Abstract
Connexin-36 (Cx36) protein forms gap junction (GJ) channels in pancreatic beta cells and is also the main Cx isoform forming electrical synapses in the adult mammalian brain. Cx36 GJs can be regulated by intracellular pH (pHi) and cytosolic magnesium ion concentration ([Mg2+]i), which can vary significantly under various physiological and pathological conditions. However, the combined effect and relationship of these two factors over Cx36-dependent coupling have not been previously studied in detail. Our experimental results in HeLa cells expressing Cx36 show that changes in both pHi and [Mg2+]i affect junctional conductance (gj) in an interdependent manner; in other words, intracellular acidification cause increase or decay in gj depending on whether [Mg2+]i is high or low, respectively, and intracellular alkalization cause reduction in gj independently of [Mg2+]i. Our experimental and modelling data support the hypothesis that Cx36 GJ channels contain two separate gating mechanisms, and both are differentially sensitive to changes in pHi and [Mg2+]i. Using recombinant Cx36 we found that two glutamate residues in the N-terminus could be partly responsible for the observed interrelated effect of pHi and [Mg2+]i. Mutation of glutamate at position 8 attenuated the stimulatory effect of intracellular acidification at high [Mg2+]i, while mutation at position 12 and double mutation at both positions reversed stimulatory effect to inhibition. Moreover, Cx36*E8Q lost the initial increase of gj at low [Mg2+]i and double mutation lost the sensitivity to high [Mg2+]i. These results suggest that E8 and E12 are involved in regulation of Cx36 GJ channels by Mg2+ and H+ ions.
Keywords: connexin-36, gap junctions, intracellular pH and Mg2+, mutants, cell culture
Introduction
Cell-to-cell coupling through gap junction (GJ) channels is essential for intercellular communication in most cell types. GJ channels serve as an intercellular pathway for ions, small metabolites such as IP3 and cAMP (Niessen et al., 2000; Bedner et al., 2006), and larger molecules such as small interfering RNAs (Valiunas et al., 2005; Antanaviciute et al., 2014) and peptides (Neijssen et al., 2005). Electrotonic coupling through the GJs ensures propagation of action potentials between cardiomyocytes (Rohr, 2004), synchronization of neuronal activity in various brain regions (Bennett and Zukin, 2004) and is an important component of retinal circuitry (Völgyi et al., 2013). GJs play an important role in non-excitable tissue as well, since intercellular cell signalling via GJs may orchestrate proliferation (Vance and Wiley, 1999; Murray et al., 2009) and apoptosis (Kameritsch et al., 2013; Akopian et al., 2014).
GJ channels consist of two apposed hemichannels from contiguous cells. In vertebrates, each hemichannel is formed by six protein subunits of the connexin (Cx) family. Structural studies have revealed that Cxs comprise four transmembrane domains (M1-M4), two extracellular loops (E1 and E2), one cytoplasmic loop (CL), and cytoplasmic N- and C-termini (NT and CT). It is well-established that GJs formed of Cxs can be regulated by transjunctional voltage (Vj) (Harris et al., 1981; Bukauskas and Verselis, 2004) or cytosolic conditions, such as intracellular pH (pHi) (Trexler et al., 1999; Palacios-Prado et al., 2010) or divalent cations (Noma and Tsuboi, 1987; Peracchia, 2004; Matsuda et al., 2010; Palacios-Prado et al., 2013). Conductance of GJs could be regulated by chemical uncouplers such as polyamines (Shore et al., 2001; Musa and Veenstra, 2003), alkanols (Weingart and Bukauskas, 1998), fenamates (Harks et al., 2001), antimalarial drugs (Srinivas et al., 2001; Cruikshank et al., 2004), and others. Cxs can also be affected by post-translational phosphorylation (Lampe and Lau, 2004; Moreno, 2005).
In humans, 21 different Cx isoforms have been identified (Söhl and Willecke, 2004). These isoforms are differentially expressed in various tissues and exhibit different biophysical and biochemical properties. Among the Cx family, Cx36 is mainly expressed in the adult mammalian central nervous system, where it forms electrical synapses. It has been shown that Cx36-containing electrical synapses play an important role in facilitating synchronous or phase-locked activity of neuronal networks, which underlie a variety of cognitive processes (Bennett and Zukin, 2004; Connors and Long, 2004; Hormuzdi et al., 2004; Saraga et al., 2006; Bissiere et al., 2011). Cx36 also forms GJs between pancreatic beta cells, where it plays an important role in insulin secretion and glycaemic control (Farnsworth and Benninger, 2014).
As compared with other Cx isoforms, Cx36 GJ channels have some distinct biophysical and regulatory properties. For example, Cx36 exhibits a very low unitary conductance and low sensitivity to transjunctional voltage (Srinivas et al., 1999; Teubner et al., 2000). Its regulation by pHi and free cytosolic Mg2+ ion concentration ([Mg2+]i) also has some distinctive characteristics. Unlike other Cx isoforms, junctional conductance (gj) of Cx36 GJs can be upregulated under low pHi (González-Nieto et al., 2008) and low [Mg2+]i (Palacios-Prado et al., 2013). In addition, [Mg2+]i can change Cx36 sensitivity to transjunctional voltage. Studies with recombinant Cx36 revealed that Mg2+-dependent regulation of gj may be explained via electrostatic interaction with a binding site located in the channel pore (Palacios-Prado et al., 2014). Preliminary results showed that the effect of [Mg2+]i and pHi on Cx36 GJs might be interrelated (Palacios-Prado et al., 2011). This raised the hypothesis that Mg2+ and H+ ions may interact on the same binding sites, as was shown for TRPM7 ion channels (Jiang et al., 2005). However, the effect on gj of Cx36 GJs produced by combined changes in [Mg2+]i and pHi has not been studied in detail.
Both pHi and [Mg2+]i are known to play an important role in normal and various pathological conditions. For example, increased neural activity may cause a shift in pHi of 0.2–0.4 units (Chesler and Kraig, 1989; Chesler and Kaila, 1992) under physiological conditions, which may subsequently modulate electrical synapses. In addition, the depletion of ATP during brain ischemia (Sato et al., 1984) may cause an increase of [Mg2+]i (Henrich and Buckler, 2008). The connection between Mg2+ deficiency and formation of epileptic seizures is well-established (Randall et al., 1959; Hanna, 1961; Nuytten et al., 1991), and the role of electrical synapses and Cx36 in epilepsy is an important topic of research (Gajda et al., 2005; Volman et al., 2011; Kohmann et al., 2016; Wu et al., 2017). Furthermore, brain [Mg2+]i is decreased in patients with Alzheimer's and Parkinson's disease (Durlach, 1990; Barbiroli et al., 1999), while patients with schizophrenia and traumatic brain injury show increased brain [Mg2+]i (Hinsberger et al., 1997). In the pancreas, low pHi plays an important role in glucose-induced insulin release, while low [Mg2+]i is associated with decreased insulin secretion (Ishizuka et al., 1994) and pancreatitis (Papazachariou et al., 2000). Thus, understanding the interaction of pHi and [Mg2+]i and their combined effects on gap junctional communication (GJC) could reveal new modulatory mechanisms of GJs in physiology and pathology.
In this study, we examined the combined effect of [Mg2+]i and pHi on gj between cells expressing Cx36-EGFP. Our data revealed that after gj was reduced by high [Mg2+]i, it could be recovered by intracellular acidification with sodium acetate (CH3COONa). In contrast, after gj was elevated by low [Mg2+]i, both alkalization or acidification with ammonium chloride (NH4Cl) or CH3COONa, respectively, induced a reduction of gj. To consider the most appropriate amino acids which could be involved in Cx36 GJ channel regulation by H+, we used homology modelling to generate a three-dimensional structure of Cx36 using the crystal structure of Cx26 (Maeda et al., 2009) as a template, which allowed us to estimate the pKa of all ionizable amino acid side chains. The calculations showed that two glutamates (E8 and E12) exhibited pKa values which were closest to physiological pH. Thus, we performed experiments with three Cx36 mutants, Cx36*E8Q-EGFP, Cx36*E12Q-EGFP and Cx36*E8Q-E12Q-EGFP, in which negatively charged glutamates were substituted to uncharged glutamines. Experimental results showed that acidification-induced gj increase at high [Mg2+]i in Cx36-EGFP was abolished in Cx36*E8Q-EGFP, while Cx36*E12Q-EGFP exhibited a small gj decrease under the same conditions. The most prominent decrease of gj at high [Mg2+]i during acidification was observed in double mutant Cx36*E8Q-E12Q-EGFP. Therefore, these amino acids could be involved in modulatory mechanisms of Cx36 GJ channels by both, [Mg2+]i and pHi.
