Abstract
Although it is becoming increasingly evident that maternal starvation during pregnancy can have permanent effects on a range of physiological processes in the offspring, scant information is available about the consequence of such condition for oogenesis and hence for lifetime reproductive success of progeny in mammals. In the present study, we address this topic by starving pregnant mice at the time of ovarian differentiation (12.5 days post coitum (dpc)) for three consecutive days and analyzed the consequence first on the survival of the fetal oocytes and their capability to progress throughout the stages of meiotic prophase I (MPI) and then on the postnatal folliculogenesis of the offspring. The results showed that maternal starvation increased apoptosis in the fetal ovaries, resulting in reduction of the oocyte number. Moreover, MPI progression was slowed down in the surviving oocytes and the expression of DNA repair players in the starved ovaries increased. Transcriptome analysis identified 61 differentially expressed genes between control and starved ovaries, the most part of these being involved in metabolic processes. A significant decrease in the percentage of oocytes enclosed in primordial follicles and the expression of oocyte genes critically involved in folliculogenesis such as Nobox, Lhx8 and Sohlh2 in the 3 days post partum (dpp) starved ovaries were found. Finally, at the time of juvenile period (21 dpp), the number of oocytes and antral follicles resulted significantly lower in the ovaries of the offspring from starved mothers in comparison to controls. Our findings support the notion that maternal starvation can affect ovary development in the offspring that could adversely affect their reproductive success in the adult life.
Introduction
Adequate and correct diet during pregnancy are critical for the health of mother and newborns1–3. As a matter of fact, it had been clearly established that some offspring pathologies (for instance, obesity, diabetes and cardiovascular disease) might have their origins in inadequate nutrition during pregnancy4,5. However, little information is available about the effect of starvation during pregnancy on the offspring reproductive functions in mammals.
In humans, three studies examined whether exposure to acute, severe famine in utero during the Dutch famine 1944–1945 affected a women’s subsequent reproduction. Lumey and Stein6 found that exposure to fetal famine was sufficient to result in a 300 g decrease in mean birthweight; however, these individuals did not suffer from adverse effects on their subsequent fecundity in adulthood, but were more likely to give birth to offspring of reduced birthweight. This reduced birthweight in the second generation was associated with a high frequency of early infant mortality. Painter et al.7, who interviewed the same sample of women at a mean age of 50 years, but used a different sample of controls, found a small but significant decrease in the prevalence of nulliparity. More recently, Yarde et al.8, in an independent sample of women born after the same famine, reported earlier menopause. Elias et al.9 found a slight decrease in age of menopause following famine exposure during early childhood. In a follow-up study of women born in England in the first half of the twentieth century, Cresswell et al.10 and Hardy and Kuh11 also found that menstruation ceased at an earlier age in those who had low weight gain during their first or the second year of life (but see Treloar et al.12, for the opposite result). Steiner et al.13 reported a weak association between birthweight and age at menopause. Some authors observed that low birthweight infants with prematurity or growth retardation tend to have fewer offspring14,15, and that retarded fetus growth can impair ovarian development, which may have implications for the timing of menopause16. Despite such apparent contradictory results in humans, animal models support the notion that starvation during pregnancy can have adverse effect on the offspring reproductive capability. A reduction of lifetime reproductive capacity after prenatal undernutrition has been reported in female mice17 and sheep18. Food restriction during the second half of pregnancy in rats resulted in premature reproductive senescence in female offspring19. In single-ovulating species, a study found evidence that maternal dietary restriction influences ovarian reserve in bovine20. Because female reproductive capacity in rodents is largely defined by the number and quality of primordial follicles developed in the ovary during the neonatal period, termed the ovarian reserve, it can be hypothesized that reduced amount of nutrients during this crucial process can impair the formation of the ovarian reserve with adverse consequence for reproduction. Actually, several studies showed that apoptosis and autophagy are part of the starvation cell response, possibly triggered by oxidative stress in damaged cells21 and that insulin-like growth factor-1 signaling plays an important role in such processes22. In this regard, we recently found that starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes21. Interestingly, diet can also cause epigenetic changes in gene expression with possible consequence for the correct development of cells and tissues23. Epigenetics involves heritable changes in gene expression via post-translational and post-transcriptional modifications without altering DNA base sequence. For instance, in the mouse, gestational nutritional restriction has been reported to alter the level of DNA methylation in the sperm of male offspring, resulting in metabolic diseases in the next generations23–25.
In the present study, pregnant mice at the time of the gonad sex differentiation (12.5 days post coitum (dpc)) experienced a starvation period of 3 days, and the consequences on the ovary development during the fetal period and early folliculogenesis were investigated.
Results and discussion
Many animals alter their reproductive strategies in response to environmental stress. For example, in female Drosophila and Caenorhabditis elegans, starvation activates apoptotic checkpoints and autophagy in oogenesis and reduces the production of mature oocytes26,27. In this regard, we recently found that nutrient deficiency at birth could generate a number of adaptive metabolic and oxidative responses in the ovaries causing increased apoptosis and autophagy in both the somatic cells and oocytes, leading to a delay of germ cell cyst breakdown and follicle assembly21,28. Here we investigated the consequences of starvation of pregnant mice for 3 days at the time of the fetal gonad sex differentiation and beginning of meiosis on the ovary development during the fetal period and early folliculogenesis.
