Abstract
The adverse outcome pathway (AOP) framework serves as a knowledge assembly, interpretation, and communication tool designed to support the translation of pathway-specific mechanistic data into responses relevant to assessing and managing risks of chemicals to human health and the environment. As such, AOPs facilitate the use of data streams often not employed by risk assessors, including information from in silico models, in vitro assays and short-term in vivo tests with molecular/biochemical endpoints. This translational capability can increase the capacity and efficiency of safety assessments both for single chemicals and chemical mixtures. Our mini-review describes the conceptual basis of the AOP framework and aspects of its current status relative to use by toxicologists and risk assessors, including four illustrative applications of the framework to diverse assessment scenarios.
Keywords: Adverse Outcome Pathway, Chemical Assessment, Human Health, Environment
1.1 Changing Face of Regulatory Toxicology: A Brief Synopsis
The past decade has witnessed an unprecedented expansion in the variety and volume of molecular and biochemical data available for taxa ranging from bacteria to humans. This has fueled significant advances in fields such as evolutionary biology, agricultural sciences, and biomedical diagnostics and technology. Other disciplines also have started to seize the opportunities provided by new data streams and tools to address past and present challenges. Toxicology is one of the disciplines that stands to significantly benefit from these new sources of biological knowledge.
Toxicology is among the most applied of the biological sciences, driven largely by mandates to assess specific aspects of chemical safety relative to human health and the environment. Historically this has been achieved mostly through the generation of data for a relative handful of high priority/visibility chemicals using well-defined animal models and apical endpoints. However, increasing societal awareness of, and concern for the number of chemicals with limited or no hazard/risk information has resulted in legislative mandates such as the REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) program in Europe and recent revisions to the Toxic Substances Control Act (TSCA) in the US, which require consideration of the possible health and ecological effects of a much larger chemical universe than in the past [1, 2]. There also is an increasing emphasis on understanding the effects of a wide variety of chemical mixtures on human health and the environment; for example, in North America the Great Lakes Restoration Initiative has identified complex mixtures of “chemicals of emerging concern” as one of the highest priority stressors in the lakes [3].
These types of newer regulatory programs and monitoring initiatives highlight the necessity of identifying and developing rapid, cost-effective approaches for predicting the potential toxicity of substances to augment (or replace) the in vivo test methods that traditionally have supported chemical risk assessments [4]. These approaches may include in silico models, in vitro assays (including those conducted in a high-throughput [HPT] format), and short-term in vivo tests with molecular/biochemical endpoints (including ‘omics) indicative of perturbation of biological pathways.
A critical challenge to using alternative tools and data types for chemical safety assessment involves translation of this information into apical responses applicable to risk assessment, such as impacts on survival, reproduction, induction of cancer, etc., in individuals and, in the case of ecological effects, populations. The adverse outcome pathway (AOP) framework was developed to address this translation challenge [5]. Herein we describe the conceptual basis and current status of the AOP framework, provide examples of its use in different types of chemical assessments, and touch on several recent developments relevant to the future of the framework.
1.2 Definition and Attributes of the AOP Framework
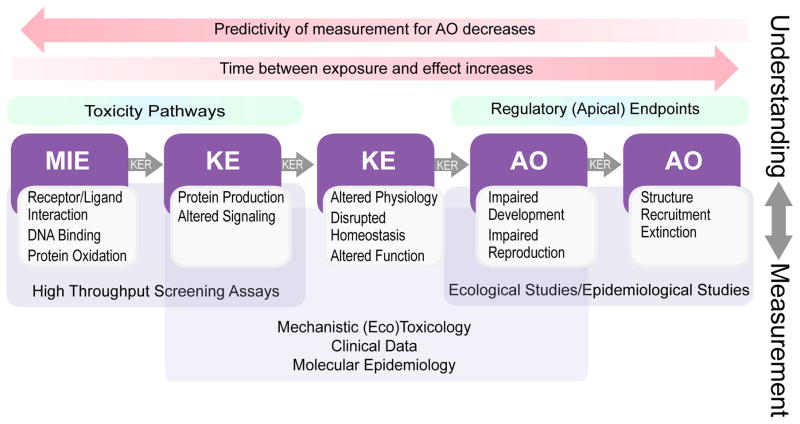
The AOP framework reflects an evolution of prior pathway-based concepts, most notably mechanism or mode of action, for assembling and depicting toxicological data across biological levels of organization [5–7]. An AOP consists of a series of measurable key events (KEs) linked to one another by key event relationships (Figure 1). The first KE generally is a molecular initiating event (MIE), which captures the interaction of a chemical with a biological macromolecule, that triggers subsequent KEs which could result in an adverse outcome (AO) at the individual or population level [8, 9]. Explicit in an AOP is that the KEs are causally linked to one-another, an attribute that can be formally assessed using weight-of-evidence analyses [10, 11]. An important property of AOPs is that they are chemically-agnostic, capturing response-response relationships that result from a given perturbation of a MIE that could be caused by any of a number of chemical (or even non-chemical) stressors [8].