Materials and methods
Structural modelling
Cx36 homology modelling was carried out with MODELLER (version 9.10) (Webb and Sali, 2014), using a Cx26 crystal structure (Maeda et al., 2009) as a template. The prediction of pKa values of ionizable groups in Cx36 was based on the 3D structure and was performed with PROPKA (version 3.0) (Olsson et al., 2011; Søndergaard et al., 2011).
Cell and culture conditions
Electrophysiological measurements were performed using HeLa (human cervix carcinoma cells, ATCC CCL2) cells transfected with wild type mouse Cx36 fused with enhanced green fluorescent protein (EGFP). Cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum. Cells were maintained in a 5% CO2 incubator in a moist atmosphere at 37 OC. Media and culture reagents were obtained from Sigma-Aldrich, Germany. Single point mutations, Cx36*E8Q-EGFP and Cx36*E12Q-EGFP, and double point mutation, Cx36*E8Q-E12Q-EGFP were generated using the QuikChange Multi Site-directed mutagenesis kit (Agilent, USA). Mutants were subcloned into pIRESPuro2 vector (Clontech, USA). Transfection procedures were performed using Lipofectamine 2000 (Life technologies, USA) following the manufacturer's protocol.
Electrophysiological measurements
For simultaneous electrophysiological and fluorescence recordings, cells grown on glass coverslips were transferred to an experimental chamber mounted on the stage of an inverted microscope Olympus IX71 (Olympus, Japan) with a constant flow-through perfusion. The gj was measured in selected cell pairs by using a dual whole-cell patch clamp. Cell-1 and cell-2 of a cell pair were voltage clamped independently with separate patch clamp amplifiers EPC-8 (HEKA Elektronik, Germany) at the same holding potential, V1 = V2. Voltages and currents were acquired and analysed using an analog-to-digital converter (National Instruments, Austin, TX) and custom-made software. By stepping the voltage in cell-1 (ΔV1) and keeping the other constant, junctional current was measured as the change in current in the unstepped cell-2, Ij = –ΔI2. Thus, gj was obtained from the ratio -Ij/ΔV1, where ΔV1 is equal to Vj and the negative sign indicating that Ij measured in cell-2 is oppositely oriented to the one measured in cell-1. To minimize the effect of series resistance on measurements of gj, we maintained recording pipette resistance below 3 MΩ. Patch pipettes were pulled from glass capillary tubes with filaments using a P-97 micropipette puller (Sutter Instrument Co., US).
Cells were perfused with modified Krebs-Ringer (MKR) solution containing (in mM): 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 2 CsCl, 1 BaCl2, 5 HEPES, 5 glucose, 2 pyruvate, pH 7.4. Changes of pHi were achieved by using NH4Cl and CH3COONa to alkalize and acidify, respectively, the intracellular milieu without a change in extracellular pH (pHo). Recording pipettes were filled with solution containing (in mM): 130 CsCl, 10 NaAsp, 0.26 CaCl2, 5 HEPES, 2 BAPTA, 1 MgCl2, pH 7.3. To investigate the effect of [Mg2+]i we used pipette solutions containing 0.01, 1 or 5 mM of MgCl2. Differences in osmolarity were compensated with the appropriate concentration of CsCl.
To prepare solutions for intracellular acidification and alkalization during experiments, we used modified Ringer's solution in which NaCl was exchanged for equal concentration of CH3COONa or NH4Cl. All extracellular solutions were adjusted to pH = 7.4. To reduce pHi to 6.5 and 6.0, we used physiological solution containing 20 and 100 mM of CH3COONa, respectively, and to increase pHi to 7.6, 7.9, and 8.2, we added to the physiological solution 1, 3, and 10 mM of NH4Cl, respectively (Table 1).
Table 1.
pHi values measured with BCECF during acidification with CH3COONa and alkalization with NH4Cl.
| Concentration | 100 mM CH3COONa | 20 mM CH3COONa | Control | 1 mM NH4Cl | 3 mM NH4Cl | 10 mM NH4Cl |
|---|---|---|---|---|---|---|
| pHi | 6.02 ± 0.05 | 6.53 ± 0.05 | 7.27 ± 0.07 | 7.64 ± 0.02 | 7.87 ± 0.03 | 8.15 ± 0.01 |
Fluorescence imaging studies
Fluorescence signals were acquired using UltraVIEW (PerkinElmerLifeSciences, Boston, MA, US) software. An excitation filter of 470 nm, and emission filter of 540 nm were used to identify the cell pairs expressing Cx36-EGFP and its mutants. For pHi measurements we used 4 μM BCECF (Invitrogen, USA), which was introduced into the cells through the patch pipettes in a whole-cell voltage clamp mode. The dye was alternately excited with 436 and 500 nm wavelengths, and the emitted light was filtered at 540 nm. The ratios of emitted light collected at excitation wavelengths of 436 and 500 nm (background subtracted) were converted to pHi values based on a calibration curve. The latter was obtained by applying the solutions of different pH (6.0, 6.5, 7.0, 7.5, 8.0, and 8.5) and using ionophore nigericin (20 μM) in the presence of potassium (140 mM) to equilibrate the pHi with an extracellular medium of different pH (Thomas et al., 1979). The pHi values at different concentrations of CH3COONa and NH4Cl are presented in Table 1. To prevent dye bleaching, imaging was performed in time-lapse mode by exposing every 15 s to a low-intensity excitation light for 500 ms.
Statistical analysis
Experimental data are reported as the representative result or as mean of at least four independent experiments ± standard error (SEM). Statistical analyses were performed using unpaired Student's t-test. Differences were considered statistically significant at p < 0.05.
Results
The effect of H+ and intracellular Mg2+ on Cx36 GJ channel function
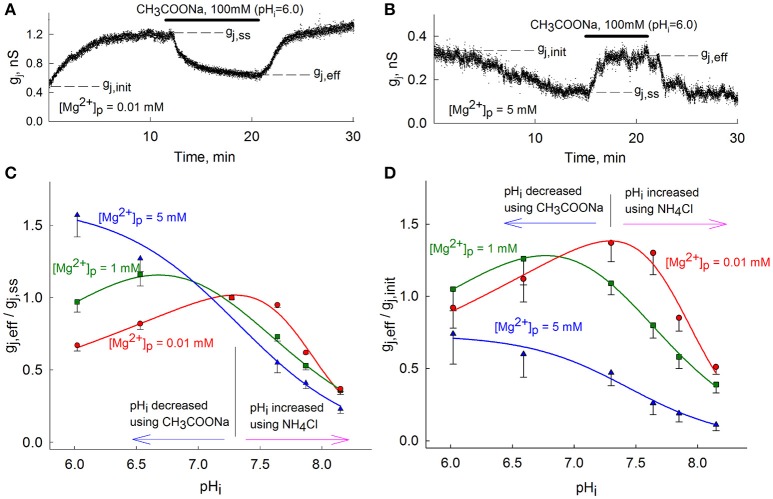
Preliminary data suggested that [Mg2+]i can substantially modulate gj-pHi dependence of Cx36-EGFP GJs (Palacios-Prado et al., 2011). To study the relation of [Mg2+]i and pHi, and their combined effect on gj more systematically, we examined gj-pHi dependence in HeLa cells expressing Cx36-EGFP cells at different concentrations of Mg2+ (0.01, 1, and 5 mM) in pipette solutions ([Mg2+]p). In these experiments, the initial gj (gj, init) at control pHi = 7.3 was registered immediately after patch opening. Typically, gj increased or decreased at low or high [Mg2+]i, respectively, until it reached a steady-state (gj, ss) (Figures 1A,B). Then, we decreased or increased pHi by applying different concentrations of CH3COONa or NH4Cl, respectively, and measured gj (gj, eff) before washing out the applied substance. To prepare these solutions, we used modified Ringer's solution in which NaCl was exchanged for equal concentration of CH3COONa or NH4Cl to maintain osmolarity. Extracellular pH (pHo) remained unchanged during the experiments. Solution and pHi measurement protocols are presented in the Methods section. The control experiments in non-transfected HeLa cells showed low conductance in very rare cases (1 in 20), which could be attributed to activity of endogenous connexins in these cells (data not shown).
Figure 1.
