Abstract
Objectives
Apathy is among the earliest and most pervasive neuropsychiatric symptoms in prodromal and mild Alzheimer’s disease (AD) dementia that correlates with functional impairment and disease progression. We investigated the association of apathy with regional 18F-fluorodeoxyglucose (FDG) metabolism in cognitively normal, mild cognitive impairment, and AD dementia subjects from the Alzheimer’s Disease Neuroimaging Initiative database.
Design
Cross-sectional and longitudinal studies.
Setting
Fifty-seven North American research sites.
Participants
Four-hundred and two community dwelling elders.
Measurements
Apathy was assessed using the Neuropsychiatric Inventory Questionnaire. Baseline FDG metabolism in five regions implicated in the neurobiology of apathy and AD was investigated in relationship to apathy at baseline (cross-sectional general linear model) and longitudinally (mixed random/fixed effect model). Covariates included age, sex, diagnosis, apolipoprotein E genotype, premorbid intelligence, cognition, and antidepressant use.
Results
Cross-sectional analysis revealed that posterior cingulate hypometabolism, diagnosis, male sex, and antidepressant use were associated with higher apathy scores. Longitudinal analysis revealed that the interaction of supramarginal hypometabolism and time, posterior cingulate hypometabolism, and antidepressant use were associated with higher apathy scores across time; only supramarginal hypometabolism was positively related to rate of increase of apathy.
Conclusions
Results support an association of apathy with hypometabolism in parietal regions commonly affected in early stages of AD, rather than medial frontal regions implicated in the neurobiology of apathy in later stages. Further work is needed to substantiate whether this localization is specific to apathy rather than to disease stage, and to investigate the potential role of AD proteinopathies in the pathogenesis of apathy.
Keywords: 18F-fluorodeoxyglucose positron emission tomography, Alzheimer’s disease, apathy, mild cognitive impairment, metabolism
Objective
Alzheimer’s disease (AD) is an epidemic with personal and public health burden. AD involves a progression of clinical symptoms and pathological processes on a spectrum from cognitively normal (CN) individuals (who may have biomarker evidence of AD) to mild cognitive impairment (MCI) and AD dementia. Strategies for prevention and treatment of AD have increasingly focused on preclinical and prodromal disease stages(1, 2).
In addition to progressive cognitive decline, neuropsychiatric symptoms (NPS) are distressing symptoms that occur across the AD spectrum(3, 4). Apathy is among the most common NPS in MCI and AD dementia, characterized by diminished interest and motivation in usual activities and in those of others(5, 6). Although it is associated with functional impairment and caregiver distress in AD dementia and with disease progression in MCI(3, 7–9), the neurobiology of apathy across the AD spectrum in preclinical and prodromal stages is incompletely understood and there are few effective prevention or treatment strategies.
Previous work has linked apathy to abnormalities in frontal-subcortical circuits(10). In older adults with AD dementia, apathy has been associated with reduced metabolism (measured by 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)) and/or perfusion (measured by single photon emission computed tomography (SPECT)) and decreased volume and thickness of the anterior cingulate cortex(11–15) and with reduced metabolism and perfusion and cortical thinning of the orbitofrontal cortex(11, 13, 15). Similarly, a study of early age of onset AD subjects using FDG-PET found that sub-syndromic apathy symptoms were associated with hypometabolism in the bilateral orbitofrontal and dorsolateral frontal cortex(16). However, investigations across the AD spectrum, including CN older adults some of whom may be in the preclinical stage of AD, have identified associations between apathy and changes in a broader range of brain regions(17–19). Studies from our group, investigating a cohort of older adults ranging from CN to mild AD dementia from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), and also a smaller cohort of CN and MCI subjects, found that reduced inferior temporal cortical thickness predicted higher apathy scores both at baseline and over time(17, 18). Studies in MCI subjects probing regional metabolism have revealed different patterns: Marshall and colleagues showed that in 24 older adults with MCI, higher apathy scores (assessed using the Apathy Evaluation Scale (AES))(20) were associated with elevated cortical amyloid (measured by Pittsburgh Compound B (PiB) PET) but not with changes in FDG-PET in regions implicated in apathy neurobiology or AD pathogenesis(21). Delrieu and colleagues, in a small subset of MCI subjects from the ADNI cohort, found MCI subjects with apathy relative to those without had significantly reduced metabolism in the posterior cingulate cortex(19). In contrast, a community based sample of 668 CN older adults from the Mayo Clinic Study of Aging, found an association between abnormal FDG PET metabolism and depression, but not apathy(22). Together, these findings suggest that apathy is a complex neurobiological construct that may be mediated by changes in cortical thickness and metabolism in a variety of brain regions, including the posterior cingulate cortex, across the AD spectrum.
To our knowledge, no studies have examined the longitudinal relationship of apathy to regional metabolism, as measured by FDG PET, across the AD continuum, from preclinical to amnestic MCI and mild AD dementia. Greater understanding of the neurobiology of apathy in the context of AD progression is needed to address prevention and treatment of this NPS in AD and to develop more effective strategies to target overall disease progression.