Figure 1.
Depiction of the role of the adverse outcome pathway (AOP) framework in linking various data streams to outcomes relevant to regulatory decision-making for chemicals. MIE – Molecular Initiating Event, KE – Key Event, KER – Key Event Relationship, AO – Adverse Outcome
The AOP framework provides a connection between mechanism-based effects measurements and apical outcomes on two levels. First, in assembling evidence supporting the proposed mechanism, AOPs provide the understanding needed to interpret data from measurements of KEs as they relate to an apical endpoint of regulatory concern (Figure 1-top). Having a structured framework for this purpose is important because the predictive utility of early KEs for the eventual AO can be limited for a variety of reasons. For example, homeostatic mechanisms will serve to obviate AOs in many cases, but modulating factors such as genetic differences, pre-existing disease, and alterations from other environmental stressors can magnify responses in some situations. Because of this, it is important to capture and organize existing knowledge and make it available for interpretation of these new data streams. In fact, once the factors influencing the propagation of the signal from the early KEs to the final AOs are sufficiently described, using early KEs as the primary assay for toxicity has the advantage of them being measurable in either in vitro or in vivo systems within hours to days, as opposed to the weeks, months or even years it can take for apical AOs to manifest.
The second role for the AOP is to serve as a scaffold for assembling data associated with a given outcome in an organized manner (Figure 1-bottom). By assembling these data in the context of an AOP, it allows for different measures of pathway perturbation to be compared with one another with regard to their predictive capacity for an AO, rather than an a priori selection of one measurement as the “gold standard”. Having all information in a structured framework also enables defined approaches for integrated toxicity assessments that incorporate information from multiple endpoints to improve the prediction of an AO.
AOPs are deliberate simplifications of normal biological pathways intended to facilitate depiction and ready communication of what can be very complex processes. A common misconception about AOPs is that they can depict KEs along a given pathway only in a linear manner, thus ignoring potentially important interactions between pathways [12]. Linear AOPs, however, can be assembled to produce AOP networks that capture shared nodes and interactions among pathways [8, 13, 14]. Furthermore, it is possible to assemble quantitative AOPs (qAOPs) that consider quantitative relationships between KEs, including feedback models designed to reflect system regulation, to predict AOs [15]. For example, Conolly et al. [16] recently described a qAOP that utilizes a feedback-controlled hypothalamic-pituitary-gonadal axis model to enable predictions of reproductive capacity in fish exposed to chemicals that inhibit sex steroid synthesis. Basically, the AOP framework is capable of capturing sufficient complexity to ensure application to a variety of assessment scenarios/challenges.
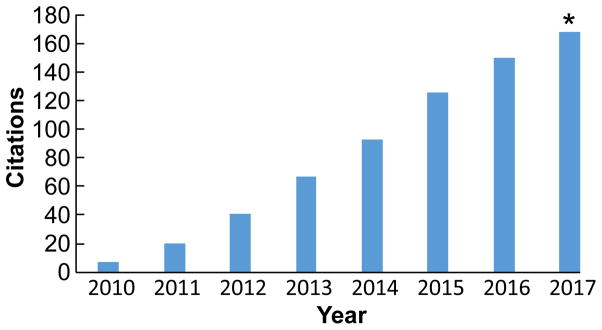
AOPs have received substantial attention as an organizing framework for toxicologically-relevant biological information, for example, in the extant scientific literature (Figure 2). A pragmatic example of interest in the AOP framework involves the Organisation for Economic Cooperation and Development (OECD) which, starting in 2012, has supported activities of a workgroup of international experts to publish harmonized guidance for the description, evaluation, and technical review of the scientific robustness of AOPs [6, 17, 18]. The AOP framework is envisioned by the OECD as a critical tool supporting the mutual acceptance of toxicological data by diverse regulatory authorities. To further enhance harmonization, the OECD also has helped facilitate development of an internationally accessible and searchable source of AOP information [19], that includes the AOP Wiki [20], an interactive knowledgebase for describing, displaying, and archiving AOPs and AOP networks. The AOP Wiki currently contains more than 200 AOPs at different stages of development, which describe processes/endpoints relevant both to human health and the environment.
