Abstract
Acromegaly is a rare but severe disease, originated in 95% of cases by a growth hormone-secreting adenoma (somatotropinoma) in the pituitary. Magnetic resonance imaging (MRI) is a non-invasive technique used for the diagnosis and prognosis of pituitary tumours. The aim of this study was to determine whether the use of T2-weighted signal intensity at MRI could help to improve the characterisation of somatotropinomas, by analysing its relationship with clinical/molecular features. An observational study was implemented in a cohort of 22 patients (mean age = 42.1 ± 17.2 years; 59% women; 95% size>10 mm). Suprasellar-extended somatotropinomas presented larger diameters vs. non-extended tumours. T2-imaging revealed that 59% of tumours were hyperintense and 41% isointense adenomas, wherein hyperintense were more invasive (according to Knosp-score) than isointense adenomas. A higher proportion of hyperintense somatotropinomas presented extrasellar-growth, suprasellar-growth and invasion of the cavernous sinus compared to isointense adenomas. Interestingly, somatostatin receptor-3 and dopamine receptor-5 (DRD5) expression levels were associated with extrasellar and/or suprasellar extension. Additionally, DRD5 was also higher in hyperintense adenomas and its expression was directly correlated with Knosp-score and with tumour diameter. Hence, T2-weighted MRI on somatotropinomas represents a potential tool to refine their diagnosis and prognosis, and could support the election of preoperative treatment, when required.
Introduction
Acromegaly is a rare disease, with a prevalence of only 35–75 cases per million inhabitants, and an incidence of 3–4 cases per million inhabitants/year1,2. It is caused by over-production of growth hormone (GH), which in ~95% of cases is due to a pituitary adenoma (PA)3, usually presenting as a macroadenoma on diagnosis4. Magnetic resonance imaging (MRI) has greatly improved the success of clinical management in recent years and is now a key element in the diagnosis of patients with acromegaly. Thus, MRI is a non-invasive diagnostic test that can help to characterise GH-secreting adenomas and could aid to predict their clinical behavior. Specifically, some studies have suggested the existence of a relationship between the intensity of the adenoma on T2-weighted imaging and its characteristics5, pathology5–7 and response to somatostatin analogues6,8. However, these studies were conducted in small series of PAs and the findings are still inconsistent and/or inconclusive, which could in part be also due to the characteristics of GH-secreting adenomas (i.e. unlike other PA types that are usually hyperintense on T2-weighted imaging, GH-secreting adenomas can present varying intensities). Yet, the reason for this variability in T2 intensity is unclear4.
The behaviour and response to treatment of GH-secreting adenomas depend on their clinical, biochemical, imaging and genetic characteristics, which cannot still be used, routinely and reliably, to predict the aggressiveness of the tumour at the time of diagnosis. Tumour markers such as securin or pituitary tumour–transforming gene (PTTG1)9 and the Ki67 proliferation marker10 can be evaluated from the tumour, as well as several receptors of central and hypothalamic hormones such as somatostatin (sst), dopamine (DRD), ghrelin (GHSR1a) or vasopressin (AVPR1b) receptors11–16. Specifically, somatostatin receptors 2 (sst2) and 5 (sst5) predominate (90%-95%) in GH-secreting tumours12,17, and the expression of these sst subtypes could provide a predictor of response to somatostatin analogue (SSA) therapy12,13,18, which would be clinically relevant as a considerable number of patients are or become totally or partially resistant to SSA therapy19–22. Interestingly, in an attempt to clarify patient response to SSAs, various studies have shown that large tumours with low sst2 expression are generally: (1) poorly granulated, (2) exhibit low positivity for GH, and (3) more invasive. While densely granulated tumours with increased sst2 expression seem to respond better to SSA23–27.
Nevertheless, the molecular analysis of receptors is only possible after surgery, and it is not available in all hospitals. Thus, molecular parameters have not been developed hitherto to predict which patients will respond to treatment, or the progression of the disease. In this context, our main objective was to determine whether the T2-weighted intensity of the adenoma correlates to the clinical and pathological characteristics of the patient at diagnosis of acromegaly and/or the genetic expression of different tumour-related receptors, in order to more precisely classify the tumours and, if required, to predict the most effective medical preoperative treatment (which could be indicated in the case of extensive cavernous sinus invasion, absence of chiasmal compression, or poor surgical candidate28).
Results
Patients characteristics
Twenty-two patients were included in the study (41% men; 59% women). Baseline clinical and analytical characteristics of the patients are shown in Supplemental Table 1 and Supplemental Table 2, respectively. Mean age at diagnosis of acromegaly was 42.09 ± 17.15 years (42.56 ± 18.08 in men vs. 41.77 ± 17.22 in women; p = 0.919). Clinical manifestations at diagnosis of acromegaly did not differ between men and women, except for headache, which was more frequent in women. Interestingly, the prevalence of obstructive sleep apnoea syndrome (OSAS) is lower than expected according to the latest review29, but similar to some national acromegaly registries30. In this sense, it should be mentioned that, in our study, patients were classified as OSAS if the Lung Specialist recommended the use of continuous positive airway pressure (CPAP) (Supplemental Table 1). Circulating Insulin-like growth factor 1 (IGF-1) levels at diagnosis were 575.45 ± 287.51 ng/ml (605.84 ± 266.64 in men vs. 607.81 ± 280.94 in women; p = 0.987), thus presenting no differences between genders (Supplemental Table 2). In addition, although there is no consensus in the current bibliography31–33, our data indicate that in this cohort, circulating basal and nadir GH level following oral glucose tolerance test (OGTT) were both significantly higher in men than in women (Supplemental Table 2).
Magnetic Resonance Imaging (MRI)
Nearly all patients (95%) presented macroadenoma when examined by MRI. Mean anteroposterior, transverse and craniocaudal diameter (APD, TD and CCD, respectively) were 18.62 ± 6.78, 18.97 ± 8.09 and 18.87 ± 8.28 mm, respectively. Interestingly, the classification of adenomas by size into 3 groups, less than 10 mm, between 10 and 20 mm, and greater than 20 mm, as previously reported4 revealed that patients with larger adenomas had numerically higher levels of IGF-1 and basal or nadir GH after OGTT, although these changes were not statistically significant within groups (Table 1).
Table 1.