Modulation of gj-pHi by [Mg2+]p. (A,B) gj measurements over time using low (A) or high (B) [Mg2+]p followed by CH3COONa-induced acidification to pHi = 6.0; (C) The (gj, eff/gj, ss)-pHi dependence of Cx36-EGFP GJs at different [Mg2+]p. Data are normalized to gj, ss at each different [Mg2+]p before changes in pHi. (D) The same as in (C), but normalized to gj, init. To avoid overlap, only lower ranges of standard error are presented in (C,D). Vertical black lines in (C,D) are located at pHi = 7.3.
Figure 1C shows gj, eff/gj, ss dependence on pHi at different [Mg2+]p. The mean values of (gj, eff/gj, ss)-pHi dependence at 0.01 and 1 mM [Mg2+]p were fitted to an equation describing biphasic effects (Swietach et al., 2007) and a sigmoid function was used to fit data at 5 mM [Mg2+]p. Alkalization to pHi = 8.2 caused a ~70% decay of gj, eff/gj, ss almost independently of [Mg2+]p. However, changes of gj during acidification highly varied depending on [Mg2+]p. At [Mg2+]p = 0.01 mM, acidification to pHi = 6.0 decreased gj, eff/gj, ss to 0.67 ± 0.04 (n = 4). At [Mg2+]p = 1 mM, acidification to pHi = 6.6 first induced an increase of gj, eff/gj, ss to 1.16 ± 0.08 (n = 8), while further acidification to pHi = 6.0 returned gj, eff/gj, ss to 0.97±0.07 (n = 12). At [Mg2+]p = 5 mM, acidification to pHi = 6.0 increased gj, eff/gj, ss to 1.57 ± 0.15 (n = 4). Figure 1D shows the ratios gj, eff/gj, init at different [Mg2+]p, to represent the effect of [Mg2+]i on gj and the consequent changes by altering pHi; at pHi = 7.3 this ratio coincides with gj, ss/gj, init. From Figure 1D it can be seen that peaks of (gj, eff/gj, init)-pHi dependencies shift toward the acidic side as the value of [Mg2+]p increases. Therefore, gj, eff/gj, init reaches a maximum value at pHi range between 7.1–7.5, 6.6–6.9 and below 6.0 for [Mg2+]p of 0.01, 1, and 5 mM, respectively. These experimental results clearly show that pHi and [Mg2+]i regulation of Cx36 is interrelated, particularly at lower pHi values.
The role of glutamates in Cx36 GJ sensitivity to pHi and [Mg2+]i
Some studies have shown that gating polarity and single channel conductance of GJ channels are determined by charged amino acids of the NT domain (Verselis et al., 1994; Musa et al., 2004; Tong and Ebihara, 2006), which forms the vestibule of the channel. We hypothesized that negatively charged amino acids of the NT domain could play an important role in Mg2+ ions' interaction with Cx36 protein as they form the path for Mg2+ ions to enter the channel and could be involved in their binding. In order to determine the amino acids which also could be sensitive to pHi, the x-ray crystal structure of Cx26 (Maeda et al., 2009) was used to generate the homology model of Cx36 with MODELLER software, and pKa values were evaluated using PROPKA software for all ionizable amino acid side chains depending on their environment. Results showed that glutamates at positions 8 and 12 (E8 and E12) have pKa values equal to 6.5 and 7.2, respectively. These pKa values were the closest to physiological pHi among other amino acids in the NT domain of Cx36, thus indicating that carboxyl groups at side chains of E8 and E12 may be sensitive to protonation and deprotonation at physiological pHi values. Based on these predictions, E8 and E12 were chosen for point mutations. As substitutes for glutamates, we chose glutamines (E8Q or E12Q, respectively), which are neutral but polar. Therefore, the substitution of glutamate by glutamine neutralizes the negative charge and abolishes channel protonation, which possibly affects the dynamic behavior of a gate that is pH-sensitive and/or Mg2+-sensitive. Moreover, double mutation, where both E8 and E12 were substituted by glutamines, was also analysed.
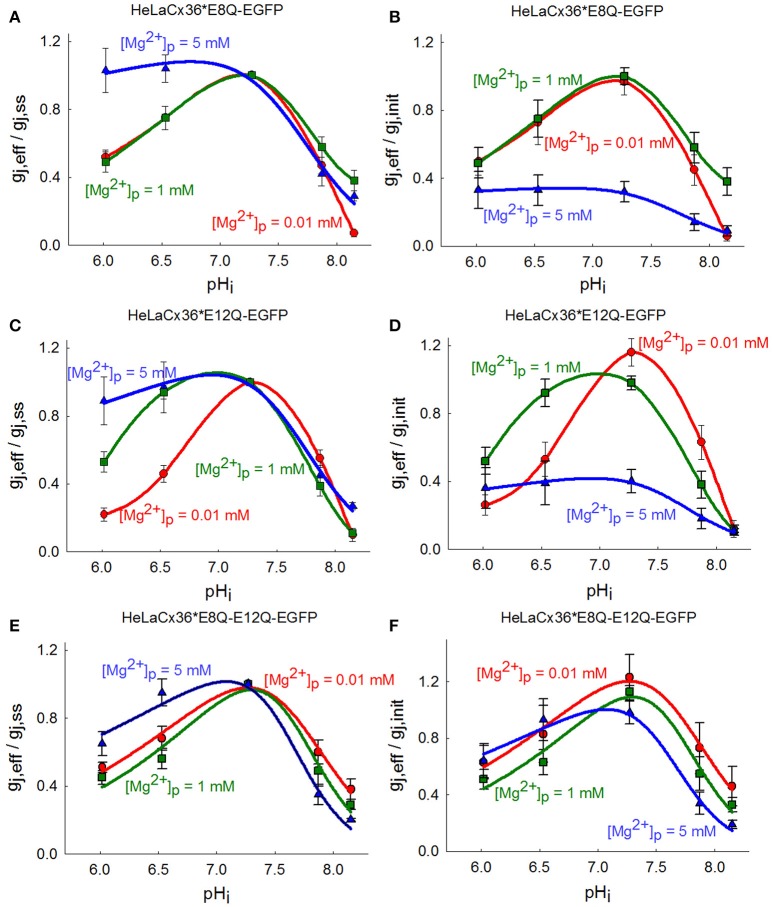
The experimental results showed that acidification to pHi 6.0 and 6.5 decreased gj, eff/gj, ss of Cx36*E8Q-EGFP approximately to the same level at 0.01 and 1 mM [Mg2+]p (Figure 2A), and at [Mg2+]p = 5 mM acidification to pHi 6.0 and 6.5 resulted in a slight increase of gj, eff/gj, ss. During alkalization to pHi = 8.2 gj, eff/gj, ss decreased ~60–90% at all [Mg2+]p. Figure 2C shows that the uncoupling effect of acidification on Cx36*E12Q-EGFP GJ channels is highly dependent on [Mg2+]p. The strongest decrease of gj, eff/gj, ss was reached at 0.01 mM [Mg2+]p, an ~50% decrease of gj, eff/gj, ss was obtained at 1 mM [Mg2+]p, and a slight decrease to 0.89 ± 0.14 (n = 10) was observed at [Mg2+]p = 5 mM. During alkalization to pHi = 8.2 the gj, eff/gj, ss decreased ~70–90% at all [Mg2+]p. Acidification to pHi 6.0 and 6.5 decreased gj, eff/gj, ss of double mutant ~50 and 40% at 0.01 and 1 mM [Mg2+]p, respectively, and ~35 and 5% at 5 mM [Mg2+]p, respectively (Figure 2E). The uncoupling of alkalization was dependent on [Mg2+]p with strongest effect at 5 mM [Mg2+]p for Cx36*E8Q-E12Q-EGFP. Figures 2B,D,F show that the shift of peaks of (gj, eff/gj, init)-pHi dependencies for all mutants were less influenced by rising [Mg2+]p than it is for Cx36-EGFP.
Figure 2.
Influence of single and double E8Q and E12Q mutations on (gj, eff/gj, ss)-pHi dependence modulated by [Mg2+]p. (A) For Cx36*E8Q-EGFP the effect of acidification is modulated by high [Mg2+]p, while alkalization—by low [Mg2+]p. (B) The increase of [Mg2+]p shifts peaks of Cx36*E8Q-EGFP (gj, eff/gj, init)-pHi curves to the left with the maximal gj, eff/gj, init values at pHi ~6.9–7.4 for 0.01 and 1 mM [Mg2+]p, and at pHi ~6.7–7.2 for 5 mM [Mg2+]p. (C) The decrease of gj, eff/gj, ss of Cx36*E12Q-EGFP during acidification depends on [Mg2+]p. (D) The peaks of (gj, eff/gj, init)-pHi dependencies shift to the left with the maximal gj, eff/gj, init values at pHi ~7.1–7.6 for 0.01 mM [Mg2+]p, and at pHi ~6.7–7.3 for 1 and 5 mM [Mg2+]p. (E) Double mutation causes decrease of gj, eff/gj, ss at high [Mg2+]p. (F) The peaks of (gj, eff/gj, init)-pHi dependencies are the same at 0.01 and 1 mM [Mg2+]p, and only slightly shift to the left with the maximal gj, eff/gj, init values at pHi ~6.9–7.3 for 5 mM [Mg2+]p.
