Abstract
Improved tools have led to a burgeoning understanding of lung regeneration in mice, but it is not yet known how these insights may be relevant to acute lung injury in humans. We report in detail two cases of fulminant idiopathic acute lung injury requiring extracorporeal membrane oxygenation in previously healthy young adults with acute respiratory distress syndrome, one of whom required lung transplantation. Biopsy specimens showed diffuse alveolar injury with a striking paucity of alveolar epithelial regeneration, rare hyaline membranes, and diffuse contiguous airspace lining by macrophages. This novel constellation was termed diffuse alveolar injury with delayed epithelization. In addition, mirroring data from murine models of lung injury/regeneration, peribronchiolar basaloid pods (previously described as squamous metaplasia) and ciliated bronchiolarization were identified in these patients and in 39% of 57 historical cases with diffuse alveolar damage. These findings demonstrate a common and clinically relevant human disease correlate for murine models of severe acute lung injury. Evidence suggests that peribronchiolar basaloid pods and bronchiolarization are related spatially and temporally and likely represent overlapping sequential stages of the response to severe distal airway injury.
The acute respiratory distress syndrome (ARDS) is a clinically important form of noncardiogenic respiratory failure characterized by rapidly developing airspace opacities and variable degrees of hypoxemia. Biopsy is rarely performed in patients with ARDS, even though it may lead to changes in management and improve survival.1, 2, 3 The most common histologic finding is diffuse alveolar damage (DAD), present in approximately half of ARDS patients, and it can be understood as a common pathway resulting from a variety of insults. DAD is divided into well-defined but overlapping early exudative and late organizing/fibroproliferative phases. The hyaline membrane is the hallmark of early DAD and is composed of a fibrin-rich inflammatory exudate and necrotic (but still strongly cytokeratin-positive) alveolar epithelium. Pulmonary interstitia are variably thickened and edematous. Within several days of injury, there is a concurrent robust regenerative proliferation of low-cuboidal alveolar epithelial type 2 cells (AEC2s) that reepithelialize the alveoli. It is has long been appreciated that the transition between these two phases occurs as the lung is reepithelialized and hyaline membranes are either cleared by macrophage phagocytosis or incorporated into the interstitium, promoting fibrosis.4, 5
Throughout all stages of DAD, there is diffuse cytokeratin reactivity seen with staining in residual alveolar epithelium, hyaline membranes, and regenerative pneumocytes. We recently provided a brief report of a novel idiopathic form of acute lung injury termed diffuse alveolar injury with delayed epithelization (DAIDE) in two young patients with clinical ARDS and aggressive clinical courses. DAIDE is distinguished from DAD by the virtual absence of hyaline membranes and diffuse alveolar injury characterized by alveolar denudation and lining by activated macrophages. Together, these features result in diffuse cytokeratin-negative alveoli in DAIDE, a stark contrast to the broad positivity seen in DAD. On the basis of the lack of epithelium and aggressive clinical course in these patients, we hypothesized that DAIDE carries a poor short-term prognosis.6
Although the stages of human DAD have been defined morphologically for decades, the mechanistic basis of repair after acute lung injury remains poorly understood. An expanding array of experimental tools has allowed examination of similar processes in mouse models of acute lung injury. Several such studies have characterized peribronchiolar nests of p63+, keratin 5+ proliferating injury-induced cells, termed pods, that originate from rare epithelial progenitors.7, 8, 9 Recent long-term lineage tracing demonstrates that pod cells are not alveolar progenitors and instead predominantly go on to form cystic spaces lined by ciliated cells with expression of some mature airway markers; however, these findings did not have a correlate in human disease.10, 11 We reported that human peribronchiolar squamous metaplasia, long appreciated in the pathology literature in a subset of DAD cases but of unknown function, is the human correlate of the well-characterized and extensively debated murine pods.6 We termed the clusters peribronchiolar basaloid pods (PBPs) to distinguish them from the distinct phenomenon of large airway squamous metaplasia and to call attention to the findings in mice. Herein, we provide more detailed characterization of the two cases of DAIDE and of 57 control patients with DAD, including details of the PBPs and bronchiolarization. Evidence suggests that bronchiolarization (lambertosis) is the human correlate of the ciliated cystic spaces generated by the murine pods and that pod-dependent bronchiolarization is a conserved regenerative response to acute lung injury.
Materials and Methods
All surgical lung wedge biopsies and autopsies with a diagnosis of DAD at the Massachusetts General Hospital (MGH; Boston, MA) from 2011 to 2016 were reviewed as control cases. Cases with a background of cancer or extensively fibrotic lung disease were excluded. Samples used as normal control slides were taken from tumor-free regions in surgical wedge resections for cancer in human never smokers. Ordinal regression and associated P values were computed using R statistical software version 3.3.2 (The R Foundation for Statistical Computing, Vienna, Austria) with the MASS version 7.3-45 (University of Oxford, Oxford, UK) and ordinal version 2015.6-28 (Technical University of Denmark, Lyngby, Denmark) packages. All studies, including data sharing, were conducted between institutions in accordance with institutional review board approval.
Histology was performed using routine clinical techniques, and slides were stained with hematoxylin and eosin. Immunohistochemistry was performed on a Leica BOND automated system (Leica Biosystems, Nussloch, Germany) with the standard chromogen 3,3′-diaminobenzidine tetrahydrochloride hydrate, using antigen retrieval solution ER1 (citrate buffer with surfactant, pH 6.0) or ER2 (EDTA buffer with surfactant, pH 9.0). Antibody was diluted as follows and incubated at room temperature: α-AE1.3/Cam5.2 [pan-cytokeratin, ER1 for 10 minutes, Leica Biosystems AE1.3, 1:1000 and BD Cam 5.2, 1:100 (BD Biosciences, San Jose, CA)]; α-TTF1 (ER2 for 30 minutes; no dilution; Cell Marque, Rocklin, CA); α-CD163 (ER1 for 20 minutes; 1:100; Leica Biosystems); α–Ki-67 (ER2 for 20 minutes; no dilution; Leica Biosystems); α-ΔNp63 (p40, ER1 for 30 minutes; 1:100; Biocare Medical, Pacheco, CA); α-D2-40 (podoplanin, ER2 for 30 minutes; no dilution; Cell Marque), and α-Muc5ac (ER2 for 2.5 minutes; no dilution; Cell Marque). Keratin 5/6 immunostains were quantified manually from three control patients and both cases.
For immunofluorescence, paraffin sections were processed in Histoclear-II solution (National Diagnostics, Atlanta, GA) and rehydrated in steps from 100% ethanol to 70% ethanol. Antigen retrieval was performed in citrate (pH 6.0) or EDTA (pH 8.0) solutions, as needed, for 10 minutes in a pressure cooker. The sections were further rehydrated with 50% ethanol and washing buffer (phosphate-buffered saline with 0.1% Tween-20) and blocked with 10% serum and 0.1% Triton- X solution for 1 hour at room temperature. Autofluorescence was quenched with 3% hydrogen peroxide solution in methanol for 15 minutes at room temperature. Primary antibodies against Krt5 (1:500; number 905504; Biolegend, San Diego, CA) and pro-SPC (1:200; ab90716; Abcam, Cambridge, MA) were applied in washing buffer at 4°C overnight. After washing, the sections were treated with 3% hydrogen peroxide solution in methanol for 15 minutes at room temperature. Fluorophore-conjugated secondary antibodies (Alexa Fluor series 594 or 647 nm; Life Technologies, Carlsbad, CA) were applied in washing buffer at 1:500 at room temperature for 1 hour. Slides were washed and mounted with DAPI mounting solution and then imaged using an Olympus (Tokyo, Japan) FluoView FV10i confocal microscope. Images were processed and analyzed using ImageJ software version 1.51g (NIH, Bethesda, MD; http://imagej.nih.gov/ij).