Mother’s starvation impairs fetal growth
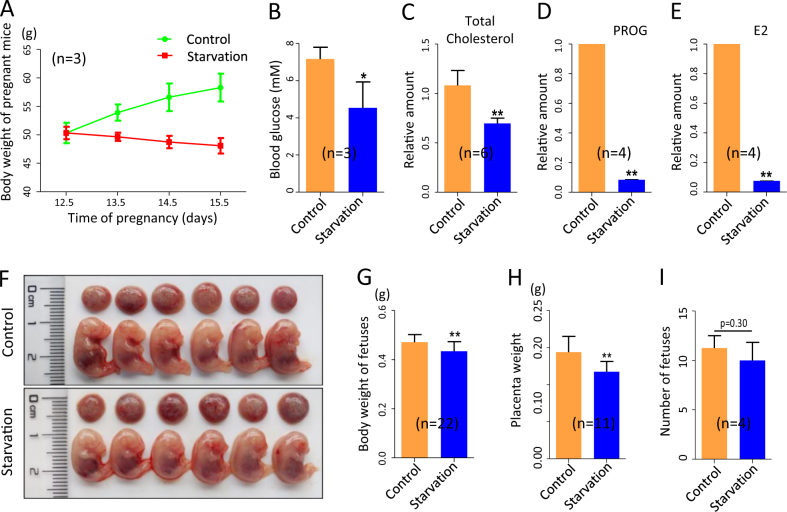
Starvation for a relative short pregnancy time, from 12.5 to 15.5 dpc, caused a significant decrease of the mother’s body weight at the end of treatment (Fig. 1a), paralleled to reduced concentrations of glucose, total cholesterol, progesterone (PROG) and E2 in the blood (Fig. 1b–e). The greatly reduced level of PROG was likely responsible for the slightly higher probability of pregnant female abortion at 15.5 dpc (data not shown). Moreover, the body weight of the surviving fetuses was significantly lower in the starved (0.43 ± 0.008 g) compared to control (0.47 ± 0.007 g) groups (Fig. 1f, g, P < 0.01). Finally, the weight of placenta also resulted significantly lower in the starved (0.12 ± 0.004 g) than in the control (0.14 ± 0.006 g) groups (Fig. 1h). For non-abortion mice after starvation, however, there was no significant difference in the number of the delivered pups compared with that of control group (11.25 ± 0.63 vs 10.00 ± 0.91) (Fig. 1i).
Fig. 1. Effects of starvation on mothers and fetuses.
a Body weight of pregnant females from 12.5 to 15.5 dpc in control and starved groups. b–e Amount of glucose, total cholesterol, PROG and E2 in the blood of 15.5 dpc pregnant females in control and starved groups. f The 15.5 dpc fetuses and placentas. g, h Body and placenta weights of 15.5 dpc fetuses. i Number of fetus of 15.5 dpc pregnant females; n = number of mothers or fetuses (g, h) used for the analyses. Results are presented as mean ± SEM. *P < 0.05; **P < 0.01
Mother’s starvation increases apoptosis in the fetal ovaries and causes reduction of the oocyte number
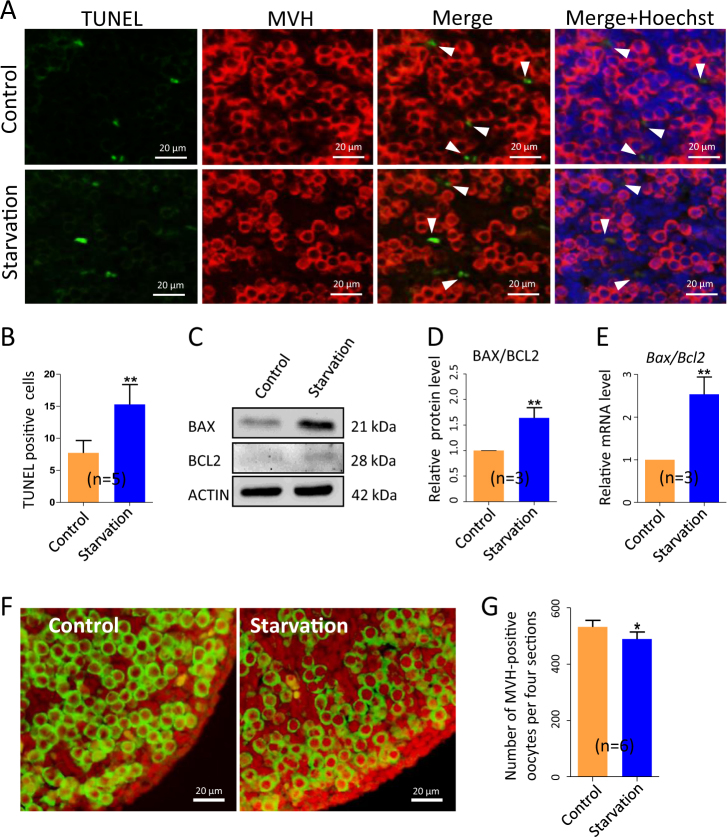
In line with our previous results reported above21 and general observation that starvation, as a result of metabolic stress, increases autophagy and/ or apoptosis in several tissues29, we found evidence of increased level of apoptosis in the ovaries of the starved 15.5 dpc fetuses. In fact, the number of TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling)-positive cells scored per unit area in tissue sections of ovaries of the starved fetuses was almost double (15.30 ± 1.39) in comparison to control (7.73 ± 0.86) (Fig. 2a, b). At the same time, the Bax/Bcl-2 ratio, evaluated at both the messenger RNA (mRNA) and protein levels, resulted significantly higher apoptosis in the ovaries of the starved fetuses in comparison to control (Fig. 2c–e).
Fig. 2. Apoptotic markers increase and oocyte numbers decrease in 15.5 dpc ovaries from fetuses of starved mothers.
a TUNEL immunohistochemistry (green) in MVH-positive oocytes (red); cell nuclei was stained with Hoechst (blue). b Number of TUNEL-positive cells. c–e WB and qRT-PCR for BAX and BCL2. f, g MVH immunofluorescence (green) and number of oocytes in control and starved ovaries; n = number of ovaries (g) or independent repeats used for the analyses. *P < 0.05; **P < 0.01
The increased levels of apoptosis markers in the starved ovaries were associated to a slight but statistically significant decreased number of oocytes assessed in tissue sections after staining with the germ cell-specific anti-mouse VASA homolog (MVH) antibody (489.30 ± 10.16 vs 532.40 ± 9.48, P < 0.05) (Fig. 2f, g). It remained to determine if such reduced oocyte number was due to increased apoptotic rate in oocytes or an indirect consequence of increased apoptosis in the surrounding somatic cells.