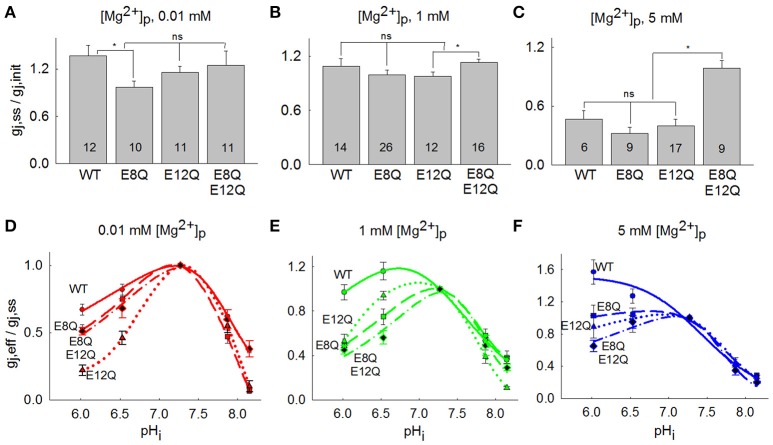
The summarized changes in gj, ss/gj, init of Cx36-EGFP, Cx36*E8Q-EGFP, Cx36*E12Q-EGFP and Cx36*E8Q-E12Q-EGFP are presented in Figures 3A–C, which shows that the effect of [Mg2+]p on gj, ss/gj, init for all mutants is comparable to Cx36-EGFP, with the exception of the E8Q mutation, which abolishes the increase of gj at low [Mg2+]p (Figure 3A), and the E8Q-E12Q mutation, which eliminates the decrease of gj at high [Mg2+]p (Figure 3C). The comparison of (gj, eff/gj, ss)-pHi between three mutants and Cx36-EGFP is represented in Figures 3D–F. Acidification at low [Mg2+]p causes the strongest uncoupling of cells transfected with Cx36*E12Q-EGFP (Figure 3D). Importantly, acidification did not cause any change of gj, eff/gj, ss of Cx36*E8Q-EGFP and decreased gj, eff/gj, ss of Cx36*E12Q-EGFP and Cx36*E8Q-E12Q-EGFP, while gj, eff/gj, ss of Cx36-EGFP is stimulated at high [Mg2+]p (Figure 3F). All mutants show an increased sensitivity to the uncoupling effect of acidification at 1 mM [Mg2+]p as compared with Cx36-EGFP (Figure 3E). Changes in sensitivity to alkalization is less visible, however alkalization causes a stronger decrease of Cx36*E12Q-EGFP gj than Cx36-EGFP at low and normal [Mg2+]p. Sensitivity of Cx36*E8Q-EGFP to alkalization is similar to Cx36-EGFP at normal [Mg2+]p and is close to Cx36*E12Q-EGFP at low [Mg2+]p. No significant changes between Cx36-EGFP and all mutants were observed in uncoupling by alkalization at high [Mg2+]p.
Figure 3.
Comparison between gj response of Cx36-EGFP, Cx36*E8Q-EGFP, Cx36*E12Q-EGFP and Cx36*E8Q-E12Q-EGFP to [Mg2+]p and pHi. (A) The increase of gj, ss/gj, init at 0.01 mM [Mg2+]p was observed for Cx36-EGFP (1.37 ± 0.13), E12Q (1.16 ± 0.08), and E8Q-E12Q (1.23 ± 0.16), while a small decrease was obtained for E8Q (0.97 ± 0.08). (B) The gj, ss/gj, init values at 1 mM [Mg2+]p were 1.09±0.08, 1.00 ± 0.05, 0.98 ± 0.04, and 1.13 ± 0.04 for Cx36-EGFP, E8Q, E12Q, and E8Q-E12Q, respectively. (C) The decrease of gj, ss/gj, init to 0.46 ± 0.09, 0.32 ± 0.06, and 0.40 ± 0.07 were obtained for Cx36-EGFP, E8Q, and E12Q, respectively, and for E8Q-E12Q mutation gj, ss/gj, init was 0.98 ± 0.08. Number of performed experiments are indicated on the bars; *p < 0.05, ns, non-significant. (D–F) All mutations enhance the uncoupling effect of acidification at 0.01 and 1 mM [Mg2+]p, single E8Q mutation abolish the stimulating effect of acidification at 5 mM [Mg2+]p, while E12Q and double mutation E8Q-E12Q reverses it to a decrease in gj, eff/gj,ss.
In summary, the effect of [Mg2+]p on gj, ss/gj, init of Cx36*E8Q-EGFP and Cx36*E12Q-EGFP remained comparable to that of Cx36-EGFP with the exception that E8Q abolished the increase of gj, ss/gj, init at low [Mg2+]p and E8Q-E12Q lost the sensitivity to high [Mg2+]p. Moreover, E8Q diminished the stimulating effect of acidification, while E12Q as well as E8Q-E12Q caused the decrease of gj, eff/gj, ss instead of stimulation, which was shown for Cx36-EGFP at high [Mg2+]p. These results show that mutations disturb the interrelated effect of [Mg2+]i and pHi on regulation of the Cx36 GJ channel.
Mathematical model of Cx36 regulation by PHi and [Mg2+]i
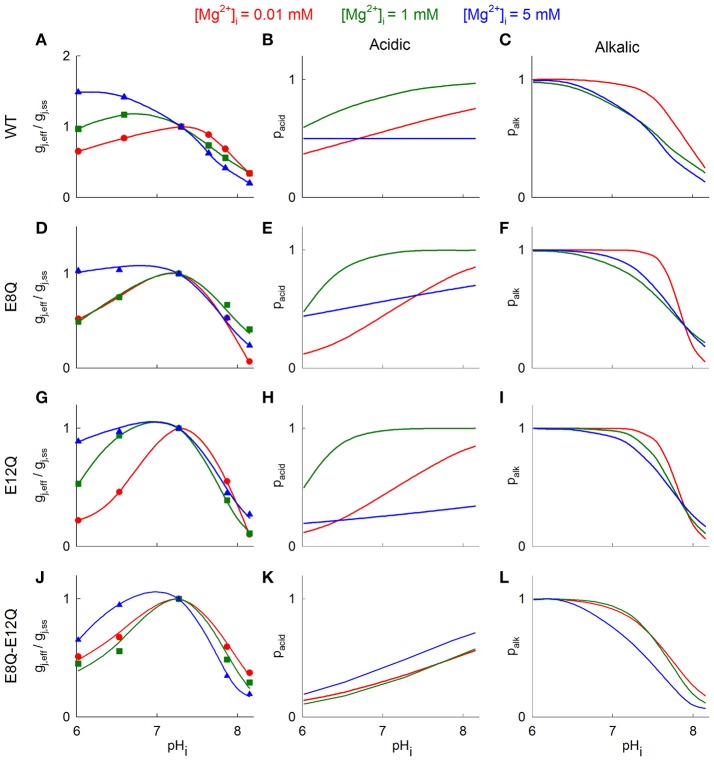
Data showing that uncoupling can be observed by both increased and decreased [H+]i suggest that Cx36 may contain pHi sensitive domains, one that leads to gj stimulation and another one that leads to gj inhibition. This hypothesis was previously raised in González-Nieto et al. (2008), where the authors postulated the existence of alkalic and acidic gates in Cx36 GJ channels. We applied this idea to model the mean values of gj, eff/gj, ss at different [Mg2+]i and pHi. We assumed that gj can be regulated by alkalic and acidic gating mechanisms, which are described by open channel probabilities in response to alkalization and acidification, palk and pacid, respectively. The probabilities palk and pacid were described by sigmoid function as follows:
| (1) |
Here, parameter A describes the steepness of the sigmoidal curve, and pH1/2 denotes the pHi level at which open channel probability is equal to 0.5. Then, overall gj can be estimated as a product of maximum junctional conductance (gmax) and open channel probabilities determined by both acidic and alkalic sensing domains:
| (2) |
Figure 4 illustrates fitted gj curves of pacid and palk under different [Mg2+]i to reproduce experimentally observed gj values. The model parameters at different levels of [Mg2+]i are presented in Table 2. Model fitting shows that pacid of Cx36-EGFP does not depend on pHi at high [Mg2+]i and increases with pHi rising at lower [Mg2+]i (Figure 4B). In contrast, palk decreased at higher pHi and this reduction does not depend on [Mg2+]i (Figure 4C).