Tissue for electron microscopy was fixed overnight at 4°C in modified Karnovsky's KII Solution (2.5% glutaraldehyde, 2.0% paraformaldehyde, 0.025% calcium chloride, in a 0.1 mol/L sodium cacodylate buffer, pH 7.4) and then processed in an Electron Microscopy Sciences Lynx automatic processor (EMS, Hatfield, PA) with post-fixation in osmium tetroxide, staining in uranyl acetate, dehydration in graded ethanol solutions, and infiltration and embedding with propylene oxide and EMS Embed 812 resin. Thin sections were cut with an LKB 8801 ultramicrotome and diamond knife, stained with Sato's lead, and examined in an FEI Morgagni transmission electron microscope (Thermo Fisher Scientific, Hillsboro, OR).
Results
We report in detail fulminant idiopathic acute lung injury requiring extracorporeal membrane oxygenation (ECMO) in two previously healthy young adults, a 27-year-old man and 35-year-old woman. Both presented with fevers and rapidly progressive hypoxemia, were empirically treated for presumptive community-acquired pneumonia, and met the Berlin criteria for severe ARDS.12 Extensive autoimmune and infectious studies did not reveal an underlying etiology. The young man underwent bilateral lung transplantation after 101 days on ECMO and has recovered and returned to work. The young woman was weaned from ECMO support after 85 days and was extubated 47 days later; she has slowly recovered, albeit with pulmonary fibrosis (Figure 1) and a restrictive ventilatory defect that persists 13 months after extubation.13 Additional clinical information is provided in Additional Clinical History.
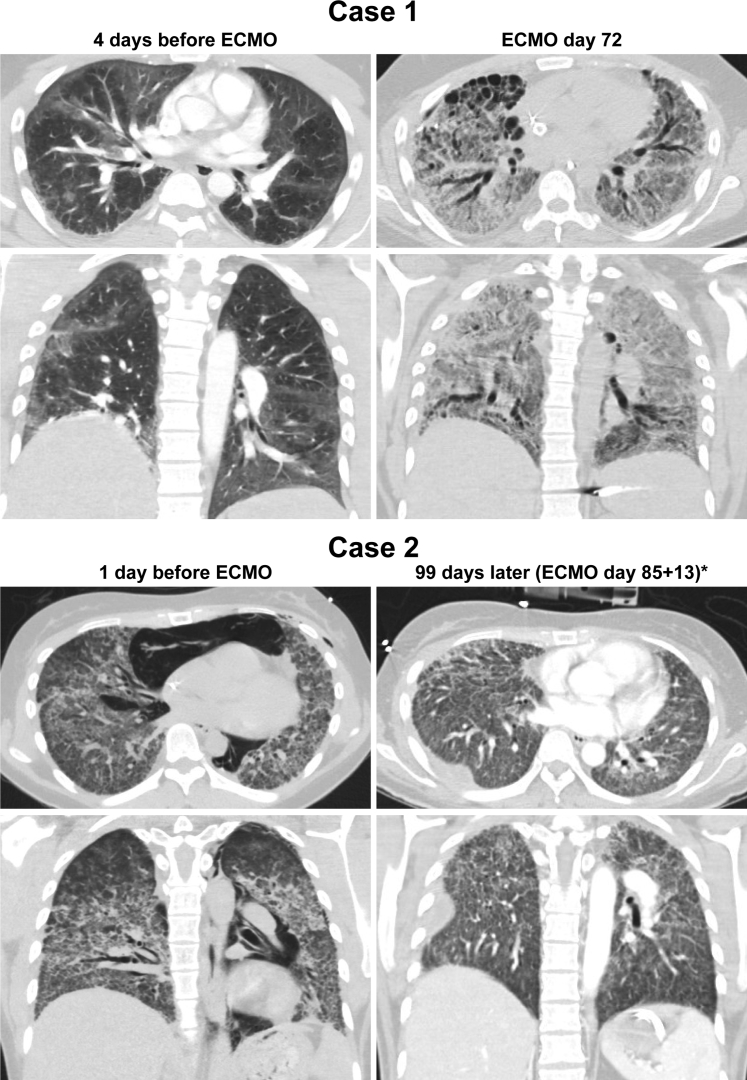
Figure 1.
Computed tomographic scans. The lungs in case 1 before extracorporeal membrane oxygenation (ECMO) show patchy bilateral ground-glass opacities and plate-like atelectasis. At ECMO day 72 and before orthotopic lung transplantation, there is diffuse interstitial fibrosis, traction bronchiectasis, and subpleural honeycomb cystic change. The lungs in case 2 before ECMO demonstrate pneumomediastinum and bilateral interstitial opacities with centrilobular accentuation. Ninety-eight days after ECMO initiation (asterisk, after 85 days of ECMO and 13 days decannulated), there is bilateral interstitial fibrosis and a loculated right effusion.
Wedge biopsy specimens were obtained 4 and 2 days after intubation, respectively, because of the patients' rapid clinical deterioration. In case 1, this was immediately before ECMO cannulation and 3 days after meeting ARDS criteria. In case 2, this was 1 day after ECMO cannulation and 1 day after meeting ARDS criteria. The biopsy specimens in both patients showed diffuse alveolar injury with rare hyaline membranes and diffuse alveolar denudation. We define alveolar denudation as a near-complete lack of epithelium at gas exchange surfaces, with absence of both type 1 alveolar epithelial cells and regenerative AEC2s, and their replacement by macrophages demonstrating extensive cytoplasmic spreading. Interstitia in both cases were mildly edematous and mildly fibrotic. These findings were in stark contrast to control cases of DAD (Figure 2), which demonstrated diffuse cytokeratin reactivity in the alveolar epithelium and hyaline membranes, confirmed by ultrastructural analysis (Supplemental Figure S1).
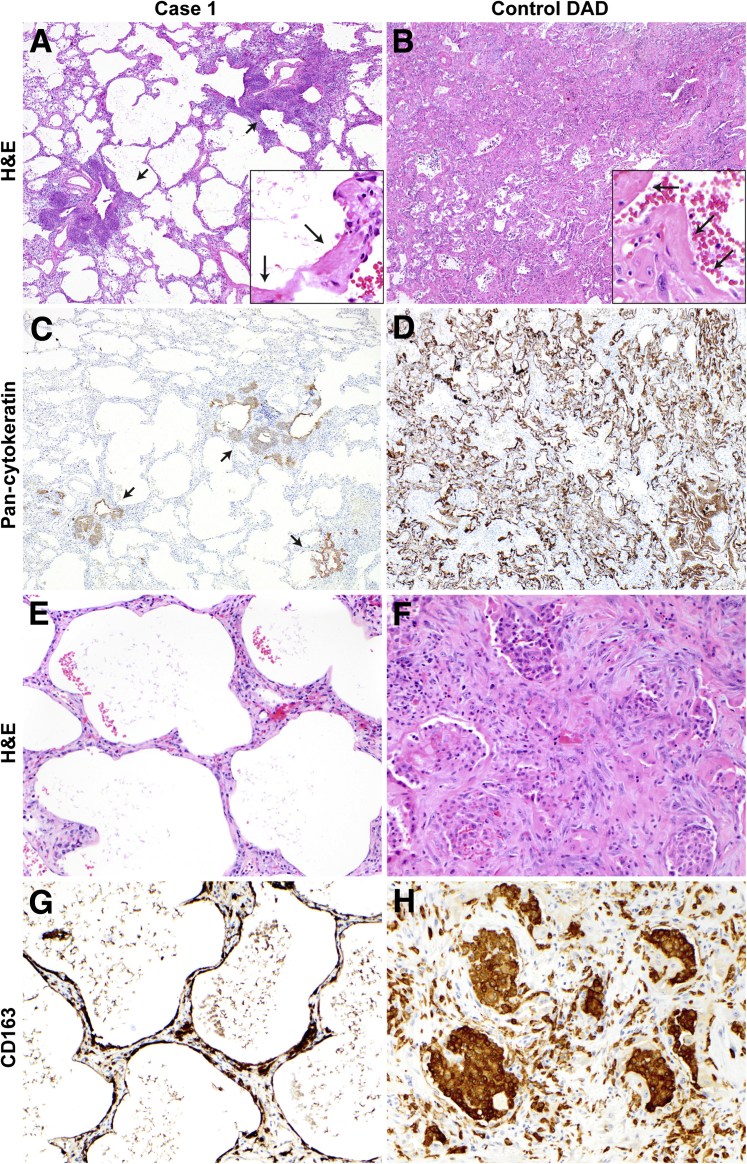
Figure 2.
