335–350 (1981), at pp. 335–336.
In Florey's laboratory, Medawar studied the toxicity of penicillin, while investigating the senescence of cultured chicken cells. See ‘Personal records of Fellows of the Royal Society’, Box 1, Folder A13, PBM.
‘Opn, Q-pair 2: homograft donor (DQ): 457’, 30 August 1945, Box 12, Folder C3, Pt2, PBM.
Interview with Leslie Brent on 31 May 2016.
‘Pinch autograft: 16’, 24 May 1943, Box 12, Folder C2, Pt3, PBM. Interview with Brent, 31 May 2016.
‘Opn, S-pair 14: homograft donor (DS): 445’, 3 June 1945, Box 12, Folder C3, Pt2, PBM.
‘Autolysis recovery test’, 30 April 1944, Box 12, Folder C2, Pt3, PBM.
‘Opn, D-pair 2: homografts (reverse in vivo): 490’, Box 12, Folder C3, Pt2, PBM.
‘Opn, Q-12: donor: 604’; ‘Opn, X-4: 607’; ‘Opn, Q-13: recipient: 609’, Box 12, Folder C3, Pt2, PBM.
‘Homograft donor’, 9 December 1943; ‘LAB No 1256’, 5 February 1944, Box 12, Folder C2, Pt3, PBM.
‘Detection of antibodies in vitro: eighth experiment’, Box 12, Folder C3, Pt1; ‘Opn, Q-pair 2: homograft recipient from 2 donors (RQ): 458’; ‘Opn, Het-3: mouse-rabbit heterografts: 508’, Box 12, Folder C3, Pt2, PBM.
Peter Medawar, ‘A second study of the behaviour and fate of skin homografts in rabbits’, J. Anat. 79, 157–177 (1945), at p. 167.
According to Medawar's usage, heterotopic grafting was the movement of a type of tissue into an organ comprising a different kind of tissue. In this case, a skin patch was grafted to the brain and the eye. See ‘Summary tables: heterotopic grafts: 3 and 7’, Box 12, Folder C3, Pt1, PBM.
‘Summary tables: heterotopic grafts: 10’, Box 12, Folder C3, Pt1, PBM.
Medawar, op. cit. (note 65), pp. 162–163.
‘Summary tables: detection of antibodies in vitro, etc’, p. 5, Box 12, Folder C3, PBM.
‘Summary tables: mitotic counts’, p. 5, Box 12, Folder C3, PBM.
Peter Medawar, ‘Immunity to homologous grafted skin. I. The suppression of cell division in grafts transplanted to immunized animals’, Brit. J. Exp. Pathol. 27, 9–24 (1946), at p. 11.
‘Summary tables: blood series: third experiment’, p. 5, Box 12, Folder C3, Pt1, PBM.
‘Summary tables: mitotic counts’, p. 4, Box 12, Folder C3, PBM; Medawar, op. cit. (note 71), p. 11.
Matthews, op. cit. (note 18). Also see Moreira and Palladino, op. cit. (note 19).
Medawar, op. cit. (note 71), p. 11.
Park, op. cit. (note 4), pp. 114–115.
Peter Medawar, ‘The behaviour and fate of skin autografts and skin homografts in rabbits’, J. Anat. 78, 176–199 (1944), at pp. 188–189. Developed by the American entomologist Chester Bliss, probit analysis is a kind of regression analysis used for identifying the relationship between two factors. To Medawar, these two were time and the death of tissue grafts. From a normal distribution curve of time and tissue mortality frequency, Medawar drew a graph showing the relationship between time and the probit mortality of grafted tissues, expressed as ‘the percentages of graft mortality as areas of the normal curve of error in terms of the normal deviate’. Medawar probably learned this analysis directly from the paper of Bliss himself, as well as Ronald Fisher, who wrote an appendix of Bliss's paper. See Ronald Fisher, ‘The case of zero survivors in probit assays’, in Chester I. Bliss, ‘The calculation of the dosage-mortality curve’, Ann. Appl. Biol. 22, 134–167 (1935), at pp. 164–165.