Figure 4.
Mathematical model of gj changes in response to pHi under different [Mg2+]i. (A) Hypothetical gj-pHi curves fitted to experimental data. Blue triangles denote gj values at [Mg2+]p = 5 mM, green squares—at [Mg2+]p = 1 mM, and red circles - at [Mg2+]p = 0.01 mM. The theoretical gj-pHi plots were obtained from the Equation (2). (B,C) shows theoretical open channel probabilities in response to acidic gating (B) and alkalic gating (C), which were estimated from (1). Red, green and blue lines denote gj-pHi dependence at [Mg2+]i = 0.01, 1, and 5 mM, respectively. (D–L) show the same results as (A–C) with Cx36 mutants.
Table 2.
Parameters of model of open channel probabilities determined by acidic and alkalic sensing domains at different levels of [Mg2+]i.
| [Mg2+]i, mM | Aacid | pH1/2, acid | Aalk | pH1/2, alk | gmax, nS | |
|---|---|---|---|---|---|---|
| Cx36-EGFP | 0.01 | 0.77 | 6.70 | 4.23 | 7.90 | 2.42 |
| 1 | 1.42 | 5.75 | 2.34 | 7.59 | 1.80 | |
| 5 | 0.00 | 5.39 | 3.00 | 7.53 | 1.39 | |
| Cx36*E8Q-EGFP | 0.01 | 1.65 | 6.16 | 9.05 | 7.84 | 1.31 |
| 1 | 1.43 | 6.77 | 2.83 | 7.70 | 1.91 | |
| 5 | 0.32 | 5.03 | 3.64 | 7.74 | 0.55 | |
| Cx36*E12Q-EGFP | 0.01 | 1.77 | 7.15 | 7.71 | 7.81 | 2.13 |
| 1 | 3.53 | 6.04 | 5.15 | 7.75 | 1.08 | |
| 5 | 0.51 | 6.49 | 3.59 | 7.71 | 0.80 | |
| Cx36*E8Q-E12Q-EGFP | 0.01 | 0.97 | 7.89 | 3.44 | 7.71 | 4.22 |
| 1 | 1.12 | 7.88 | 4.16 | 7.67 | 3.99 | |
| 5 | 1.10 | 7.32 | 3.41 | 7.40 | 3.32 |
The pacid of all mutants gained the dependence on pHi at high [Mg2+]i (Figures 4E,H,K blue lines) with most prominent difference for Cx36*E8Q-E12Q-EGFP. In addition, overall pacid values were significantly reduced for Cx36*E12Q-EGFP at [Mg2+]i = 5 mM and for double mutant these values were reduced at 1 and 5 mM [Mg2+]i. The dependence of palk on pHi remains comparable to that of Cx36-EGFP and does not significantly change at all [Mg2+]i for all three mutants (Figures 4C,F,I,L).
Discussion
The distinct effects of pHi and divalent ion concentrations on the conductance of GJ channels and hemichannels have been known for a long time and have been reported in many studies. The effect of pHi on electrical coupling was demonstrated even before the sequencing of the Cx gene family (Rose and Loewenstein, 1975; Turin and Warner, 1977; Giaume and Korn, 1982). Most Cx isoforms have been found to be modulated by pHi (Hermans et al., 1995; Wang and Peracchia, 1996; Palacios-Prado et al., 2010). Intracellular divalent cations have also been shown to modulate gj of GJ channels (Peracchia, 1990; Matsuda et al., 2010; Harris and Contreras, 2014). The interaction between pHi and [Ca2+]i and their consequent effect on gj have been previously studied (Peracchia, 2004). Nonetheless, we recently found that changes in [Mg2+]i strongly affect gj in Cx36 (Palacios-Prado et al., 2013); (Palacios-Prado et al., 2014), which encouraged us to study the possible interaction between pHi and [Mg2+]i and their interrelated effect on Cx36-dependent coupling. In this study, we present data demonstrating that pHi and [Mg2+]i have an interrelated effect on the function of Cx36 GJ channels, and showed that two glutamate residues in NT domain are involved in this modulation.
Free intracellular H+ and Mg2+ ions interact with channel residues
Mg2+ and H+ are known to participate in a variety of physiological processes and could exert differential effects on many cellular targets. There are many different ways by which both pHi and [Mg2+]i could affect conductance of GJ channels and hemichannels. Therefore, it is not surprising that a variety of possible mechanisms have been proposed to account for their effect. For example, in Bevans and Harris (1999) and Tao and Harris (2004) it was proposed that H+ ions affect gating of Cx26 GJ channels and hemichannels via protonation of taurine, which can inhibit GJ channel activity. In addition, Peracchia (2004) suggested that the uncoupling effect of pHi in most cases could be explained through an increase in [Ca2+]i. However, some studies have also shown that H+ could affect conductance of GJ channels and hemichannels via direct protonation of Cx residues. For example, Spray et al. (1981) concluded that gj in amphibian blastomeres directly depends on pHi. In addition, recent studies have shown that pHi modulates Cx36 GJ channel activity through a direct effect on the channel (González-Nieto et al., 2008). In the same study, it was shown that the H18 residue in the NT domain is crucial for the uncoupling effect produced by alkalization. Moreover, others have also reported that low pHi could have a direct effect on Cx hemichannels (Trexler et al., 1999; Sanchez and Verselis, 2014).
Direct interaction of Mg2+ ions with Cx36 GJ channels was suggested in Palacios-Prado et al. (2013, 2014). It was proposed that Mg2+-dependent gating of Cx36 GJs is a distinct regulatory mechanism, in which the sensitivity of Cx36 GJ channels to high [Mg2+]i is determined by aspartate (D47), located in the channel pore (Palacios-Prado et al., 2014).
Our results show that gj of the Cx36 GJ channel during acidification strongly depends on [Mg2+]i (Figure 1), but is less affected by alkalization. It is likely that during an intracellular acidification, H+ reduces the affinity of Mg2+ for its binding site, thus causing an increase of gj at high [Mg2+]i. This view is supported by the fact that acidic pHi causes the decrease of gj only at low [Mg2+]i, when the inhibiting effect of Mg2+ would be reduced and changes in gj should be mainly determined by H+. One possible explanation for such a reduction of affinity could be an interaction between H+ and Mg2+ for the same negatively charged binding sites. Such an interaction has a strong chemical basis and was previously reported in various studies. For example, Russell and Brodwick (1988) demonstrated that competition of H+ and Mg2+ ions can affect Cl− fluxes in giant barnacle muscle cells. Mg2+ and pHi interaction was also demonstrated for TRPM7 cation channels (Jiang et al., 2005) or P2X7 receptors (Acuña-Castillo et al., 2007). It was proposed that TRPM7 channels have two binding sites for the Mg2+ ion, one of which could also be the site for H+ binding (Chokshi et al., 2012). Experimental data of this study show that alkalization decreases gj to a similar degree at all levels of [Mg2+]i, which suggests that the effect of alkalization does not significantly depend on [Mg2+]i. Moreover, this supports the hypothesis that alkalization of Cx36 has a distinct gating mechanism, as was proposed in González-Nieto et al. (2008). The lack of strong dependence between high pHi and [Mg2+]i probably does not require an explicit explanation if one assumes that the competition of H+ and Mg2+ ions is the driving factor for the combined effect of pHi and [Mg2+]i. On the other hand, one cannot exclude the possibility that Mg2+ ions interact more efficiently with the Cx36 protein under alkaline conditions. Under such a hypothesis, the binding affinity of Mg2+ would increase due to reduced [H+]i, which could stabilize the protein channel in a closed conformation even at low [Mg2+]i.
The mathematical model used in this study explain the biphasic nature of the gj-pHi relationship, assuming the presence of two separate gating mechanisms sensitive to acidic and alkalic conditions, as was proposed by González-Nieto et al. (2008). We presume that the activity of the channel could be modulated by separate sensing domains, which provide different sensitivity through the same gate. Our modelling results (Figure 4) suggest that Mg2+ ions might affect both alkalic and acidic gating mechanisms, particularly at low pHi.
