Abstract
Aim: The present study was designed to investigate the effect of amino acids and their dipeptides in the medium related to the urea cycle on the motility, viability, acrosome reaction (AR) and accumulation of ammonia in the medium over different incubation periods in porcine spermatozoa and to assess the utilization of glucose.
Methods: Porcine spermatozoa were washed, swim‐up and incubated at 37°C for 0–4 h in mTALP medium supplemented with 75–600 µmol/L ammonia. Amino acids (1.0 mmol) or their dipeptides (2.0 mmol) were added individually to the mTALP medium containing either no ammonia or 300 µmol/L of ammonia. The viability and AR of porcine spermatozoa were assessed using the triple‐staining technique and the accumulation of ammonia in the medium was measured using the indophenol method.
Results: The motility, viability and AR were adversely affected (P < 0.05) by concentrations of ammonia ≥300 µmol/L compared with the control. Supplementation of l‐alanyl‐l‐glutamine (AlaGln), l‐glycyl‐l‐glutamine (GlyGln) and AlaGln + GlyGln in the presence of 300 µmol/L ammonia significantly increase (P < 0.05) the rate of motility, viability, AR, incorporation, accumulation of ammonia and oxidation of 14C(U)‐glucose compared with the ammonia supplement control.
Conclusion: AlaGln and GlyGln in mTALP medium were more stable and effective than the individual amino acids in reducing the accumulation of ammonia, and subsequently increasing the rate of AR and the utilization of glucose in porcine spermatozoa. (Reprod Med Biol 2008; 7: 123–131)
Keywords: amino acids, ammonia, acrosome reaction, dipeptides, porcine spermatozoa
INTRODUCTION
AMINO ACIDS PLAY an important role in chemically defined medium for the culture of in vitro fertilization (IVF) in several mammalian species, including porcine species. 1 It has been suggested that some of the amino acids that are detected in seminal plasma enhance spermatozoa metabolism and motility. 2 Sperm motility, viability and acrosome reaction (AR) depend strongly on the medium used during the process of in vitro manipulation.
Ammonia toxicity for the living cell is well recognized in vitro and in vivo. 3 , 4 Ammonia levels increase significantly during the culture period because of the deamination and spontaneous breakdown of amino acids, particularly glutamine, in the medium. 5 , 6 The increased levels of ammonia decrease the pH and increase the osmolality, leading to a progressive loss in sperm motility. 7 The toxic effect of glutamine can be avoided by making use of the fact that dipeptides can be directly used by mammalian cells in vitro. 8 Dipeptides such as l‐alanyl‐l‐glutamine (AlaGln) and l‐glycyl‐l‐glutamine (GlyGln) are stable in solution and are suitable as a source of glutamine. 9 Glutamine‐containing dipeptides such as AlaGln and GlyGln have also been considered as a replacement for glutamine in culture systems, 10 and the addition of these dipeptides might achieve higher rates of embryo development in the mouse. Biggers et al. 11 reported that the addition of the dipeptides AlaGln and GlyGln can play an important role in in vitro preimplantation in mouse embryos. Our previous study showed that the accumulation of ammonia can be reduced in the medium by supplementation with the dipeptides AlaGln and GlyGln, which can play an important role in in vitro maturation and fertilization of porcine oocytes. 12
The AR involves the fusion of the outer acrosomal membrane with the plasma membrane and is of fundamental importance for the spermatozoa to fertilize oocytes. Many substances, such as bovine serum albumin, 13 relaxin 14 and fatty acids, 15 have been used for the induction of in vitro AR in porcine spermatozoa. Amino acids containing medium that is commonly used with bull spermatozoa has been found to have effects on motility and AR. 16 Flaherty et al. 17 reported that the addition of some amino acids induced AR without altering the progressive motility and viability of bovine spermatozoa. However, there have been no reports concerning the effect of amino acids and dipeptides on the AR in porcine spermatozoa. As an exogenous energy source, glucose has various effects on mammalian spermatozoa and is required for the stimulation of hyperactivated sperm motility and AR in some species. 18
The present study was designed to investigate the effect of amino acids and their dipeptides related to the urea cycle on the motility, viability, AR and accumulation of ammonia in the medium over different incubation periods in porcine spermatozoa and to assess the utilization of glucose.
