Abstract
Diabetes Mellitus (DM) is one of the biggest healthcare and financial problems worldwide. The disease is strongly associated with microvascular and macrovascular complications, causing co-existing diseases like Diabetic Retinopathy, Diabetic Neuropathy and Diabetic Nephropathy. Annual healthcare expenditures for diabetes treatment and complications prevention cost 727 billion USD in year 2017.
Diabetes Mellitus, Diabetic Retinopathy and Diabetic Retinal Neuropathy are closely related diseases - originating from incorrectly controlled glycemia, blood pressure and lipid levels in the course of increasing resistance of the body tissues to insulin.
Irrespectively of thorough programs for Diabetes Mellitus prevention and treatment, Diabetic Retinopathy management requires targeted treatment strategies for both microvasculopathy and retinal neurodegeneration, to delay disease severity course and risk of blindness.
The study and conclusions in this article are based on web-available data and officially published articles related to the diabetes mellitus and associated diseases – Diabetic Retinopathy and Diabetic Retinal Neuropathy. The articles have been reviewed and analyzed to assess mutual relations between the discussed diseases.
Keywords: Diabetes, Mellitus, Diabetic Retinopathy, DR, Diabetic Retinal Neuropathy, DRN
1. Introduction
Undeniably, Diabetes Mellitus (DM) is one of the biggest healthcare and financial problems worldwide that drains private and healthcare budgets every year. According to the International Diabetes Federation, in year 2017 there were 424.9 million adults with Diabetes Mellitus globally. Despite countless medical studies, publications and treatment guidelines related to DM, hundreds of thousands physicians engaged, 50 percent (212.4 million) of DM patients are unaware of their disease. Annual worldwide healthcare costs for diabetes treatment and complications prevention are calculated at 727 billion USD in year 2017 and 776 billion USD for year 2045 respectively. In the following years significant growth of expenditures on DM treatment is expected, mainly due to growing incidence and prevalence of mellitus. The disease incidence is a natural consequence of unhealthy nutrition, lack of physical activity leading to obesity and resistance of the body tissues to insulin over a long period of time. Continuously increasing life expectancy additionally impacts prevalence and duration of DM, so undoubtedly the overall number of DM patients treated every year will increase worldwide [2].
Diabetes Mellitus is strongly associated with microvascular and macrovascular complications [3]. Diabetic Retinopathy, Diabetic Neuropathy and Diabetic Nephropathy are present in up to 50 percent of patients with diabetes mellitus [3, 4].
The rationale for the study is to review recent publications related to Diabetic Mellitus, Diabetic Retinopathy and Diabetic Retinal Neuropathy and confirm direct associations and mutual dependency between the DR and DRN. Concluding research studies, Diabetic Retinopathy is not just the disease of microvascular degeneration, but also neurons atrophy in the retina. Consequently, a twofold strategy for DR treatment, neural and vascular protection from structural and functional damage or degeneration is required.
2. Materials and methods
The study and conclusions in this article are based on web-available data, officially published articles, related to diabetes mellitus and associated diseases – Diabetic Retinopathy and Diabetic Retinal Neuropathy. The articles have been reviewed and analyzed to assess mutual relations between the discussed diseases.
3. Results
Diabetic Retinopathy (DR) is an eye disease, a long-term developing microvascular complication as a natural course of Diabetes Mellitus. The incidence of DR equals to 33.2 percent of Diabetes Mellitus cases - 4 years after the onset of the DM, 45.1 percent after five years and 67.1 percent after 10 years respectively. Diabetic Retinopathy significantly increases risk of visual impairment. After 4 years of DM course 8.7 percent of patients develop Proliferative Diabetic Retinopathy (PDR) and 24 percent Diabetic Macular Edema (DME). 10 percent of DM patients are diagnosed as having severe visual loss (SVL) [5, 6]. There are several risk factors, which play an important role in DR development - improperly controlled glycemia, blood pressure and lipid levels during the diabetes mellitus course. The longer DM lasts, the higher probability of DR development is confirmed [7].
Treatment costs of DR play an important role in the overall expenses of diabetes management. According to Heintz, Wirehn et al., the annual average healthcare cost for PDR is calculated at 257 EUR and for DME 216 EUR per person per year. The annual costs for DR treatment equals to 106 thousand EUR per 100 thousand inhabitants [8].