Silverstein, op. cit. (note 2), p. 242; Anderson and Mackay, op. cit. (note 2), pp. 80–81. Medawar himself discussed this episode in detail. See Peter Medawar, ‘Burnet and immunological tolerance’, in Walter and Eliza Hall Institute of Medical Research annual review: a tribute to Sir Macfarlane Burnet, pp. 31–33 (Exchange Press, Melbourne, 1979).
D. Anderson, R. E. Billingham, G. H. Lampkin and P. B. Medawar, ‘The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle’, Heredity 5, 379–397 (1951); R. Billingham, G. Lampkin, P. Medawar and H. Williams, ‘Tolerance to homografts, twin diagnosis and the freemartin condition in cattle’, Heredity 6, 201–212 (1952).
Frank Macfarlane Burnet and Frank Fenner, The production of antibodies (Macmillan, London, 1949), p. 102.
Ray Owen, ‘Immunogenetic consequences of vascular anastomoses between bovine twins’, Science 102, 401–402 (1945). Medawar then ignored another key paper by Erich Traub that Burnet cited. See Erich Traub, ‘Epidemiology of lymphocytic choriomeningitis in a mouse stock observed for four years’, J. Exp. Med. 69, 801–817 (1939).
Park, op. cit. (note 4).
Medawar did not separate growth and ageing in his evolutionary theory of ageing. As the hypothetical organisms in his theory were subject to the pressure of natural selection right after birth, the evolutionary changes for the creation of senile symptoms could take place at the earliest phases of life. For more details, see Park, op. cit. (note 33), pp. 186–190.
‘Miss Whitby's aged rabbit: SNR-1’, 22 December 1951, Box 13, Folder C4, PBM.
‘The theory of the differences between individuals’, 1946, p. 103, Box 36, Folder E23, PBM.
‘Young to old: SnM-1’, Mar–May 1952, Box 13, Folder C4; ‘Series ‘IMM’, November 1952–March 1953, Box 43, Folder C155, PBM.
‘Skin storage experiments (Exp. 2): SnR-2.5’, 26 May 1953, Box 13, Folder C4, PBM; R. E. Billingham and P. B. Medawar, ‘The freezing, drying and storage of mammalian skin’, J. Exp. Biol. 29, 454–468 (1952). The use of freezers brought about a complex set of problems and prospects in biomedicine. See, for example, Joanna Radin, ‘Latent life: concepts and practices of human tissue preservation in the international biological program’, Soc. Stud. Sci. 43, 484–508 (2013).
R. E. Billingham and P. B. Medawar, ‘The “cytogenics” of black and white guinea pig skin’, Nature 159, 115–117 (1947); ‘Pigment spread and cell heredity in guinea-pigs’ skin', Heredity 2, 29–47 (1948).
Jan Sapp, Beyond the gene: cytoplasmic inheritance and the struggle for authority in genetics (Oxford University Press, 1987).
Rupert E. Billingham, ‘Reproduction of cytoplasmic particles: “infective” cellular transformations’, in VIIth international congress of the International Society for Cell Biology at Yale University (4–8 September 1950), Box 503464, Folder VII International Congress for Cell Biology, George Gey Papers, Alan Mason Chesney Medical Archives of the Johns Hopkins Medical Institution, Baltimore, MD, USA.
Burnet and Fenner, op. cit. (note 80), pp. 105–106. See also Hyung Wook Park, ‘Germs, hosts and the origin of Frank Macfarlane Burnet's concept of “self” and “tolerance”, 1936–1949’, J. Hist. Med. All. Sci. 61, 492–534 (2006), at pp. 519–522.