Alveolar epithelialization in diffuse alveolar injury with delayed epithelization case 1 versus control diffuse alveolar damage (DAD), matched in timing relative to the onset of clinical acute respiratory distress syndrome. A: Lung sections stained and examined at low magnification demonstrate diffuse alveolar injury and peribronchiolar basaloid pods (short arrows). Hyaline membranes are rare (long arrows, inset), and the lung interstitium is mildly thickened. B: Control case of DAD in the proliferative phase shows diffuse hyaline membranes (arrows, inset) and thickened lung interstitium. C: The absence of TTF1+/cytokeratin+ alveolar epithelium in case 1 is highlighted by the lack of cytokeratin cellular immunostaining; peribronchiolar basaloid pods are cytokeratin positive (arrows). D: In contrast, there is diffuse cytokeratin immunostaining of alveolar lining cells and hyaline membranes in the control. E: Denuded alveoli in case 1 show a lack of alveolar epithelium, with no hyaline membranes in this region. F and H: In control DAD, there are abundant CD163+ intra-alveolar and interstitial macrophages. G: CD163 immunohistochemistry demonstrates macrophages contiguously lining alveoli and in the interstitium. Original magnification: ×40 (A–D); ×200 (E–H); ×400 (insets). H&E, hematoxylin and eosin.
In both cases, the diffuse denudation was accompanied by exuberant peribronchiolar proliferations of atypical basaloid p63+ keratin 5/6+ epithelial cells (Figure 3, A–C), a pattern previously called squamous metaplasia in the pathology literature.14, 15 These proliferating nests of cells were termed PBPs to distinguish them from the distinct phenomenon of large airway squamous metaplasia and to call attention to analogous findings in mice (Discussion). PBPs were organized as stratified polarized epithelia with a consistent gradient of cellular differentiation (by p40/p63 staining) and with a higher proliferation rate (by Ki-67 staining) in the peripheral/basal compartment than the central/apical one (Figure 3, A–C, and Supplemental Figure S2). Clusters abutted >90% of bronchioles, were confined to the bronchiolar interstitium, and were sometimes contiguous with ciliated bronchiolar epithelium (Figure 3A). Serial sectioning demonstrated that many PBPs appeared to centrally canalize, opening to ciliated lumens, some of which were contiguous with larger airspaces (Supplemental Figure S3). PBPs were stained for evidence of expression of differentiation markers, and no expression of SPC (a marker of AEC2s) (Supplemental Figure S4) was noted. Heterogeneous cytoplasmic staining for podoplanin (D2-40) was found to be most prominent in the pod periphery (Supplemental Figure S5), similar to staining seen in control airway basal cells (Supplemental Figure S6). Rare pod cells were positive for Muc5ac, typically centrally/apically positioned and abutting areas of canalization (Supplemental Figure S5). In contrast, the large airway epithelium was unremarkable and without airway squamous metaplasia both at the time of biopsies and in the explanted lungs in case 1 (Supplemental Figure S7), suggesting injury was confined to the distal lung.
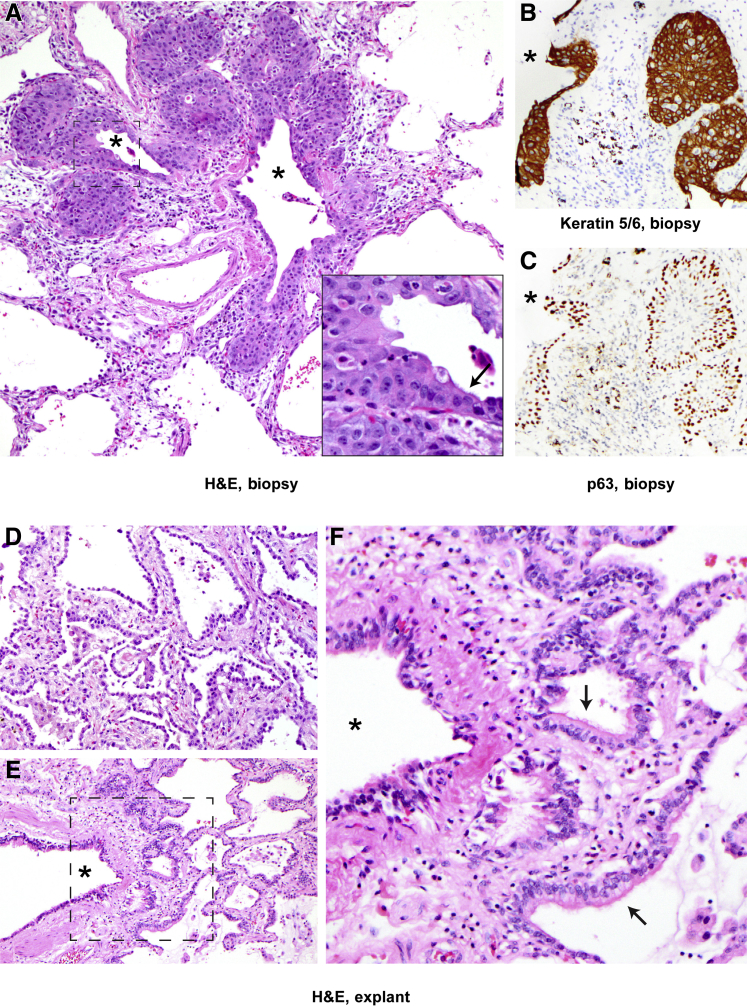
Figure 3.
Peribronchiolar basaloid pods (previously referred to as squamous metaplasia in the pathology literature5, 14, 15) and bronchiolarization in biopsy and explants, case 1. A: Proliferating basaloid/squamoid cells are adjacent to a terminal bronchiole and are focally contiguous with ciliated bronchiolar epithelium (arrow indicates ciliated bronchiolar cells; asterisks, bronchiolar lumens; inset, higher magnification of boxed area). B and C: Pod cells are immunopositive for TTF1 (not shown) and keratin 5/6 (B), and basally positive for p63 (C). Asterisks indicate bronchiolar lumens. D: Explanted lungs in case 1 after 101 days on extracorporeal membrane oxygenation demonstrate robust alveolar reepithelialization and fibrosis. E and F: There is also prominent peribronchiolar metaplasia (bronchiolarization), with ciliated bronchiolar-type epithelium in peribronchiolar small air spaces lacking smooth muscle, as seen in F, which is a higher magnification of the boxed area in E. E and F: Arrows indicate ciliated bronchiolar epithelium; asterisk, bronchiolar lumens. Original magnification, ×200 (A–F); ×400 (inset). H&E, hematoxylin and eosin.