Mother’s starvation delays meiotic progression in fetal oocytes and increases the expression of DNA repair players in the starved ovaries
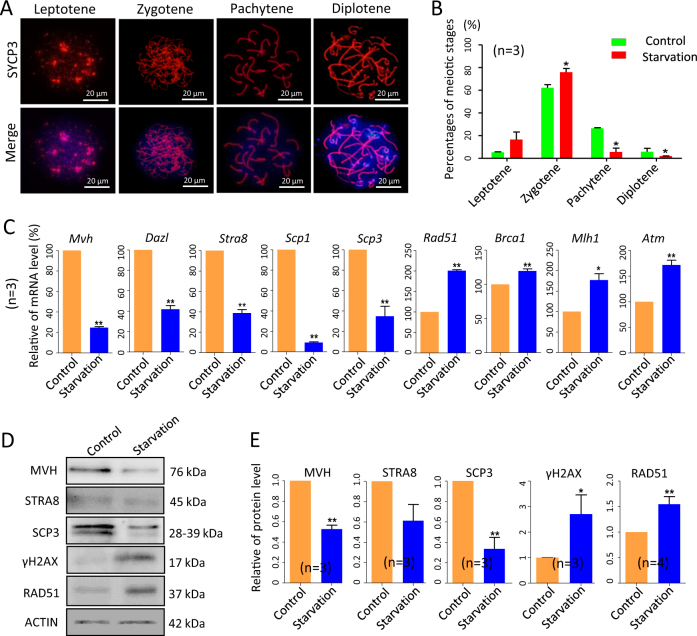
In the fetal ovaries of the CD-1 mice, between 12.5 and 14.5 dpc, most part of germ cells enter meiosis and begin to progress through the meiosis prophase I (MPI) stages30. To evaluate if starvation affects these processes, we analyzed MPI stages by oocyte cytospreads. The results showed that MPI progression was altered in starved (oocytes at zygotene 75.92 ± 3.25%; oocytes at pachytene: 5.61 ± 3.41%) compared with control ovaries (oocytes at zygotene: 62.21 ± 2.73%; oocytes at pachytene: 26.56 ± 0.37%) (Fig. 3a, b). Moreover, quantitative real-time PCR (qRT-PCR) analyses showed that the transcripts for the germ cell-specific genes, Mvh and deleted in azoospermia-like (Dazl), and of the meiosis-related genes, stimulated by retinoic acid 8 (Stra8), synaptonemal complex protein 1 and 3 (Scp1 and Scp3), were all significantly decreased in the starved ovaries (Fig. 3c). These results were also confirmed at the protein level for MVH, STRA8 and SCP3 (Fig. 3d, e). Reduction of the oocyte number and delay in MPI progression in the starved ovaries could both explain such decreased gene expressions. The absence of significant difference in the percentages of oocytes at different MPI stages in the ovary of control and treated groups at 18.5 dpc (see below), suggested, however, a recovery in meiotic progression after the mother resuming the correct diet in the last period of pregnancy.
Fig. 3. Slowed MPI progression and increased DNA repair players in 15.5 dpc ovaries from fetuses of starved mothers.
a SCP3 immunofluorescence (red) of oocyte cytospread at various MPI stages; nuclei was counterstained with Hoechst (blue). b Percentages of oocyte at different MPI of 15.5 dpc ovaries. c–e qRT-PCR and WB analyses of the expressions of germ cell-specific, meiotic- and DNA damage-related genes in control and starved ovaries; n = number of independent repeats used for the analyses. *P < 0.05; **P < 0.01
Interestingly, western blot (WB) analyses showed significant higher levels of γH2AX (a marker of DNA double-strand breaks) and RAD51 (involved in DNA double-strand breaks repair), in the starved 15.5 dpc ovaries (Fig. 3d, e). Moreover, besides Rad51, we found that starvation increased the transcript levels of other genes involved in DNA repair and homologous recombination such as Atm (ataxia telangiectasia mutated), Mlh1 (mutL homolog 1) and Brca1 (breast cancer type 1 susceptibility protein) (Fig. 3c).
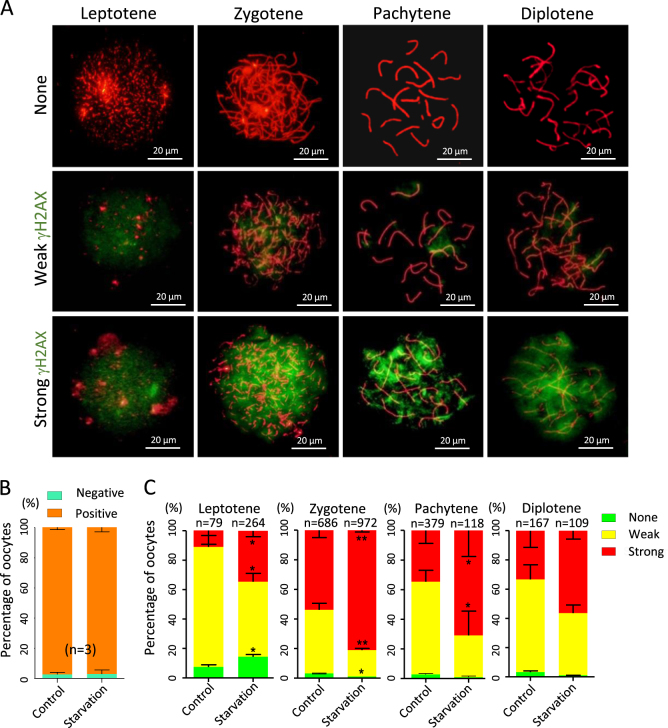
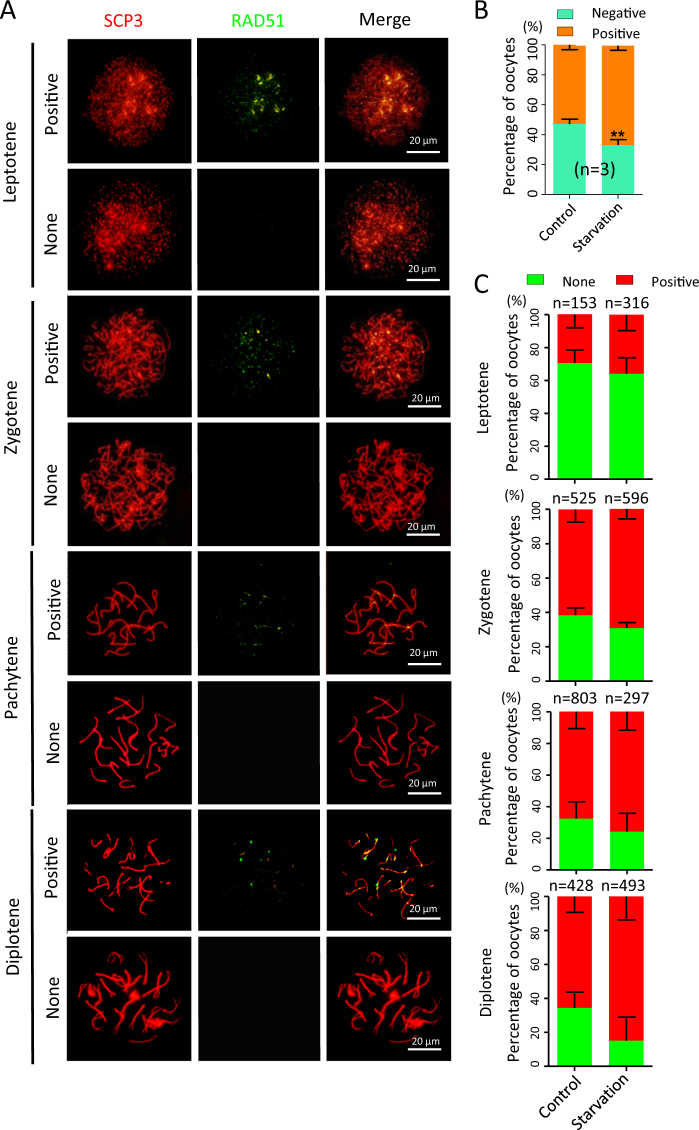
Double staining of oocyte cytospreads for SCP3 and γH2AX (Fig. 4a) showed that while there was no significant difference in the total percentage of γH2AX-positive oocytes between the control (97.34 ± 0.80%) and the starved (97.11 ± 1.60%) groups (Fig. 4b), in the latter, at all MPI stages analyzed, the percentages of oocytes showing strong γH2AX was much higher at varying degrees except diplotene stage (Fig. 4c). Finally, double staining for SCP3 and RAD51 revealed that RAD51-positive rate significantly increased (P < 0.01; Fig. 5a, b), but there were not significant differences in the number of positive oocytes between the control and starved groups at each MPI stage (Fig. 5c).