To fill this gap in the literature, we investigated the cross-sectional and longitudinal relationship of glucose metabolism measured by FDG-PET and apathy in the well-defined ADNI cohort of older adults across the AD continuum (CN, MCI, and mild AD dementia). We included the CN group in our analyses since this group includes some subjects in the preclinical stage of AD. We hypothesized that hypometabolism in regions previously implicated in the neurobiology of apathy as well as other, less-explored regions relevant to early AD progression (anterior cingulate cortex, medial orbitofrontal cortex, inferior temporal cortex, posterior cingulate cortex and supramarginal gyrus) would predict the presence and worsening of apathy over time, independent of diagnosis, age, sex, apoliporotein E (APOE) genotype, premorbid intelligence, memory performance, processing speed, antidepressant use, and (for longitudinal analyses) baseline dependent variables.
Methods
Subjects
Data analyzed were obtained from the ADNI database (adni.loni.usc.edu, PI Michael W. Weiner) (see Supplemental Digital Content 1;(23)). The study sample consisted of 402 subjects with the following baseline diagnoses: CN: 104; amnestic MCI: 203; mild AD dementia: 95. As per ADNI design, subjects underwent baseline FDG PET and clinical assessments every 6 to 12 months up to three years. The mean follow-up time for all subjects was 2.3 +/− 0.9 years. CN and MCI subjects had up to 3 years follow-up and AD dementia subjects up to 2 years follow-up (maximum follow-up: 4.02 years; see Supplemental Digital Content 1–3, Tables 1a–1b). At screening, all subjects met the following criteria: between ages 55–89 (inclusive), no acute medical problems, no significant neurological disorders (with the exception of MCI or AD dementia for subjects in those groups), no significant cerebrovascular disease, no active psychiatric illness and not significantly depressed (score on the Geriatric Depression Scale short form(24) less than or equal to 5); (see Supplemental Digital Content 1)(3, 25).
Clinical assessments
All subjects underwent clinical assessments as described previously(26), including the Neuropsychiatric Inventory Questionnaire brief form (NPI-Q) informant version(27). The NPI-Q has twelve symptom items, including an apathy item, which was used to assess apathy across all subjects. The NPI-Q apathy item asks the following question of the subject’s informant: “Does the patient seem less interested in his or her usual activities and in the activities and plans of others?”(27). Each NPI-Q symptom item is rated for presence and severity over the past month, and scored on an ordinal scale of 0–3; a higher score indicates greater symptom severity. Other relevant assessments included: APOE genotype, the American National Adult Reading Test (AMNART) intelligence quotient (IQ) (a proxy measure of premorbid verbal IQ), the Rey Auditory Verbal Learning Test (RAVLT) total learning score (a measure of episodic memory), and the Wechsler Adult Intelligence Scale-Revised Digit Symbol (measures processing speed, working memory, and visual scanning)(Supplemental Digital Content 1).
FDG PET acquisition
FDG PET images were obtained using scanners located throughout the US as previously described (see Supplemental Digital Content 1)(26).
FDG region of interest (ROI) generation and selection
Cortical FDG metabolism was expressed as the standard uptake value (SUV) and normalized to an aggregate of gray, pons, and primary (sensorimotor) cortex for each region of interest(28). Regions were sampled using the Harvard-Oxford probabilistic atlas (http://neuro.debian.net/pkgs/fsl-harvard-oxford-atlases.html). Five bilateral ROIs were selected based on prior evidence for a role in the neurobiology of apathy (orbitofrontal cortex, anterior cingulate cortex)(11–13, 29) and in AD pathogenesis but less commonly apathy (inferior temporal, posterior cingulate gyrus, supramarginal gyrus)(30).
Statistical Analyses
Analyses were carried out using SAS Version 9.4 (SAS, Cary, NC).
Subject demographics and characteristics
Analysis of variance (ANOVA) was used to evaluate the associations between diagnostic groups and demographic and clinical variables. Post-hoc comparisons were carried out using a pairwise t test with Bonferroni correction for continuous variables and a chi-square test for categorical variables.
Cross-sectional analyses
The five FDG ROIs were entered as simultaneous predictors in a backward elimination general linear model (GLM) (p<0.05 cut off), regressing the baseline NPI-Q apathy item score on these ROIs, diagnosis at baseline, and the interaction of each of these FDG ROIs and diagnosis. The following were also initial covariates: sex, interaction of sex and diagnosis, baseline age (linear and quadratic terms), use of antidepressant medication (yes or no), RAVLT total learning score at baseline, Digit Symbol score at baseline, number of APOE4 alleles, and AMNART IQ. Significance test results (p values) for the model as a whole and individual predictors were complemented with partial regression coefficient estimates (β) with 95% confidence intervals (CI), and percent variance accounted for in the dependent variable for the model as a whole and for each individual predictor uniquely. Residuals were checked for conformance to assumptions of normality and homoscedasticity as well as for assessment of model fit.
Longitudinal Analyses
Longitudinal analyses were run across time (linear year in the study) for the dependent variable NPI-Q apathy. A mixed fixed and random-coefficient regression model was used with a backward elimination algorithm (p<0.05 cut off) on an initial pool of fixed predictors and variances/covariances of random terms. The fixed predictors were diagnostic group and its interaction with time, the FDG ROIs and their interactions with time, and the same covariates used in the cross-sectional analyses. Random terms were subject intercepts and their linear slope effect of time (initially allowed to be correlated). Residuals from the predicted values of both fixed and random terms were checked for model fit, and conformance to assumptions of normality and homoscedasticity (See Supplemental Digital Content 1, 4–5, tests of liberal bias).