Assuming the exchange rate EUR to USD 1.2:1, PDR treatment costs might represent up to 10 percent and DME 8 percent of the overall diabetes management costs.
Diabetic Neuropathy (DN) is a nerve damaging disease that develops over time due to Diabetes Mellitus. DN furthers mortality and morbidity of DM patients. The disease is the cause of 50-75 percent non-traumatic amputations and up to 25 percent neuropathic pain in mellitus patients [9]. A quarter of mellitus patients attending diabetic clinics report Diabetic Neuropathy symptoms by themselves and over half of them have been diagnosed with DN after basic clinical tests [9, 10, 11].
According to the US National Institute of Diabetes and Digestive and Kidney Diseases, 50 to 70 percent of DM patients have some form of neuropathy [12].
There are four basic types of Diabetic Neuropathy [12]:
Peripheral neuropathy that affects the upper and lower limbs, causing pain and or loss of feeling.
Autonomic neuropathy impacting heart and blood vessels, digestive systems, urinary tracts, sex organs and eyes.
Proximal neuropathy causing pain in thighs, hips, buttocks or legs predominantly on the side of the body.
Focal neuropathy affecting selective areas of the body including eyes, facial and ear muscles, chest, abdomen and lower limbs [12].
Just Diabetic Peripheral Neuropathy might represent up to 27 percent of overall diabetes management cost, and in the United States alone, these costs are estimated at between 10.9 to 13.7 billion USD [13].
Dworkin et al. calculated the cost for painful diabetic peripheral neuropathy at 6.2 thousand USD per patient, which is even higher than the average costs for DM treatment [14].
Diabetic Retinopathy is commonly recognized as microvascular degeneration as a result of chronic hyperglycemia. But retina is not just a network of blood vessels that undergo structural and functional changes during the primary disease course. It is vascularized neural tissue,; therefore Diabetic Retinopathy management requires targeted strategies for both microvasculopathy and neurodegeneration of the retina [15].
The loss of neurons in histopathologic studies among DR patients was reported more than forty years ago [15]. Recent studies with short-wave automated perimetry and frequency doubling perimetry tests confirm neural degeneration in DM patients with no or limited vascular retinopathy [15]. It is suggested that DM patients’ eyes neurodegeneration has sources in the inflammatory process, where antibodies, inflammatory cells and amino acids damage vessels and neurons. Consequently, imbalanced inflammation leads to over-expression of growth factors like VEGF, causing visual impairment [15].
Imbalance between retinal cell survival neutrophins and inflammatory mediators among DM patients is presented in the Figure 3.
Figure 3.
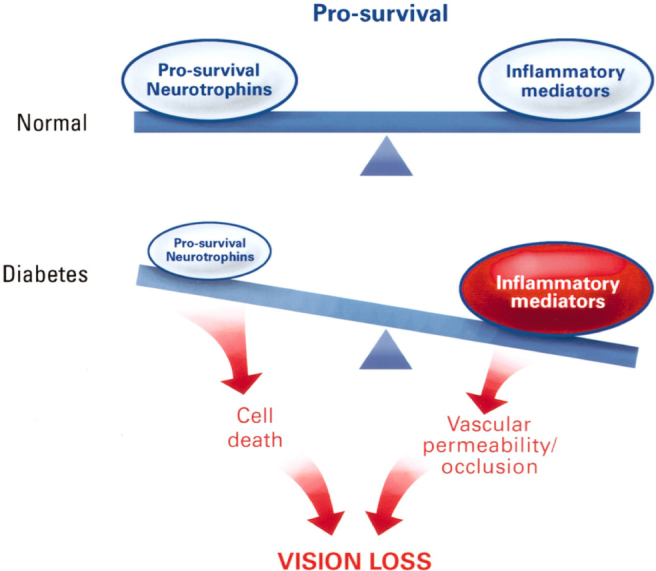
Concept of imbalance between pro-survival factors and inflammatory mediators [15]. Diabetes disturbs the homeostatic equilibrium of the retina. Under normal conditions, there is an equilibrium in which pro-survival and anti-inflammatory stimuli maintain retinal cell survival and function. In diabetes, pro-survival (neurotrophic) inputs may be reduced and pro-inflammatory cytokines, chemokines, and cellular responses increased. Together, these processes accelerate retinal cell death and increase vascular permeability and occlusion, thus impairing vision [15].