The explanted lungs in case 1 (101 days on ECMO) showed diffuse interstitial fibrosis; alveolar denudation was replaced by widespread active reepithelialization by hyperplastic cuboidal AEC2s (Figure 3D). PBPs were no longer present, but there was prominent bronchiolarization (peribronchiolar bronchiolar metaplasia with ciliated bronchiolar-type epithelium lacking surrounding smooth muscle bundles, a pattern previously described as lambertosis5) (Figure 3, E and F). Hemosiderin deposition and foci of interstitial emphysema were present, likely related to prolonged ECMO support.16 Sections were again examined for evidence of differentiation marker expression, and it was noted that a subset of nonciliated epithelial cells in bronchiolarized regions expressed the airway mucin gene MUC5AC and that cells in the basolateral positions exhibited heterogeneous D2-40 expression comparable to that seen in PBPs and native airway basal cells. Even in regions with adjacent AEC2-rich reepithelialization, SPC-positive cells within bronchiolarized epithelium were never encountered (Supplemental Figures S4 and S8). Krt5/6 staining patterns between normal airways, PBPs, and regions of bronchiolarization were also compared (Supplemental Figure S9). As expected, basal cells of both native airways and bronchiolarized regions were positive for Krt5/6. Pod cells were uniformly and strongly Krt5/6+ (block positive). In native uninjured airways, only a subset (4% to 27%; mean, 11%) of suprabasal cells retained Krt5/6 staining, with varying intensity. Regions of bronchiolarization had a staining pattern intermediate between pods and native airways, with some regions uniformly Krt5/6+ (block positive, identical to pods) and others with Krt5/6 marking subsets of suprabasal cells in a graduated manner (17% to 100% of suprabasal cells positive; mean, 78%). p63 uniformly marks basal cells in all three groups, additionally marking central/suprabasal cells in pods in a graduated manner (Figures 3C and 4E).
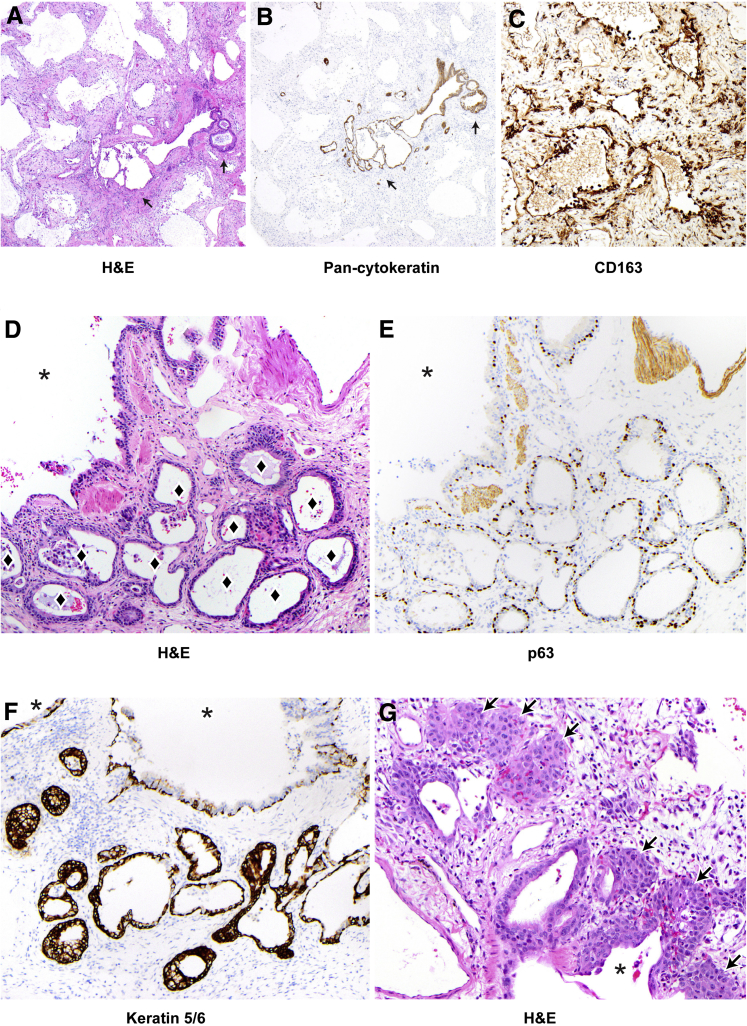
Figure 4.
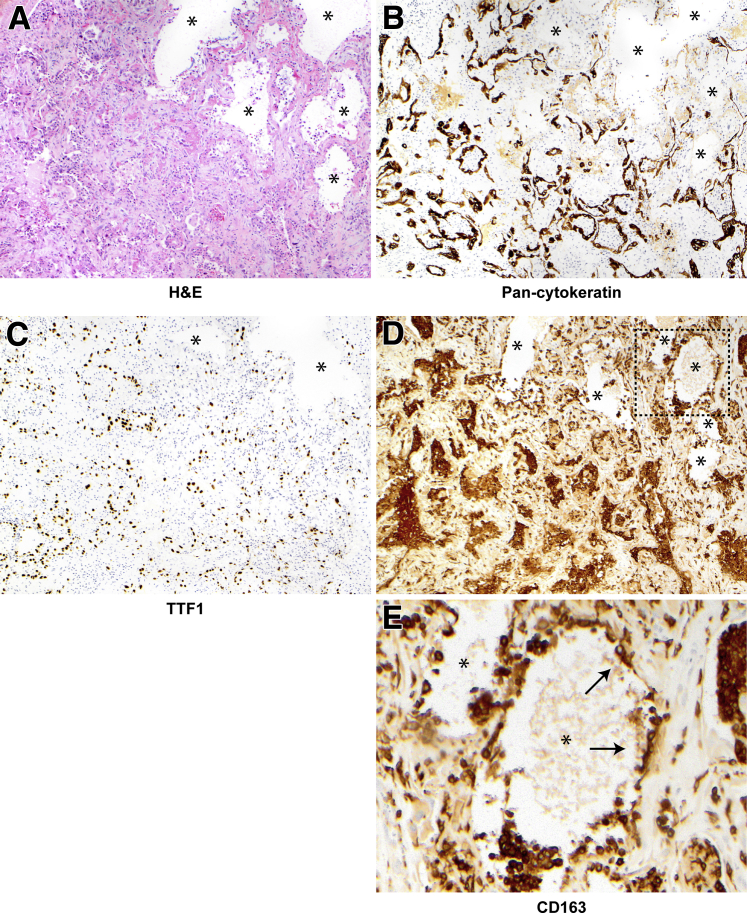
Alveolar epithelization, peribronchiolar basaloid pods (PBPs), and bronchiolarization in case 2. A: Lung sections stained and examined at low power show diffuse alveolar injury with diffuse alveolar denudation and peribronchiolar proliferation (arrows). Hyaline membranes are rare (not shown), and the interstitium is thickened. B: Pan-cytokeratin staining demonstrates diffuse denudation with rare residual epithelium in denuded alveoli and highlights the peribronchiolar proliferation (arrows). C: CD163 staining shows alveoli are lined by macrophages, many of which demonstrate cytoplasmic spreading. D–G: Peribronchiolar metaplasia at higher power. Diamonds indicate lumens in areas of bronchiolarization; asterisks, bronchiolar lumens. E and F: Bronchiolarization consists of a bilayered epithelium with p63 (E) staining basal cells and keratin 5/6 (F) showing diffuse positivity (see also Supplemental Figures S8 and S9). G: PBPs are also present in some foci (arrows). Original magnifications: ×40 (A and B); ×200 (C–G). H&E, hematoxylin and eosin.
The biopsy specimens in case 2, like those in case 1, showed diffuse alveolar denudation, with rare residual keratin-positive alveoli and macrophages instead lining alveolar walls (Figure 4, A–C). As in case 1, there was prominent peribronchiolar p63+, keratin 5/6+ epithelial proliferation, characterized predominantly by ciliated bronchiolarization (Figure 4, D–F), but also containing foci of confluent, squamoid PBPs (Figure 4G).
As controls, seven cases of surgical wedge biopsies and 50 autopsy cases demonstrating DAD were identified; the patients are summarized in Supplemental Tables S1 and S2. In all control cases, alveolar surfaces were diffusely lined by regenerating AEC2s, with conspicuous hyaline membranes and rare residual type 1 alveolar epithelial cells. CD163 stains highlighted predominantly intra-alveolar macrophages and interstitial macrophages phagocytosing hyaline membranes (Figures 2 and 5). Rare foci of alveolar denudation could be identified in less than half of control cases (27/57) (Figure 5); however, these comprised no more than 0.1% to 1% of any specimen, markedly distinct from the extensive denudation seen in >90% of the biopsy area in the cases.