Results
Descriptive Statistics
Table 1 provides baseline demographic and clinical data for all subjects and across diagnostic groups. Groups did not differ in age or sex, but did differ in education and AMNART IQ: CN and MCI subjects had significantly higher levels of education than subjects with AD dementia, and CN subjects had significantly higher levels of pre-morbid intelligence than either MCI or AD dementia groups (Table 1). There were significant differences between diagnostic groups for cognitive test variables in the expected directions. NPI-Q apathy was significantly different across groups, with CN subjects having significantly less apathy than MCI subjects, and those having significantly less apathy than AD dementia subjects (Table 1). Antidepressant use also differed significantly across groups, with increasing proportions of subjects taking antidepressants in groups spanning CN to MCI to AD dementia (12%, 23%, and 34% respectively) (Table 1).
Table 1.
Baseline Demographic and Clinical Data for Subjects
| Group | All subjects | CN | MCI | AD dementia | F or χ2 | df | P |
|---|---|---|---|---|---|---|---|
| N | 402 | 104 | 203 | 95 | |||
| Age (years) | 75.4 +/− 6.7 (Median: 75.5) | 75.9 +/− 4.8 (Median: 75.8) | 75.0 +/− 7.2 (Median: 75.0) | 75.6 +/− 7.4 (Median: 75.8) | 0.652 | 2, 399 | 0.521 |
| Sex (% male) | 63.9 | 61.5 | 67.5 | 59.0 | 2.40 | 2 | 0.302 |
| Education (years) | 15.5 +/− 3.1 (Median: 16.0) | 15.9 +/− 3.1 (Median: 16.0) | 15.8 +/− 2.9 (Median: 16.0) | 14.6 +/− 3.2 (Median: 16.0) | 5.48 | 2, 399 | <0.05* |
| AMNART IQ | 117.5 +/− 11.5 (Median: 121.0) | 120.7 +/− 11.3 (Median: 123.0) | 117.2 +/− 11.0 (Median: 121.0) | 114.4 +/− 11.9 (Median: 115.0) | 7.72 | 2, 398 | <0.01** |
| APOE4(%non-carrier/heterozygous carrier/homozygous carrier) | 51.0/38.3/10.7 | 75.0/23.1/1.9 | 46.8/40.4/12.8 | 33.7/50.5/15.8 | 38.9 | 4 | <0.0001**** |
| MMSE | 26.8+/− 2.6 (Median: 27.0) | 29.0 +/− 1.1 (Median: 29.0) | 27.2 +/− 1.7 (Median: 27.0) | 23.5 +/− 2.1 (Median: 24.0) | 280.69 | 2, 399 | <0.0001*** |
| RAVLT total learning | 32.2 +/− 11.1 (Median: 31.0) | 42.2 +/− 9.8 (Median: 41.0) | 31.4 +/− 9.1 (Median: 30.0) | 22.9+/− 6.9 (Median: 22.0) | 120.61 | 2, 399 | <0.0001*** |
| Digit Symbol | 36.5 +/− 13.0 (Median: 37.5) | 44.5 +/− 10.4 (Median: 43.0) | 37.4 +/− 10.9 (Median: 38.0) | 25.9 +/−12.4 (Median: 26.0) | 69.99 | 2, 399 | <0.0001*** |
| NPI-Q Apathy (% present) | 0.25 +/− 0.6 (18.2) (Median: 0.0) | 0.02 +/− 0.1 (1.9) (Median: 0.0) | 0.2 +/− 0.5 (15.3) (Median: 0.0) | 0.6 +/− 0.8 (42.1) Median: 0.0) | 28.88 | 2, 399 | <0.001++ |
| Antidepressant use (% present) | 22.6 | 11.5 | 23.2 | 33.7 | 13.97 | 2 | <0.001+ |
All values (except N, sex, APOE4, and antidepressant use) represent mean ± standard deviation. Medians are given in parentheses. AD (Alzheimer’s disease), AMNART IQ (American National Adult Reading Test intelligence quotient), APOE4 (Apolipoprotein E ε4), CN (cognitively normal elderly), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NPI-Q (Neuropsychiatric Inventory Questionnaire), RAVLT (Rey Auditory Verbal Learning Test).
Significance tests used: χ2 test (categorical variables), analysis of variance (continuous variables).
Additional post-hoc pairwise comparisons of diagnostic groups were carried out using Bonferroni corrected t-tests:
For education: df = 399, error mean square = 9.201 for all comparisons; for AMNART IQ: df = 398, error mean square = 127.76 for all comparisons; for MMSE: df = 399, error mean square = 2.767 for all comparisons; for RAVLT: df = 399, error mean square = 77.707 for all comparisons; for Digit Symbol: df = 399, error mean square = 124.773 for all comparisons; for NPI-Q apathy: df = 399, error mean square = 0.302 for all comparisons. The p values given in the table pertain to the overall main effect of group whereas the * and + symbols indicate the results of the pertinent Bonferroni corrected post hoc pairwise comparisons as follows:
p<0.05 for CN vs. AD and MCI vs. AD;
p<0.05 for CN vs. MCI and CN vs. AD;
p< 0.0001 for CN vs. MCI; CN vs. AD and MCI vs. AD;
p<0.0001 for CN vs. MCI and CN vs. AD;
p<0.05 for CN vs. MCI, CN vs. AD and MCI vs. AD,
p<0.001 for CN vs. MCI and CN vs. AD.