Particular characteristics of GH-secreting adenomas stratified by maximum tumour diameter.
| Tumour size | ≤10 mm | 10–20 mm | >20 mm | P value |
|---|---|---|---|---|
| Number | 1 | 12 | 9 | — |
| Sex (♂/♀) | 0 vs. 1 | 6 vs. 7 | 3 vs. 5 | 0.551 |
| Age at diagnosis | 36 | 41.55 ± 17.74 | 41.13 ± 19.28 | 0.961 |
| IGF-1 (nd/dL) | 500.43 | 568.49 ± 276.66 | 626.34 ± 307.09 | 0.673 |
| Basal GH (ng/dL) | 5.05 | 7.27 ± 8.07 | 11.39 ± 9.80 | 0.537 |
| Nadir GH (ng/dL) | 5.53 | 6.37 ± 7.88 | 12.00 ± 11.36 | 0.311 |
GH-secreting adenomas were classified into 3 groups by maximum tumour diameter (less than 10 mm, between 10 and 20 mm, and greater than 20 mm) and demographic (sex and age at diagnosis) and clinical (levels of IGF-1 and basal and nadir GH after OGTT) parameters were evaluated.
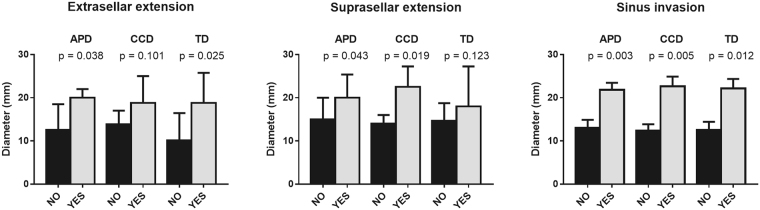
In total, 79% of patients presented extrasellar tumour extension, wherein 63% exhibited suprasellar growth. As expected, tumours with extrasellar/suprasellar growth and those that invaded the sinuses were larger than the tumours without extension and/or invasion (Fig. 1). No differences in tumour size was found in terms of sex, age at diagnosis, IGF-1 level, and basal or nadir GH after OGTT.
Figure 1.
Association between extrasellar/suprasellar extension and invasion and the diameter of somatotropinomas. The diameter of GH secreting adenomas was measured in millimetres. APD: anteroposterior diameter; CCD: craniocaudal diameter; TD: transverse diameter. Data represent median ± interquartile range in extrasellar/suprasellar extension and mean ± SEM in invasion graphs. Differences were evaluated by Student t or Mann-Whitney U tests depending on the normality of each data distribution.
Additionally, right cavernous sinus space invasion was observed in 26% of patients, left sinus invasion in 21%, and invasion of both spaces in 16%. A higher Knosp score was associated with larger tumour diameter (Table 2), but not with any other study parameter, including IGF-1 levels.
Table 2.
Characteristics of pituitary adenomas based on Knosp score.
| Knosp score | 0 | 1 | 2 | 3 | 4 | P value |
|---|---|---|---|---|---|---|
| % | 17.6% | 17.6% | 23.5% | 11.8% | 29.4% | — |
| Age (years) | 35.67 ± 7.51 | 29.33 ± 14.50 | 29.50 ± 13.03 | 62.00 ± 15.56 | 47.40 ± 18.68 | 0.116 |
| IGF-1 (ng/mL) | 547.80 ± 116.25 | 423.18 ± 264.93 | 516.47 ± 300.18 | 493.05 ± 311.95 | 597.91 ± 401.64 | 0.956 |
| Nadir GH (ng/mL) | 11.41 ± 11.80 | 10.95 ± 9.32 | 9.79 ± 10.46 | 8.84 ± 7.98 | 8.75 ± 10.08 | 0.861 |
| Sex (♂/♀) | 3/1 | 1/3 | 3/3 | 0/2 | 2/4 | 0.067 |
| APD (mm) | 10.00 ± 4.58 | 15.20 ± 4.70 | 18.70 ± 3.50 | 16.90 ± 1.56 | 26.50 ± 5.54 | 0.004 |
| TD (mm) | 8.77 ± 2.85 | 17.87 ± 3.01 | 18.15 ± 4.57 | 17.65 ± 3.32 | 27.12 ± 9.36 | 0.022 |
| CCD (mm) | 11.10 ± 4.76 | 15.17 ± 4.76 | 16.97 ± 5.98 | 17.90 ± 4.38 | 29.60 ± 6.42 | 0.003 |
APD: anteroposterior diameter; TD: transverse diameter; CCD: craniocaudal diameter.
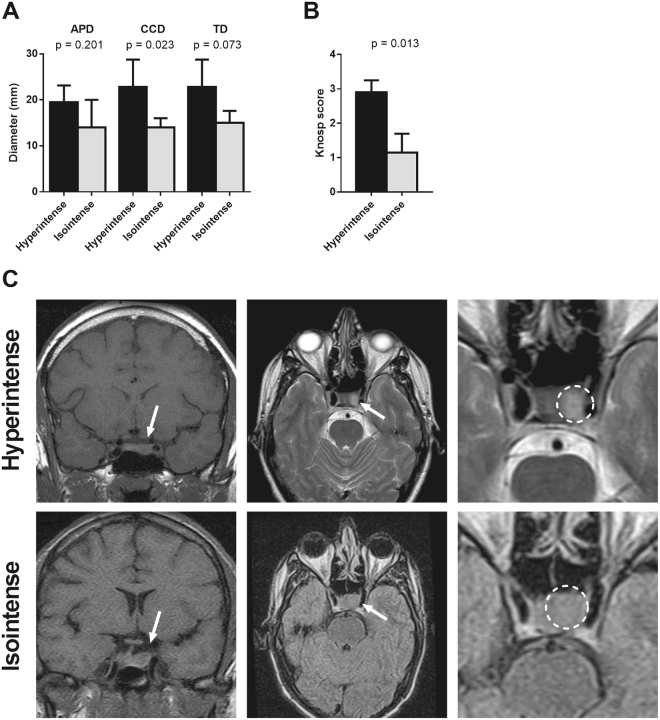
Finally, T2 imaging indicated that 59% of adenomas were hyperintense and 41% isointense. No hypointense adenomas were found in our series. T2 intensity (hyperintense vs. isointense groups) did not differ significantly when compared with sex, age, IGF-1 levels, or nadir GH at diagnosis. On T2-weighted MRI, CCD and TD of hyperintense adenomas were, or tended to be, greater than isointense tumours (p = 0.023 and p = 0.073, respectively) (Fig. 2A). Moreover, mean Knosp score for hyperintense adenomas was higher than in isointense tumours (Fig. 2B). Representative images of hyperintense and isointense adenomas are shown in Fig. 2C.