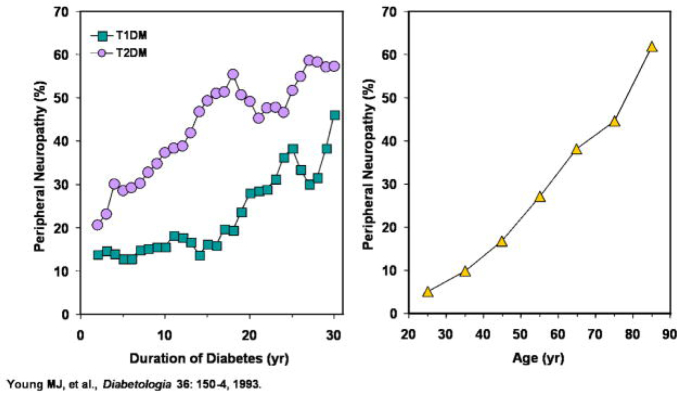
Figure 1.
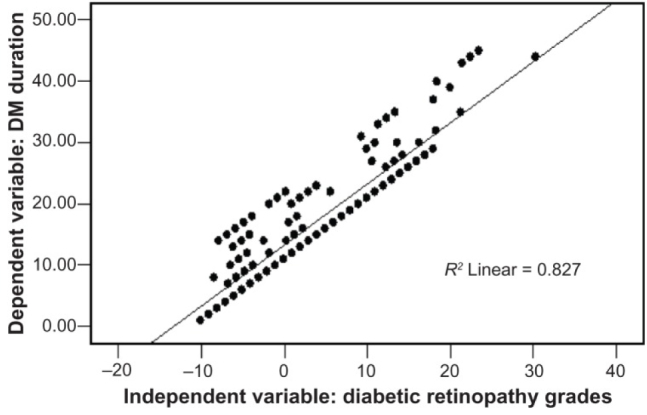
Correlation of the Diabetic Retinopathy with duration of Diabetes Mellitus [7].
Figure 2.
Effect of disease duration and age on prevalence of Diabetic Neuropathy [11].
For both T1DM and T2DM, duration of diabetes (left) as well as age of patient (right) is correlated to the incidence of diabetic neuropathy [11].
Sohn et al. recognize Retinal Diabetic Neuropathy (RDN) as independent from retina vessels ischemia. In the groundbreaking longitudinal study with human eye and mice tissue, researches proved that retinal neurodegeneration precedes microvascular changes in the Diabetes Mellitus eye. The neurodegeneration is independent from glycated hemoglobin, age and gender [16].
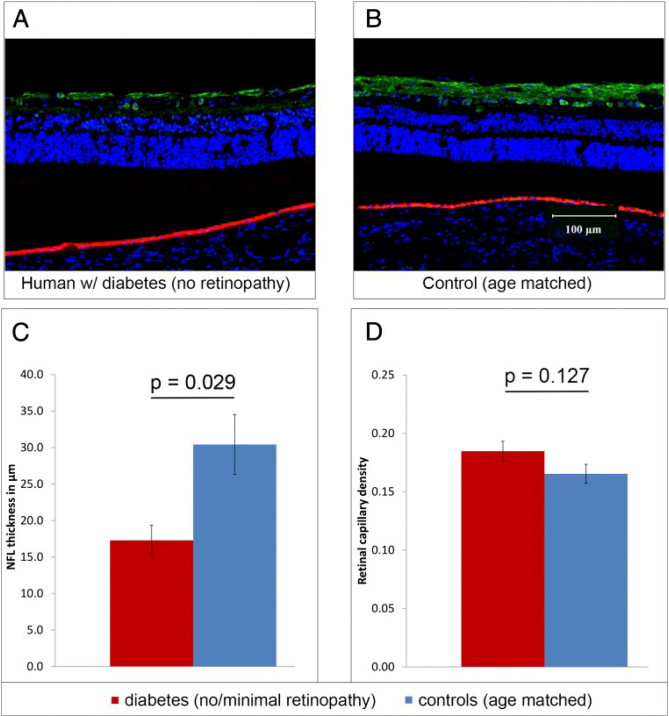
In the study among 45 patients with Diabetes Mellitus and with lack of minimal Diabetic Retinopathy diagnosed, using optical coherence tomography, researchers observed progressive thickness loss of the nerve fiber layer (NFL), the ganglion cell (GC) and inner plexiform layer (IPL). NFL thickness among DM patients without retinopathy is presented in Figure 4 [16].
