Abstract
Background and Aims: Sperm motility is regulated by protein phosphorylation. The 66 kDa protein obtained from hamster sperm flagella was phosphorylated at serine residues associated with the motility initiation. In order to understand the regulatory mechanism of sperm motility, the 66 kDa protein was identified in the present study.
Methods: The 66 kDa protein was purified by 2‐D gel electrophoresis and identified by matrix‐assisted laser desorption ionization mass spectrometry, liquid chromatography‐tandem mass spectrometry and peptide sequencer.
Results: The 66 kDa protein was tubulin β chain.
Conclusion: The 66 kDa protein is one of the tubulin β chain isoforms and phosphorylated in relation to the motility initiation. (Reprod Med Biol 2004; 3: 133–139)
Keywords: hamster, liquid chromatography‐tandem mass spectrometry, peptide mass finger printing, phosphorylation, spermatozoa, tubulin
INTRODUCTION
PROTEIN PHOSPHORYLATION and/or dephosphorylation are a very important event in the signal transduction regulated sperm motility. 1 , 2 , 3 Many protein phosphorylations regulated sperm motility occurrs by extracellular activation factors such as calcium and/or bicarbonate, and cyclic adenosine monophosphate (cAMP). Extracellular activation factors stimulate adenylate cyclase resulting in cAMP production. 4 , 5 , 6 Cyclic AMP activates a cAMP‐dependent protein kinase and introduces protein phosphorylation and/or dephosphorylation. 7 , 8 , 9 , 10 , 11 , 12 , 13 Eventually, the interaction between dynein adenine triphosphatase and axoneme generates the bending motion in flagella.
In hamster spermatozoa, the extracellular activation factor is calcium. 14 It is assumed that the pathways for intracellular signal transduction related to motility activation in the hamster spermatozoa are basically the same as described above. In our previous studies, 11 , 12 , 13 , 15 , 16 , 17 , 18 , 19 we have detected many phosphoproteins associated with hamster sperm motility. We recently proposed that sperm motility should be regulated through two types of phosphorylation cascade in hamster spermatozoa, 15 because that the hamster spermatozoa moved slowly even when extracellular calcium was chelated 11 and that the spermatozoa swam vigorously when calcium was present in the medium. 11 From these observations, we redefined sperm motility as four states: preinitiation, initiation, activation and hyperactivatiom. 15 We detected four flagellar phosphoproteins associated with sperm initiation and activation. 13 , 15 At the motility initiation, the 66 kDa protein and the 58 kDa protein were phosphorylated at serine residues. The 58 kDa protein was identified as adenosine triphosphate (ATP) synthase F1 component β subunit that existed in sperm flagellum. 18 On activation, two types of 36 kDa proteins, which were designated as the 36K‐A protein and the 36K‐B protein, were phosphorylated at serine residues in a cAMP‐dependent manner. 13 , 19 The 36K‐A protein was identified as a pyruvate dehydrogenase E1 component β subunit localized at the fibrous sheath of the principal piece of sperm flagellum. 19 However, the 36K‐B protein was also identified as a pyruvate dehydrogenase E1 component β subunit and localized at the middle piece of sperm flagellum. 13
In the present study, we identified the 66 kDa protein, which was one of four phosphoproteins associated with hamster sperm motility.
MATERIALS AND METHODS
Reagents
AGAROSE FOR ISOELECTRIC focusing (IEF) and ampholine were purchased from Amersham‐Biosciences (Buckingham, UK). Trypsin was purchased from Promega (Madison, WI, USA). Other chemicals were of reagent grade from Wako Pure Chemical Industries (Osaka, Japan).