Fig. 4. γH2AX staining in oocytes at various MPI stages.
a Representative SCP3 (red) and γH2AX (green) staining of oocyte cytospread at various MPI stages. b, c Quantification of γH2AX-positive oocytes in the ovaries of fetuses from control and starved mothers; n = number of independent repeats or counting oocytes (c) used for the analyses. *P < 0.05; **P < 0.01
Fig. 5. RAD51 staining in oocytes at various MPI stages.
a Representative SCP3 (red) and RAD51 (green) staining of oocyte cytospread at various MPI stages. b, c Quantification of RAD51-positive oocytes in the ovaries of fetuses from control and starved mothers; n = number of independent repeats or counting oocytes (c) used for the analyses. **P < 0.01
These last results evidence that defects in MPI likely occur because of alteration of homologous recombination and DNA repair in the oocytes of the starved ovaries. This supports the important notion that nutrients might modulate cellular DNA damage response. The relevance of diet and nutrition in human health and disease is well established. Actually, basic laboratory researches, clinical trials and epidemiological studies demonstrated that nutrient-rich bioactive foods can induce epigenetic changes and alter gene expression by the alteration of the histone structure, DNA methylation, miRNA modulation and DNA repair31,32.
Transcriptome analysis and comparison identify differentially expressed genes between control and starved ovaries
In transcriptome analyses, we found that control and starved ovaries showed 61 significant differentially expressed genes (DEGs) (Fig. S1A-S1C) and that most part of these genes were involved in metabolic process (Fig. S1D).
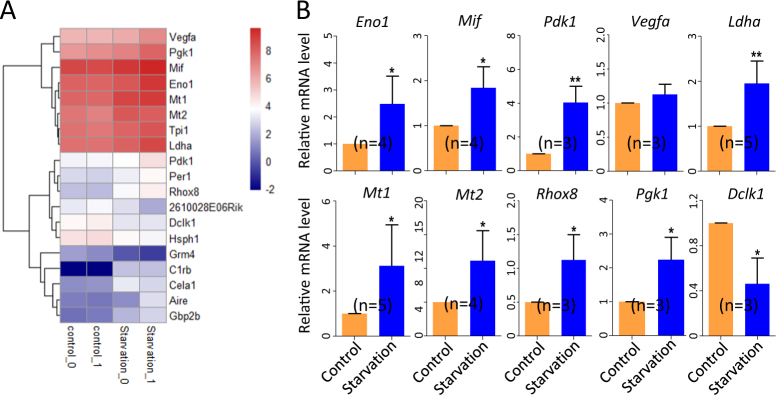
For DEGs that resulted more constantly affected by maternal starvation, a heatmap was generated (Fig. 6a). qRT-PCR was then performed for 10 of these genes potentially relevant for reproductive processes (Fig. 6b). The transcripts of most of these genes, such those for enolase 1 (Eno1)33, macrophage migration inhibitory factor (Mif)34, pyruvate dehydrogenase kinase 1 (Pdk1)35, lactate dehydrogenase A (Ldha)36, melatonin receptors Mt1 and Mt237, reproductive homeobox 8 (Rhox8)38 and phosphoglycerate kinase 1 (Pgk1)39, were significantly increased. Conversely, mRNA amount of doublecortin-like kinase 1 (Dclk1)40 was decreased while those of vascular endothelial growth factor A (Vegfa)41 was not affected.
Fig. 6. Transcriptome analysis of 15.5 dpc ovaries from fetuses of control and starved mothers.
a Heatmap of differentially expressed genes (DEGs) constantly affected by maternal starvation. b qRT-PCR analyses of 10 genes showed in the heatmap in 15.5 dpc control and starved ovaries; n = number of independent repeats used for the analyses. *P < 0.05; **P < 0.01
Mother’s starvation causes reduction in the number of the primordial follicles and impairs follicle development in the offspring ovaries
To investigate whether the defects in the oogenic process found in the starved 15.5 dpc fetal ovaries impaired the ovary development at later stages, we examined MPI in oocytes obtained from 18.5 dpc starved ovaries, and folliculogenesis in 3 and 21 days post partum (dpp) ovaries of offspring of starved mothers.