Figure 2.
Temporal citation analysis of an initial paper describing the adverse outcome pathway (AOP) framework (Ankley et al. 2010). “Final” data for 2017 are extrapolated from 6-month values. Analysis was conducted using the Web of Science (Clarivate Analytics).
1.3 Examples of AOP Uses
The AOP framework is intended to be flexible relative to potential research and regulatory applications. Below we provide four comparatively brief, illustrative examples of application of the concept to diverse assessment scenarios.
1.3.1 Case Example 1: Predicting Skin Sensitization
Skin sensitization involves covalent modification of cellular proteins in skin by electrophilic compounds, which subsequently can enhance reactions to allergens. As such, this is an important endpoint for safety assessments involving personal care products, which historically has been evaluated using in vivo assays. However, legislation in the European Union dictated moving away from whole animal tests for evaluating sensitization, resulting in the need for an alternative assessment approach [21]. This has been addressed by developing an AOP for skin sensitization that includes description of several intermediate KEs related to induction of inflammatory cytokines and proliferation of T-cells [20 (AOP 40), 22]. This AOP, which has been supported by extensive technical review [23, 24], provides the basis for identifying and validating a suite of in vitro assays reflecting these intermediate KEs. Data from this assay suite for test chemicals of interest can be assessed using modeling approaches such as Bayesian network analysis to combine/weight data from different biological levels of organization, captured in the AOP, to produce categorical predictions of the potential for skin sensitization [25, 26]. This effort shows how capturing pathway-based data in an AOP can facilitate the use of alternative data streams as a replacement for conventional test methods [27].
1.3.2 Case Example 2: Prioritizing Endocrine Disrupting Chemicals
Chemicals that exert adverse effects in humans and wildlife through their ability to alter endocrine function have been a topic of scientific and regulatory concern for almost 25 years [28]. The US Environmental Protection Agency (USEPA) has a legislated mandate to develop a screening and testing program to identify potential adverse endocrine-mediated effects of more than 10,000 chemicals [29], a task that cannot plausibly be accomplished in a reasonable timeframe solely through in vivo testing [30, 31]. To address this challenge, in vitro HTP data and models are being used to prioritize the list of target chemicals for those likely to act via endocrine MIEs of regulatory concern, such as activation or antagonism of estrogen or androgen receptors and inhibition of specific enzymes involved in sex steroid or thyroid hormone synthesis. Browne et al. [30] recently described this approach using estrogen receptor activation as an example. In this context the AOP framework provides demonstrable linkages between in silico or in vitro measures of bioactivity and potential adverse effects in vivo [31], thus supporting both identification of assays suitable for detecting MIEs of concern, and providing conceptual “phenotypic anchoring” supporting their use in the prioritization process.
1.3.3 Case Example 3: Evaluating Pesticide Toxicity to Pollinators
Key pollinator species, such as honeybees, have experienced significant worldwide declines, resulting in concerns for possible effects on global food production. In the US, for example, a national strategy has been developed to assess the significance and causes of pollinator declines [32]. A number of chemical and non-chemical stressors have been proposed as contributing to declines, one of the more prominent of which are neonicotinoid pesticides [33, 34]. However, significant uncertainties exist as to the biological plausibility of a link between the molecular action (MIE) of neonicotinoids—activation of the nicotinic acetylcholine receptor—and impacts on honeybee colonies. To help assess the veracity of hypothesized effects of neonicotinoids on honeybees, LaLone et al. [14] assembled an AOP network based on molecular, biochemical, physiological, behavioral, and population data from more than 220 papers in the open literature. Not only were they able to demonstrate a plausible linkage between perturbation of nicotinic acetylcholine receptor signaling and adverse effects in honeybees, but the analysis highlighted areas of uncertainty that would benefit from focused research and/or monitoring [14]. In this example, the AOP framework supported integration of a complex, biologically-diverse dataset in the context of evaluating causal relationships among endpoints at different levels of organization, and served as a basis for generating hypotheses to test these interactions.