An indirect effect of alkalization on gj could also be considered. For example, it is known that high pHi increases [Ca2+]i via release of Ca2+ from the endoplasmic reticulum (Li et al., 2012). However, this is unlikely to be the only mechanism involved, because our results showed no significant difference between gj decreases in control experiments with pipette solutions containing cytosolic Ca2+ buffer BAPTA, 10 mM (data not shown). In Palacios-Prado et al. (2013), it was shown that the increase of gj at low [Mg2+]i can be seen even in phosphomimetic mutants of Cx36 and using pipette solutions containing BAPTA. This excluded the role of CaMKII kinase, which was previously reported to cause a “run-up” phenomenon in Cx36 GJs (Del Corsso et al., 2012).
The possible role of NT domain in H+ and Mg2+ regulation
Mg2+ and H+ ions have many possible binding sites through which they could affect gj. Potential candidates might include various negatively charged residues for divalent cations or histidine residues, which can be protonated at physiological pHi values. For example, computational analysis and crystallography indicate that two glutamates (E42 and E47), residing in the Cx26 GJ channel pore near the extracellular gap could coordinate Ca2+ binding (Bennett et al., 2016), which might also be targeted by Mg2+. Negatively charged glutamates of the NT domain, which forms the vestibule of the GJ channel (Purnick et al., 2000), could be involved in binding Mg2+ ions or facilitate their pass through the channel. At high [Mg2+]i, E8Q and E12Q mutations abolished the stimulating effect of acidification and double mutation of these two amino acids even enhanced uncoupling in the same conditions. In addition, all mutations increased the uncoupling effect at low [Mg2+]i. The effect of [Mg2+]i on gj at control pHi remained comparable to Cx36-EGFP, with the exception of the E8Q mutation, which eliminated the increase of gj at low [Mg2+]i and the most prominent difference was obtained with double mutation, which basically abolished the uncoupling effect of high [Mg2+]i. Our modelling results indicate that both mutations modified the open channel probability determined by the acidic sensing domain, particularly at high [Mg2+]i. In particularly, sensitivity of acidic gating to high [Mg2+]i is gained in mutants with most prominent manifestation in double mutant (Figures 4E,H,K blue line), while in Cx36 WT no acidic gating sensitivity is observed (Figure 4B blue line). Moreover, double mutation decreased dependence of open channel probability on pHi at different [Mg2+]i as compared with Cx36 WT and both single mutations.
The most plausible interpretation of these results might include the direct binding of Mg2+ and H+ ions to E8 and E12. However, the remaining sensitivity of Cx36*E12Q-EGFP to Mg2+ ions at control pHi imply that E12 is not a part of the Mg2+-binding site. Moreover, the lost sensitivity of Cx36*E8Q-EGFP to low [Mg2+]i and the remaining response to high [Mg2+]i at control pHi, suggest that Cx36 could have two or more binding sites with different affinity to Mg2+, similar to TRPM7 channels (Chokshi et al., 2012). We could not exclude indirect involvement of E8 and E12 residues in channel modulation by Mg2+. These residues are located in the NT domain, which forms the entrance into the channel (Beyer et al., 2012), and their negative charges could favour Mg2+ ions to reach the D47 residue, located at the first extracellular loop. Thus, the substitution of E8 or E12 could disturb local [Mg2+]i at the channel pore, which might explain why the E8Q mutation causes the loss in sensitivity to high [Mg2+]i and the E12Q mutation led to a decrease of gj during acidification at high [Mg2+]i. The loss of double mutant sensitivity to high [Mg2+]i and the increased uncoupling during acidification also imply that these two amino acids are important for Cx36 GJ channels regulation by Mg2+ and H+.
Overall, the exact mechanism of pHi and [Mg2+]i effect on gj and the role of the NT domain are unclear. Further investigations are needed to determine the regulatory sites for acidification and their dependence on Mg2+.
Functional role of [Mg2+]i, [H+]i, and their interaction
Our data show that [Mg2+]i can modulate the sensitivity of Cx36 channels to pHi. The low sensitivity of Cx36 GJs to low pHi was proposed to act as a preventive mechanism of the function of electrical synapses during brain ischemia (González-Nieto et al., 2008). Our results suggest that such mechanism would require normal levels of [Mg2+]i. There are a number of studies which have demonstrated therapeutic effect of Mg2+ in the treatment of brain ischemia (Westermaier et al., 2013), thus it is possible that [Mg2+]i and pHi effect on Cx36 plays at least a partial role in the protective mechanisms. For example, the depletion of ATP during brain ischemia (Sato et al., 1984) could induce an increase of [Mg2+]i (Henrich and Buckler, 2008), therefore these factors together might coordinate the regulation of electrical coupling. Presumably, under a mild ischemia it might be beneficial to maintain the normal electrical coupling, while the closure of Cx36 GJ channels during a severe ischemia could isolate the damaged regions of cells, thus preventing the further spread of apoptosis.
Author contributions
LR, VS, and FB: conception of the work, design of experiments, collection, analysis and interpretation of data, drafting of manuscript; TK and NP-P: recorded and analysed the experimental data; MS: constructed and applied mathematical models; NP-P and MS: critically revised the manuscript; VJ: performed experiments.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Alina Marandykina-Prakiene for generating mutants of Cx36 and Vytautas Raskevicius for performing structural modelling and evaluation of pKa.
Footnotes
Funding. This study was supported by grant No. MIP-76/2015 from the Research Council of Lithuania to FB, and partially supported by grant ICM-Economía P09-022-F Centro Interdisciplinario de Neurociencias de Valparaíso and Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) grants No. 3180272 to NP-P.
References
- Acuña-Castillo C., Coddou C., Bull P., Brito J., Huidobro-Toro J. P. (2007). Differential role of extracellular histidines in copper, zinc, magnesium and proton modulation of the P2X7 purinergic receptor. J. Neurochem. 101, 17–26. 10.1111/j.1471-4159.2006.04343.x [DOI] [PubMed] [Google Scholar]
- Akopian A., Atlasz T., Pan F., Wong S., Zhang Y., Völgyi B., et al. (2014). Gap junction-mediated death of retinal neurons is connexin and insult specific: a potential target for neuroprotection. J. Neurosci. 34, 10582–10591. 10.1523/JNEUROSCI.1912-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antanaviciute I., Rysevaite K., Liutkevicius V., Marandykina A., Rimkute L., Sveikatiene R., et al. (2014). Long-distance communication between laryngeal carcinoma cells. PLoS ONE 9:e99196. 10.1371/journal.pone.0099196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbiroli B., Martinelli P., Patuelli A., Lodi R., Iotti S., Cortelli P., et al. (1999). Phosphorus magnetic resonance spectroscopy in multiple system atrophy and Parkinson's disease. Mov. Disord. 14, 430–435. [DOI] [PubMed] [Google Scholar]
- Bedner P., Niessen H., Odermatt B., Kretz M., Willecke K., Harz H. (2006). Selective permeability of different connexin channels to the second messenger cyclic AMP. J. Biol. Chem. 28, 6673–6681. 10.1074/jbc.M511235200 [DOI] [PubMed] [Google Scholar]
- Bennett B. C., Purdy M. D., Baker K. A., Acharya C., McIntire W. E., Stevens R. C., et al. (2016). An electrostatic mechanism for Ca(2+)-mediated regulation of gap junction channels. Nat. Commun. 7, 8770. 10.1038/ncomms9770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Zukin R. S. (2004). Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron 41, 495–511. 10.1016/S0896-6273(04)00043-1 [DOI] [PubMed] [Google Scholar]