Figure 2.
Association between T2-signal and the diameter (A) and Knosp score (B) of somatotropinomas. The diameter of GH secreting adenomas was measured in millimetres. APD: anteroposterior diameter; TD: transverse diameter; CCD: craniocaudal diameter. Data represent median ± interquartile range. (C) Classification of T2-weighted signal of GH secreting pituitary adenomas: T2-hyperintense adenomas, upper-panel and T2-isointense adenomas, lower-panel. Tumour region was indicated with an arrow in coronal MRI (left), and in T2-weighted axial MRI (middle), the tumour area was indicated by a circle in right panel.
All hyperintense adenomas presented extrasellar growth vs. only 43% of isointense adenomas (p = 0.023). Furthermore, tumours that presented suprasellar growth or those that invaded the cavernous sinus space were more commonly hyperintense compared to isointense adenomas (in both cases, 90% vs. 42.9%; p = 0.036). Specifically, in the case of hyperintense adenomas, 10% were only invasive, 10% were only suprasellar, and 80% were both. However, no statistically significant differences were found between hyperintense and isointense tumours with regard to treatment or disease control, as specified in Methods section (Table 3).
Table 3.
Treatment overview of the patients according to T2-intensity.
| Pharmacological Treatment | Number (%) | Radiotherapy | Disease control (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Iso | Hyper | Total | Iso | Hyper | Total | Iso | Hyper | |
| No treatment | 5 (22.7) | 1 | 4 | 0 | 0 | 0 | 5 | 1 | 4 |
| Oct | 2 (9.1) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Lan | 5 (22.7) | 3 | 2 | 3 | 2 | 1 | 2 | 1 | 1 |
| Cab | 3 (13.6) | 2 | 1 | 0 | 0 | 0 | 3 | 2 | 1 |
| Oct + Cab | 2 (9.1) | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lan + Cab | 3 (13.6) | 1 | 2 | 1 | 1 | 0 | 3 | 1 | 2 |
| Pas + Cab | 1 (4.5) | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 |
| Peg + Cab | 1 (4.5) | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Total | 22 (100) | 9 | 13 | 7 | 5 | 2 | 14 (63.7) | 6 | 8 |
Iso: isointense adenomas; Hyper: hyperintense adenomas; Oct: octreotide; Lan: lanreotide; Cab: cabergoline; Peg: pegvisomant.
Molecular analysis of the pituitary adenomas
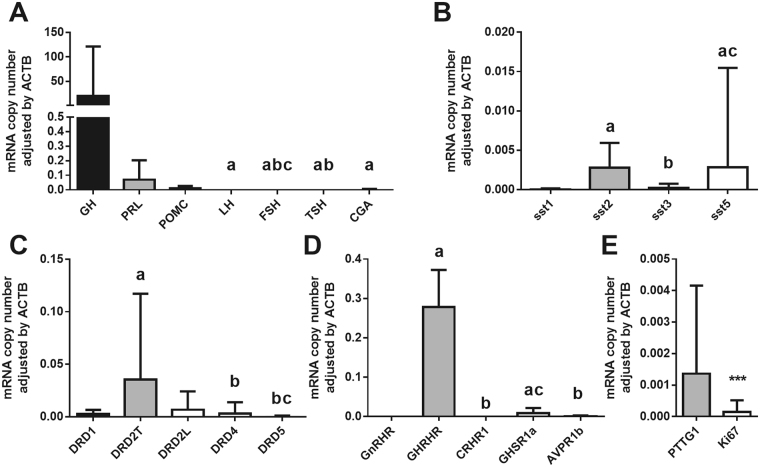
The analysis of the molecular profile (Fig. 3A) confirmed that somatotropinomas expressed high levels of GH (19.586 ± 113.743 copies/adjusted by ACTB) and low levels of other hormones, being PRL (0.07 ± 0.147 copies/adjusted by ACTB) and POMC (0.01 ± 0.017 copies/adjusted by ACTB) the most expressed among them.
Figure 3.
Molecular characterisation of the somatotropinomas. Seventeen somatotropinomas were available to determine by quantitave real-time PCR (qPCR) the expression profile of: (A) pituitary hormones [growth hormone (GH), prolactin (PRL), proopiomelanocortin (POMC), luteinizing hormone (LH), follicle stimulating hormone (FSH), thyroid-stimulating hormone (TSH) and the alpha subunit of the glycoproteins (CGA)]; (B) the five somatostatin receptors subtypes (sst1-5); (C) the five dopamine receptors subtypes (DRD1-5), including the total and large isoform of DRD2 (DRD2T and DRD2L, respectively); (D) other receptors [gonadotropin releasing hormone receptor (GnRHR), growth hormone releasing hormone receptor (GHRHR), corticotropin releasing hormone receptor (CRHR1), ghrelin receptor (GHSR1a), arginine-vasopressin receptor 1b (AVPR1b); and (E) and two proliferation markers [securin (PTTG1) and Ki67]. Data represent median ± interquartile range of absolute expression levels (copy number) of each transcript adjusted by the expression levels of a control gene (ACTB). Values in A-D that do not share a common letter are statistically different (P < 0.05) using a Kruskal-Wallis test followed by a Dunn’s multiple comparisons test. Mann-Whitney test was used for E (*** indicates P < 0.001).
Somatostatin and dopamine receptor subtypes were differently expressed (Fig. 3B,C). Particularly, sst5 and sst2 were the most frequently expressed (95% of the tumours in both cases), followed by sst3 (74%) and sst1 (59%). In the case of dopamine system, DRD2 was most frequently expressed receptor (100% of the cases), followed by DRD4 (89%), DRD1 (87%) and DRD5 (80.0%). As expected, GH-releasing hormone receptor (GHRHR) and ghrelin receptor (GHSR1a) were also expressed at high levels in these somatotropinomas (100% and 92% of the cases, respectively; Fig. 3D), being GHRH-R mRNA levels significantly higher than those of GHSR1a. In contrast, other receptors subtypes involved in the function of different pituitary cell types, such as gonadotropin-releasing hormone receptor (GnRHR), corticotropin-releasing hormone receptor (CRHR1) or vasopressin receptor (AVPR1b) were not noticeably expressed in somatotropinoma samples (Fig. 3D). We also measured two well-known proliferation markers in our cohort of somatotropinoma samples, and found that PTTG1 mRNA levels were higher than Ki67 levels (Fig. 3E).