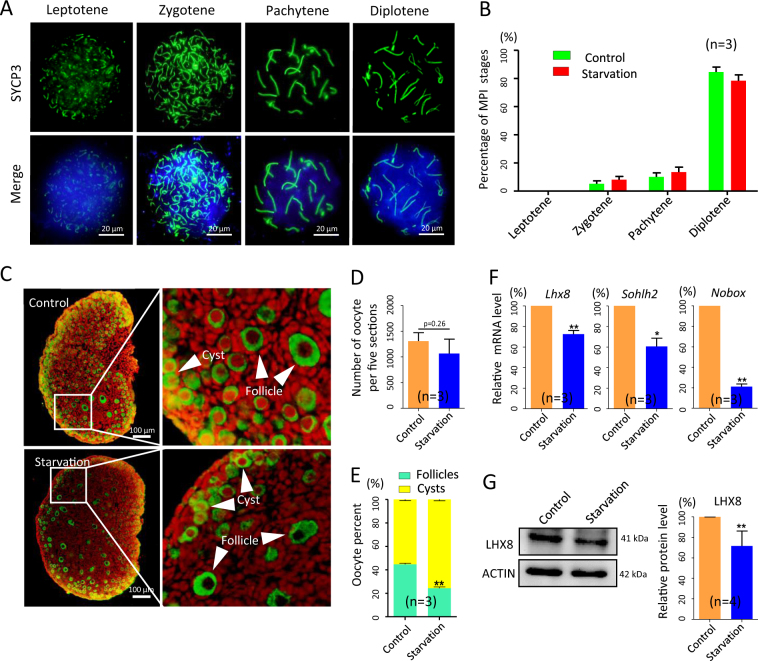
While, as reported above, at 18.5 dpc, no significant difference in the oocyte MPI stages between control and starved ovaries was found (Fig. 7a, b), in the latter, at 3 dpp, we scored a slight decrease in the oocyte number (Fig. 7c, d), but above all a marked lower percentage of oocytes enclosed in primordial follicles in comparison to the control (24.31 ± 1.15% vs 44.65 ± 1.01%, P < 0.01; Fig. 7e). The parallel decreased amount of mRNA of oocyte genes involved in the germ cell cyst breakdown and primordial follicle formation such as Nobox (newborn ovary homeobox), Sohlh2 (spermatogenesis and oogenesis specific basic helix-loop-helix2) (Fig. 7f) and Lhx8 (LIM homeobox 8, protein level in Fig. 7g) in the 3 dpp starved ovaries offered a likely explanation of this defect (P < 0.05 or P < 0.01).
Fig. 7. MPI oocyte stages and folliculogenesis in the ovaries of 18.5 dpc fetuses and 3 dpp pups from control and starved mothers.
a Representative oocyte cytospreads stained for SCP3 (green); nuclei counterstained with Hoechst (blue). b Percentages of oocyte in MPI stages. c Sections of 3 dpp ovaries showing MVH-positive oocytes (green) in cysts (white arrows) or in primordial follicles (white arrowheads). d, e Number of oocytes and percentages of oocyte in cysts or in primordial follicles in 3 dpp ovaries. f, g qRT-PCR and WB of oocyte-specific genes involved in folliculogenesis; n = number of ovaries (d, e) or independent repeats used for the analyses. *P < 0.05; **P < 0.01
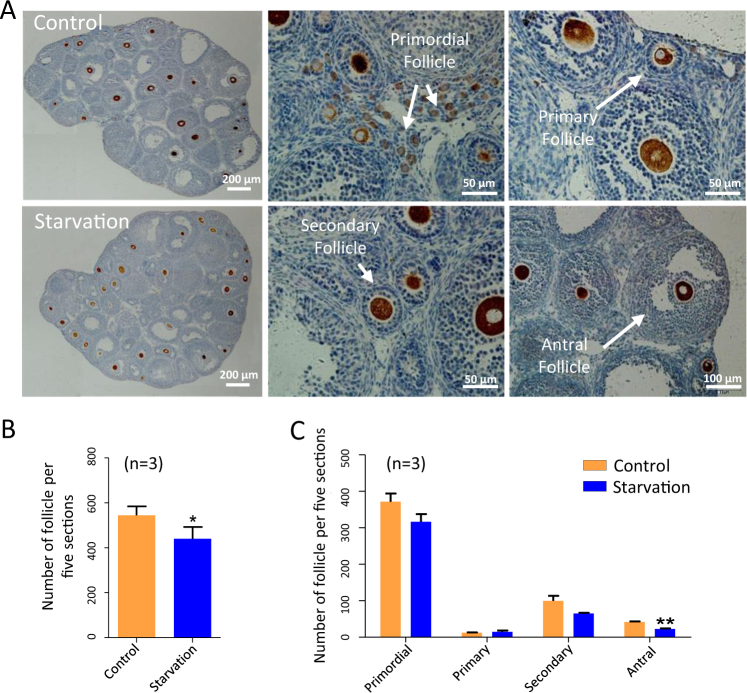
Finally, at 21 dpp, the number of follicles in tissue sections resulted significantly lower in starved ovaries in comparison to control (440.5 ± 25.94 vs 544.6 ± 17.67) (P < 0.05; Fig. 8a, b), while the number of antral follicles showed a significant decrease of almost one half in the ovaries of the offspring from starved mothers (22.67 ± 1.67 vs 42.00 ± 1.53) (P < 0.01; Fig. 8c). Although these last observations are predictive of reduced fertility performance, at the moment no detailed information is available about such possible defect.
Fig. 8. Analysis of folliculogenesis in 21 dpp ovaries of offspring of starved pregnant mothers.
a Representative immunohistochemistry of MVH-positive oocytes (dark brown) in 21 dpp ovaries. b, c Quantification of the number of oocytes and different classes of follicles in 21 dpp ovaries of offspring of control and starved mothers; n = number of ovaries used for the analyses. *P < 0.05; **P < 0.01
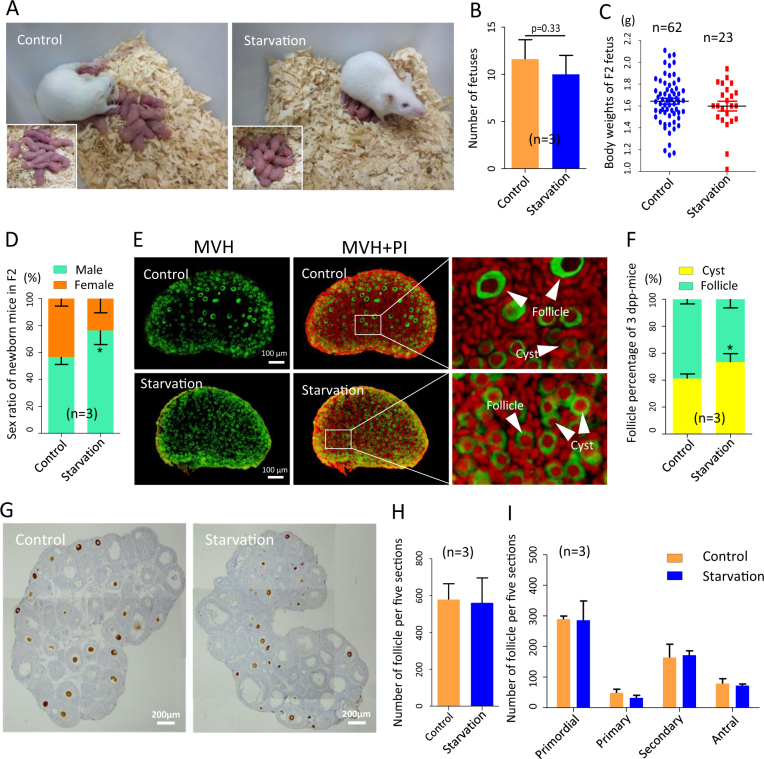
On the other hand, the litter size and birthweight of F2 offspring produced from F1 females scarcely declined (Fig. 9a–c), while there was an apparent imbalance in the sex ratio at birth (P < 0.05; Fig. 9d). Moreover, the percentages of oocyte enclosed in cysts and follicles suggested a small depletion in the formation of primordial follicles at 3 dpp (P < 0.05; Fig. 9e, f), whereas the total number of follicles and progression of follicular growth between starved and control groups had no significant defects at 21 dpp (Fig. 9g–i).