Figure 5.
Example focus of denuded alveoli in control diffuse alveolar damage. A–E: Hematoxylin and eosin (H&E) staining shows a cluster of alveoli lacking epithelium (asterisks), with absent staining for pan-cytokeratin (B) and TTF1 (C), which highlight residual and regenerating epithelial components and hyaline membranes in the remainder of the field. D and E: In the denuded alveoli, CD163+ macrophages instead line the alveolar wall (boxed area in D is shown at higher magnification in E), with areas of cytoplasmic spreading (some highlighted by arrows). These areas comprised at most 1% of any control biopsy, but confluent diffuse denudation characterized >95% of the cases. Original magnification: ×100 (A–D); ×400 (E).
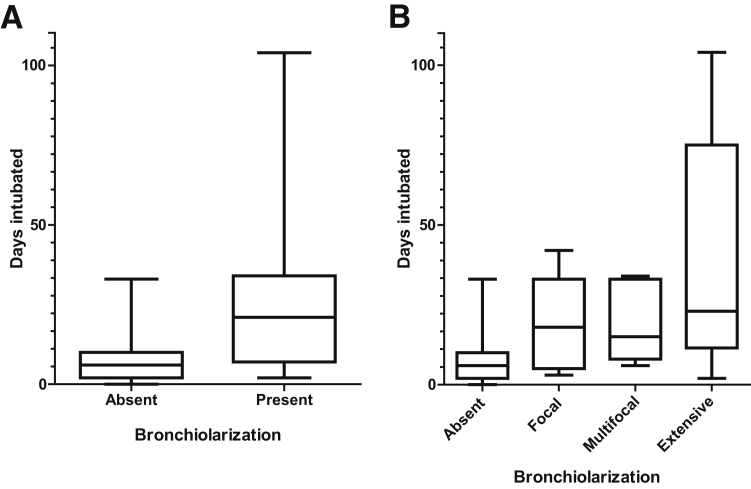
Five control surgical cases and 10 autopsies demonstrated PBPs; in two surgical cases and two autopsies, this was extensive (Figure 6). There was no correlation between the abundance of PBPs and the degree of alveolar denudation, and no apparent spatial relationship between these elements was seen. Bronchiolarization was present in one surgical control case and 13 autopsies, occurring with PBPs in the surgical case and in six of the autopsy cases. In total, either PBPs or bronchiolarization were identified in 22 of 57 control cases (39%). Bronchiolarization, particularly extensive bronchiolarization, tended to occur in late DAD. Supporting this, using intubation time as a surrogate for duration of illness before biopsy or autopsy, it was found that the degree of bronchiolarization was highly statistically correlated with illness duration (P = 0.00053, ordinal regression model) (Figure 7). There was no statistically significant association between presence of PBPs and intubation time, and neither PBP abundance nor degree of bronchiolarization had any association with cause of death.
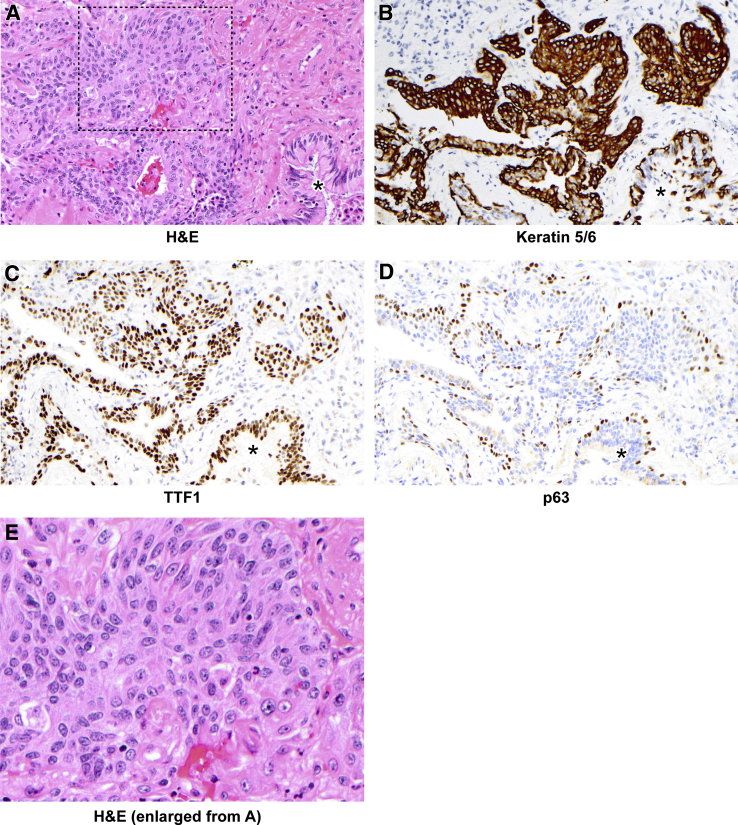
Figure 6.
Peribronchiolar basaloid pods (squamous metaplasia) in control diffuse alveolar damage. A: Hematoxylin and eosin (H&E) staining demonstrates an exuberant peribronchiolar basaloid/squamoid proliferation. B–D: These cells are positive for keratin 5/6 (B) and TTF1 (C), and basally positive for p63 (D). A mixed inflammatory infiltrate is also present. A–D: Asterisks indicate bronchiole. E: Higher-power view of boxed area from A. This case also demonstrates robust hyaline membranes (Figure 1). Original magnification: ×200 (A–D), ×400 (E).
Figure 7.
Bronchiolarization increases with longer intubation times. See Supplemental Table S2 for original data. A: Degree of bronchiolarization represented as binary data (P = 0.0016, binomial logistic regression). B: Degree of bronchiolarization represented as ordinal data (P = 0.00053, ordinal model). n = 44 absent (A and B); n = 16 present (A); n = 6 focal (B); n = 5 multifocal and extensive (B).
Additional Clinical History
Case 1
The index patient was a 28-year-old man who initially presented to another hospital with several days of dry cough, chills, and fever to 39°C. He was admitted and treated empirically with broad-spectrum antibiotics, including trimethoprim-sulfamethoxazole, and with corticosteroids, but hypoxemic respiratory failure progressed and he required intubation. Computed tomographic imaging revealed bilateral ground glass opacities (Figure 1). An extensive initial laboratory evaluation was unrevealing for infection or malignancy, and he was transferred to MGH on hospital day 6. Medical history was notable for treatment of latent tuberculosis with isoniazid 10 years before presentation, recent contact with coworkers experiencing upper respiratory tract infections, and recurrent rectal abscesses, drained and treated with trimethoprim-sulfamethoxazole 2 weeks before symptom onset. There were no rashes or evidence of epidermal exfoliation. There had been no international travel in the year before admission. He was a never smoker without recognized inhalational exposures.
Imaging and physiological findings met Berlin criteria for severe ARDS, with <7 days development of bilateral airspace opacities, consistent with pulmonary edema and accompanied by severe hypoxemia, with PaO2/FiO2 ratio <100 on mechanical ventilation with positive end-expiratory pressure exceeding 5 cm H2O. Extensive infectious and autoimmune studies were unrevealing. Hypoxemia worsened despite maximal medical therapy, including low tidal volume ventilation (<6 mL/kg), paralysis, recruitment maneuvers, positive end-expiratory pressure titration, pulmonary vasodilators, and prone positioning. Lung wedge biopsy specimens were obtained at a bedside thoracotomy, and the patient was placed on venovenous extracorporeal membrane oxygenation on MGH admission day 2, 4 days after both intubation and meeting Berlin severe ARDS criteria. The hospital course was subsequently complicated by persistent vasopressor requirement, heparin-induced thrombocytopenia, recurrent hemopneumothorax, and progression of chest computed tomographic findings to diffuse fibrotic lung disease with traction bronchiectasis, severe honeycombing, and diffuse cystic changes (Figure 1). ECMO support could not be weaned, and bilateral orthotopic lung transplantation was performed after 101 days on ECMO. Postoperatively, he was liberated from ECMO and mechanical ventilation and has returned to work.