Preparation of demembranated sperm flagellar extracts
Demembranated sperm flagellar extracts were prepared according to the method described in our previous studies 11 with some modifications. 15 Spermatozoa obtained from the cauda epididymis of sexually mature male golden hamsters (Mesocricetus auratus) were suspended in the homogenization buffer containing 200 mM sucrose, 25 mM glutamic acid, 25 mM potassium hydroxide and 20 mM Tris‐HCl (pH 7.9) supplemented with 10 mM phenylmethylsulfonyl fluoride (PMSF) and 20 µg/mL leupeptine and homogenized with a Teflon homogenizer. After the homogenate was diluted into a fourfold volume of the homogenization buffer supplemented with 2.5 mM PMSF and 5 µg/mL leupeptine, they were centrifuged at 750 g for 5 min at 4°C to separate the sperm head and flagellum and the supernatant including flagella was collected. Flagella were demembranated by the homogenization buffer supplemented 1 mM dithiothireitol (DTT), 0.1% (w/v) Triton X‐100 for 30 s at a ambient temperature. Demembranated flagella were collected by centrifugation at 5500 g for 5 min at 4°C and were adjusted to a final protein concentration of 1 mg/mL with the homogenization buffer. After they were precipitated by 10% trichloroacetic acid the precipitate was suspended at 1 mg/mL in a guanidine solution containing 8 M guanidine hydrochroride, 10 mM sodium pyrophosphate, 10% (v/v) 2‐mercaptoethanol, 2% (v/v) Nonidet P‐40 and 0.5 M Tris‐HCl (pH 7.5). The suspension was dialyzed in a urea solution containing 7 M urea and 1% (v/v) 2‐mercaptoethanol before electrophoresis.
Purification of the 66 kDa protein with electrophoresis
In order to purify the 66 kDa protein, demembranated sperm flagellar extracts were subjected to 2‐D gel electrophoresis after the extracts were carried out by sucrose density gradient IEF.
Sucrose density gradient IEF was carried out according to the method of Vesterberg 20 with some modifications. 15 Sucrose density gradient was made from 0 to 50% (v/v) together with an ampholine (pH 3.5–10) as the carrier ampholite. Urea was added to all solutions at 3 M as a final concentration.
2‐D gel electrophoresis combined with agarose‐IEF and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) was carried out according to the method of Hirabayashi 21 with some modifications. 22 First dimension IEF was performed on cylindrical agarose gels at 4°C with a mixture of two types of ampholines (pH 4–6 and pH 3.5–10 used at 2 : 1) as carrier ampholite. SDS‐PAGE was carried out according to the method of Laemmli. 23 The separating gel used was 10% (w/v) polyacrylamide containing 0.1% (w/v) SDS.
Peptide mass finger printing
Peptide mass finger printing was carried out according to our previous study. 13 After 2‐D gel electrophoresis, a spot of the 66 kDa protein was dried with acetonitoril and then soaked in 100 mM ammonium bicarbonate containing 10 µg/mL trypsin for in‐gel digestion. After the incubation, the supernatant was subjected to mass spectrometry with MALDI‐TOF‐MASS (M@LDI, Micromass, Manchester, UK). Based on results obtained by mass spectrometry, the 66 kDa protein was analyzed by means of mass spectrometry (MS)‐Fit (http://prospector.ucsf.edu/ucsfhtml4.0/msfit.htm).
Amino acid sequence analysis with LC‐MS/MS
Amino acid sequence analysis with LC‐MS/MS was carried out according to our previous study. 13 After 2‐D gel electrophoresis, a spot of the 66 kDa protein was soaked in 40 µL of 100 mM ammonium bicarbonate containing 10 µg/mL trypsin. After digested peptides were extracted from spots, they were subjected to mass spectrometry with an LC‐MS/MS (MAGIC2002 high‐performance liquid chromatography ultra violet, Michrom Bioresources, Auburn, CA, USA; LC‐Q ion‐trap mass spectrometer; Thermo Finnigan, San Jose, CA, USA). Based on the results obtained by mass spectrometry, the 66 kDa protein was analyzed with a TurboSequest (Thermo Finnigan). Furthermore, several amino acid sequences obtained by mass spectrometry were subjected to a blast search (http://blast.genome.ad.jp/).
Amino acid sequence analysis with a peptide sequencer
Amino acid sequence analysis with a peptide sequencer was carried out according to our previous study. 13 Amino acid sequence analysis of prepared peptides was performed with a peptide sequencer (PPSQ‐21; Shimadzu, Kyoto, Japan). After 2‐D gel electrophoresis, spots were transferred to a polyvinylidene fluoride (PVDF) membrane (Immobilone Psq; Millipore, Bedford, MA, USA) and were subjected to amino acid sequence analysis. Based on the amino acid sequence analysis results, the 66 kDa protein was identified by means of FASTA (http://fasta.genome.ad.jp/).