‘Application by Professor P. B. Medawar’, 9 April 1948, p. 1, FD 1/6960 (Medawar's skin grafting, 1944–1948), TNA; Peter Medawar, ‘Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates’, Symp. Soc. Exp. Biol. 7, 320–338 (1953). For the wartime transfusion service and the significance of the Rh factor, see Jenny Bangham, ‘Between the transfusion services and blood group research: human genetics in Britain during World War II’, in Human heredity in the twentieth century (ed. Bernd Gausemeier, Staffan Müller-Wille and Edmund Ramsden), pp. 69–83 (Pickering & Chatto, London, 2013). For Medawar's comments on the wartime blood transfusion, see ‘Report of M.R.C. 1952–3: the grafting of tissues’, p. 24, Box 36, Folder E47, PBM.
Medawar to Jones, 10 April 1952, Box 17, Folder C28.
‘Experiment SnR-2: SnR-2.2’, 3 August 1952, Box 13, Folder C4, PBM. Also see ‘Skin storage experiment: SnR-2.6’, 30 June 1954, Box 13, Folder C4, PBM. Although Medawar did not detail his problems, he had probably failed to put an adequate amount of glycerol–Ringer as the cryoprotectant before freezing a piece of skin.
‘Skin storage experiments (exp. 2): SnR-2.5’, 17 June 1953, Box 13, Folder C4, PBM; ‘Repeated triple inoculation of newborns, adult cells (cba to A): EMB-57’, 24 November 1952, Box 43, Folder C156, PBM; ‘Intra-embryonic inoculation of foetal mice: EMB-73’, 11 September 1953, Box 43, Folder C156, PBM.
‘Effect of cortisone on mice: AD1-13’, 24 October–1 December 1952; ‘Cortisone dosage experiment: AD14-29’, 9 June–24 June 1953, Box 13, Folder C4, PBM.
‘Adrenalectomy: delayed homografting (A to aaUU): AD.125-132’, 24 November 1953; 10 December 1953, Box 13, Folder C4, PBM.
‘Skin storage experiment SnR-2’, 12 June 1952, Box 13, Folder C4, PBM.
‘Experiment SnR-2: SnR-2.2’, 15 August 1952, Box 13, Folder C4, PBM.
‘Opn, Chorio-allantoic grafts of node and lymph: S-46’, 16–21 April 1948, Box 13, Folder C4, PBM.
Many philosophers, sociologists and historians have explored why not every step in an experiment could be explicitly discussed. Probably, the troublesome steps in Medawar's experiments were intertwined with either ‘somatic tacit knowledge’ or ‘collective tacit knowledge’, as described in Harry Collins, Tacit and explicit knowledge (University of Chicago Press, 2010), chapters 5 and 6.
‘Experiment, Repeated retransplantation of skin grafts: animal no. R.28’, 20 March 1950, Box 13, Folder C4, PBM.
‘NB-8 inoculation of newborns with cell suspensions: EMB-16’, 30 July 1952; ‘F-23 inoc. of foetal mice (A to CBA): EMB-41’, 12 September 1952, Box 43, Folder C156, PBM.
Medawar, op. cit. (note 6), p. 132.
‘Intra-embryonic inoculation of embryos (CBA to A) with foetal cells: EMB-68’, 23 December 1952, Box 43, Folder C156, PBM.
‘Injection of foetal mice (cba to A) intraembryonically with adult cell brei: EMB-64’, 11 December 1952; ‘Inoculation of newborn mice: EMB-8’, 15 July 1952; ‘Intra-embryonic inoculation of embryos (CBA to A) with foetal cells: EMB-68’, 23 December 1952; ‘Inoculation of newborns with adult cell suspension: EMB-24’, 18 August 1952, Box 43, Folder C156, PBM. In the last case, Medawar erroneously injected cortisone into a newborn mouse inoculated with foreign cells, although he wanted to see if it developed tolerance to extraneous tissues without the use of an immunosuppressant.
‘Intra-embryonic inoculation of foetal mice: EMB-73’, 9 July 1953, Box 43, Folder C156, PBM.