1.3.4 Case Example 4: Evaluating Hazards of Complex Chemical Mixtures
Newer approaches being advocated/used in predictive toxicology for single chemicals can, in conjunction with the AOP framework as a translator, also be employed to help assess risks of complex mixtures of chemicals. A significant challenge in assessing complex mixtures is predicting the possible biological effects of hundreds or even thousands of contaminants, many of which may be unknown. Schroeder et al. [35] recently described how suites of HTP assays can be used to measure diverse bioactivities of complex contaminant mixtures in surface waters to effectively augment more targeted instrumental analyses. Measurements from HTP assays often correspond to MIEs or early KEs (e.g., receptor activation, enzyme inhibition, etc.), so it is possible to query/cross-reference knowledgebases such as the AOP Wiki to translate bioactivity data generated from complex mixtures into potential hazards in exposed organisms, such as fish [35, 36]. The AOP framework also can serve as the basis for translating molecular/biochemical data from field-collected animals exposed to complex mixtures into endpoints useful for inferring hazard/risk [37]. Miller et al. [38] recently described a study in which an AOP construct was linked to a population model for white suckers, a large cyprinid species indigenous to the Great Lakes, to predict population status based on observed changes in sex steroid synthesis in fish exposed to a complex pulp and paper mill effluent. In this application, it was not necessary to know the identity of the chemicals responsible for decreasing steroid synthesis, only that they consistently affected an early KE in an AOP relating depressed steroid synthesis to decreased egg production and, hence, population status [20 (AOP 25); 38].
1.4 Concluding Thoughts
The AOP concept has matured from a largely conceptual construct to an increasingly practical and sophisticated knowledge-assembly/communication tool with multiple applications. In addition to the types of uses illustrated above, the AOP framework is being applied to more novel scenarios, such as consideration of contaminant interactions with environmental variables associated with climate change [39], evaluation of environmental and/or human health effects of nanomaterials and ionizing radiation [40–43], chemical life-cycle assessment and alternatives analysis [44, 45], and the design of hypothesis-driven environmental monitoring programs [46]. Furthermore, AOPs are being considered by the biomedical community as a means to support drug discovery/development and understand disease initiation/progression [e.g., 47–50].
In addition to novel applications, innovative scientific approaches are being identified/employed in support of basic AOP development. For example, recent efforts have focused on the identification, development and evaluation of new AOPs based on ‘omic and/or HTP data, systems/network modeling, and/or repositories of curated toxicity information [52–55]. Increasing the “library” of available AOPs is critically important to supporting the varied applications of the framework in the future.
Many of the technical and practical advancements in the AOP framework have occurred as a result of recommendations from different international fora [e.g., 15, 53, 56, 57], including a recent SETAC Pellston meeting in Cornwall, ON, Canada (April 2017). This meeting utilized a novel “horizon scanning” approach to identify upcoming/priority issues for AOP development and use based on input from the broader scientific and regulatory communities [12]. Topics included development and practical implementation of AOP networks and qAOPs, case examples of regulatory use of AOPs, and development of a roadmap for a long-term, sustainable model supporting AOP development and use [58].
Evolution of the AOP concept thus far has been facilitated through the individual and joint efforts of a variety of research and regulatory organizations around the world, representing governmental, business, and academic interests. It is this multi-sectorial interest that has advanced the AOP concept and hopefully will continue to support its development as a flexible and practical tool supporting 21st century toxicology.
Acknowledgments
This paper has been reviewed in accordance with USEPA guidelines, but does not necessarily reflect the views or policies of the Agency. We thank Dan Villeneuve for comments on an earlier version of the paper.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
AUTHOR DECLARATION TEMPLATE
We wish to confirm that there are no known conflicts of interest ass ociated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
References
- 1.European Commission. Regulation (EC) 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the registration, evaluation, authorisation and restriction of chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) 793/93 and Commission Regulation (EC) No. 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Official J Eur Union. 2006;L396:374–375. [Google Scholar]
- 2.US Congress. The Frank R. Lautenberg Chemical Safety for the 21st Century Act. Pub. L. No. 114–182. 2016 Jun 22; Available from: https/ www.epa.gov/assessing-and-managing-chemicals-under-tsca/frank-r-lautenberg-chemical-safety-21st-century-act.