RESULTS
IN ORDER TO identify the 66 kDa protein, it was purified using sucrose density gradient IEF (Fig. 1) and 2‐D gel electrophoresis (Fig. 2). The fractions of pH 4.5–6 15 of the 66 kDa protein were collected (Fig. 1) and carried out with 2‐D gel electrophoresis (Fig. 2). The 66 kDa protein was purified as a protein spot from those fractions using 2‐D gel electrophoresis (Fig. 2). As the spot of the 66 kDa protein consisted of a single component, 15 it was analyzed by peptide mass finger printing after the spot of the 66 kDa protein was digested by trypsin (Fig. 3). As shown in Fig. 3a, many peptide materials of the 66 kDa protein and trypsin were detected. Sixteen materials were obtained and matched to the tubulin β chain from the result of database search (Fig. 3b,c). As shown in Fig 3b, 16 materials corresponded to sequences of tubulin β chain, respectively. The corresponding sequences of the 16 materials were overlapped to human tubulin β chain (Fig. 3c). The coverage of those 16 peaks was 33.93% from the results of the database search. For precision, the 66 kDa protein was analyzed using LC‐MS/MS (Fig. 4). As shown in Fig. 4a, many peptide materials of the 66 kDa protein and trypsin were detected. From the results of the analysis of MS/MS for each materials, 20 materials were originated in the 66 kDa protein (Fig. 4a,b). From the results of the database search, amino acid sequences of those materials also matched to human tubulin β chain (Fig. 4c). Coverage of those 20 peaks was 51% by amino acid count.
Figure 1.
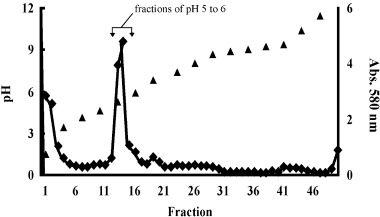
Sucrose density gradient isoelectric focusing (IEF) of flagellar extracts. After sucrose density gradient IEF and fractionation of 2 mL, the pH and the absorbance at 580 nm of each fraction were measured. Most proteins were recovered in acidic pH fractions, and the pH of fractions 13, 14 and 15 was from pH 4.5 to pH 6. (▴), pH; (◆), absorbance.
Figure 2.
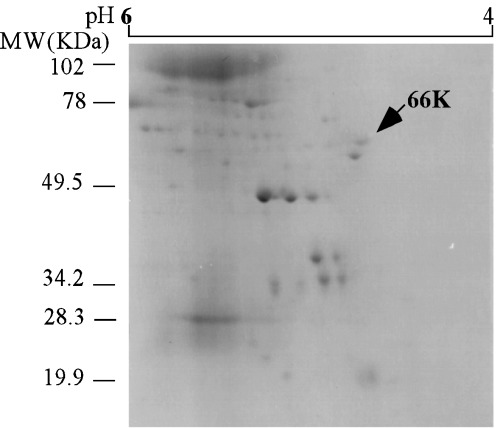
2‐D gel electrophoresis of pH 5 fraction of sucrose density gradient isoelectric focusing. The arrow on the photograph indicates the 66 kDa protein. Bars on the left side of photograph show molecular weight standards. Bar on the top of photograph shows the isoelectric point range.
Figure 3.

Peptide mass finger printing of the 66 kDa protein. (a) Mass spectrogram of the 66 kDa protein digested by trypsin. Numbers on spectrogram indicate molecular mass of 16 peaks originated from the 66 kDa protein. (b) Results of data base search using mass spectrometry‐fit. Amino acid sequences of 16 peaks originated from the 66 kDa protein were estimated. All estimated amino acid sequences were involved in the tubulin β chain. (c) Amino acid sequences of human tubulin β chain. Netted sequences indicate area covered by the results of peptide mass finger printing.
Figure 4.