Figure 4.
Nerve fiber layer thickness in DM patients with no retinopathy [16].
(A and B) Sections of eye from human donors with DM and no retinopathy (A) and from age-matched controls (B) stained with γ-synuclein antibody to immunolabel GCs and the NFL (green); DAPI nuclear counterstaining is blue; the red-orange signal at the level of the RPE is caused by auto fluorescence. (C) The NFL was significantly thinner in the diabetic eye (n = 6) than in controls (n = 5). (D) Retinal capillary density was measured from whole-mount sections; vessels were immunolabeled with biotinylated UEA-I followed by avidin conjugated to Texas Red (Fig. S4). No significant difference in retinal capillary density was found in the eye from diabetic donors (n = 6) and from controls (n= 5) [16].
The most recent studies suggest neurodegeneration in DM patients’ eyes as not only accompanying Diabetic Retinopathy, but also having a direct correlation. Lynch and Abramoff argue that DRN is a cause of DR, but further studies are required to prove this hypothesis [17].
Araszkiewicz and Ziolkiewicz directly associate retinal neurodegeneration with a course Diabetes Mellitus, where neurons apoptosis is first observed in retinal ganglion cells (RGC) [18]. The neurodegeneration in diabetic patients co-occurs with symptomatic changes in glial cells. It results in over-expression of glial acidic fibrillar protein, weakens astrocyte functions and activation of microglial cells. Also it initiates pro-inflammatory cytokines release and finally neuronal cell death [18].
The retina endothelial cells of micro-vessels determine a blood retinal barrier, which protects neural tissue from damage. An imbalance in metabolic processes in the retina can cause both neurodegeneration and vasculopathy resulting in structural changes in the retina, visual acuity impairment and finally, structural diabetic retinopathy [17, 18].
Parallel to DR consequences of neurovascular disorders in the retina might result in open-angle glaucoma (OAG). DM patients increase the incidence of primary glaucoma by 36 percent [19, 20]. From 32 percent to 43 percent of DM patients with secondary glaucoma is caused by Proliferative Diabetic Retinopathy [21].
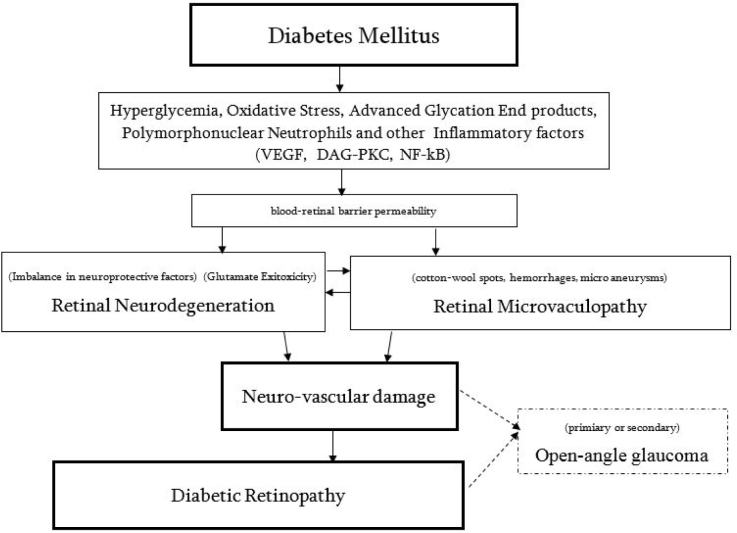
Key mechanisms and associations leading to the Diabetic Retinopathy are presented in Figure 5 [18,19,20,21,22].
Figure 5.
Key mechanisms leading to the DR [18].
Schematic representation of the main mechanisms leading to Diabetic Retinopathy, where neural and vascular damage leads to Diabetic Retinopathy. In addition to DR development neuro-vascular damage might cause open-angle glaucoma [18].
4. Discussion
Diabetic Retinopathy management requires targeted treatment strategies for both microvasculopathy and retinal neurodegeneration to delay disease severity course and risk of blindness [22]. Retina neuroprotection should be considered a crucial part of the overall diabetic retinopathy treatment. There are several experimental studies, suggesting Angiotensin II Type 1 blockers, Lutein, Green Tea and Ascorbic Acid as potent neuroprotective agents [23].