Cross-Sectional Analysis
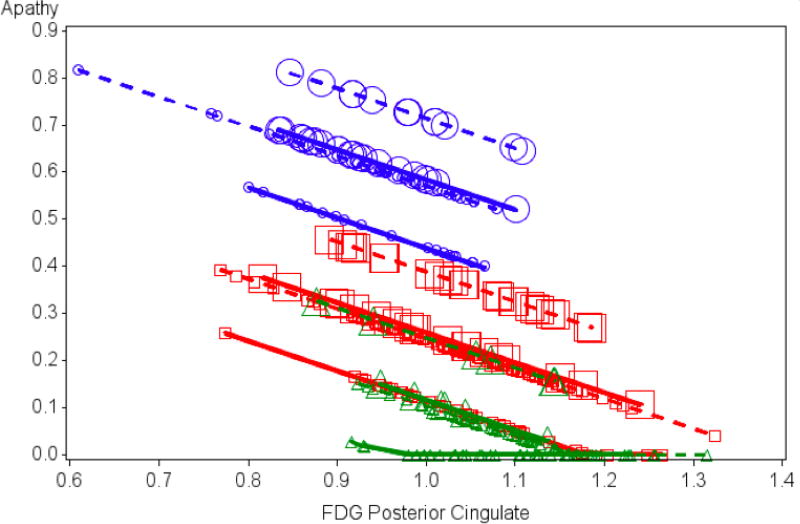
The five FDG ROIs were included in the initial predictor pool subjected to backward elimination in the cross-sectional GLM. Following backward elimination, posterior cingulate metabolism (hypometabolism was associated with more apathy), diagnosis (adjusted mean for AD>MCI>CN), sex (males had more apathy), and antidepressant medication use (medication users had more apathy) remained significant (Table 2). The model as a whole linearly accounted for about 15% of the variance in apathy scores, whereas each predictor uniquely accounted for only about 1% of the variance, except for diagnostic group at about 6%. No other ROIs were retained in the model (all dropped out with p>0.35). Residuals conformed reasonably to normality and homoscedasticity, although with some positive skewing due to floor effects for the CN group. See Table 2 and Figure 1.
Table 2. Results from Cross-Sectional Model.
Cross-sectional general linear model of the association of apathy to regional FDG metabolism and covariates displaying predictors retained in the final model
| Model: R2=0.154, (df=5, 396) F=14.40, p<0.0001 |
β (Adjusted Mean) |
95% CI for β | F | df | p | % Variance Total | % Variance Partial |
|---|---|---|---|---|---|---|---|
| Baseline diagnosis | 14.89 | (2, 396) | <0.0001 | 5.9 | 6.5 | ||
| AD | 0.47 (0.56) | (0.29, 0.64) | |||||
| MCI | 0.14 (0.23) | (0.01, 0.27) | |||||
| CN | 0.00 (0.09) | ||||||
| Posterior cingulate | −0.63 | (−1.22, −0.044) | 4.46 | (1, 396) | 0.035 | 0.74 | 0.85 |
| Sex | |||||||
| Female | −0.13(0.23) | (−0.24, −0.016) | 5.04 | (1, 396) | 0.025 | 0.86 | 1.00 |
| Male | 0.00 (0.36) | ||||||
| Antidepressant medication use | |||||||
| No | −0.14 (0.22) | (−0.28, −0.011) | 4.55 | (1, 396) | 0.034 | 0.76 | 0.87 |
| Yes | 0.00 (0.37) |
Cross-sectional general linear model of the association of apathy to regional FDG metabolism and covariates. AD (Alzheimer's disease), β (partial unstandardized regression coefficient estimate), CI (confidence interval), CN (cognitively normal elderly), MCI (mild cognitive impairment), F: F test statistic, df: degrees of freedom. CN was used as a reference group in predictors coding diagnosis. For antidepressant medication use “yes” was the reference group of subjects taking antidepressant medications. For sex, male sex was the reference group. For diagnosis, sex, and antidepressant use, β weights as well as Adjusted Means (group means adjusted for other predictors) are reported. For Post Hoc Pairwise Tests of Diagnostic Groups: AD vs CN: t=5.32, df=396, p<0.0001; MCI vs CN: t=2.11, df=396, p<0.035; AD vs MCI: t=4.44, df=396, p<0.0001.
“%Variance Total” represents percent of total variance of NPI-Q Apathy (Neuropsychiatric Inventory Questionnaire brief form, apathy item) uniquely associated with the indicated predictor (unbiased population estimate), “%Variance Partial” represents percent of variance of NPI-Q Apathy with portion associated with other predictors pre-removed, which is uniquely associated with the indicated predictor (unbiased population estimate).
Figure 1.
Results from Cross-Sectional Model. Values for baseline apathy (scores on the NPI-Q apathy item) as predicted by the reduced cross-sectional general linear model including posterior cingulate FDG metabolism (F=4.46; df=1,396; p=0.035), diagnosis (F=14.89; df=2,396; p<0.0001), sex (F=5.04; df=1,396; p=0.025), and baseline antidepressant medications (presence or absence; F=4.55; df=1,396; p=0.034). Symbols designate values. Variation in the symbols and lines connecting them differentiate various groupings. To indicate diagnosis: circles = AD dementia; squares = MCI; triangles = CN; for sex: dashed line = male; solid line = female; for antidepressant medication use: large symbol = presence of antidepressant medication; small symbol = absence of antidepressant medication. NPI-Q: Neuropsychiatric Inventory Questionnaire; FDG: 18F-fluorodeoxyglucose; AD: Alzheimer’s disease; MCI: mild cognitive impairment; CN: cognitively normal.