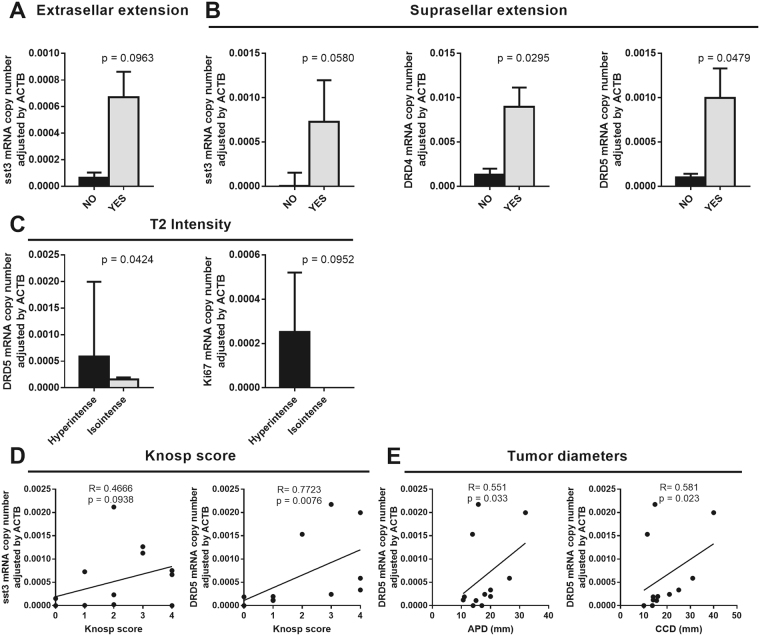
Interestingly, adenomas with extrasellar and suprasellar extension tended to present higher sst3 levels (Fig. 4A,B). In the same line, adenomas with suprasellar extension presented higher levels of DRD4 and DRD5 than adenomas without suprasellar extension (Fig. 4B). No differences were found concerning the invasion of cavernous sinuses. Moreover, compared to isointense adenomas, hyperintense adenomas at T2 imaging presented higher expression levels of DRD5, and a trend for enhanced Ki67 expression (Fig. 4C). Nevertheless, no other receptors or hormones were different in hyperintense vs. isointense adenomas at mRNA level. Additionally, a direct correlation was also found between expression of DRD5 and the Knosp score, which also showed a trend to associate with the expression of sst3 (Fig. 4D). Finally, DRD5 expression was directly correlated with both APD and CCD (Fig. 4E).
Figure 4.
Association between molecular markers and tumour phenotype. (A and B) Association between the presence of extrasellar and suprasellar extension and mRNA levels of sst3, DRD4 and DRD5. (C) Association between T2 signal and mRNA levels of DRD5 and Ki67. (D and E) Correlation study between Knosp score and tumour diameter and mRNA level of sst3 and DRD5.
Discussion
The main objective of this study was to determine whether the T2-weighted intensity of the adenoma correlates to the patient’s clinical and pathological characteristics at diagnosis of acromegaly, or to the genetic expression of different tumour-relevant receptors and tumour-related markers. To that end, we retrospectively analysed a series of 22 patients bearing a GH-producing tumour, in whom T2-weighted intensity analysis of the adenoma were available. Interestingly, we found a predominance of hyperintense adenomas in our series (59%), and no evidence of hypointense tumours, even though these latter have been previously reported to account for between 27% and 50% of all adenomas4–8. This discrepancy is probably related to differences in the definition of adenoma intensity on T2-weighted MRI among published studies. Our approach to intensity definition is similar to that used by Potorac et al., who compared T2-weighted signal intensity of adenomas with that of normal pituitary tissue, and on this basis classified them as hypo-, iso-, or hyper-intense4. When normal tissue was concealed, signal intensity was compared with that of temporal lobe grey matter. In other studies, signal intensity of adenomas on T2-weighted MRI was classified as hyperintense when higher than grey matter intensity, hypointense when lower than white matter intensity, and isointense when higher than white matter intensity but lower than grey matter intensity5,6,8,34,35. However, it seems reasonable to conceive that studies involving MRI evaluation of pituitary tumours should compare T2-weighted signal intensity of adenoma tissue with that of normal pituitary tissue whenever possible, as we have performed in this study.
In our series, hyperintense adenomas tended to be larger than isointense tumours, especially if APD is considered, and more invasive, presenting a higher Knosp score. In fact, all hyperintense adenomas presented extrasellar growth, wherein 90% of them were suprasellar. Invasion of the cavernous sinus space was also more common in hyperintense vs. isointense adenomas. These findings are consistent with those of other published reports4,6,8, and suggest that adenomas that are hyperintense on T2-weighted MRI are potentially more proliferative than non-hyperintense tumours. Additionally, Heck et al., reported that adenomas that are hyperintense on T2-weighted MRI tend to be larger but secrete less GH and hence are associated with lower IGF-1 levels, which could be explained by the finding that all sparsely granulated adenomas studied were hyperintense, while the densely granulated ones were mainly hypo or isointense6,8. In this sense, it has been reported that sparsely granulated somatotropinomas are typically larger, more invasive, less responsive to treatment and more frequently found in younger patients, compared to densely granulated tumours36, which indicates that hyperintense adenomas (which usually correspond to sparsely granulated adenomas) may be more aggressive. Additionally, it has also been reported that hyperintense adenomas differed significantly in GH and IGF-1 levels from the hypo- and isointense group37, wherein the absolute IGF-1 values tended to be lower in the hyperintense group despite a trend towards younger age. Moreover, as tumour size tended to be larger in the hyperintense group, the amount of GH secreted respective to the tumour volume, also known as GH-index, was lower in the hyperintense adenomas and, furthermore, the response to an octreotide test was blunted in these patients37. Remarkably, a similar phenotype has been reported in sparsely-granulated adenomas, wherein a trend towards lower GH-index and blunted response to octreotide test dose has been described27,38. Therefore, these data indicate that the hypo and hyperintense tumours might represent biologically different subgroups with a divergent secretory behaviour. Further supporting this idea, recent guidelines for acromegaly management have also noted the potential utility of using T2 intensity to optimize patient management28.