‘Intra-embryonic inoculation of embryos (CBA to A) with foetal cells through body wall: EMB-68’, 22 December 1952, Box 43, Folder C156, PBM; Billingham et al., op. cit. (note 1), p. 604.
‘Intraembryonic inoculation of foetal mice with adult cells through body wall (A to aaUU): EMB-100’, 8 June 1953, Box 43, Folder C156, PBM.
‘Inoculation of foetal mice (A to cba) with embryonic cell suspension through body wall: EMB-85’, 5 March 1953, Box 43, Folder C156, PBM.
‘Inoc. of foetal mice (A to cba), adult cells, subcut.: EMB-41’, 13 September 1952; ‘Intra-embryonic inoculation of foetal mice (A to cba): EMB-73’, 5 August 1953, Box 43, Folder C156, PBM.
‘Intra-embryonic inoculation of foetal mice (aaUU to A) with adult cell suspension through body wall: EMB-69’, 7 February 1953, Box 43, Folder C156, PBM.
Interview with Brent, 31 May 2016; P. B. Medawar, ‘Skin homografts’, 1 September 1944–1 September 1945, p. 3, FD 1/6960 (Medawar's skin grafting 1944–48), TNA. Medawar also used Fisher's mice. See Fisher to Medawar, 22 November 1943, RAF. On the origin of the inbred mice of the Jackson Laboratory, see Karen Rader, Making mice: standardizing animals for American biomedical research, 1900–1955 (Princeton University Press, 2004).
‘Mouse homograft survival times: B series’, p. ii, Box 43, Folder C155, PBM.
‘Genetical divergence test in A-line’, undated, Box 43, Folder C155, PBM. But mutation can just be another factor increasing ‘residual heterozygosity’.
‘Genetical divergence test in C-line’, Box 43, Folder C155, PBM; Billingham et al., op. cit. (note 1), pp. 603–604.
‘Genetical divergence test in A-line’, undated, Box 43, Folder C155, PBM.
‘Mouse homograft survival times: B series’, pp. iii–iv, Box 43, Folder C155, PBM.
Ibid.
‘Effect of cortisone on mice: AD-1 … 6’, 8 November 1952, Box 13, Folder C4, PBM.
‘Histology: X-series’; ‘Histology: Y-1: difficulties to decide between 5% survival & total breakdown’, Box 43, Folder C155, PBM.
As Peter Dear has shown, chronological details in a scientific paper increased its trustworthiness in the seventeenth-century Royal Society. Peter Dear, ‘Totius in verba: rhetoric and authority in the early Royal Society’, Isis 76, 144–161 (1985). However, Medawar, working in the mid twentieth century, chose to make his discovery look like a timeless truth by removing all chronological details from his paper.
Billingham et al., op. cit. (note 1), p. 604.
‘Intra-embryonic inoculation of foetal mice: EMB-73’, 31 March–21 May 1953, Box 43, Folder C156, PBM.
Billingham et al., op. cit. (note 1), p. 604.
‘Intra-embryonic inoculation of foetal mice: EMB-73’, 23 July 1953, Box 43, Folder C156, PBM. Through this experiment, Medawar did demonstrate that tolerance was due to a change in the host body rather than the graft. But the long survival time of the A-line skin was still considered unusual as he thought that it was due to his own mistake.
‘Foetal inoculations (A to cba): EMB-130’, 25 September 1953, Box 43, Folder C156, PBM.
The word in italics is my emphasis. See Billingham et al., op. cit. (note 1), p. 604.
My count of Medawar's newborn injections is quite close to his own count in the 1953 paper. I found that eight out of 90 mice that had been inoculated immediately after birth showed tolerance to the original donor's skin. But four among them tolerated grafts just for 12–14 days.