Case 2
The second patient was a 37-year-old woman who presented with 1 week of dry cough, chills, headache, nausea, vomiting, and fever to 39°C. She was admitted to another hospital for multifocal pneumonia and slowly improved; her therapy included glucocorticoids and broad-spectrum antibiotics, including trimethoprim-sulfamethoxazole. After 3 weeks, she developed a new and rapidly progressive oxygen requirement and was transferred to MGH. At the time of transfer, she required high-flow nasal cannula oxygenation and had developed a pneumomediastinum. The medical history was notable only for childhood asthma. A daughter had a recent upper respiratory tract infection. Social history was notable for birth in Japan and personal travel to Cuba in the previous month. She was a never smoker without recognized inhalational exposures.
Chest computed tomography at MGH initially showed bilateral ground glass opacities, but subsequently showed diffuse centrilobular nodules predominating in the bilateral lower lobes (Figure 1). Extensive infectious and autoimmune studies were unrevealing. She was intubated, and hypoxemia worsened despite maximal medical therapy, including low tidal volume ventilation (<6 mL/kg), paralysis, recruitment maneuvers, positive end-expiratory pressure titration, pulmonary vasodilators, and prone positioning. She met Berlin criteria for severe ARDS, as described in Results. She was subsequently cannulated for venovenous ECMO on MGH hospital day 2. The patient underwent a video-assisted thoracoscopic surgical left lung wedge biopsy on ECMO day 1, 2 days after intubation and meeting Berlin criteria for severe ARDS. Her hospital course was complicated by loculated hemopneumothorax, intercostal arterial bleeding, and two episodes of ventilator-associated pneumonia. She required ECMO support for 85 days, but had slow recovery and was successfully decannulated. She was discharged after a 5-month hospitalization and was recovering well with room air oxygen saturations >94% at 4-month follow-up. Computed tomographic findings (Figure 1) were notable for resolving diffuse ground glass opacities and diffuse interstitial fibrosis with patchy honeycomb change.
Discussion
We have termed a novel pathologic pattern of acute lung injury diffuse alveolar injury with delayed epithelization (DAIDE), a pattern that we hypothesize may portend a poor short-term prognosis given the critical requirement for gas exchange across the alveolar membrane and the aggressive clinical course in these patients despite maximal medical therapy. Of the findings described, we believe that the combination of a paucity of hyaline membranes and diffuse alveolar denudation with lining by activated macrophages is sufficient to describe the pattern; PBPs are not specific to DAIDE and likely reflect the severity and distribution of injury (discussed later in this section). Key limitations of this study include predominantly peripheral sampling in one to four wedge biopsy specimens in just two cases; additional cases and, if possible, more extensive sampling will be critical to refine diagnostic criteria and to determine prognostic and clinical implications of these findings.
It is known from morphologic and murine lineage tracing studies that AEC2s typically proliferate after alveolar injury and can differentiate into type 1 alveolar epithelial cells.17, 18 In combination, the clinical courses of these two patients and the rare hyaline membranes and well-established PBPs seen on biopsy would be suggestive of a late exudative to early organizing phase of DAD; however, DAD at this stage also demonstrates prominent AEC2s. In contrast, the diffuse and near complete absence of alveolar epithelium in these two patients at a time when AEC2s would have normally been prominent is markedly distinct from any control DAD case examined and suggests that alveolar reepithelialization was profoundly delayed or, formally, that alveolar damage was unremitting because of ongoing injury. The cause of this injury and the source of the eventual reepithelialization are unclear. It is possible that severe injury obliterated nearly all of the preexisting AEC2s or that there was some persistent toxicity to AEC2s or alternate AEC2 precursors. The extensive negative infectious workup argues against, but does not rule out, an infectious trigger for this process. Indeed, both patients presented with a viral-like illness, raising the strong possibility of an abnormally severe response to an unidentified known or novel respiratory virus. The inability to identify a specific viral agent despite PCR and next-generation sequencing attempts is difficult to interpret in light of wholesale epithelial destruction, which would likely substantially reduce assay sensitivity. Likewise, we were unable to perform gene expression analysis by RNA sequencing of lung epithelium because there were insufficient epithelial cells to recover for laser capture microdissection.
In the absence of AEC2 proliferation, macrophages normally involved in hyaline membrane resolution and alveolar remodeling were instead tightly apposed to alveolar surfaces. Macrophages are known to adhere to denuded surfaces during early wound healing; these cases suggest macrophages can line denuded alveolar surfaces and persist until reepithelialization. Although macrophages appear to provide a provisional barrier function, it is uncertain to what extent they allow gas exchange.
The prominent peribronchiolar epithelial metaplasia in the cases was striking, but it did not exceed that seen in a subset of control cases. Although the finding of peribronchiolar squamous metaplasia accompanying DAD has been recognized for decades, to our knowledge, no specific function has been attributed to these cells in human pathology.15, 19, 20 Indeed, they are largely discussed because they may be mistaken for malignant or virally infected cells given their sometimes marked cytologic atypia. These foci reportedly disappear over the course of weeks, with complete resolution at autopsy in two patients 13 and 15 days after biopsy.15 These foci were termed PBPs. The PBPs seen in case 1 were absent in the explanted lungs, which instead showed bronchiolarization in an identical anatomical distribution. Indeed, bronchiolarization generally occurred more frequently in later DAD, exemplified by case 2's later biopsy, demonstrating both patterns again in the same distribution, and in multiple autopsy cases. In several cases, pods demonstrate direct contiguity with ciliated bronchiolar epithelium (Figure 3A and Supplemental Figures S2 and S3). PBPs stain as expected for airway basal cells, with rare cells staining for Muc5ac showing limited evidence of mature airway differentiation. Conversely, regions of bronchiolarization exhibited histologic (cilia) and marker (mucin expression) evidence of airway differentiation with associated loss of basal cell markers. Taken together, these findings argue that peribronchiolar basaloid pods and bronchiolarization are temporally, spatially, and developmentally related. They likely represent overlapping, but sequential, stages of postinjury proliferation and differentiation after distal airway injury, mirroring findings from murine studies (described in the next paragraph).10, 11
We note the similarity between human peribronchiolar basaloid pods and a reactive murine p63+ cell population referred to as Krt5 pods9, 10, 21, 22, 23, 24 that proliferate in a peribronchiolar distribution soon after infection with influenza virus or, with variable frequency, after bleomycin-mediated injury.21 These clusters appear to originate from rare airway epithelial progenitor populations based on stringent indelible lineage tracing.10, 11, 24 To our knowledge, no human correlate of these has previously been described. The roles of these pods after injury remain uncertain; they may subtend a barrier function, perform some function related to immune modulation, provide a source for regenerating distal airways and/or alveoli, or represent abortive or maladaptive regenerative structures. The most recent murine findings suggest minimal alveolar contribution. Instead, they demonstrate that pods go on to form ciliated lumens with expression of some mature airway cell differentiation markers,11 a phenotype essentially identical to the bronchiolarization seen late in the course of the reported cases and historical controls. Together, the data suggest that these pods arise from severely injured distal airway and go on to form bronchiolarization in both mice and humans. More important, although this pathway may provide a barrier early in injury, the end result of bronchiolarization may well be a scar-like response rather than true regeneration.11
The present findings suggest a previously undescribed form of alveolar injury and repair. The observed failure of AEC2-mediated reepithelialization (and associated macrophage lining) may represent the extreme of a phenomenon occurring with spatiotemporal heterogeneity among patients with DAD when AEC2s are, for whatever reason, unable to efficiently repopulate injured epithelium. Which stem cells are recruited in a particular injury setting is likely a complex plastic decision dependent on the nature, severity, time course, and distribution of a particular insult.9, 11 It is likely, therefore, that these two patients with DAIDE and the subset of control patients with PBPs sustained similarly severe distal airway injuries. Pod-dependent bronchiolarization appears to represent a common and evolutionarily conserved response to severe distal lung injury, although it remains unclear if this process is maladaptive or, if not, how it might contribute to productive lung regeneration. We suggest that the parallel analyses of biopsy tissues in patients experiencing ARDS and mechanistic studies in model organisms can, together, further illuminate the cellular and tissue-level responses to mammalian lung injury.