Few studies have explored to date the potential association between MRI features of pituitary adenomas and their molecular profile. In our series of somatotropinomas, the overall pattern of gene expression appeared to be in line with that reported in comparable previous studies. Thus, specifically, GH was the most abundantly expressed hormone, followed by PRL and POMC17. Similarly, sst5 and sst2, and DRD2 were the mainly expressed somatostatin and dopamine receptors in tumoural somatotropes12,16,17,39,40. As previously reported, the most expressed receptors for hypothalamic regulators were those for GHRH41 and ghrelin14,42–46, while the expression of receptors for other hypothalamic modulators such as GnRHR, AVPR1b and CRHR1 are virtually lacking, as previously observed47. A recent study has evaluated the relation between T2 intensity and sst2 and sst5 immunoreactivity, and, interestingly, the level of sst5, but not sst2, was inversely correlated with T2 intensity35. In our series, no apparent associations were found between sst2 or sst5 expression and T2 intensity. However, the molecular study revealed, for the first time, that although the expression of sst3, DRD4 and DRD5 is low in these samples, it is increased in adenomas with suprasellar extension compared with the tumours with no suprasellar growth. Of note, these receptors are not currently targeted with specific pharmacological therapies for pituitary adenomas, as there are no available selective agonists for these receptors. Our present results suggest that this possibility is worth to be explored, due to its putative therapeutic implications, and, therefore, further studies should be implemented in this regard. Moreover, we found that T2 hyperintense tumours presented an increased expression of DRD5 and Ki67 at mRNA level, together with the larger size and invasive nature, lending credence to the notion that DRD5 might be a marker of poor prognosis. In support to this contention is the direct correlation between DRD5 and APD and CCD diameter, which is also further reinforced by the direct association between Knosp score and DRD5 and sst3 expression. These findings, which are reported for the first time in the literature, suggest that a combined analysis of T2 intensity and expression of these molecular markers could help to guide therapeutic choices at the time of diagnosis. Indeed, our results could suggest that larger tumours, characterised by poorer prognosis for surgical resolution and bearing a higher expression of DRD5 and sst3, could be possibly treated before surgery using alternative DRD agonists (i.e. with high affinity for DRD5) or pasireotide (a multireceptor ligand with affinity for sst1, sst2, sst3 and sst5), insofar the mRNA of these receptors is correctly transcribed to protein, as previously reported in neuroendocrine tumours48.
In conclusion, in this study we found that on T2-weighted imaging, hyperintense GH-secreting pituitary adenomas are larger and tend to be more aggressive due to the invasion of adjacent structures, and these features may be linked to the expression of specific molecular markers. Hence, detailed preoperative imaging studies might help to predict the ideal preoperative treatment and management in these patients. However, further studies in larger cohorts of patients using the same criteria for defining pituitary adenoma characteristics on MRI, collecting the pharmacological and/or radiotherapy treatment outcome and analysing the molecular profile are required to clarify the implications of MRI in somatotropinoma characterization and in the choice of medical treatment, if required.
Methods
Patients
This is a retrospective, single-centre study (Reina Sofía University Hospital in Cordoba, Spain) including all patients diagnosed with acromegaly that had a preliminary MRI of the pituitary prior to the start of treatment, and with a molecular expression profile of the tumour immediately after surgery. Only patients with a confirmed diagnosis of acromegaly were included. This study was approved by the Hospital Ethic Committee and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Diagnosis of acromegaly was based on the presence of classic clinical features and on a result of GH ≥1 ng/dl in a 75 mg OGTT, elevated IGF-1 levels according to the patient’s reference range for age and sex and/or on the presence of a pituitary adenoma by MRI. In all cases, histopathology of the surgical specimen confirmed the presence of a GH-producing adenoma. Criteria to consider successful resolution of acromegaly (cured patients) are disappearance of the pituitary adenoma, normalisation of GH and IGF-1 levels, and suppression of GH levels following OGTT (below 1 or 0.4 ng/l), without the need for medical treatment. Additionally, acromegaly controlled with medication was defined as GH and IGF-1 levels within normal ranges in a patient under treatment49. Demographic variables such as age, sex and race were collected, together with clinical characteristics and results of different tests at diagnosis. Exclusion criteria were pregnancy or breastfeeding and refusal to sign the informed consent form.
Magnetic Resonance Imaging
Imaging studies were performed using high resolution 1.5 T MRI instrument (MAGNETOM Aera 1.5 Tesla, Siemens). To qualify for inclusion in the study, patients must have at least 1 coronal and 1 sagittal T1- and T2-weighted sequence. MRI images were independently analyzed by two neuro-radiologists expert in the interpretation of MRI pituitary images, wherein their evaluations were 100% concurrent. The following parameters were recorded: anteroposterior diameter (APD), transverse diameter (TD) and craniocaudal diameter (CCD), extrasellar growth, presence or absence of suprasellar contact with optic chiasm, invasion of cavernous sinus space, and T1 and T2 signal intensity. T2-weighted intensity was classified as hypo-, iso- or hyper-intense compared with normal pituitary tissue (n = 17/22 cases). When the pituitary tissue was hidden, as in the case of pituitary macroadenomas, the intensity was compared with that of temporal lobe grey matter (n = 5/22 cases), as previously reported4. Invasion of cavernous sinus spaces was classified according to the system of grading invasion of cavernous sinus by pituitary adenomas (grade 0–4) developed by Knosp et al.50
Hormonal analysis
Absolute IGF-1 levels at diagnosis were determined by an immunoradiometric assay (#A15729 Immunotech SAS, Marseille, France) and compared to the upper limit of normal adjusted by age and sex. To avoid ambiguity, IGF-1 levels are expressed as a standard deviation score with respect to the reference range for age, sex, pubertal stage, and determination method. GH was determined by chemiluminescence by immunoradiometric assay (#HGH-RIACT, Cisbio Bioassays, Codolet, France). All the information regarding specificity, detectability, and reproducibility for each of the assays can be accessed at the website of the companies.