- 3.US Environmental Protection Agency. [accessed 29.09.17];Great Lakes Restoration Initiative. https://www.glri.us/actionplan/pdfs/glri-action-plan-2.pdf.
- 4.National Academy of Sciences (NAS) Toxicity testing in the 21st century: A vision and strategy. National Academies Press; 2007. [Google Scholar]
- 5.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Technol Chem. 2010;29:730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 6.Perkins E, Garcia-Reyero N, Edwards S, Wittwehr C, Villeneuve D, Lyons D, Ankley G. The adverse outcome pathway: A conceptual framework to support toxicity testing in the twenty-first century. In: Hoeng J, Peitsch MC, editors. Computational Systems Toxicology. Humana Press; Totowa, New Jersey: 2015. pp. 1–26. [Google Scholar]
- 7.Vinken M, Knapen D, Vergauwen L, Hengstler JG, Angrish M, Whelan M. Adverse outcome pathways: A concise introduction for toxicologists. Arch Toxicol. 2017 doi: 10.1007/s00204-017-2020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lattieri T, Munn S, Nepelska M, Ottinger MA, Vergauwen L, Whelan M. Adverse outcome pathway (AOP) development 1: Strategies and principles. Toxicol Sci. 2014;142:312–320. doi: 10.1093/toxsci/kfu199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen TE, Goodman JM, Gutsell S, Russell PJ. A history of the molecular initiating event. Chem Res Toxicol. 2016;29:2060–2070. doi: 10.1021/acs.chemrestox.6b00341. [DOI] [PubMed] [Google Scholar]
- 10.Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, Watanabe H, Barton-Maclaren TS. Increasing scientific confidence in adverse outcome pathways: Application of tailored Bradford-Hill considerations for evaluating weight of evidence. Reg Toxicol Pharmacol. 2015;72:514–537. doi: 10.1016/j.yrtph.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Collier ZA, Gust KA, Gonzalez-Morales B, Gong P, Wilbanks MS, Linkov I, Perkins EJ. A weight of evidence assessment approach for adverse outcome pathways. Reg Toxicol Pharmacol. 2016;75:46–57. doi: 10.1016/j.yrtph.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 12.LaLone CA, Ankley GT, Belanger SE, Embry MR, Hodges G, Knapen D, Munn S, Perkins EJ, Rudd MA, Villeneuve DL, Whelan M, Willett C, Zhang X, Hecker M. Advancing the adverse outcome pathway framework-An international horizon scanning approach. Environ Toxicol Chem. 2017;36:1411–1421. doi: 10.1002/etc.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knapen D, Vergauwen L, Villeneuve DL, Ankley GT. The potential of AOP networks for reproductive and developmental toxicity assay development. Repro Toxicol. 2015;56:52–55. doi: 10.1016/j.reprotox.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 14.LaLone CA, Villeneuve DL, Wu-Smart J, Milsk RY, Sappington K, Garber KV, Housenger J, Ankley GT. Weight of evidence evaluation of a network of adverse outcome pathways linking activation of the nicotinic acetylcholine receptor in honey bees to colony death. Sci Total Environ. 2017;584–585:751–775. doi: 10.1016/j.scitotenv.2017.01.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittwehr C, Aladjov H, Ankley G, Byrne HJ, de Knecht J, Heinzle E, Klambauer G, Landsmann B, Luijten M, MacKay C, Maxwell G, Meek ME, Paini A, Perkins E, Sobanski T, Villeneuve D, Waters KM, Whelan M. How adverse outcome pathways can aid the development and use of computational prediction models for regulatory toxicology. Toxicol Sci. 2017;155:326–336. doi: 10.1093/toxsci/kfw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conolly R, Ankley GT, Cheng W, Mayo M, Miller DH, Perkins EJ, Villeneuve DL, Watanabe KH. Quantitative adverse outcome pathways and their application to predictive toxicology. Environ Sci Technol. 2017;51:4661–4672. doi: 10.1021/acs.est.6b06230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delrue N, Sachana M, Sakuratani Y, Gourmelon A, Leinala E, Diderich R. The adverse outcome pathway concept: A basis for developing regulatory decision-making tools. Altern Lab Anim. 2016;44:417–429. doi: 10.1177/026119291604400504. [DOI] [PubMed] [Google Scholar]
- 18.Organisation for Economic Cooperation and Development. [accessed 15.08.17]; http://www.oecd.org/chemicalsafety/testing/adverse-outcome-pathways-molecular-screening-and-toxicogenomics.htm.