Fig. 9. Analysis of folliculogenesis F2 offspring of starved pregnant mothers.
a Photograph of F2 offspring of control and starved mothers. b Litter size of F1 mothers. c Body weight of F2 fetus at 0 dpp. d Sex ratio of F2 offspring. e, f MVH-positive oocytes (green) in cysts (white arrows) or in primordial follicles (white arrows) in 3 dpp ovaries. g–i Quantification of the number of follicles and percentages of different classes of follicles in 21 dpp ovaries from control and F2 offspring of starved ovaries; n = number of ovaries or fetuses (c) or independent repeats (b, d) used for the analyses. *P < 0.05
In conclusion, our study represents a first indication that even a relatively short period of mother starvation during critical stages of the ovary development in the fetuses can disturb oogenic processes with adverse consequence for folliculogenesis.
Materials and methods
Animal breeding and treatment
All animals used in this study were CD-1 mice, which were kept in self-help feeding way with light and dark cycle of half day and raised according to the national guideline and Ethical Committee of Qingdao Agricultural University. Mating was arranged regularly at 4:30–5:30 p.m., vaginal plug was checked at 8:30–9:30 a.m. on next morning; the presence of vaginal plug was considered as 0.5 dpc.
Due to the sensibility to food deprivation between 12.5 and 15.5 dpc, which is crucial for the ovarian sex differentiation and meiosis initiation, starvation began at 11:00–12:00 a.m. at 12.5 dpc and ceased at the same time at 15.5 dpc. During this process of starvation, the water was provided as usual and food supply was cut down, and after that the diet of starved females came back to normal. The total number of starved mothers was 120. These mothers, unless otherwise indicated, were killed to remove the fetuses.
Detection of blood sugar, total cholesterol and sex hormone levels
Biochemical indicators designed to validate the effect of starvation on the pregnant mothers were: blood sugar, total cholesterol, PROG and 17β-estradiol (E2) levels. The blood sugar concentration was determined by collecting blood samples with the ACCU-CHEK® Active of Roche (Roche, Germany) and corresponding test strips. The levels of total cholesterol (Comin Biotechnology, Suzhou, China), PROG (LanpaiBIO, shanghai, China) and E2 (Jinma Biotechnology, Shanghai, China) were tested following the manufacturer’s instructions.
Quantitative real-time PCR
The complementary DNA (cDNA) prepared for PCR was synthesized by TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, AT311-03, Beijing, China) from total extracted RNA using RNAprep pure Micro Kit (Aidlab, RN07, Beijing, China). All primers used for analysis were listed in Table S1. PCR amplification was conducted with SYBR® Premix Ex Taq™ II (TAKARA, RR820A, Japan) and Light Cycler real-time PCR instrument of Roche 480 (Roche, Germany). Each sample extracted from 3 to 6 ovaries was amplified in triplicate to normalize the system and pipetting error, and the relative mRNA expression levels of all genes were calculated by the formula of 2−ΔΔCt and normalized with β-actin.
Analysis of meiotic stages
The meiotic stages of fetal oocytes were analyzed using cytospreads as previously described42,43. Fixed samples were blocked with antibody dilution buffer (ADB) at 37 °C for 30 min and incubated with primary antibodies (Table S2) at 37 °C for 8 h in a 1:200 dilution. After three washes with Tris-buffered saline (TBS), they were blocked at 4 °C overnight in ADB. Finally, the cells were incubated in dark with secondary antibodies of CY3 (anti-rabbit, Beyotime, A0562, Nantong, China; anti-mouse, Beyotime, A0568) or fluorescein isothiocyanate (FITC)-labeled goat IgG (anti-rabbit, Beyotime, A0516; anti-mouse, Beyotime, A0521) at 37 °C for 1.5 h and stained with Hoechst 33342 (Beyotime, C1022) for 5 min. Slides were sealed and prepared for fluorescence microscope observation (Olympus, BX51, Japan). The number of ovaries of each sample was 3 to 4 and every independent experiment for oocyte counting was at least 300.
Immunohistochemistry and TUNEL assay
Ovaries were fixed in 4% paraformaldehyde (Solaibio, P1110, Beijing, China) and processed according to standard methods for paraffin samples. Sections were taken every 5 µm and immunohistochemistry performed on deparaffinated sections after antigen retrieval at 96 °C. Sections were blocked and incubated with anti-MVH protein antibody as reported above for primary antibodies, then FITC (CY3 in TUNEL assay)-labeled goat anti-rabbit IgG and nucleic acid was stained with propidium iodide (Solaibio, P8080-10, Beijing, China) or Hoechst 33342 (in TUNEL assay). For immunohistochemistry with enzyme substrates, the samples must be treated with 3% H2O2 before primary antibody incubation, and then the secondary antibody was horse radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Beyotime, A0258); the DAB kit was purchased from ZSGB-BIO company (Beijing, China). Considering that the oocyte number fluctuated largely in ovaries of different size, the oocyte of each experimental ovary was counted in every five sections and at total three sections from different fetuses or six for pups. Then, Image J software was used to calculate areas of these ovary sections, and accordingly the oocyte counting was adjusted to the same level on the basis of ovarian section areas.