Longitudinal Analysis
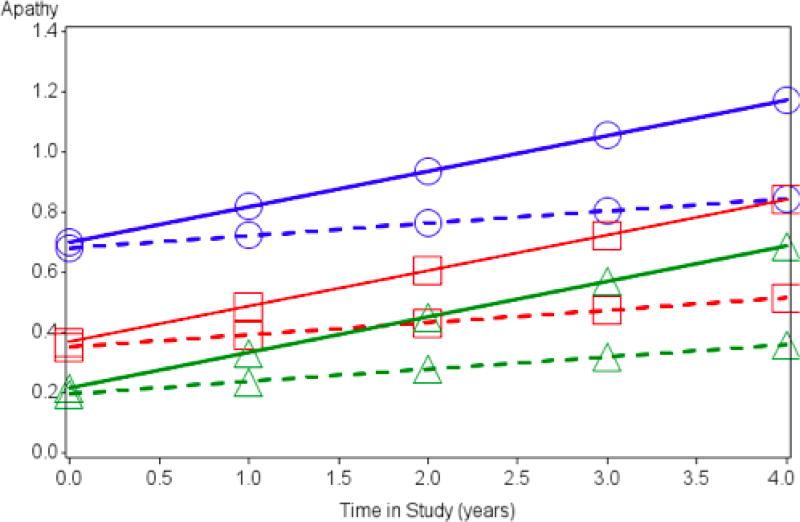
The fixed effects remaining after backward elimination were: the interaction of supramarginal gyrus metabolism and time (p=0.01; reduction of slope across time by −0.6 units of apathy per year, CI= −1,−0.13, for each unit increase of suprmarginal gyrus metabolism; i.e., baseline supramarginal hypometabolism was positively associated with apathy over time), a main effect for posterior cingulate metabolism (baseline posterior cingulate hypometabolism was positively associated with apathy on average across time), a main effect for diagnosis (adjusted mean for AD>MCI>CN), male sex (males had more apathy), and antidepressant medication use (users had more apathy) (Table 3). Only baseline supramarginal hypometabolism was positively associated with rate of increase in apathy over time in all subjects. See Table 3 and Figure 2.
Table 3. Longitudinal Mixed Effects Model of Association of Apathy and Regional FDG metabolism over time, displaying predictors retained in the final model.
Model: r =0.37, R2 =0.14, p<0.0001 for actual vs. predicted values (fixed); r=0.77, R2= 0.59, p<0.0001 for actual vs. predicted values (fixed and random)
| Predictor | Partial β | 95% CI for β | F | df | p |
|---|---|---|---|---|---|
| Diagnosis | 25.1 | (2, 396) | <0.0001 | ||
| AD | 0.49 | (0.35, 0.62) | |||
| MCI | 0.15 | (0.05, 0.26) | |||
| CN | 0.0 | ||||
| Posterior Cingulate | −0.66 | (−1.18, −0.14) | 6.22 | (1, 1047) | 0.013 |
| Supramarginal gyrus × time | −0.6 | (−1, −0.13) | 6.45 | (1, 1047) | 0.01 |
| Supramarginal gyrus | −0.14 | (−1, 0.71) | 0.11 | (1, 1047) | 0.74 |
| Time | 0.68 | (0.21, 1.16) | 8.18 | (1, 388) | 0.004 |
| Sex | |||||
| Female | −0.10 | (−0.19, −0.0090) | 4.68 | (1, 396) | 0.03 |
| Male | 0.00 | ||||
| Antidepressant medication use | |||||
| No | −0.12 | (−0.23, −0.02) | 5.55 | (1, 396) | 0.02 |
| Yes | 0.00 |
CI: confidence interval, df: degrees of freedom, F: F test statistic, AD (Alzheimer's disease), β (partial unstandardized regression coefficient estimate), CN (cognitively normal elderly), MCI (mild cognitive impairment). CN was used as a reference group in predictors coding diagnosis. For antidepressant medication use “yes” was the reference group of subjects taking antidepressant medications. For sex, male sex was the reference group. For Post Hoc Pairwise Tests of Diagnostic Groups: AD vs CN: t=6.96, df=396, p<0.0001; MCI vs CN: t=2.96, df=396, p<0.003; AD vs MCI: t=5.69, df=396, p<0.0001.
Figure 2.
Results from Longitudinal Model. Apathy (NPI-Q apathy item scores) as predicted by the fixed effects predictors supramarginal FDG metabolism and diagnostic groups from the longitudinal model across time (in years). The fixed effects model shows a longitudinal positive association between hypometabolism and rate of progression of apathy over time. For purposes of the graph, other covariates in the model were set at the values: FDG metabolism in the posterior cingulate =1 (mean); sex = male; Antidepressant medication = yes (presence of antidepressant medication). Circles = AD dementia; squares = MCI; triangles = CN. Solid line = low FDG metabolism for the supramarginal gryus (1 standard deviation below the mean); Dashed line = high FDG metabolism for the supramarginal gryus (1 standard deviation above the mean). NPI-Q: Neuropsychiatric Inventory Questionnaire; FDG: 18F-fluorodeoxyglucose; AD: Alzheimer’s disease; MCI: mild cognitive impairment; CN: cognitively normal.