Specimen analysis
GH-secreting pituitary adenomas tissue samples were obtained during transsphenoidal surgery. The fact that the tissue piece collected by our laboratory corresponded to somatotropinoma tissue was confirmed by 3 separate methods, as previously reported15: by the examination of an anatomopathologist expert in pituitary, by testing of the hormonal phenotype using cell-blotting assays, and by assessing the molecular screening using qPCR evaluating the genes described above, which has been repeatedly proven in our laboratory11. Subsequently, a portion of the tumour was retained for pathological examination and the remaining fragments were snap frozen in liquid nitrogen until RNA extraction. Total RNA was isolated with the AllPrep DNA/RNA/Protein Mini Kit with deoxyribonuclease treatment using RNase-Free DNase Set (Qiagen, Limburg, Netherlands), following the instructions of the manufacturer. In all cases, total RNA concentration and purity was assessed using Nanodrop 2000 spectrophotometer (Thermo Scientific). Total RNA was retro-transcribed using random hexamer primers and the cDNA First Strand Synthesis kit (Thermo Scientific). Details regarding the quantitative PCR (qPCR) procedure used to determine the absolute expression levels of the different transcripts included in this study have been previously reported by our laboratory11–15. Specifically, the genes analysed in this study were: growth hormone (GH), prolactin (PRL), proopiomelanocortin (POMC), follicle stimulating hormone (FSH), luteinising hormone (LH), thyroid-stimulating hormone (TSH) and the alpha subunit of the glycoproteins (CGA); somatostatin receptor subtypes (sst1, sst2, sst3, sst5), dopamine receptor subtypes (DRD1, DRD2T, DRD2L, DRD4, DRD5), other hormonal receptors [gonadotropin releasing hormone receptor (GnRHR), growth hormone releasing hormone receptor (GHRHR), corticotropin releasing hormone receptor (CRHR1), ghrelin receptor (GHSR1a) and arginine-vasopressin receptor 1b (AVPR1b)] and two proliferation markers (Ki67 and PTTG1). Specific sets of primers for these genes have been previously validated and reported by our group11. To control for variations in the amount of RNA used in the retro-transcription reaction and the efficiency of the retro-transcription reaction, mRNA copy numbers of the different transcripts analysed were adjusted by beta-actin (ACTB) expression (used as housekeeping gene, as recently reported39).
Statistical analysis
The descriptive analysis of qualitative variables in each category was expressed in terms of absolute frequencies and percentage. Quantitative variables are expressed as mean ± standard variation, using the Shapiro-Wilk analysis to test for normality. mRNA data adjusted by ACTB expression were expressed as median ± interquartile range. Wherever non-normal variables were detected, the corresponding non-parametric test was used. The chi-square test was used to associate qualitative variables. Means were compared using the Student’s t test for parametric variables, and the Mann-Whitney U test for non-parametric variables. In the case of multiple comparison, a Kruskal-Wallis test followed by a Dunn’s multiple comparisons test was performed. Spearman’s correlation coefficient was used as a measure of correlation between non-parametric variables. Statistical significance was set at 5%, and the statistical analysis was performed using SPSS version 22.0 for Windows, and GraphPad Prism 7.0.
Electronic supplementary material
Acknowledgements
This work has been funded by the following grants: Junta de Andalucía (CTS-1406, BIO-0139); Ministerio de Economía y Competitividad, Gobierno de España (BFU2013-43282, BFU2016-80360-R), Instituto de Salud Carlos III, co-funded by European Union (ERDF/ESF, “Investing in your future”; PI16/00264, CP15/00156), Ayuda Merck Serono 2013 and CIBERobn. CIBER is an initiative of Instituto de Salud Carlos III, Ministerio de Sanidad, Servicios Sociales e Igualdad, Spain. Part of the analyses included in this study was carried out within the REMAH (‘Spanish molecular registry of pituitary adenomas’) project, supported by Novartis Oncology as well as by the Andalusian and Spanish Societies of Endocrinology and Nutrition (SAEDYN and SEEN).
Author Contributions
Conception of the work: M.R.A.-E., J.P.C., M.A.G., R.M.L.; Design of research: M.R.A.-E., A.I.-C., J.P.C., M.A.G., R.M.L.; Performed experiments: M.R.A.-E., A.I.-C., P.M.-M., E.R.-C., M.C.V.-B.; Data analysis: M.R.A.-E., A.I.-C., P.M.-M., E.R.-C., M.C.V.-B., R.M.L.; Interpreted results of experiments: M.R.A.-E., A.I.-C., M.D.G., J.P.C., M.A.G., R.M.L.; Prepared figures: M.R.A.-E., A.I.-C., M.D.G., R.M.L.; Acquisition of clinical data and samples: M.R.A.-E., P.M.-M., C.B.-A., A.T.-D.; Acquisition of radiological data: M.S.L.-G., J.A.V.-C.; Draft the work: M.R.A.-E., A.I.-C., M.D.G., J.P.C., M.A.G., R.M.L.; Wrote the manuscript: M.R.A.-E., A.I.-C., M.D.G., J.P.C., M.A.G., R.M.L.; Critically revised the manuscript for important intellectual content and approved final version of manuscript, M.R.A.-E., A.I.-C., P.M.-M., E.R.-C., M.C.V.-B., C.B.-A., A.T.-D., M.S.L.-G., J.A.V.-C., M.D.G., J.P.C., M.A.G., R.M.L.
Competing Interests
M.A. Gálvez, J.P. Castaño and R.M. Luque have received lecture fees and/or research grant support from Ipsen and Novartis. The rest of the authors have nothing to disclose. Novartis Oncology was not involved in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript but the financial support of the REMAH database. Additionally, we declare no non-financial involvement of any Novartis Oncology, or any company, employee concerning the publication or preparation of the present manuscript.