Other potential neurotrophic components, such as pigment epithelium-derived factor (PEDF), nerve growth factors (NGF), brain-derived neurotrophic factor (BDNF) might also enhance the number of treatment options for neuroprotection in diabetic retinopathy patients.
Some experimental studies suggest neuroprotection role in the diabetic’s eye retina by Memantine - glutamate receptor antagonist, Pentazocine - a specific sigma receptor-1 ligand and Gabapentin - a specific inhibitor of the neuronal, cytosolic isoform of branched chain amino-transferase (BCATc) [23]. Randomized and clinically controlled studies are required to prove retinal neuroprotection efficacy and safety of the mentioned above therapeutic options [23].
In the multi-center, two years prospective, randomized and controlled clinical study Eurocondor (project ID 278040) with target population of a representative group of male and female type two diabetic patients (n= 450) the somatostatin selective agonists (SST) and brominide have been assessed to check their aptitude of neurodegeneration prevention in early stages of Diabetic Retinopathy. Somatostatin and bromonide have shown a positive effect inhibiting the progression in those patients in whom some degree of neurodegeneration is already present. However this therapeutic option does not seem to be useful for the prevention of development of neurodegeneration in patients with best retinal function. A secondary result of the study, somatostatin proved an efficacy in arresting vasculopathy progression compared to a placebo [24].
Irrespectively of new prospective therapies that would allow to step up with prevention or subjugation neuro and vascular damage in diabetic eye and better Diabetic Retinopathy control, standardization of neural abnormalities detection methods are needed. Current multifocal electroretinography (mf-ERG) and frequency domain optical coherence tomography (fd-OCT) are used to observe morphological and functional changes of nerves in the retina, even before microvascular abnormality. Evaluation of changes in the nerve fiber layer, ganglion cell density, photoreceptor changes, thickness of the retina will be crucial in further perspective of the two fold strategy for DR treatment - neural and vascular protection from structural and functional damage or degeneration [24].
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
References
- [1].Cho N.H., Kirigia J., Mbanya J.C., Ogurstova K., Guariguata L., Rathmann W.. et al. IDF Diabetes Atlas. (Eighth Edition) 2017 https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html [Google Scholar]
- [2].Kontis V., Bennet E.J., Mathers D.C., Li G., Foreman K., Ezzatiet M.. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. http://dx.doi.org/10.1016/S0140-6736(16)32381-32389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cade WT.. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Physical Therapy. 2008;88(11):1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia. 1991;34:877–890. https://www.ncbi.nlm.nih.gov/pubmed/1778353 [PubMed] [Google Scholar]
- [5].Zheng Y., He M., Congdon N.. The worldwide epidemic of diabetic retinopathy. Indian Journal of Ophthalmology. 2012;60(5):428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wong T.Y., Mwamburi M., Klein R., Larsen M., Flynnc H., Hernandez-Medina M.. et al. Rates of Progression in Diabetic Retinopathy During Different Time Periods: A systematic review and meta-analysis. Diabetes Care. 2009;32(12):2307–2313. doi: 10.2337/dc09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].El-Bab M.F., Shawky N., Al-Sisi A., Akhtar M.. Retinopathy and risk factors in diabetic patients from Al-Madinah Al-Munawarah in the Kingdom of Saudi Arabia. Clinical Ophthalmology (Auckland, NZ) 2012;6:269–276. doi: 10.2147/OPTH.S27363. Reprinted from. Material from this publication has been used under CC-BY-NC Dove Medical Press Limited license. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Heintz E., Wirèhn A.B., Peebo B.B., Rosenqvist U., Levin L.A.. Diabetologia. 2010;53:22147. doi: 10.1007/s00125-010-1836-3. https://doi.org/10.1007/s00125-010-1836-3 [DOI] [PubMed] [Google Scholar]