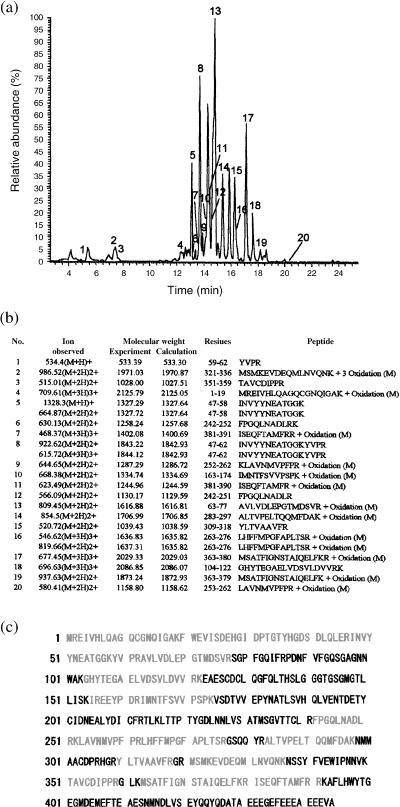
Liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) analysis of the 66‐Da protein. (a) Chromatogram of the 66 kDa protein digested by trypsin. Many peaks originated from the 66 kDa protein and peaks of self‐digested peptides of trypsin were detected. Twenty‐one peaks of those originated from the 66 kDa protein. Numbers on spectrogram indicate peaks originated from the 66 kDa protein, respectively. (b) Molecular mass and amino acid sequences of each peak originated from the 66 kDa protein. All estimated amino acid sequences were identical to the tubulin β chain from the results of data base search. (c) Amino acid sequences of human tubulin β chain. Red letters indicate sequences covered by the results of LC‐MS/MS analysis.
Furthermore, the spot of the 66 kDa protein was analyzed at the N‐terminal sequences using peptide sequencer. N‐terminal amino acid sequences of the 66 kDa protein were MREIVHLQAGQ. They corresponded to the N‐terminal sequences of tubulin β chain from the result of the database search.
DISCUSSION
PROTEIN PHOSPHORYLATION IS the essential event in the signal transduction which regulates sperm motility. It has been accepted that protein phosphorylation associated with sperm motility is generally a result of cAMP and cAMP‐dependent protein kinase as sperm motility depends on cAMP. In many previous studies it was found that many proteins were cAMP‐dependent phosphorylated in association with sperm motility. For example, a 58 kDa protein, named axokinin, in dog spermatozoa, 7 65 kDa proteins in mouse spermatozoa, 10 36 kDa and 10 kDa proteins in hamster spermatozoa, 11 , 12 , 13 20 kDa and 26 kDa proteins in ascidian spermatozoa 24 and 40 kDa, 20 kDa and 15 kDa proteins in salmon spermatozoa. 25 , 26 , 27 , 28 However, it was also found that many cAMP‐independent protein phosphorylation is associated with sperm motility in several animals, for example, boar, 29 goat, 30 hamster, 15 , 31 , 32 , 33 , 34 , 35 human, 36 , 37 , 38 mouse, 39 and sea urchin 40 spermatozoa. Those phosphoproteins were associated with activation and/or hyperactivation of sperm motility. Recently, it was demonstrated that the small G‐protein was associated with the regulatory mechanism of sperm motility. 41 It was demonstrated that approximately 80 kDa tyrosine phosphoprotein detected in several mammalian spermatozoa was A‐kinase anchoring protein. 42 , 43 Furthermore, it was suggested that 58 kDa and 36 kDa proteins detected in hamster spermatozoa was ATP synthase F1 component β subunit 18 and pyruvate dehydrogenase E1 component β subunit, 13 , 19 respectively. In salmon and trout spermatozoa, 26 it was shown that 40 kDa and 20 kDa proteins was catalytic subunit of A‐kinase and dynein light chain, respectively. However, other phosphoproteins were not identified.
In our previous studies, 11 , 15 we suggested that hamster sperm motilities, the slow movement and the vigorous movement, are regulated through two types of phosphorylation cascade. The former slow movement was triggered independently of extracellular activation factors and was essential for starting sperm motility. We defined the start of motility as ‘initiation’. The latter vigorous movement was extracellular activation factor‐dependent. We defined the activation of motility as ‘activation’. We detected four flagellar phosphoproteins associated with sperm initiation and activation. 15 At the motility initiation, the 66 kDa protein and the 58 kDa protein were phosphorylated at serine residues. In the present experiment, the 66 kDa protein was identified as tubulin β chain. The 58 kDa protein was identified as ATP synthase F1 component β subunit in a previous study. 18 On activation, two types of 36 kDa proteins, which were designated as the 36K‐A protein and the 36K‐B protein, were phosphorylated at serine residues in a cAMP‐dependent manner. 13 Both the 36K‐A protein and the 36K‐B protein were identified as a pyruvate dehydrogenase E1 component β subunit in previous studies. 13 , 19 Proteins detected and identified in our previous studies 13 , 18 , 19 were phosphoproteins associated with metabolism, although they were detected as phosphorylation associated with sperm motility. We understand that those proteins were phosphorylated associating with ATP supply when spermatozoa move. Only the 66 kDa protein was not related to metabolism as it was identified as tubulin.