Conclusions
Apathy is among the earliest and most distressing NPS in AD. Despite its clinical significance, its neural correlates across the AD continuum remain poorly understood. We examined cerebral glucose metabolism in regions previously associated with apathy (anterior cingulate cortex, medial orbitofrontal cortex)(11–13, 29) and those associated with early AD but less commonly with apathy (inferior temporal, posterior cingulate, and supramarginal gyrus)(30) in relation to apathy at baseline and over time in a cohort of older adults spanning the AD continuum.
We found a cross-sectional relationship between posterior cingulate hypometabolism and higher apathy scores. In longitudinal analysis, baseline posterior cingulate hypometabolism was associated with higher apathy scores on average across time, and baseline supramarginal gyrus (lateral parietal) hypometabolism was positively associated with rate of change in apathy over time. In contrast, we did not find a significant relationship between apathy and metabolism in the inferior temporal lobe, anterior cingulate cortex or medial orbitofrontal cortex.
Our findings of a relationship between apathy and hypometabolism in AD-related medial and lateral parietal regions are in contrast to prior studies in subjects with AD dementia that have shown associations between apathy and reduced cortical thickness, perfusion, and/or metabolism in medial frontal regions(11–15). These findings, are however, more consistent with studies from our group and others that investigated the relationship between apathy and regional integrity (measured by cortical metabolism and thickness) and included CN and MCI subjects(17–19). Delrieu and colleagues focused on regional metabolism in a cross-sectional study of a much smaller subset of MCI subjects from the ADNI cohort (65 total, 11 with apathy and 54 without apathy)(19) which represents a small subset of the ADNI MCI subjects included in our analyses. Consistent with our findings, the authors reported reduced metabolism in the posterior cingulate cortex in MCI subjects with apathy, and no associations with medial frontal regions. Donovan and colleagues found a longitudinal relationship between apathy and reduced inferior temporal thickness across the 802 ADNI cohort subjects (CN, MCI, AD dementia) who had MRI data available, but no relationship with thickness of the rostral anterior cingulate or medial orbitofrontal cortex(17). Guercio and colleagues, using the AES in a cross-sectional study of CN and MCI subjects, similarly found that reduced inferior temporal cortical thickness predicted higher apathy scores(18). We did not identify a significant relationship between inferior temporal cortex metabolism and apathy. This may be in part due to different measurements employed (cortical thickness vs. metabolism), with atrophy and regional hypometabolism possibly representing different pathogenic mechanisms across brain regions and/or different stages in pathogenic change (metabolic change preceding atrophy or vice versa). However, our results are overall concordant with those studies including both CN and MCI subjects(17–19) in that we found an association between changes in medial and lateral parietal regions and apathy, rather than medial frontal regions.
In contrast to our study, a population based cross-sectional study of 668 CN older adults from the Mayo Clinic Study of Aging found an association between FDG PET hypometabolism in a cortical aggregate region (comprised of the bilateral angular gyrus, posterior cingulate, precuneus, and inferior temporal cortical regions) and depression, but not apathy(22). However, the study sample was comprised of CN elders (unlike ours, which also contained early AD subjects), and was population based, while the ADNI cohort is referral-based. In addition, it is possible that analysis of an aggregate ROI may have limited the ability to detect associations between apathy and specific regions such as the posterior cingulate. Further studies are needed to distinguish between these possibilities, and to investigate whether region specific metabolic changes related to apathy differ between preclinical and prodromal AD stages.
Another potential explanation for one set of findings implicating parietal regions with apathy while another implicating frontal regions is that those regions are connected. Our group recently explored the cross-sectional functional connectivity correlates of NPS in MCI(31). We found a positive association between reduced frontoparietal control network connectivity and NPS, in particular apathy. Therefore, it is possible that both frontal and parietal regions relate to apathy, and that alterations in neural network activity (altered metabolic activity of one or several network nodes, or in the connections between nodes) contribute to the pathophysiology of apathy. So, too, may alterations in structural connectivity in frontal-subcortical or cortical-cortical circuits, as may be mediated by disruption of white matter tracts (32). More studies are needed to investigate these neurobiological mechanisms and the relationship between apathy, network connectivity measures, and AD associated proteinopathies in early AD.
Our study adds to previous findings by examining regional metabolism and its relationship to apathy across the full AD continuum in an ADNI sample with sufficient power to include potential confounding factors related to disease progression in analyses. Our work and the larger body of studies above raise the possibility that while apathy in AD dementia is mediated by abnormalities in medial frontal circuitry, the neural correlates of apathy differ at preclinical and prodromal disease stages. Future longitudinal studies examining apathy at each stage of the AD spectrum with more sensitive assessment measures and with multimodal imaging—including amyloid and tau PET imaging—are needed to differentiate between these alternatives.
As a related finding, in cross sectional analyses, diagnosis, male sex, and antidepressant use were independently associated with more apathy, and in longitudinal analyses, each variable at baseline was associated with higher apathy scores on average across time. We did not find an association between apathy and the interaction of sex and diagnostic group. Our findings related to diagnosis are consistent with previous work from our group and others showing that apathy is more severe with AD progression (increasing in severity from MCI to severe dementia)(3, 5, 33, 34) and that it worsens over time across a spectrum of CN elderly at risk for AD and in MCI when assessed through a combination of self and informant measures(35). Perhaps more striking are our findings related to male sex and higher apathy scores. We previously reported higher apathy scores over time in CN aging males compared to females(35). Similarly, Brodaty and colleagues evaluating apathy longitudinally in a cohort of healthy elderly using the AES-informant scale and Geda and colleagues using the NPI-Q in a cross-sectional analysis of NPS at baseline in a cohort of 1587 CN elderly, found higher apathy scores in males compared to females(36, 37). However, differences in apathy between sexes in MCI and AD dementia have not been consistently described(6). The mechanisms underlying differential expression of apathy in males and females in aging and, as our study suggests, possibly also in the AD spectrum, warrant further investigation.