Footnotes
Justo P. Castaño, María A. Gálvez and Raúl M. Luque contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-24260-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Justo P. Castaño, Email: justo@uco.es
María A. Gálvez, Email: mariaa.galvez.sspa@juntadeandalucia.es
Raúl M. Luque, Email: raul.luque@uco.es
References
- 1.Puig Domingo M. Treatment of acromegaly in the era of personalized and predictive medicine. Clin. Endocrinol. (Oxf.) 2015;83:3–14. doi: 10.1111/cen.12731. [DOI] [PubMed] [Google Scholar]
- 2.Capatina C, Wass JA. 60 YEARS OF NEUROENDOCRINOLOGY: Acromegaly. J. Endocrinol. 2015;226:T141–160. doi: 10.1530/JOE-15-0109. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Shlomo, A. & Melmed, S. Acromegaly. Endocrinol. Metab. Clin. North Am. 37, 101-122, viii, 10.1016/j.ecl.2007.10.002 (2008). [DOI] [PMC free article] [PubMed]
- 4.Potorac I, et al. Pituitary MRI characteristics in 297 acromegaly patients based on T2-weighted sequences. Endocr. Relat. Cancer. 2015;22:169–177. doi: 10.1530/ERC-14-0305. [DOI] [PubMed] [Google Scholar]
- 5.Hagiwara A, et al. Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology. 2003;228:533–538. doi: 10.1148/radiol.2282020695. [DOI] [PubMed] [Google Scholar]
- 6.Heck A, et al. Intensity of pituitary adenoma on T2-weighted magnetic resonance imaging predicts the response to octreotide treatment in newly diagnosed acromegaly. Clin. Endocrinol. (Oxf.) 2012;77:72–78. doi: 10.1111/j.1365-2265.2011.04286.x. [DOI] [PubMed] [Google Scholar]
- 7.Bakhtiar Y, et al. Geometric survey on magnetic resonance imaging of growth hormone producing pituitary adenoma. Pituitary. 2014;17:142–149. doi: 10.1007/s11102-013-0479-z. [DOI] [PubMed] [Google Scholar]
- 8.Heck A, Emblem KE, Casar-Borota O, Bollerslev J, Ringstad G. Quantitative analyses of T2-weighted MRI as a potential marker for response to somatostatin analogs in newly diagnosed acromegaly. Endocrine. 2016;52:333–343. doi: 10.1007/s12020-015-0766-8. [DOI] [PubMed] [Google Scholar]
- 9.Melmed S. Acromegaly pathogenesis and treatment. J. Clin. Invest. 2009;119:3189–3202. doi: 10.1172/JCI39375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao D, Tomono Y, Nose T. Expression of P27kip1 and Ki-67 in pituitary adenomas: an investigation of marker of adenoma invasiveness. Acta Neurochir. (Wien.) 1999;141:187–192. doi: 10.1007/s007010050285. [DOI] [PubMed] [Google Scholar]
- 11.Luque RM, et al. The Molecular Registry of Pituitary Adenomas (REMAH): A bet of Spanish Endocrinology for the future of individualized medicine and translational research. Endocrinol Nutr. 2016;63:274–284. doi: 10.1016/j.endonu.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Taboada GF, et al. Quantitative analysis of somatostatin receptor subtype (SSTR1-5) gene expression levels in somatotropinomas and non-functioning pituitary adenomas. Eur. J. Endocrinol. 2007;156:65–74. doi: 10.1530/eje.1.02313. [DOI] [PubMed] [Google Scholar]
- 13.Taboada GF, et al. Quantitative analysis of somatostatin receptor subtypes (1-5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAR. Eur. J. Endocrinol. 2008;158:295–303. doi: 10.1530/EJE-07-0562. [DOI] [PubMed] [Google Scholar]
- 14.Ibáñez-Costa A, et al. In1-ghrelin splicing variant is overexpressed in pituitary adenomas and increases their aggressive features. Sci. Rep. 2015;5:8714. doi: 10.1038/srep08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luque RM, et al. A cellular and molecular basis for the selective desmopressin-induced ACTH release in Cushing disease patients: key role of AVPR1b receptor and potential therapeutic implications. J. Clin. Endocrinol. Metab. 2013;98:4160–4169. doi: 10.1210/jc.2013-1992. [DOI] [PubMed] [Google Scholar]
- 16.Neto LV, et al. Expression analysis of dopamine receptor subtypes in normal human pituitaries, nonfunctioning pituitary adenomas and somatotropinomas, and the association between dopamine and somatostatin receptors with clinical response to octreotide-LAR in acromegaly. J. Clin. Endocrinol. Metab. 2009;94:1931–1937. doi: 10.1210/jc.2008-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibáñez-Costa A, et al. Octreotide and pasireotide (dis)similarly inhibit pituitary tumor cells in vitro. J. Endocrinol. 2016;231:135–145. doi: 10.1530/JOE-16-0332. [DOI] [PubMed] [Google Scholar]
- 18.Casarini AP, et al. Acromegaly: correlation between expression of somatostatin receptor subtypes and response to octreotide-lar treatment. Pituitary. 2009;12:297–303. doi: 10.1007/s11102-009-0175-1. [DOI] [PubMed] [Google Scholar]
- 19.Colao A, Auriemma RS, Lombardi G, Pivonello R. Resistance to somatostatin analogs in acromegaly. Endocr. Rev. 2011;32:247–271. doi: 10.1210/er.2010-0002. [DOI] [PubMed] [Google Scholar]
- 20.Theodoropoulou M, Stalla GK. Somatostatin receptors: from signaling to clinical practice. Front. Neuroendocrinol. 2013;34:228–252. doi: 10.1016/j.yfrne.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Cuevas-Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J. Mol. Endocrinol. 2014;52:R223–240. doi: 10.1530/JME-14-0011. [DOI] [PubMed] [Google Scholar]
- 22.Brzana J, Yedinak CG, Gultekin SH, Delashaw JB, Fleseriu M. Growth hormone granulation pattern and somatostatin receptor subtype 2A correlate with postoperative somatostatin receptor ligand response in acromegaly: a large single center experience. Pituitary. 2013;16:490–498. doi: 10.1007/s11102-012-0445-1. [DOI] [PubMed] [Google Scholar]
- 23.Kiseljak-Vassiliades K, et al. Clinical implications of growth hormone-secreting tumor subtypes. Endocrine. 2012;42:18–28. doi: 10.1007/s12020-012-9660-9. [DOI] [PubMed] [Google Scholar]
- 24.Horvath E, Kovacs K. Pathology of acromegaly. Neuroendocrinology. 2006;83:161–165. doi: 10.1159/000095524. [DOI] [PubMed] [Google Scholar]
- 25.Larkin S, et al. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur. J. Endocrinol. 2013;168:491–499. doi: 10.1530/EJE-12-0864. [DOI] [PubMed] [Google Scholar]