In hamster spermatozoa, the 66 kDa protein was tubulin β chain. However, the 66/65 kDa phosphoprotein was also detected from mouse spermatozoa. 10 , 39 Is mouse 66/65 kDa protein also tubulin β chain? It was shown that mouse 66/65 kDa protein was phosphorylated at serine residues in a cAMP dependent manner. 10 , 39 In contrast, phosphorylation of hamster 66 kDa protein did not depend on cAMP, although this protein was phosphorylated at serine residues. 15 Therefore, it seems that mouse 66/65 kDa protein differs from hamster 66 kDa protein.
Tubulin is the most major component of axoneme in sperm flagellum. We could not show the isoform type of tubulin from data obtained in the present experiment. Although tubulin can be phosphorylated, it is not clear whether the phosphorylation of tubulin is associated with sperm motility. The phosphorylation of 66 kDa protein increased associated with the initiation of sperm motility, but it occurred at a low level before the initiation of sperm movement. 15 Recently, it was reported that the 15 kDa phosphoprotein detected in salmon sperm contained tubulin‐like sequences 28 and localized at the basal portion of sperm flagellum with protein kinase. 44 , 45 As tubulin is a major cytoskeleton protein in sperm flagellum, function of tubulin have not been examined in signal transduction regulated sperm motility. However, tubulin may play a role in sperm motility regulation as phosphoproteins associated with sperm motility were tubulin and/or tubulin‐like protein.
ACKNOWLEDGMENTS
WE THANK STAFF of the Laboratory Animal Research Center of Dokkyo University School of Medicine for their support, and were supported by a Grant‐in‐Aid for Scientific Research (No.15790860) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1. Morisawa M. Cell signaling mechanisms for sperm motility. Zool Sci 1994; 11: 647–662. [PubMed] [Google Scholar]
- 2. Yanagimachi R. Mammalian fertilization In: Knobil E, Neill JD. (eds). The Physiology of Reproduction, Vol. 1 New York: Raven Press, 1994; 189–317. [Google Scholar]
- 3. Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zool Sci 2003; 20: 1043–1056. [DOI] [PubMed] [Google Scholar]
- 4. Hyne RV, Garbers DL. Regulation of guinea pig sperm adenylate cyclase by calcium. Biol Reprod 1979; 21: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 5. Okamura N, Sugita Y. Activation of spermatozoan adenylate cyclase by a low molecular weight factor in porcine seminal plasma. J Biol Chem 1983; 258: 13056–13062. [PubMed] [Google Scholar]
- 6. Wade MA, Jones RC, Murdoch RN, Aitken RJ. Motility activation and second messenger signaling in spermatozoa from rat cauda epididymidis. Reproduction 2003; 125: 175–183. [DOI] [PubMed] [Google Scholar]
- 7. Tash JS, Kakar SS, Means AR. Flagellar motility requires the cAMP‐dependent phosphorylation of a heat‐stable NP‐40‐soluble 56 kd protein, axokinin. Cell 1984; 38: 551–559. [DOI] [PubMed] [Google Scholar]
- 8. Tash JS, Krinks M, Patel J, Means RL, Klee CB, Means AR. Identification, characterization, and functional correlation of calmodulin‐dependent protein phosphatase in sperm. J Cell Biol 1988; 106: 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tash JS, Bracho GE. Regulation of sperm motility: emerging evidence for a major role for protein phosphatases. J Androl 1994; 15: 505–509. [PubMed] [Google Scholar]