Our finding of an association between antidepressant use and higher apathy scores also needs to be explored. Depression and apathy are distinct syndromes that commonly co-occur and are difficult to distinguish clinically. Antidepressants have efficacy in treatment of depression in CN elders(38), though data are mixed regarding their efficacy for depression in AD and in targeting apathy(39). Thus, the use of antidepressants to target depression or apathy (or other comorbid symptoms) in our study sample could explain this association between antidepressant use and apathy.
Our study has a number of strengths and limitations. We used a hypothesis driven approach to focus on the relationship of apathy to cerebral metabolism in five bilateral cortical regions. Thus, we may have missed other regions associated with apathy that an exploratory approach might have identified. We focused on the relationship of apathy to metabolism, but did not take into account vascular disease burden, which may also contribute to the pathophysiology of apathy. One of our objectives was to investigate neural correlates of apathy across the AD continuum. However, the ADNI cohort is enriched in MCI and AD dementia subjects with mild disease severity. Although we co-varied for diagnosis and correlates of disease severity in our models, given that apathy may be differentially mediated as pathophysiology progresses, our results may more closely reflect neural correlates of apathy at early disease stages, rather than mechanisms that underlie apathy during late stages of AD. Our study is limited in relying on the NPI-Q informant to measure apathy across diagnoses, and the overall apathy “signal” across subjects is low. Previous studies have indicated that self-report of cognitive symptoms and NPS may be more reliable than informant report in CN subjects(35, 40), thus, we may not be detecting the full symptom range across all subjects. Future studies incorporating more specialized apathy assessment instruments may provide greater sensitivity to detect this symptom across a range of subjects. Finally, referral bias within the ADNI cohort presents an additional major limitation of our study. Replication of our findings in population-based studies is needed to substantiate their validity.
In conclusion, we found that regional hypometabolism in the posterior cingulate was associated with higher apathy scores at baseline and that baseline hypometabolism of the suparmarginal gyrus positively predicted rate of increase of apathy over time across a cohort of older adults from the ADNI database that included CN, MCI and AD dementia subjects. These findings highlight the importance of posterior brain regions in association with apathy rather than frontal-subcortical structures more typically associated with apathy in later stages of AD. As such, they provide novel insight into the neurobiology of this symptom in CN older adults and in early stages of AD. Future investigation across different disease stages incorporating amyloid and tau PET imaging in relation to apathy is needed to more fully elucidate the neurobiology of this devastating symptom in AD and to develop more effective prevention and treatment interventions.
Supplementary Material
Acknowledgments
This study was supported by the Massachusetts Alzheimer’s Disease Research Center Neurodiscovery Grant, the Harvard Medical School Department of Psychiatry Dupont Warren Fellowship (JRG), Rogers Family Foundation (JRG), the Muriel Silberstein Alzheimer’s Disease Research Fund (NJD) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) “(National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012)(See Supplementary Data). The authors have received research salary support from Eisai, Inc. (GAM, NJD), Eli Lilly and Company (GAM, NJD), and Avid Radiopharmaceuticals (KAJ).
Footnotes
Presented as an abstract at the Alzheimer’s Association International Conference (AAIC) 2016, Toronto, Canada; July 22–28, 2016 (Gatchel JR, Donovan NJ, Locascio JJ, et al: Regional 18F-fluorodeoxyglucose hypometabolism is associated with greater apathy over time in early Alzheimer’s disease).
List of Supplemental Digital Content:
Supplemental Digital Content 1. doc (text)
Supplemental Digital Content 2. doc (table)
Supplemental Digital Content 3. doc (table)
Supplemental Digital Content 4. doc (table)
Supplemental Digital Content 5. doc (table)
References
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer's disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadsworth LP, Lorius N, Donovan NJ, et al. Neuropsychiatric symptoms and global functional impairment along the Alzheimer's continuum. Dement Geriatr Cogn Disord. 2012;34:96–111. doi: 10.1159/000342119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry. 2014;22:1642–1651. doi: 10.1016/j.jagp.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 6.Apostolova LG, Cummings JL. Neuropsychiatric manifestations in mild cognitive impairment: a systematic review of the literature. Dement Geriatr Cogn Disord. 2008;25:115–126. doi: 10.1159/000112509. [DOI] [PubMed] [Google Scholar]
- 7.Palmer K, Di Iulio F, Varsi AE, et al. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis. 2010;20:175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 8.Starkstein SE, Jorge R, Mizrahi R, et al. A prospective longitudinal study of apathy in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77:8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle PA, Malloy PF, Salloway S, et al. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:214–221. [PubMed] [Google Scholar]
- 10.Cummings JL. Frontal-subcortical circuits and human behavior. J Psychosom Res. 1998;44:627–628. doi: 10.1016/s0022-3999(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 11.Lanctot KL, Moosa S, Herrmann N, et al. A SPECT study of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:65–72. doi: 10.1159/000103633. [DOI] [PubMed] [Google Scholar]