For the estimate of the number of the different classes of follicles in postnatal ovaries, we defined the primordial and primary follicle of a follicle containing an intact MVH-positive oocyte surrounded by a single layer of flat or cuboidal granulosa cells, the secondary and mature follicles were two or multiple layers of granulosa cells, and the antral follicle was a kind of follicle with a fluid-filled cavity adjacent to the oocyte44: the total number of each follicle class was counted in every five sections for a total of 8 or 10 sections for each of three ovaries from different mice.
The TUNEL assay was performed on paraffin ovary sections as before43, using the BrightGreen Apoptosis Detection Kit (Vazyme, A112-02, Nanjing, China) according to the manufactures’ instructions.
Western blot
For each sample, proteins were extracted from 3 to 4 ovaries using the RIPA lysis solution (Beyotime, P0013C) for sodium dodecyl sulfate–polyacrylamide gel electrophoresis. They were then transferred onto a polyvinylidene fluoride membrane (immobilon-PSQ transfer membranes, Millipore, ISEQ00010, USA). After blocking in TBST buffer (TBS with Tween-20) containing 5% bovine serum albumin, the membrane was incubated in primary antibody (Table S2) overnight at 4 °C, washed and incubated with HRP-conjugated goat anti-rabbit (Beyotime, A0208) or anti-mouse IgG (Beyotime, A0216) diluted in TBST at room temperature for 1.5 h. Ultimately, the BeyoECL Plus Kit (Beyotime, A0018) was used for chemiluminescence; β-ACTIN was used as housekeeping protein as previous reported21.
Transcription data analysis
A total of 10–12 ovaries were collected from fetuses, subjected to total RNA extraction and sequenced. The latter was obtained from ANOROAD company with Illumina Hiseq 2000 sequencing system (San Diego, CA, USA). The bioinformatic analysis was carried out as previously described45–47. With the transcriptome data assembly with Cufflinks and calculation of gene expression levels with Cuffdiff, the differentially expressed mRNAs were defined as the expression levels of mRNAs with q-value < 0.05, and the data visualization was conducted with R Bioconductor/CummeRbund packages48. In addition, the biofunction of these differentially expressed genes was analyzed via Gene Ontology (GO) enrichment based on GO Database49.
Statistical analysis
Results obtained from at least three independent experiments were expressed as mean ± SEM. Data were statistically analyzed with Graph Pad Prism software and the significant difference was determined with Student’s unpaired t-test of independent sample. P < 0.05 was considered as significantly different, while P < 0.01 was a highly significant difference.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31471346 and 31671554) and National Basic Research Program of China (973 Program, 2012CB944401).
Authors' contributions
J.-J.W., X.-W.Y, R.-Y.W., X.-F.S., S.-F.C., W.G., J.-C.L., Y.-P.L., J.L. and S.-H..Z. conducted the experiments; J.-J.W and X.-W.Y. analyzed the data; J.-J.W., W.S. and M.D.F. wrote the manuscript; W.S. and M.D.F. designed the experiments. All authors reviewed the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
These authors contributed equally: Jun-Jie Wang, Xiao-Wei Yu.
Edited by E. Candi
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41419-018-0492-2.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lundqvist A, et al. Reported dietary intake in early pregnant compared to non-pregnant women – a cross-sectional study. BMC Pregnancy Childbirth. 2014;14:1–10. doi: 10.1186/s12884-014-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies PS, et al. Early life nutrition and the opportunity to influence long-term health: an Australasian perspective. J. Dev. Orig. Health Dis. 2016;7:440–448. doi: 10.1017/S2040174415007989. [DOI] [PubMed] [Google Scholar]
- 3.Qureshi Z, Khan R. Diet intake trends among pregnant women: in rural area of Rawalpindl, Pakistan. J. Ayub Med. Coll. Abbottabad. 2015;27:684–688. [PubMed] [Google Scholar]
- 4.Gillman MW, Richedwards JW. The fetal origin of adult disease: from sceptic to convert. Paediatr. Perinat. Epidemiol. 2000;14:192–193. doi: 10.1046/j.1365-3016.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaiserman A. Early-life origin of adult disease: evidence from natural experiments. Exp. Gerontol. 2011;46:189–192. doi: 10.1016/j.exger.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Lumey LH, Stein AD. Offspring birth weights after maternal intrauterine undernutrition: a comparison within sibships. Am. J. Epidemiol. 1997;146:810–819. doi: 10.1093/oxfordjournals.aje.a009198. [DOI] [PubMed] [Google Scholar]
- 7.Painter RC, et al. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 8.Yarde F, et al. Prenatal famine, birthweight, reproductive performance and age at menopause: the Dutch hunger winter families study. Hum. Reprod. 2013;28:3328–3336. doi: 10.1093/humrep/det331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias SG, van Noord PA, Peeters PH, Den TI, Grobbee DE. Caloric restriction reduces age at menopause: the effect of the 1944-1945 Dutch famine. Menopause. 2003;10:399–405. doi: 10.1097/01.GME.0000059862.93639.C1. [DOI] [PubMed] [Google Scholar]
- 10.Cresswell JL, et al. Is the age of menopause determined in-utero? Early Human. Dev. 1997;49:143–148. doi: 10.1016/S0378-3782(97)00028-5. [DOI] [PubMed] [Google Scholar]
- 11.Hardy R, Kuh D. Change in psychological and vasomotor symptom reporting during the menopause. Soc. Sci. Med. 2002;55:1975–1988. doi: 10.1016/S0277-9536(01)00326-4. [DOI] [PubMed] [Google Scholar]
- 12.Treloar SA, Sadrzadeh S, Do KA, Martin NG, Lambalk CB. Birth weight and age at menopause in Australian female twin pairs: exploration of the fetal origin hypothesis. Hum. Reprod. 2000;15:55–59. doi: 10.1093/humrep/15.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Steiner AZ, D’Aloisio AA, Deroo LA, Sandler DP, Baird DD. Association of intrauterine and early-Life exposures with age at menopause in the sister study. Am. J. Epidemiol. 2010;172:140–148. doi: 10.1093/aje/kwq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swamy GK, Ostbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA. 2008;299:1429–1436. doi: 10.1001/jama.299.12.1429. [DOI] [PubMed] [Google Scholar]
- 15.Dekeyser N, et al. Premature birth and low birthweight are associated with a lower rate of reproduction in adulthood: a Swedish population-based registry study. Hum. Reprod. 2012;27:1170–1178. doi: 10.1093/humrep/der478. [DOI] [PubMed] [Google Scholar]