Since Burnet and Medawar shared the Nobel Prize in 1960, many people have believed that Medawar's experiment directly proved Burnet's theory. But historian Arthur Silverstein has recently suggested that there was no good reason to nominate Burnet and Medawar together in 1960. Silverstein argues that they shared the prize ‘for the wrong reason’. See Arthur M. Silverstein, ‘The curious case of the 1960 Nobel Prize to Burnet and Medawar’, Immunology 147, 269–274 (2016).
‘Mouse homograft survival times: B series’, p. iii, Box 43, Folder C155, PBM.
Medawar, op. cit. (note 6), p. 132.
Leslie Brent, A history of transplantation immunology (Academic Press, San Diego, 1997), p. 191. The existence of ‘hidden variables’ was a controversial topic in twentieth-century quantum mechanics. See James Cushing, Quantum mechanics: historical contingency and the Copenhagen hegemony (University of Chicago Press, 1994), pp. 58, 77, 134. Indeed, immunologists later found that there was a hidden factor, the graft-versus-host disease, which considerably lowered the success rate of transplantation. See Hamilton, op. cit. (note 3), pp. 240–241.
Billingham et al., op. cit. (note 1), p. 604.
Ibid.
Medawar and his team later had to question the whole theoretical scheme, including the null period. See Brent, op. cit. (note 133), pp. 197–200.
These numbers were only about the mice showing a long-term tolerance over 50 days. These success rates were higher than those of the experiments conducted between 1952 and 1953, indicating their improving skill. See Rupert Billingham, Leslie Brent and Peter Medawar, ‘Quantitative studies on tissue transplantation immunity. III: actively acquired tolerance’, Philos. Trans. R. Soc. Lond. B 239, 357–414 (1956), at p. 367.
Ibid., p. 373. Jack Cannon and William Longmire, ‘Studies of successful skin homografts in the chicken’, Ann. Surg. 135, 60–68 (1952).
Billingham et al., op. cit. (note 1), p. 603. The term ‘acquired’ also means that it was not ‘natural’ or ‘inherited’. Medawar's ‘acquired tolerance’ was not a sort of ‘natural immunity’ observed in the transfusion of unmatched blood. This immunity would immediately develop, while acquired immunity needed a ‘latent period’. ‘Actively acquired tolerance is clearly not transferrable to the offspring’, as the offspring of the chimeric mice never tolerated tissues from the original donor strain. See Peter Medawar, ‘General problems of immunity’ in Preservation and transplantation of normal tissues (ed. G. E. W. Wolstenholme and Margaret P. Cameron), pp. 1–20 (Little, Boston, 1954), at p. 4; ‘Intra-embryonic inoculation of foetal mice: EMB-73’, 27 July 1953, Box 43, Folder C156, PBM.
Brent, op. cit. (note 133), p. 191; Rupert Billingham and Leslie Brent, ‘A simple method for inducing tolerance of skin homografts in mice’, Transplant Bull. 4, 67–71 (1957). Morten Simonsen seemed already to have noticed the disease in the early 1950s. See Hamilton, op. cit. (note 3), p. 239.
Billingham et al., op. cit. (note 1), p. 603.
The italicized word is Medawar's. See Medawar, op. cit. (note 139), p. 19; Billingham et al., op. cit. (note 1), p. 603. For some readers' clinical attempts, see M. F. Woodruff, ‘Can tolerance to homologous skin be induced in the human infant at birth?’, Transplant Bull. 4, 26–28 (1957); R. Fowler Jr, W. K. Schubert and C. D. West, ‘Acquired partial tolerance to homologous skin grafts in the human infant at birth’, Ann. NY Acad. Sci. 87, 403–428 (1960).
Joseph Murray, Surgery of the soul: reflections on a curious career (Science History Publications, Sagamore Beach, 2001), pp. 89–98.
Ilana Löwy, ‘The strength of loose concepts: boundary concepts, federative experimental strategies and disciplinary growth’, Hist. Sci. 30, 371–396 (1992).
Hamilton, op. cit. (note 3), p. 241.