- 26.Mayr B, et al. Molecular and functional properties of densely and sparsely granulated GH-producing pituitary adenomas. Eur. J. Endocrinol. 2013;169:391–400. doi: 10.1530/EJE-13-0134. [DOI] [PubMed] [Google Scholar]
- 27.Fougner SL, Casar-Borota O, Heck A, Berg JP, Bollerslev J. Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin. Endocrinol. (Oxf.) 2012;76:96–102. doi: 10.1111/j.1365-2265.2011.04163.x. [DOI] [PubMed] [Google Scholar]
- 28.Katznelson L, et al. Acromegaly: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014;99:3933–3951. doi: 10.1210/jc.2014-2700. [DOI] [PubMed] [Google Scholar]
- 29.Pivonello R, et al. Complications of acromegaly: cardiovascular, respiratory and metabolic comorbidities. Pituitary. 2017;20:46–62. doi: 10.1007/s11102-017-0797-7. [DOI] [PubMed] [Google Scholar]
- 30.Maione L, et al. Changes in the management and comorbidities of acromegaly over three decades: the French Acromegaly Registry. Eur. J. Endocrinol. 2017;176:645–655. doi: 10.1530/EJE-16-1064. [DOI] [PubMed] [Google Scholar]
- 31.Sze L, et al. Gender dependence of serum soluble Klotho in acromegaly. Clin. Endocrinol. (Oxf.) 2014;80:869–873. doi: 10.1111/cen.12385. [DOI] [PubMed] [Google Scholar]
- 32.Eden Engstrom B, Burman P, Karlsson FA. Men with acromegaly need higher doses of octreotide than women. Clin. Endocrinol. (Oxf.) 2002;56:73–77. doi: 10.1046/j.0300-0664.2001.01440.x. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka S, Fukuda I, Hizuka N, Takano K. Gender differences in serum GH and IGF-I levels and the GH response to dynamic tests in patients with acromegaly. Endocr. J. 2010;57:477–483. doi: 10.1507/endocrj.K09E-342. [DOI] [PubMed] [Google Scholar]
- 34.Puig-Domingo M, et al. Magnetic resonance imaging as a predictor of response to somatostatin analogs in acromegaly after surgical failure. J. Clin. Endocrinol. Metab. 2010;95:4973–4978. doi: 10.1210/jc.2010-0573. [DOI] [PubMed] [Google Scholar]
- 35.Shen M, et al. Predictive value of T2 relative signal intensity for response to somatostatin analogs in newly diagnosed acromegaly. Neuroradiology. 2016;58:1057–1065. doi: 10.1007/s00234-016-1728-4. [DOI] [PubMed] [Google Scholar]
- 36.Ibáñez-Costa, A. & Korbonits, M. AIP and the somatostatin system in pituitary tumours. J. Endocrinol., 10.1530/JOE-17-0254 (2017). [DOI] [PubMed]
- 37.Potorac I, et al. T2-weighted MRI signal predicts hormone and tumor responses to somatostatin analogs in acromegaly. Endocr. Relat. Cancer. 2016;23:871–881. doi: 10.1530/ERC-16-0356. [DOI] [PubMed] [Google Scholar]
- 38.Obari A, et al. Clinicopathological features of growth hormone-producing pituitary adenomas: difference among various types defined by cytokeratin distribution pattern including a transitional form. Endocr. Pathol. 2008;19:82–91. doi: 10.1007/s12022-008-9029-z. [DOI] [PubMed] [Google Scholar]
- 39.Ibáñez-Costa A, et al. BIM-23A760 influences key functional endpoints in pituitary adenomas and normal pituitaries: molecular mechanisms underlying the differential response in adenomas. Sci. Rep. 2017;7:42002. doi: 10.1038/srep42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gatto F, et al. beta-Arrestin 1 and 2 and G protein-coupled receptor kinase 2 expression in pituitary adenomas: role in the regulation of response to somatostatin analogue treatment in patients with acromegaly. Endocrinology. 2013;154:4715–4725. doi: 10.1210/en.2013-1672. [DOI] [PubMed] [Google Scholar]
- 41.Garcia EA, et al. Characterization of SNARE proteins in human pituitary adenomas: targeted secretion inhibitors as a new strategy for the treatment of acromegaly? J. Clin. Endocrinol. Metab. 2013;98:E1918–1926. doi: 10.1210/jc.2013-2602. [DOI] [PubMed] [Google Scholar]
- 42.Gahete MD, et al. Ghrelin gene products, receptors, and GOAT enzyme: biological and pathophysiological insight. J. Endocrinol. 2014;220:R1–R24. doi: 10.1530/JOE-13-0391. [DOI] [PubMed] [Google Scholar]
- 43.Barlier A, et al. Expression of functional growth hormone secretagogue receptors in human pituitary adenomas: polymerase chain reaction, triple in-situ hybridization and cell culture studies. J. Neuroendocrinol. 1999;11:491–502. doi: 10.1046/j.1365-2826.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- 44.Korbonits M, et al. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2001;86:881–887. doi: 10.1210/jcem.86.2.7190. [DOI] [PubMed] [Google Scholar]
- 45.Rubinfeld H, et al. Novel ghrelin analogs with improved affinity for the GH secretagogue receptor stimulate GH and prolactin release from human pituitary cells. Eur. J. Endocrinol. 2004;151:787–795. doi: 10.1530/eje.0.1510787. [DOI] [PubMed] [Google Scholar]
- 46.Korbonits M, et al. Expression of the growth hormone secretagogue receptor in pituitary adenomas and other neuroendocrine tumors. J. Clin. Endocrinol. Metab. 1998;83:3624–3630. doi: 10.1210/jcem.83.10.5210. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Tejada L, et al. Contribution of molecular analysis to the typification of the non-functioning pituitary adenomas. PLoS One. 2017;12:e0180039. doi: 10.1371/journal.pone.0180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaemmerer D, et al. Somatostatin receptors in bronchopulmonary neuroendocrine neoplasms: new diagnostic, prognostic, and therapeutic markers. J. Clin. Endocrinol. Metab. 2015;100:831–840. doi: 10.1210/jc.2014-2699. [DOI] [PubMed] [Google Scholar]
- 49.Cordido F, Garcia Arnes JA, Marazuela Aspiroz M, Torres Vela E. & grupo de Neuroendocrinologia de la Sociedad Espanola de Endocrinologia y, N. [Practical guidelines for diagnosis and treatment of acromegaly. Grupo de Neuroendocrinologia de la Sociedad Espanola de Endocrinologia y Nutricion] Endocrinol Nutr. 2013;60:457 e451–457 e415. doi: 10.1016/j.endonu.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Knosp, E., Steiner, E., Kitz, K. & Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery33, 610–617; discussion 617–618 (1993). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.