Acknowledgments
We thank our patients and their families who participated and Dr. Teri Franks for locating prior published cases of diffuse alveolar damage with extensive squamous metaplasia.
M.S.T., R.R.C., L.C.M., B.T.T., J.R., and R.L.K. designed the study, interpreted the results, wrote the manuscript, and prepared figures; W.J.O. and C.F.F. cared for the patients, interpreted the results, and critically reviewed the manuscript; P.R.T. interpreted results and critically reviewed the manuscript; M.K.S. performed and interpreted electron microscopy; A.W. performed and interpreted immunofluorescence microscopy and designed and interpreted immunohistochemical experiments; all authors approved the final version of the manuscript.
Footnotes
Supported in part by NIH grants R01HL116756 (J.R.), R01HL118185 (J.R.), and K99HL127181 (P.R.T). J.R. is a Howard Hughes Medical Institute Faculty Scholar, a New York Stem Cell Foundation Robertson Investigator, a Maroni Research Scholar at Massachusetts General Hospital, and a member of the Ludwig Cancer Institute at Harvard Medical School.
M.S.T. and R.R.C. contributed equally to this work.
Disclosures: None declared.
Current address of P.R.T., Department of Cell Biology, Duke University Medical Center, Durham, NC.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.01.021.
Supplemental Data
Electron microscopy confirms that the lining cells in case 1 are activated macrophages. Arrowheads indicate basement membrane; asterisk, alveolar space. Inset: Activated macrophage cytoplasm with pinocytotic vesicles (arrows) and abundant rough endoplasmic reticulum and mitochondria. Original magnification: ×3500 (main image); ×18,000 (inset). E, endothelial cell nucleus; M, macrophage nucleus.
Additional characterization of peribronchiolar basaloid pods. A: Pod cells are TTF1 positive, and surrounding denuded alveoli are TTF negative. B and C: High-power hematoxylin and eosin (H&E) staining of pod cells shows atypical basaloid/squamoid cells with mitotic figures (arrow) and a high Ki-67 proliferation rate at pod periphery. Asterisks indicates lumens. Original magnification: ×200 (A); ×400 (B and C).
Serial 5u sections of peribronchiolar basaloid pods (PBPs), case 1. A–D: Serial sections show PBPs in contiguity with an injured bronchiole (asterisks). Arrows indicate new lumens forming or disappearing throughout levels; arrowheads, pods that appear separated from bronchioles on individual levels but that are contiguous on others. E: p63 stain highlights pod and airway nuclei. Original magnification, ×100 (A–E).
Immunofluorescence assay for pro-SPC and Krt5, case 1 and controls. A–C: Case 1 biopsy specimen shows no SPC staining in KRT5 pods. D–F: Case 1 explants show pro-SPC staining in low cuboidal airspace lining cells, confirming their morphologic identity as AEC2s, with KRT5 staining in areas of bronchiolarization; no overlapping areas of staining are identified. G–I: Staining in control human lung. Scale bars = 100 μm (B–I).
Additional characterization of peribronchiolar basaloid pods, case 2. A–C: Muc5ac stain shows residual staining in airways (asterisks) and rare centrally located cells in pods. B: Higher-power view of right boxed area in A. C: Higher-power view of left boxed area in A. D and E: Podoplanin staining of pod and adjacent airway (asterisks) shows heterogeneous cytoplasmic staining with accentuation at the periphery of pods. Original magnification: ×200 (A and E); ×400 (B and C); ×40 (D).
Control immunostains, in normal human lung. A–D: Representative staining of the same small airway. B: Keratin 5/6 stains all basal nuclei and a subset of suprabasal cells (24% in this example; range, 4% to 27%). C: Muc5ac staining of secretory (goblet) cells; the amount of staining shows heterogeneity in different size airways (data not shown). D and E: Podoplanin (D2-40) stains a subset of basal cells at varying intensities in small and large airways, respectively. F: Podoplanin shows moderate-intensity staining of AT1 cells. Original magnification, ×200 (A–F). H&E, hematoxylin and eosin.
Large airways. A and B: Bronchial epithelium in case 1 at biopsy (A) and in the explanted lungs (B) shows mild basaloid hyperplasia in both. C: Bronchial epithelium in case 2 shows mild basaloid hyperplasia with an inflammatory exudate in the lumen. Original magnification, ×200 (A–C).
Additional characterization of case 1 explants and bronchiolarization. A–D: Case 1, explant. A: Low-power hematoxylin and eosin (H&E) stain of regions shown. B: Podoplanin shows no staining of AT2 cell lined areas (enlarged from top boxed area in A). C and D: Podoplanin shows similar staining in areas of bronchiolarization to that seen in control airways; C, the bottom boxed area in A at higher magnification; D, the boxed area in C at higher magnification. E: Muc5ac shows heterogeneous staining in areas of bronchiolarization. F: Podoplanin staining in bronchiolarization in case 2 is similar to that in the explants of case 1. Asterisks designate bronchiolar lumens. Original magnification: ×40 (A); ×200 (B, C, E, F); ×400 (D).
Keratin 5/6 staining is enriched but heterogeneous in bronchiolarization. A and B: Hematoxylin and eosin (H&E) staining of regions shown in C–F. C–F: Keratin 5/6. Asterisks designate bronchiolar lumens. Bronchiolarized regions vary from block positive (100% of cells staining) to staining similar to airways (all basal cells and approximately 20% of suprabasal cells, example marked by a diamond). Original magnification: ×40 (A); ×200 (B); ×100 (C and D); ×400 (E and F).