- 19. [accessed 15.09.17]; https://aopkb.org/
- 20. [accessed 15.09.17]; https://aopwiki.org.
- 21.European Chemicals Agency. [accessed 15.08.17]; https://echa.europa.eu/view-article/-/journal_content/title/registrants-to-use-alternative-test-methods-for-skin-sensitisation.
- 22.Maxwell G, MacKay C, Cubberley R, Davies M, Gellatly N, Glavin S, Gouin T, Jacquoilleot S, Moore C, Pendlington R. Applying the skin sensitization adverse outcome pathway (AOP) to quantitative risk assessment. Toxicol In Vitro. 2014;28:8–12. doi: 10.1016/j.tiv.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Patlewicz G, Simon TW, Rowlands JC, Budinsky RA, Becker RA. Proposing a scientific confidence framework to help support the application of adverse outcome pathways for regulatory purposes. Reg Toxicol Pharmacol. 2015;71:463–477. doi: 10.1016/j.yrtph.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Perkins EJ, Antczak P, Burgoon L, Falciani F, Garcia-Reyero N, Gutsell S, Hodges G, Kienzler A, Knapen D, McBride M, Willett C. Adverse outcome pathways for regulatory applications: Examination of four case studies with different degrees of completeness and scientific confidence. Toxicol Sci. 2015;148:14–25. doi: 10.1093/toxsci/kfv181. [DOI] [PubMed] [Google Scholar]
- 25.Jaworska J, Dancik Y, Kern P, Gerberick F, Natsch A. Bayesian integrated testing strategy to assess skin sensitization potency: From theory to practice. J Appl Toxicol. 2013;33:1353–1364. doi: 10.1002/jat.2869. [DOI] [PubMed] [Google Scholar]
- 26.Pirone JR, Smith M, Kleinstreuer NC, Burns TA, Strickland J, Dancik Y, Morris R, Rinckel LA, Casey W, Jaworska JS. Open source software implementation of an integrated testing strategy for skin sensitization potency based on a Bayesian network. Altex. 2014;31:336–340. doi: 10.14573/altex.1310151. [DOI] [PubMed] [Google Scholar]
- 27.Schultz TW, Dimitrova G, Dimitrov S, Mekenyan OG. The adverse outcome pathway for skin sensitization: Moving closer to replacing animal testing. Altern Lab Anim. 2016;44:453–460. doi: 10.1177/026119291604400515. [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread” – environmental endocrine disrupters and human and wildlife health: Where we are today and where we need to go. Toxicol Sci. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.US Environmental Protection Agency. [accessed 20.08.17];Endocrine Disruptor Screening and Testing Program. https://www.epa.gov/chemical-research/endocrine-disruption-screening-program-21st-century.