- Bevans C. G., Harris A. L. (1999). Regulation of connexin channels by pH. Direct action of the protonated form of taurine and other aminosulfonates. J. Biol. Chem. 274, 3711–3719. 10.1074/jbc.274.6.3711 [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Lipkind G. M., Kyle J. W., Berthoud V. M. (2012). Structural organization of intercellular channels II. Amino terminal domain of the connexins: sequence, functional roles, and structure. Biochim. Biophys. Acta 1818, 1823–1830. 10.1016/j.bbamem.2011.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissiere S., Zelikowsky M., Ponnusamy R., Jacobs N. S., Blair H. T., Fanselow M. S. (2011). Electrical synapses control hippocampal contributions to fear learning and memory. Science 331, 87–91. 10.1126/science.1193785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas F. F., Verselis V. K. (2004). Gap junction channel gating. Biochim. Biophys. Acta 1662, 42–60. 10.1016/j.bbamem.2004.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M., Kaila K. (1992). Modulation of pH by neuronal activity. Trends Neurosci. 15, 396–402. 10.1016/0166-2236(92)90191-A [DOI] [PubMed] [Google Scholar]
- Chesler M., Kraig R. P. (1989). Intracellular pH transients of mammalian astrocytes. J. Neurosci. 9, 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chokshi R., Matsushita M., Kozak J. A. (2012). Detailed examination of Mg2+ and pH sensitivity of human TRPM7 channels. Am. J. Physiol. Cell Physiol. 302, C1004–C1011. 10.1152/ajpcell.00422.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Long M. A. (2004). Electrical synapses in the mammalian brain. Annu. Rev. Neurosci. 27, 393–418. 10.1146/annurev.neuro.26.041002.131128 [DOI] [PubMed] [Google Scholar]
- Cruikshank S. J., Hopperstad M., Younger M., Connors B. W., Spray D. C., Srinivas M. (2004). Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc. Natl. Acad. Sci. U.S.A. 101, 12364–12369. 10.1073/pnas.0402044101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corsso C., Iglesias R., Zoidl G., Dermietzel R., Spray D. C. (2012). Calmodulin dependent protein kinase increases conductance at gap junctions formed by the neuronal gap junction protein connexin36. Brain Res. 1487, 69–77. 10.1016/j.brainres.2012.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlach J. (1990). Magnesium depletion and pathogenesis of Alzheimer's disease. Magn. Res. 3, 217–218. [PubMed] [Google Scholar]
- Farnsworth N. L., Benninger R. K. (2014). New insights into the role of connexins in pancreatic islet function and diabetes. FEBS Lett. 588, 1278–1287. 10.1016/j.febslet.2014.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajda Z., Szupera Z., Blazso G., Szente M. (2005). Quinine, a blocker of neuronal cx36 channels, suppresses seizure activity in rat neocortex in vivo. Epilepsia 46, 1581–1591. 10.1111/j.1528-1167.2005.00254.x [DOI] [PubMed] [Google Scholar]
- Giaume C., Korn H. (1982). Ammonium sulfate induced uncouplings of crayfish septate axons with and without increased junctional resistance. Neuroscience 7, 1723–1730. 10.1016/0306-4522(82)90030-6 [DOI] [PubMed] [Google Scholar]
- González-Nieto D., Gómez-Hernández J. M., Larrosa B., Gutiérrez C., Muñoz M. D., Fasciani I., et al. (2008). Regulation of neuronal connexin-36 channels by pH. Proc. Natl. Acad. Sci. U.S.A. 105, 17169–17174. 10.1073/pnas.0804189105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna S. (1961). Plasma magnesium in health and disease. J. Clin. Pathol. 14, 410–414. 10.1136/jcp.14.4.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harks E. G., de Roos A. D., Peters P. H., de Haan L. H., Brouwer A., Ypey D. L., et al. (2001). Fenamates: a novel class of reversible gap junction blockers. J. Pharmacol. Exp. Ther. 298, 1033–1041. [PubMed] [Google Scholar]
- Harris A. L., Contreras J. E. (2014). Motifs in the permeation pathway of connexin channels mediate voltage and Ca2+ sensing. Front. Physiol. 5:113 10.3389/fphys.2014.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. L. (1981). Kinetic properties of a voltage-dependent junctional conductance. J. Gen. Physiol. 77, 95–117. 10.1085/jgp.77.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich M., Buckler K. J. (2008). Effects of anoxia, aglycemia, and acidosis on cytosolic Mg2+, ATP, and pH in rat sensory neurons. Am. J. Physiol. Cell Physiol. 294, C280–C294. 10.1152/ajpcell.00345.2007 [DOI] [PubMed] [Google Scholar]
- Hermans M. M., Kortekaas P., Jongsma H. J., Rook M. B. (1995). pH sensitivity of the cardiac gap junction proteins, connexin 45 and 43. Pflugers Arch. 431, 138–140. 10.1007/BF00374389 [DOI] [PubMed] [Google Scholar]
- Hinsberger A. D., Williamson P. C., Carr T. J., Stanley J. A., Drost D. J., Densmore M., et al. (1997). Magnetic resonance imaging volumetric and phosphorus 31 magnetic resonance spectroscopy measurements in schizophrenia. J. Psychiatry Neurosci. 22, 111–117. [PMC free article] [PubMed] [Google Scholar]
- Hormuzdi S. G., Filippov M. A., Mitropoulou G., Monyer H., Bruzzone R. (2004). Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks. Biochim. Biophys. Acta 1662, 113–137. 10.1016/j.bbamem.2003.10.023 [DOI] [PubMed] [Google Scholar]
- Ishizuka J., Bold R. J., Townsend C. M., Jr., Thompson J. C. (1994). In vitro relationship between magnesium and insulin secretion. Magnes. Res. 7, 17–22. [PubMed] [Google Scholar]
- Jiang J., Li M., Yue L. (2005). Potentiation of TRPM7 inward currents by protons. J. Gen. Physiol. 126, 137–150. 10.1085/jgp.200409185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameritsch P., Khandoga N., Pohl U., Pogoda K. (2013). Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis. 4, e584. 10.1038/cddis.2013.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmann D., Lüttjohann A., Seidenbecher T., Coulon P., Pape H. C. (2016). Short-term depression of gap junctional coupling in reticular thalamic neurons of absence epileptic rats. J. Physiol. 594, 5695–5710. 10.1113/JP271811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe P. D., Lau A. F. (2004). The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 36, 1171–1186. 10.1016/S1357-2725(03)00264-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Hao B., Lu Y., Yu P., Lee H. C., Yue J. (2012). Intracellular alkalinization induces cytosolic Ca2+ increases by inhibiting sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). PLoS ONE 7:e31905. 10.1371/journal.pone.0031905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Nakagawa S., Suga M., Yamashita E., Oshima A., Fujiyoshi Y., et al. (2009). Structure of the connexin 26 gap junction channel at 3.5 A resolution. Nature 458, 597–602. 10.1038/nature07869 [DOI] [PubMed] [Google Scholar]
- Matsuda H., Kurata Y., Oka C., Matsuoka S., Noma A. (2010). Magnesium gating of cardiac gap junction channels. Prog. Biophys. Mol. Biol. 103, 102–110. 10.1016/j.pbiomolbio.2010.05.009 [DOI] [PubMed] [Google Scholar]
- Moreno A. P. (2005). Connexin phosphorylation as a regulatory event linked to channel gating. Biochim. Biophys. Acta 1711, 164–171. 10.1016/j.bbamem.2005.02.016 [DOI] [PubMed] [Google Scholar]
- Murray S. A., Nickel B. M., Gay V. L. (2009). Gap junctions as modulators of adrenal cortical cell proliferation and steroidogenesis. Mol. Cell. Endocrinol. 300, 51–56. 10.1016/j.mce.2008.09.027 [DOI] [PubMed] [Google Scholar]
- Musa H., Fenn E., Crye M., Gemel J., Beyer E. C., Veenstra R. D. (2004). Amino terminal glutamate residues confer spermine sensitivity and affect voltage gating and channel conductance of rat connexin40 gap junctions. J. Physiol. 557(Pt 3), 863–878. 10.1113/jphysiol.2003.059386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa H., Veenstra R. D. (2003). Voltage-dependent blockade of connexin40 gap junctions by spermine. Biophys. J. 84, 205–219. 10.1016/S0006-3495(03)74843-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssen J., Herberts C., Drijfhout J. W., Reits E., Janssen L., Neefjes J. (2005). Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434, 83–88. 10.1038/nature03290 [DOI] [PubMed] [Google Scholar]
- Niessen H., Harz H., Bedner P., Krämer K., Willecke K. (2000). Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J. Cell Sci. 113(Pt 8), 1365–1372. [DOI] [PubMed] [Google Scholar]
- Noma A., Tsuboi N. (1987). Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J. Physiol. 382, 193–211. 10.1113/jphysiol.1987.sp016363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuytten D., Van Hees J., Meulemans A., Carton H. (1991). Magnesium deficiency as a cause of acute intractable seizures. J. Neurol. 238, 262–264. 10.1007/BF00319737 [DOI] [PubMed] [Google Scholar]
- Olsson M. H., Søndergaard C. R., Rostkowski M., Jensen J. H. (2011). PROPKA3: consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 7, 525–537. 10.1021/ct100578z [DOI] [PubMed] [Google Scholar]
- Palacios-Prado N., Bennett M. V. L., Bukauskas F. F. (2011). H+- and Mg2+-dependent modulation of cell-cell coupling and voltage gating in gap junction channels, in International Gap Junction Conference (Ghent). [Google Scholar]