- 10. Si Y, Okuno M. Activation of mammalian sperm motility by regulation of microtubule sliding via cyclic adenosine 5′‐monophosphate‐dependent phosphorylation. Biol Reprod 1995; 53: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 11. Si Y, Okuno M. Regulation of microtubule sliding by a 36‐kDa phosphoprotein in hamster sperm flagella. Mol Reprod Dev 1999; 52: 328–334. [DOI] [PubMed] [Google Scholar]
- 12. Fujinoki M, Ohtake H, Okuno M. Tyrosine phosphorylation and dephosphorylation associated with motility of hamster spermatozoa. Biomed Res 2001; 22: 147–155. [Google Scholar]
- 13. Fujinoki M, Kawamura T, Toda T et al. Identification of 36k‐Da flagellar phosphoproteins associated with hamster sperm motility. J Biochem 2003; 133: 361–369. [DOI] [PubMed] [Google Scholar]
- 14. Feng B, Bhattacharyya A, Yanagimachi R. Ca2+ is essential for the motility of plasma membrane‐intact, but not of demembranated, hamster spermatozoa. Andrologia 1987; 20: 155–162. [DOI] [PubMed] [Google Scholar]
- 15. Fujinoki M, Ohtake H, Okuno M. Serine phosphorylation of flagellar proteins associated with the motility activation of hamster spermatozoa. Biomed Res 2001; 22: 45–58. [Google Scholar]
- 16. Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol Reprod 1999; 61: 240–246. [DOI] [PubMed] [Google Scholar]
- 17. Si Y. Hyperactivation of hamster sperm motility by temperature‐dependent tyrosine phosphorylation of an 80‐kDa protein. Biol Reprod 1999; 61: 247–252. [DOI] [PubMed] [Google Scholar]
- 18. Fujinoki M, Kawamura T, Toda T et al. Identification of the 58k‐Da phosphoprotein associated with motility initiation of hamster spermatozoa. J Biochem 2003; 134: 559–565. [DOI] [PubMed] [Google Scholar]
- 19. Fujinoki M, Kawamura T, Toda T et al. Identification of 36k‐Da phosphoprotein in fibrous sheath of hamster spermatozoa. Comp Biochem Physiol 2004; 137B: 509–520. [DOI] [PubMed] [Google Scholar]
- 20. Vesterberg O. Isoelectric focusing of proteins In: Methods in Enzymology, Vol. 22. New York: Academic Press, 1971; 399–412. [Google Scholar]
- 21. Hirabayashi T. Two‐dimensional gel electrophoresis of chicken skeletal muscle proteins with agarose gels in the first dimension. Anal Biochem 1981; 117: 443–451. [DOI] [PubMed] [Google Scholar]
- 22. Fujinoki M, Tomiyama T, Ishimoda‐Takagi T. Tropomyosin isoforms presents in the sea anemone, Anthopleura japonica (Anthozoan, Cnidaria). J Exp Zool 2002; 293: 649–663. [DOI] [PubMed] [Google Scholar]
- 23. Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 24. Nomura M, Inaba K, Morisawa M. Cyclic AMP‐ and calmodulin‐dependent phosphorylation of 21 and 26 kDa proteins in axoneme is a prerequisite for SAAF‐induced motile activation in ascidian spermatozoa. Dev Growth Differ 2000; 42: 129–138. [DOI] [PubMed] [Google Scholar]
- 25. Morisawa M, Hayashi H. Phosphorylation of a 15K axonemal protein is the trigger initiate trout sperm motility. Biomed Res 1985; 6: 181–184. [Google Scholar]
- 26. Inaba K, Morisawa S, Morisawa M. Proteasomes regulate the motility of salmonid fish sperm through modulation of cAMP‐dependent phosphorylation of an outer arm dynein light chain. J Cell Sci 1998; 111: 1105–1115. [DOI] [PubMed] [Google Scholar]
- 27. Itoh A, Inaba K, Fujinoki M, Morisawa M. Motility‐associated and cyclic AMP‐dependent protein phosphorylation in the sperm of the chum salmon, Oncorhynchus keta . Biomed Res 2001; 22: 241–248. [Google Scholar]
- 28. Itoh A, Fujinoki M, Kawamura T et al. Purification and characterization of the 15‐kDa protein from the sperm flagella of salmonid fishes. Biomed Res 2003; 24: 153–164. [Google Scholar]