References
- 1.Cardinal-Fernandez P., Bajwa E.K., Dominguez-Calvo A., Menendez J.M., Papazian L., Thompson B.T. The presence of diffuse alveolar damage on open lung biopsy is associated with mortality in patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Chest. 2016;149:1155–1164. doi: 10.1016/j.chest.2016.02.635. [DOI] [PubMed] [Google Scholar]
- 2.Papazian L., Doddoli C., Chetaille B., Gernez Y., Thirion X., Roch A., Donati Y., Bonnety M., Zandotti C., Thomas P. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. 2007;35:755–762. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- 3.Patel S.R., Karmpaliotis D., Ayas N.T., Mark E.J., Wain J., Thompson B.T., Malhotra A. The role of open-lung biopsy in ARDS. Chest. 2004;125:197–202. doi: 10.1378/chest.125.1.197. [DOI] [PubMed] [Google Scholar]
- 4.Thompson B.T., Guerin C., Esteban A. Should ARDS be renamed diffuse alveolar damage? Intensive Care Med. 2016;42:653–655. doi: 10.1007/s00134-016-4296-5. [DOI] [PubMed] [Google Scholar]
- 5.Katzenstein A.-L.A. Katzenstein and Askin's surgical pathology of non-neoplastic lung disease. In: Katzenstein A.-L.A., Askin F.B., Livolsi V.A., editors. Major Problems in Pathology. ed 4. Saunders/Elsevier; Philadelphia: 2006. [Google Scholar]
- 6.Taylor M.S., Chivukula R.R., Myers L.C., Jeck W.R., Tata P.R., O'Donnell W.J., Farver C.F., Thompson B.T., Rajagopal J., Kradin R.L. Delayed alveolar epithelization: a distinct pathology in diffuse acute lung injury. Am J Respir Crit Care Med. 2018;197:522–524. doi: 10.1164/rccm.201706-1094LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotton D.N., Morrisey E.E. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan B.L., Barkauskas C.E., Chapman H.A., Epstein J.A., Jain R., Hsia C.C., Niklason L., Calle E., Le A., Randell S.H., Rock J., Snitow M., Krummel M., Stripp B.R., Vu T., White E.S., Whitsett J.A., Morrisey E.E. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tata P.R., Rajagopal J. Plasticity in the lung: making and breaking cell identity. Development. 2017;144:755–766. doi: 10.1242/dev.143784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughan A.E., Brumwell A.N., Xi Y., Gotts J.E., Brownfield D.G., Treutlein B., Tan K., Tan V., Liu F.C., Looney M.R., Matthay M.A., Rock J.R., Chapman H.A. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517:621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanegai C.M., Xi Y., Donne M.L., Gotts J.E., Driver I.H., Amidzic G., Lechner A.J., Jones K.D., Vaughan A.E., Chapman H.A., Rock J.R. Persistent pathology in influenza-infected mouse lungs. Am J Respir Cell Mol Biol. 2016;55:613–615. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Herridge M.S., Tansey C.M., Matte A., Tomlinson G., Diaz-Granados N., Cooper A., Guest C.B., Mazer C.D., Mehta S., Stewart T.E., Kudlow P., Cook D., Slutsky A.S., Cheung A.M., Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 14.Beasley M.B. Acute lung injury. In: Tomashefski J.F., Cagle P.T., Farver C.F., Fraire A.E., editors. Dail and Hammar's Pulmonary Pathology. ed 3. Springer; New York: 2008. pp. 64–83. [Google Scholar]
- 15.Ogino S., Franks T.J., Yong M., Koss M.N. Extensive squamous metaplasia with cytologic atypia in diffuse alveolar damage mimicking squamous cell carcinoma: a report of 2 cases. Hum Pathol. 2002;33:1052–1054. doi: 10.1053/hupa.2002.128246. [DOI] [PubMed] [Google Scholar]
- 16.Chou P., Blei E.D., Shen-Schwarz S., Gonzalez-Crussi F., Reynolds M. Pulmonary changes following extracorporeal membrane oxygenation: autopsy study of 23 cases. Hum Pathol. 1993;24:405–412. doi: 10.1016/0046-8177(93)90089-y. [DOI] [PubMed] [Google Scholar]
- 17.Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., Randell S.H., Noble P.W., Hogan B.L. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai T.J., Brownfield D.G., Krasnow M.A. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shenoy E.S., Lai P.S., Shepard J.A., Kradin R.L. CASE RECORDS of the MASSACHUSETTS GENERAL HOSPITAL: case 39-2015: a 22-year-old man with hypoxemia and shock. N Engl J Med. 2015;373:2456–2466. doi: 10.1056/NEJMcpc1507212. [DOI] [PubMed] [Google Scholar]
- 20.Yeldandi A.V., Colby T.V. Pathologic features of lung biopsy specimens from influenza pneumonia cases. Hum Pathol. 1994;25:47–53. doi: 10.1016/0046-8177(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 21.Kumar P.A., Hu Y., Yamamoto Y., Hoe N.B., Wei T.S., Mu D., Sun Y., Joo L.S., Dagher R., Zielonka E.M., Wang de Y., Lim B., Chow V.T., Crum C.P., Xian W., McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo W., Zhang T., Wu D.Z., Guan S.P., Liew A.A., Yamamoto Y., Wang X., Lim S.J., Vincent M., Lessard M., Crum C.P., Xian W., McKeon F. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517:616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng D., Yin L., Chen J. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol. 2014;50:595–604. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- 24.Ray S., Chiba N., Yao C., Guan X., McConnell A.M., Brockway B., Que L., McQualter J.L., Stripp B.R. Rare SOX2+ airway progenitor cells generate KRT5+ cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Reports. 2016;7:817–825. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electron microscopy confirms that the lining cells in case 1 are activated macrophages. Arrowheads indicate basement membrane; asterisk, alveolar space. Inset: Activated macrophage cytoplasm with pinocytotic vesicles (arrows) and abundant rough endoplasmic reticulum and mitochondria. Original magnification: ×3500 (main image); ×18,000 (inset). E, endothelial cell nucleus; M, macrophage nucleus.
Additional characterization of peribronchiolar basaloid pods. A: Pod cells are TTF1 positive, and surrounding denuded alveoli are TTF negative. B and C: High-power hematoxylin and eosin (H&E) staining of pod cells shows atypical basaloid/squamoid cells with mitotic figures (arrow) and a high Ki-67 proliferation rate at pod periphery. Asterisks indicates lumens. Original magnification: ×200 (A); ×400 (B and C).
Serial 5u sections of peribronchiolar basaloid pods (PBPs), case 1. A–D: Serial sections show PBPs in contiguity with an injured bronchiole (asterisks). Arrows indicate new lumens forming or disappearing throughout levels; arrowheads, pods that appear separated from bronchioles on individual levels but that are contiguous on others. E: p63 stain highlights pod and airway nuclei. Original magnification, ×100 (A–E).
Immunofluorescence assay for pro-SPC and Krt5, case 1 and controls. A–C: Case 1 biopsy specimen shows no SPC staining in KRT5 pods. D–F: Case 1 explants show pro-SPC staining in low cuboidal airspace lining cells, confirming their morphologic identity as AEC2s, with KRT5 staining in areas of bronchiolarization; no overlapping areas of staining are identified. G–I: Staining in control human lung. Scale bars = 100 μm (B–I).
Additional characterization of peribronchiolar basaloid pods, case 2. A–C: Muc5ac stain shows residual staining in airways (asterisks) and rare centrally located cells in pods. B: Higher-power view of right boxed area in A. C: Higher-power view of left boxed area in A. D and E: Podoplanin staining of pod and adjacent airway (asterisks) shows heterogeneous cytoplasmic staining with accentuation at the periphery of pods. Original magnification: ×200 (A and E); ×400 (B and C); ×40 (D).
Control immunostains, in normal human lung. A–D: Representative staining of the same small airway. B: Keratin 5/6 stains all basal nuclei and a subset of suprabasal cells (24% in this example; range, 4% to 27%). C: Muc5ac staining of secretory (goblet) cells; the amount of staining shows heterogeneity in different size airways (data not shown). D and E: Podoplanin (D2-40) stains a subset of basal cells at varying intensities in small and large airways, respectively. F: Podoplanin shows moderate-intensity staining of AT1 cells. Original magnification, ×200 (A–F). H&E, hematoxylin and eosin.
Large airways. A and B: Bronchial epithelium in case 1 at biopsy (A) and in the explanted lungs (B) shows mild basaloid hyperplasia in both. C: Bronchial epithelium in case 2 shows mild basaloid hyperplasia with an inflammatory exudate in the lumen. Original magnification, ×200 (A–C).
Additional characterization of case 1 explants and bronchiolarization. A–D: Case 1, explant. A: Low-power hematoxylin and eosin (H&E) stain of regions shown. B: Podoplanin shows no staining of AT2 cell lined areas (enlarged from top boxed area in A). C and D: Podoplanin shows similar staining in areas of bronchiolarization to that seen in control airways; C, the bottom boxed area in A at higher magnification; D, the boxed area in C at higher magnification. E: Muc5ac shows heterogeneous staining in areas of bronchiolarization. F: Podoplanin staining in bronchiolarization in case 2 is similar to that in the explants of case 1. Asterisks designate bronchiolar lumens. Original magnification: ×40 (A); ×200 (B, C, E, F); ×400 (D).
Keratin 5/6 staining is enriched but heterogeneous in bronchiolarization. A and B: Hematoxylin and eosin (H&E) staining of regions shown in C–F. C–F: Keratin 5/6. Asterisks designate bronchiolar lumens. Bronchiolarized regions vary from block positive (100% of cells staining) to staining similar to airways (all basal cells and approximately 20% of suprabasal cells, example marked by a diamond). Original magnification: ×40 (A); ×200 (B); ×100 (C and D); ×400 (E and F).