- [9].Vinik A., Casellini C., Nevoret M.L.. Diabetic Neuropathies. 2015 https://www.ncbi.nlm.nih.gov/books/NBK279175 [Google Scholar]
- [10].Vinik A. Diabetic Neuropathy: Pathogenesis and Therapy. Am. J. Med. 1999;107(2B):17S–26S. doi: 10.1016/s0002-9343(99)00009-1. [DOI] [PubMed] [Google Scholar]
- [11].Edwards J.L., Vincent A., Cheng T., Feldman E.L.. Diabetic Neuropathy: Mechanisms to Management. Pharmacology & therapeutics. 2008;120(1):1–34. doi: 10.1016/j.pharmthera.2008.05.005. Reprinted from. with permission from Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nerve Damage (Diabetic Neuropathies), National Institute of Diabetes and Digestive and Kidney Diseases. NIH Publication. 2009:09–3185. https://www.niddk.nih.gov/health-in-formation/diabetes/overview/preventing-problems/nerve-damage-diabetic-neuropathies [Google Scholar]
- [13].Gordois A., Scuffham P., Shearer A., Oglesby A., Janet Ash Tobian A.J.. The Health Care Costs of Diabetic Peripheral Neuropathy in the U.S. Diabetes Care. 2003;26(6):1790–1795. doi: 10.2337/diacare.26.6.1790. https://doi.org/10.2337/diacare.26.6.1790 [DOI] [PubMed] [Google Scholar]
- [14].Dworkin R., Panarites C., Chen J.. Impact of postherpetic neuralgia and painful diabetic peripheral neuropathy on healthcare costs. The Journal of Pain. 2009;10(4):S17. doi: 10.1016/j.jpain.2009.08.005. http://dx.doi.org/10.1016/j.jpain.2009.01.075 [DOI] [PubMed] [Google Scholar]
- [15].Antonetti D.A., Barber J.A., Bronson K.S., Freeman M.W., Gardner W.T., Jefferson S.L.. Diabetic Retinopathy - Seeing Beyond Glucose-Induced Microvascular Disease; American Diabetes Association. Diabetes. 2006;55(9):2401–2411. doi: 10.2337/db05-1635. https://doi.org/10.2337/db05-1635 Reprinted from. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association. [DOI] [PubMed] [Google Scholar]
- [16].Sohn E.H., van Dijk H.W., Jiao C., Kok H.B.P., Jeong W., Demirkaya N.. et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. PNAS. 2016;113(19):E2655–E2664. doi: 10.1073/pnas.1522014113. Reprinted from. Material from this publication has been used with the permission of Proceedings of the National Academy of Sciences of the United States of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lynch K.S., Abràmoff D.M.. Diabetic retinopathy is a neurodegenerative disorder. Vision Research. 2017;139:101–107. doi: 10.1016/j.visres.2017.03.003. https://doi.org/10.1016/j.visres.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Araszkiewicz A., Zozulinska-Ziolkiewicz D.. Retinal Neurodegeneration in the Course of Diabetes-Pathogenesis and Clinical Perspective. Current Neuropharmacology. 2016;14(8):805–809. doi: 10.2174/1570159X14666160225154536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mitchell P., Smith W., Chey T., Healey P.R.. Open-angle glaucoma and diabetes: the Blue Mountains eye study. Ophthalmology. 1997;104(4):712–718. doi: 10.1016/s0161-6420(97)30247-4. http://dx.doi.org/10.1016/S0161-6420(97)30247-4 [DOI] [PubMed] [Google Scholar]
- [20].Zhao Y-X., Chen X-W.. Diabetes and risk of glaucoma: systematic review and a Meta-analysis of prospective cohort studies. International Journal of Ophthalmology. 2017;10(9):1430–1435. doi: 10.18240/ijo.2017.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jeganathany V.S.E., Wang J.J., Wong T.Y.. Ocular Associations of Diabetes Other Than Diabetic Retinopathy. Diabetes Care. 2008;31(9):1905–1912. doi: 10.2337/dc08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Simό R., Hernández C.. Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives. Trends Endocrinol. Metab. 2014;25(1):2333. doi: 10.1016/j.tem.2013.09.005. http://dx.doi.org/10.1016/j.tem.2013.09.005 [DOI] [PubMed] [Google Scholar]
- [23].Ola M.S., Nawaz M.I., Khan H.A., Alhomida A.S.. Neurodegeneration and Neuroprotection in Diabetic Retinopathy. International Journal of Molecular Sciences. 2013;14(2):2559–2572. doi: 10.3390/ijms14022559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].European Consortium for the Early Treatment of Diabetic Retinopathy. http://cordis.europa.eu/result/rcn/189871_pl.html Project ID: 278040. [Google Scholar]