- 29. Kaláb P, Peknicová J, Geussová G, Moos J. Regulation of protein tyrosine phosphorylation in boar sperm through a cAMP‐dependent pathway. Mol Reprod Dev 1998; 51: 304–314. [DOI] [PubMed] [Google Scholar]
- 30. Nath D, Majumder GC. Maturation‐dependent modification of the protein phosphorylation profile of isolated goat sperm plasma membrane. J Reprod Fertil 1999; 115: 29–37. [DOI] [PubMed] [Google Scholar]
- 31. Uma Devi K, Jha K, Patil SB, Padma P, Shivaji S. Inhibition of motility of hamster spermatozoa by protein tyrosine kinase inhibitors. Andrologia 2000; 32: 95–106. [DOI] [PubMed] [Google Scholar]
- 32. Kula Nand J, Shivaji S. Capacitation‐associated changes in protein tyrosine phosphorylation, hyperactivation and acrosome reaction in hamster spermatozoa. Andrologia 2001; 33: 95–104. [DOI] [PubMed] [Google Scholar]
- 33. Patil SB, Kula Nand J, Padma P, Shivaji S. Reactivation of motility of demembranated hamster spermatozoa: role of protein tyrosine kinase and protein phosphatases. Andrologia 2002; 34: 74–86. [DOI] [PubMed] [Google Scholar]
- 34. Kula Nand J, Shivaji S. Identification of the major tyrosine phosphorylated protein of capacitated hamster spermatozoa as a homologue of mammalian sperm A kinase anchoring protein. Mol Reprod Dev 2002; 61: 258–270. [DOI] [PubMed] [Google Scholar]
- 35. Kula Nand J, Shivaji S. Protein serine and threonine phosphorylation, hyperactivation and acrosome reaction in in vitro capacitated hamster spermatozoa. Mol Reprod Dev 2002; 63: 119–130. [DOI] [PubMed] [Google Scholar]
- 36. Naz RK. Involvement of protein serine and threonine phosphorylation in human sperm capacitation. Biol Reprod 1999; 60: 1402–1409. [DOI] [PubMed] [Google Scholar]
- 37. Bajpai M, Doncel GF. Involvement of tyrosine kinase and cAMP‐dependent kinase cross‐talk in the regulation of human sperm motility. Reproduction 2003; 126: 183–195. [DOI] [PubMed] [Google Scholar]
- 38. Ficarro S, Chertihin O, Westbrook VA et al. Phosphoproteome analysis of capacitated human sperm. J Biol Chem 2003; 278: 11579–11589. [DOI] [PubMed] [Google Scholar]
- 39. Tash JS, Bracho GE. Identification of phosphoproteins coupled to initiation of motility in live epididymal mouse sperm. Biochem Biophys Res Commun 1998; 251: 557–563. [DOI] [PubMed] [Google Scholar]
- 40. Bracho GE, Fritch JJ, Tash JS. Identification of flagellar proteins that initiate the activation of sperm motility in vivo . Biochem Biophys Res Commun 1998; 242: 231–237. [DOI] [PubMed] [Google Scholar]
- 41. NagDas SK, Winfrey VP, Olson GE. Identification of Ras and its downstream signaling elements and their potential role in hamster sperm motility. Biol Reprod 2002; 67: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 42. Carrera A, Moos J, Ning XP et al. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin‐dependent mechanism. Identification of A kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol 1996; 180: 284–296. [DOI] [PubMed] [Google Scholar]
- 43. Visconti PE, Johnson LR, Oyaski M et al. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol 1997; 192: 351–363. [DOI] [PubMed] [Google Scholar]
- 44. Hayashi H, Yamamoto K, Yonekawa H, Morisawa M. Involvement of tyrosine protein kinase in the initiation of flagellar movement in rainbow trout spermatozoa. J Biol Chem 1987; 262: 16692–16698. [PubMed] [Google Scholar]
- 45. Jin ZX, Inaba K, Manaka K, Morisawa M, Hayashi H. Monoclonal antibodies against the protein complex that contains the flagellar movement‐initiating phosphoprotein of Onchorhynchus keta . J Biochem 1994; 115: 885–890. [DOI] [PubMed] [Google Scholar]