- 30.Browne P, Judson RS, Casey WM, Kleinstreuer NC, Thomas RS. Screening chemicals for estrogen receptor bioactivity using a computational model. Environ Sci Technol. 2015;49:8804–8814. doi: 10.1021/acs.est.5b02641. [DOI] [PubMed] [Google Scholar]
- 31.Browne P, Noyes PD, Casey WM, Dix DJ. Application of adverse outcome pathways to US EPA’s endocrine disruptor screening program. Environ Health Perspect. 2017 doi: 10.1289/EHP1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White House. National strategy to promote the health of honey bees and other pollinators. Pollinator Health Task Force; Washington, DC: 2015. [Google Scholar]
- 33.Godfray HCJ, Blacquiere T, Field LM, Hails RS, Potts SG, Raine NE, Vanbergen AJ, McLean AR. A restatement of recent advances in the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc Royal Soc B. 2015;282:1818–1821. doi: 10.1098/rspb.2015.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Environmental Protection Agency. Preliminary pollinator assessment to support the registration review of imidacloprid. Office of Pesticide Programs, Environmental Fate and Effects Division; Washington, DC: 2016. https://www.epa.gov/pesticides/epa-release-first-four-preliminary-risk-assessments-insecticides-potentially-harmful. [Google Scholar]
- 35.Schroeder AL, Ankley GT, Houck K, Villeneuve DL. Environmental surveillance and monitoring – The next frontiers for high-throughput toxicology. Environ Toxicol Chem. 2016;35:513–525. doi: 10.1002/etc.3309. [DOI] [PubMed] [Google Scholar]
- 36.Blackwell BR, Ankley GT, Corsi SR, DeCicco LA, Houck K, Judson R, Li S, Martin M, Murphy E, Schroder AL, Smith ET, Swintek J, Villeneuve DL. An “EAR” on environmental surveillance and monitoring: a case study on the use of exposure-activity ratios (EARs) to prioritize sites, chemicals, and bioactivities of concern in Great Lakes waters. Environ Sci Technol. 2017;51:8713–8724. doi: 10.1021/acs.est.7b01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer VJ, Etterson MA, Hecker M, Murphy CA, Roesijadi G, Spade DJ, Ankley GT. Adverse outcome pathways and ecological risk assessment: Bridging to population-level effects. Environ Toxicol Chem. 2011;30:64–76. doi: 10.1002/etc.375. [DOI] [PubMed] [Google Scholar]
- 38.Miller DH, Tietge JE, McMaster ME, Munkittrick KR, Xia X, Griesmer DA, Ankley GT. Linking mechanistic toxicology to population models in forecasting recovery from chemical stress: A case study from Jackfish Bay, Ontario, Canada. Environ Toxicol Chem. 2015;34:1623–1633. doi: 10.1002/etc.2972. [DOI] [PubMed] [Google Scholar]
- 39.Hooper MJ, Ankley GT, Cristol DA, Maryoung LA, Noyes PD, Pinkerton KE. Interactions between chemical and climate stressors: A role for mechanistic toxicology in assessing climate change risks. Environ Toxicol Chem. 2013;32:32–48. doi: 10.1002/etc.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labib S, Williams A, Yauk CL, Nikota JK, Wallin H, Vogel U, Halappanavar S. Nano-risk science: Application of toxicogenomics in an adverse outcome pathway framework for risk assessment of multi-walled carbon nanotubes, Part. Fibre Toxicol. 2016;13 doi: 10.1186/s12989-016-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyes WK, Thornton BLM, Al-Abed SR, Andersen CP, Bouchard DC, Burgess RM, Hubal EAC, Ho KT, Hughes MF, Kitchin K, Reichman JR, Rogers KR, Ross JA, Rygiewicz PT, Scheckel KG, Thai SF, Zepp RG, Zucker RM. A comprehensive framework for evaluating the environmental health and safety implications of engineered nanomaterials. Crit Rev Toxicol. 2017;47:767–810. doi: 10.1080/10408444.2017.1328400. [DOI] [PubMed] [Google Scholar]
- 42.Mirshafiee V, Jiang W, Sun B, Wang X, Xia T. Facilitating translational nanomedicine via predictive safety assessment. Mol Ther. 2017;25:1522–1530. doi: 10.1016/j.ymthe.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Preston RJ. Integrating basic radiobiological science and epidemiological studies: Why and how. Health Phys. 2015;108:125–30. doi: 10.1097/HP.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 44.Gust KA, Collier ZA, Mayo ML, Stanley JK, Gong P, Chappell MA. Limitations of toxicity characterization in life cycle assessment: Can adverse outcome pathways provide a new foundation? Integ Environ Assess Manage. 2016;12:580–590. doi: 10.1002/ieam.1708. [DOI] [PubMed] [Google Scholar]