- 12.Robert PH, Darcourt G, Koulibaly MP, et al. Lack of initiative and interest in Alzheimer's disease: a single photon emission computed tomography study. Eur J Neurol. 2006;13:729–735. doi: 10.1111/j.1468-1331.2006.01088.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall GA, Monserratt L, Harwood D, et al. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64:1015–1020. doi: 10.1001/archneur.64.7.1015. [DOI] [PubMed] [Google Scholar]
- 14.Apostolova LG, Akopyan GG, Partiali N, et al. Structural correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:91–97. doi: 10.1159/000103914. [DOI] [PubMed] [Google Scholar]
- 15.Tunnard C, Whitehead D, Hurt C, et al. Apathy and cortical atrophy in Alzheimer's disease. Int J Geriatr Psychiatry. 2011;26:741–748. doi: 10.1002/gps.2603. [DOI] [PubMed] [Google Scholar]
- 16.Ballarini T, Iaccarino L, Magnani G, et al. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer's disease. Hum Brain Mapp. 2016 doi: 10.1002/hbm.23305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donovan NJ, Wadsworth LP, Lorius N, et al. Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry. 2014;22:1168–1179. doi: 10.1016/j.jagp.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guercio BJ, Donovan NJ, Ward A, et al. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J Neuropsychiatry Clin Neurosci. 2015;27:e22–27. doi: 10.1176/appi.neuropsych.13060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delrieu J, Desmidt T, Camus V, et al. Apathy as a feature of prodromal Alzheimer's disease: an FDG-PET ADNI study. Int J Geriatr Psychiatry. 2015;30:470–477. doi: 10.1002/gps.4161. [DOI] [PubMed] [Google Scholar]
- 20.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 21.Marshall GA, Donovan NJ, Lorius N, et al. Apathy is associated with increased amyloid burden in mild cognitive impairment. J Neuropsychiatry Clin Neurosci. 2013;25:302–307. doi: 10.1176/appi.neuropsych.12060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krell-Roesch J, Ruider H, Lowe VJ, et al. FDG-PET and Neuropsychiatric Symptoms among Cognitively Normal Elderly Persons: The Mayo Clinic Study of Aging. J Alzheimers Dis. 2016;53:1609–1616. doi: 10.3233/JAD-160326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2012;8:S1–68. doi: 10.1016/j.jalz.2011.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheikh JI, Yesavage JA, Brooks JO, 3rd, et al. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr. 1991;3:23–28. doi: 10.1017/s1041610291000480. [DOI] [PubMed] [Google Scholar]
- 25.Marshall GA, Rentz DM, Frey MT, et al. Executive function and instrumental activities of daily living in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement. 2011;7:300–308. doi: 10.1016/j.jalz.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landau SM, Harvey D, Madison CM, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 28.Roy K, Pepin LC, Philiossaint M, et al. Regional fluorodeoxyglucose metabolism and instrumental activities of daily living across the Alzheimer's disease spectrum. J Alzheimers Dis. 2014;42:291–300. doi: 10.3233/JAD-131796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JW, Lee DY, Choo IH, et al. Microstructural alteration of the anterior cingulum is associated with apathy in Alzheimer disease. Am J Geriatr Psychiatry. 2011;19:644–653. doi: 10.1097/JGP.0b013e31820dcc73. [DOI] [PubMed] [Google Scholar]
- 30.McDonald CR, McEvoy LK, Gharapetian L, et al. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munro CE, Donovan NJ, Guercio BJ, et al. Neuropsychiatric Symptoms and Functional Connectivity in Mild Cognitive Impairment. J Alzheimers Dis. 2015;46:727–735. doi: 10.3233/JAD-150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn C, Lim HK, Won WY, et al. Apathy and white matter integrity in Alzheimer's disease: a whole brain analysis with tract-based spatial statistics. PLoS One. 2013;8:e53493. doi: 10.1371/journal.pone.0053493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caputo M, Monastero R, Mariani E, et al. Neuropsychiatric symptoms in 921 elderly subjects with dementia: a comparison between vascular and neurodegenerative types. Acta Psychiatr Scand. 2008;117:455–464. doi: 10.1111/j.1600-0447.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 34.Tschanz JT, Corcoran CD, Schwartz S, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the Cache County Dementia Progression study. Am J Geriatr Psychiatry. 2011;19:532–542. doi: 10.1097/JGP.0b013e3181faec23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guercio BJ, Donovan NJ, Munro CE, et al. The Apathy Evaluation Scale: A Comparison of Subject, Informant, and Clinician Report in Cognitively Normal Elderly and Mild Cognitive Impairment. J Alzheimers Dis. 2015;47:421–432. doi: 10.3233/JAD-150146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodaty H, Altendorf A, Withall A, et al. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. Int Psychogeriatr. 2010;22:426–436. doi: 10.1017/S1041610209991335. [DOI] [PubMed] [Google Scholar]
- 37.Geda YE, Roberts RO, Mielke MM, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–581. doi: 10.1176/appi.ajp.2014.13060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexopoulos GS, Katz IR, Reynolds CF, 3rd, et al. The expert consensus guideline series. Pharmacotherapy of depressive disorders in older patients. Postgrad Med. 2001:1–86. Spec No Pharmacotherapy. [PubMed] [Google Scholar]
- 39.Leong C. Antidepressants for depression in patients with dementia: a review of the literature. Consult Pharm. 2014;29:254–263. doi: 10.4140/TCP.n.2014.254. [DOI] [PubMed] [Google Scholar]
- 40.Caselli RJ, Chen K, Locke DE, et al. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement. 2014;10:93–98. doi: 10.1016/j.jalz.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.