- 16.de Bruin JP, et al. Fetal growth retardation as a cause of impaired ovarian development. Early Hum. Dev. 1998;51:39–46. doi: 10.1016/S0378-3782(97)00073-X. [DOI] [PubMed] [Google Scholar]
- 17.Meikle D, Westberg M. Maternal nutrition and reproduction of daughters in wild house mice (Mus musculus) Reproduction. 2001;122:437–442. doi: 10.1530/rep.0.1220437. [DOI] [PubMed] [Google Scholar]
- 18.Rae MT, et al. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim. Reprod. Sci. 2002;72:63–71. doi: 10.1016/S0378-4320(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 19.Khorram O, Keenrinehart E, Chuang TD, Ross MG, Desai M. Maternal undernutrition induces premature reproductive senescence in adult female rat offspring. Fertil. Steril. 2015;103:291–298. doi: 10.1016/j.fertnstert.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mossa F, et al. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 2013;88:92. doi: 10.1095/biolreprod.112.107235. [DOI] [PubMed] [Google Scholar]
- 21.Wang YY, et al. Starvation at birth impairs germ cell cyst breakdown and increases autophagy and apoptosis in mouse oocytes. Cell Death Dis. 2017;8:e2613. doi: 10.1038/cddis.2017.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibshman JD, Hung A, Baugh LR. Maternal diet and insulin-like signaling control intergenerational plasticity of progeny size and starvation resistance. PLoS Genet. 2016;12:e1006396. doi: 10.1371/journal.pgen.1006396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 24.Radford EJ, et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science. 2014;345:1255903. doi: 10.1126/science.1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumey LH, Stein AD, Susser E. Prenatal famine and adult health. Annu. Rev. Public. Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barth JMI, Szabad J, Hafen E, Köhler K. Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ. 2011;18:915–924. doi: 10.1038/cdd.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidel HS, Kimble H. The oogenic germline starvation response in C. elegans. PLoS One. 2011;6:e28074. doi: 10.1371/journal.pone.0028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JJ, et al. Complete in vitro oogenesis: retrospects and prospects. Cell Death Differ. 2017;24:1845–1852. doi: 10.1038/cdd.2017.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman BJ, Rathmell JC. Metabolic stress in autophagy and cell death pathways. Cold Spring Harb. Perspect. Biol. 2012;4:a008763. doi: 10.1101/cshperspect.a008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Felici M, et al. Establishment of oocyte population in the fetal ovary: primordial germ cell proliferation and oocyte programmed cell death. Reprod. Biomed. Online. 2005;10:182–191. doi: 10.1016/S1472-6483(10)60939-X. [DOI] [PubMed] [Google Scholar]
- 31.Choi SW, Friso S. Epigenetics: a new bridge between nutrition and health. Adv. Nutr. 2010;1:8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felicia C, et al. How diet intervention via modulation of DNA damage response through microRNAs may have an effect on cancer prevention and aging, an in silico study. Int. J. Mol. Sci. 2016;17:752. doi: 10.3390/ijms17050752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang B, Jiang DM, Bai L, He H, Ma R. Molecular characterisation and expression profiling of the ENO1 gene in the ovarian follicle of the Sichuan white goose. Mol. Biol. Rep. 2014;41:1927–1935. doi: 10.1007/s11033-014-3039-3. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Nishihira J, Koyama Y, Kanagawa H. The role of macrophage migration inhibitory factor in pregnancy and development of murine embryos. Biochem. Mol. Biol. Int. 1996;38:409–416. [PubMed] [Google Scholar]
- 35.Reddy P, et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- 36.Sugiura K, et al. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 37.Wang SJ, et al. Melatonin suppresses apoptosis and stimulates progesterone production by bovine granulosa cells via its receptors (MT1 and MT2) Theriogenology. 2012;78:1517–1526. doi: 10.1016/j.theriogenology.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 38.Brown RM, Davis MG, Hayashi K, Maclean JA. Regulated expression of Rhox8 in the mouse ovary: evidence for the role of progesterone and Rhox5 in granulosa cells. Biol. Reprod. 2013;88:832–839. doi: 10.1095/biolreprod.112.103267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singersam J, Goldstein L, Dai A, Gartler SM, Riggs AD. A potentially critical Hpa II site of the X chromosome-linked PGK1 gene is unmethylated prior to the onset of meiosis of human oogenic cells. Proc. Natl. Acad. Sci. USA. 1992;89:1413–1417. doi: 10.1073/pnas.89.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verissimo CS, et al. Silencing of the microtubule-associated proteins doublecortin-like and doublecortin-like kinase-long induces apoptosis in neuroblastoma cells. Endocr. Relat. Cancer. 2010;17:399–414. doi: 10.1677/ERC-09-0301. [DOI] [PubMed] [Google Scholar]
- 41.Roberts AE, et al. Neutralization of endogenous vascular endothelial growth factor depletes primordial follicles in the mouse ovary. Biol. Reprod. 2007;76:218–223. doi: 10.1095/biolreprod.106.050880. [DOI] [PubMed] [Google Scholar]
- 42.Liu KH, et al. The impact of Zearalenone on the meiotic progression and primordial follicle assembly during early oogenesis. Toxicol. Appl. Pharmacol. 2017;329:9–17. doi: 10.1016/j.taap.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Liu JC, et al. Di (2-ethylhexyl) phthalate exposure impairs meiotic progression and DNA damage repair in fetal mouse oocytes in vitro. Cell Death Dis. 2017;8:e2966. doi: 10.1038/cddis.2017.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang ZP, et al. Growth of mouse oocytes to maturity from premeiotic germ cells in vitro. PLoS One. 2012;7:e41771. doi: 10.1371/journal.pone.0041771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge W, et al. Cutaneous applied nano-ZnO reduce the ability of hair follicle stem cells to differentiate. Nanotoxicology. 2017;11:465–474. doi: 10.1080/17435390.2017.1310947. [DOI] [PubMed] [Google Scholar]
- 47.Lai FN, et al. Di (2-ethylhexyl) phthalate impairs steroidogenesis in ovarian follicular cells of prepuberal mice. Arch. Toxicol. 2017;91:1–14. doi: 10.1007/s00204-016-1790-z. [DOI] [PubMed] [Google Scholar]
- 48.Huber W, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods. 2015;12:115–121. doi: 10.1038/nmeth.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ashburner M, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.