- Palacios-Prado N., Briggs S. W., Skeberdis V. A., Pranevicius M., Bennett M. V., Bukauskas F. F. (2010). pH-dependent modulation of voltage gating in connexin45 homotypic and connexin45/connexin43 heterotypic gap junctions. Proc. Natl. Acad. Sci. U.S.A. 107, 9897–9902. 10.1073/pnas.1004552107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N., Chapuis S., Panjkovich A., Fregeac J., Nagy J. I., Bukauskas F. F. (2014). Molecular determinants of magnesium-dependent synaptic plasticity at electrical synapses formed by connexin36. Nat. Commun. 5:4667. 10.1038/ncomms5667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N., Hoge G., Marandykina A., Rimkute L., Chapuis S., Paulauskas N., et al. (2013). Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J. Neurosci. 33, 4741–4753. 10.1523/JNEUROSCI.2825-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazachariou I. M., Martinez-Isla A., Efthimiou E., Williamson R. C., Girgis S. I. (2000). Magnesium deficiency in patients with chronic pancreatitis identified by an intravenous loading test. Clin. Chim. Acta 302, 145–154. 10.1016/S0009-8981(00)00363-6 [DOI] [PubMed] [Google Scholar]
- Peracchia C. (1990). Increase in gap junction resistance with acidification in crayfish septate axons is closely related to changes in intracellular calcium but not hydrogen ion concentration. J. Membr. Biol. 113, 75–92. 10.1007/BF01869608 [DOI] [PubMed] [Google Scholar]
- Peracchia C. (2004). Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 1662, 61–80. 10.1016/j.bbamem.2003.10.020 [DOI] [PubMed] [Google Scholar]
- Purnick P. E., Benjamin D. C., Verselis V. K., Bargiello T. A., Dowd T. L. (2000). Structure of the amino terminus of a gap junction protein. Arch. Biochem. Biophys. 381, 181–190. 10.1006/abbi.2000.1989 [DOI] [PubMed] [Google Scholar]
- Randall R. E., Jr., Rossmeisl E. C., Bleifer K. H. (1959). Magnesium depletion in man. Ann. Intern. Med. 50, 257–287. 10.7326/0003-4819-50-2-257 [DOI] [PubMed] [Google Scholar]
- Rohr S. (2004). Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc. Res. 62, 309–322. 10.1016/j.cardiores.2003.11.035 [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. (1975). Permeability of cell junction depends on local cytoplasmic calcium activity. Nature 254, 250–252. 10.1038/254250a0 [DOI] [PubMed] [Google Scholar]
- Russell J. M., Brodwick M. S. (1988). The interaction of intracellular Mg2+ and pH on Cl- fluxes associated with intracellular pH regulation in barnacle muscle fibers. J. Gen. Physiol. 91, 495–513. 10.1085/jgp.91.4.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez H. A., Verselis V. K. (2014). Aberrant Cx26 hemichannels and keratitis-ichthyosis-deafness syndrome: insights into syndromic hearing loss. Front. Cell. Neurosci. 8:354. 10.3389/fncel.2014.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraga F., Ng L., Skinner F. K. (2006). Distal gap junctions and active dendrites can tune network dynamics. J. Neurophysiol. 95, 1669–1682. 10.1152/jn.00662.2005 [DOI] [PubMed] [Google Scholar]
- Sato M., Paschen W., Pawlik G., Heiss W. D. (1984). Neurologic deficit and cerebral ATP depletion after temporary focal ischemia in cats. J. Cereb. Blood Flow Metab. 4, 173–177. 10.1038/jcbfm.1984.25 [DOI] [PubMed] [Google Scholar]
- Shore L., McLean P., Gilmour S. K., Hodgins M. B., Finbow M. E. (2001). Polyamines regulate gap junction communication in connexin 43-expressing cells. Biochem. J. 357(Pt 2), 489–495. 10.1042/bj3570489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söhl G., Willecke K. (2004). Gap junctions and the connexin protein family. Cardiovasc. Res. 62, 228–232. 10.1016/j.cardiores.2003.11.013 [DOI] [PubMed] [Google Scholar]
- Søndergaard C. R., Olsson M. H., Rostkowski M., Jensen J. H. (2011). Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J. Chem. Theory Comput. 7, 2284–2295. 10.1021/ct200133y [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. (1981). Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 211, 712–715. 10.1126/science.6779379 [DOI] [PubMed] [Google Scholar]
- Srinivas M., Hopperstad M. G., Spray D. C. (2001). Quinine blocks specific gap junction channel subtypes. Proc. Natl. Acad. Sci. U.S.A. 98, 10942–10947. 10.1073/pnas.191206198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M., Rozental R., Kojima T., Dermietzel R., Mehler M., Condorelli D. F., et al. (1999). Functional properties of channels formed by the neuronal gap junction protein connexin36. J. Neurosci. 19, 9848–9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swietach P., Rossini A., Spitzer K. W., Vaughan-Jones R. D. (2007). H+ ion activation and inactivation of the ventricular gap junction: a basis for spatial regulation of intracellular pH. Circ. Res. 100, 1045–1054. 10.1161/01.RES.0000264071.11619.47 [DOI] [PubMed] [Google Scholar]
- Tao L., Harris A. L. (2004). Biochemical requirements for inhibition of Connexin26-containing channels by natural and synthetic taurine analogs. J. Biol. Chem. 279, 38544–38554. 10.1074/jbc.M405654200 [DOI] [PubMed] [Google Scholar]
- Teubner B., Degen J., Söhl G., Güldenagel M., Bukauskas F. F., Trexler E. B., et al. (2000). Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J. Membr. Biol. 176, 249–262. 10.1007/s00232001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. (1979). Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18, 2210–2218. 10.1021/bi00578a012 [DOI] [PubMed] [Google Scholar]
- Tong J. J., Ebihara L. (2006). Structural determinants for the differences in voltage gating of chicken Cx56 and Cx45.6 gap-junctional hemichannels. Biophys. J. 91, 2142–2154. 10.1529/biophysj.106.082859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler E. B., Bukauskas F. F., Bennett M. V. L., Bargiello T. A., Verselis V. K. (1999). Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J. Gen. Physiol. 113, 721–742. 10.1085/jgp.113.5.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turin L., Warner A. E. (1977). Carbon dioxide reversibly abolishes ionic communication between cells of early amphibian embryo. Nature 270, 56–57. 10.1038/270056a0 [DOI] [PubMed] [Google Scholar]
- Valiunas V., Polosina Y. Y., Miller H., Potapova I. A., Valiuniene L., Doronin S., et al. (2005). Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J. Physiol. 568, 459–468. 10.1113/jphysiol.2005.090985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance M. M., Wiley L. M. (1999). Gap junction intercellular communication mediates the competitive cell proliferation disadvantage of irradiated mouse preimplantation embryos in aggregation chimeras. Radiat. Res. 152, 544–551. 10.2307/3580152 [DOI] [PubMed] [Google Scholar]
- Verselis V. K., Ginter C. S., Bargiello T. A. (1994). Opposite voltage gating polarities of two closely related connexins. Nature 368, 348–351. 10.1038/368348a0 [DOI] [PubMed] [Google Scholar]
- Völgyi B., Pan F., Paul D. L., Wang J. T., Huberman A. D., Bloomfield S. A. (2013). Gap junctions are essential for generating the correlated spike activity of neighboring retinal ganglion cells. PLoS ONE 8:e69426. 10.1371/journal.pone.0069426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V., Perc M., Bazhenov M. (2011). Gap junctions and epileptic seizures–two sides of the same coin? PLoS ONE 6:e20572. 10.1371/journal.pone.0020572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. G., Peracchia C. (1996). Connexin 32/38 chimeras suggest a role for the second half of inner loop in gap junction gating by low pH. Am. J. Physiol. 271(5 Pt 1), C1743–C1749. 10.1152/ajpcell.1996.271.5.C1743 [DOI] [PubMed] [Google Scholar]
- Webb B., Sali A. (2014). Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinformatics Chapter 5:Unit-5.6. 10.1002/0471250953.bi0506s47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingart R., Bukauskas F. F. (1998). Long-chain n-alkanols and arachidonic acid interfere with the Vm-sensitive gating mechanism of gap junction channels. Pflugers Arch. 435, 310–319. 10.1007/s004240050517 [DOI] [PubMed] [Google Scholar]
- Westermaier T., Stetter C., Kunze E., Willner N., Raslan F., Vince G. H., et al. (2013). Magnesium treatment for neuroprotection in ischemic diseases of the brain. Exp. Transl. Stroke Med. 5:6. 10.1186/2040-7378-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. M., Wang G. L., Hao X. S., Feng J. C. (2017). Dynamic expression of CX36 protein in kainic acid kindling induced epilepsy. Transl. Neurosci. 8, 31–36. 10.1515/tnsci-2017-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]