- 45.Malloy T, Zaunbrecher V, Beryt E, Judson R, Tice R, Allard P, Blake A, Cote I, Godwin H, Heine L, Kerzic P, Kostal J, Marchant G, McPartland J, Moran K, Nel A, Ogunseitan O, Rossi M, Thayer K, Tickner J, Whittaker M. Advancing alternatives analysis: The role of predictive toxicology in selecting safer chemical products and processes. Integ Environ Assess Manage. 2017;13:915–925. doi: 10.1002/ieam.1923. [DOI] [PubMed] [Google Scholar]
- 46.Arciszewski TJ, Munkittrick KR, Scrimgeour GJ, Dube MG, Wrona FJ. Using adaptive processes and adverse outcome pathways to develop meaningful, robust, and actionable environmental monitoring programs. Integ Environ Assess Manage. 2017;13:877–891. doi: 10.1002/ieam.1938. [DOI] [PubMed] [Google Scholar]
- 47.Horii I. The principle of safety evaluation in medicinal drug – how can toxicology contribute to drug discovery and development as a multidisciplinary science? J Toxicol Sci. 2016;41:49–67. doi: 10.2131/jts.41.SP49. [DOI] [PubMed] [Google Scholar]
- 48.Morgan MM, Johnson BP, Livingston MK, Schuler LA, Alarid ET, Sung KE, Beebe DJ. Personalized in vitro cancer models to predict therapeutic response: Challenges and a framework for improvement. Pharmacol Ther. 2016;165:79–92. doi: 10.1016/j.pharmthera.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Issa NT, Wathieu H, Ojo A, Byers SW, Dakshanamurthy S. Drug metabolism in preclinical drug development: a survey of the discovery process, toxicology, and computational tools. Curr Drug Metab. 2017;18:556–565. doi: 10.2174/1389200218666170316093301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelkonen O, Terron A, Hernandez AF, Menendez P, Bennekou SH. EFSA WG EP11, Chemical exposure and infant leukemia: Development of an adverse outcome pathway (AOP) for aetiology and risk assessment research. Arch Toxicol. 2017;91:2763–2780. doi: 10.1007/s00204-017-1986-x. [DOI] [PubMed] [Google Scholar]
- 51.Grafström RC, Nymark P, Hongisto V, Spjuth O, Ceder R, Willighagen E, Hardy B, Kaski S, Kohonen P. Toward the replacement of animal experiments through the bioinformatics-driven analysis of ‘omics’ data from human cell cultures. Altern Lab Anim. 2015;43:325–32. doi: 10.1177/026119291504300506. [DOI] [PubMed] [Google Scholar]
- 52.Oki NO, Nelms MD, Bell SM, Mortensen HM, Edwards SW. Accelerating adverse outcome pathway development using publicly available data sources. Curr Environ Health Rep. 2016;3:53–63. doi: 10.1007/s40572-016-0079-y. [DOI] [PubMed] [Google Scholar]
- 53.Brockmeier EK, Hodges G, Hutchinson TH, Butler E, Hecker M, Tollefsen KE, Garcia-Reyero N, Kille P, Becker D, Chipman K, Colbourne J, Collette TW, Cossins A, Cronin M, Graystock P, Gutsell S, Knapen D, Katsiadaka I, Lange A, Marshall S, Owen SF, Perkins EJ, Plaistow S, Schroeder A, Taylor D, Viant M, Ankley G, Falciani F. The role of omics in the application of adverse outcome pathways for chemical risk assessment. Toxicol Sci. 2017;158:252–262. doi: 10.1093/toxsci/kfx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fay KA, Villeneuve DL, LaLone CA, Song Y, Tollefsen K-E, Ankley GT. Practical approaches to adverse outcome pathway (AOP) development and weight-of-evidence evaluation as illustrated by ecotoxicological case studies. Environ Toxicol Chem. 2017;36:1429–1449. doi: 10.1002/etc.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wathieu H, Ojo A, Dakshanamurthy S. Prediction of chemical multi-target profiles and adverse outcomes with systems toxicology. Curr Med Chem. 2017;24:1705–1720. doi: 10.2174/0929867323666161214115540. [DOI] [PubMed] [Google Scholar]
- 56.Villeneuve DL, Garcia-Reyero N. Predictive ecotoxicology in the 21st century. Environ Toxicol Chem. 2011;30:1–8. doi: 10.1002/etc.396. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Reyero N. Are adverse outcome pathways here to stay? Environ Sci Toxicol. 2015;49:3–9. doi: 10.1021/es504976d. [DOI] [PubMed] [Google Scholar]
- 58. [accessed 01.10.17]; http://www.saaop.org/workshops/pellston2017.html.