METHODS
Chemicals
RADIOACTIVE GLUCOSE WAS purchased from American Radiolabeled Chemicals (St Louis, MO, USA). Ammonia, glutamine (Gln), alanine (Ala), glycine (Gly), l‐alanyl‐l‐glutamine (AlaGln), l‐glycyl‐l‐glutamine (GlyGln) and all other chemicals were analytical grade and were purchased from Nacalai Tesque (Kyoto, Japan).
Semen collection and preparation
Semen was collected from 2‐year‐old Duroc boars using the gloved‐hand technique at the Nagano Animal Industry Experiment Station, Nagano, Japan. The semen was diluted according to the method of Johnson et al. 19 with Modena extender to produce a sperm concentration of 1 × 108/mL at room temperature. The tube of semen was carried to the laboratory within 30 min using a cork box to keep it at 21°C. The uppermost 10 mL of the semen sample was removed from the tube and incubated for 10 min in a water bath at 37°C for anabiosis. Then, 3 mL of the anabiosed sperm sample was washed twice with mTALP medium 20 and centrifuged at 900 g for 5 min. The sperm pellet was suspended in mTALP medium and pre‐incubated at 37°C, 95% humidified air and 5% CO2 for 1 h to swim‐up. A 50 µL sperm aliquot obtained from the swim‐up separation was resuspended into the previously prepared drop with the specified medium to give a final concentration of spermatozoa of 5 × 106/mL. The sperm was than incubated at 37°C, 95% humidified air and 5% CO2 for 0–4 h to evaluate motility. To evaluate the viability and acrosome status, swim‐up spermatozoa were resuspended separately at a concentration of 20 × 106/mL and incubated at 37°C for 0–4 h using the same conditions.
Semen treatments
The effect of exogenous ammonia on the motility, AR and viability of spermatozoa was examined by adding 0 (control), 75, 150, 300, 450 and 600 µmol/L ammonium chloride (ammonia) to mTALP medium. The effect of individual amino acids (1.0 mmol), dipeptides (2.0 mmol) and their combination (1.0 mmol + 1.0 mmol) was examined over a 0–4 h incubation period. The accumulation of ammonia, and the motility, AR and viability were evaluated by adding amino acids (1.0 mmol), dipeptides (2.0 mmol) and their combination (1.0 mmol + 1.0 mmol) to medium containing 300 µmol/L ammonia. A 50 µL sperm aliquot obtained from swim‐up separation was resuspended in previously prepared drops of medium containing ammonia, amino acids and dipeptides to produce a final sperm concentration of 5 × 106/mL and was incubated at 37°C for 0–4 h to evaluate sperm motility. To evaluate acrosome status, the swim‐up spermatozoa were resuspended separately in mTALP medium with or without ammonia, amino acids and dipeptides to produce a final concentration of 20 × 106/mL and were incubated at 37°C for 0–4 h. After supplementation of all the chemicals, the pH (HM‐30 s; Tokyo TOA Electronics, Tokyo, Japan) and osmolarity (Model 3300; Advanced Instruments, Norwood, MA, USA) of these medium were adjusted to 7.4 with 1 N HCl or 1 N NaOH and to 270 mOsm with NaCl, respectively.
Progressive motility
After completion of the 0–4 h incubation period, three subsamples (10 µL) of each sample were transferred to an examination chamber (Fujihire Industry, Tokyo, Japan), placed on a warmer set (MPF‐10‐SZX; Kitazato Supply, Tokyo, Japan) kept at 37°C, and the motility was examined under a light microscope (100×). Sperm motility was assessed by determining the percentage of spermatozoa in each of the following four categories of movement: (a) rapid progressive motility; (b) slow or sluggish progressive motility; (c) non‐progressive motility; and (d) immotility. Motility was then expressed as a progressive motility percentage. The percentage of spermatozoa with progressive motility was determined subjectively by scoring 400 individual spermatozoa in each sample.
Acrosome reaction and viability
To evaluate the AR and viability, spermatozoa were stained using the triple‐staining technique as described by Ooba et al. 21 Namely, dead spermatozoa were stained with trypan blue at the postacrosomal region, whereas live spermatozoa were not stained with this dye, but were stained with Bismark brown, staining light brown at the postacrosomal region. The acrosome was stained by Rose Bengal. Therefore, intact acrosomes or unreacted spermatozoa were pink at the acrosomal cap, but reacted spermatozoa or spermatozoa without an acrosome were white. The triple‐stained slides were examined by light microscopy under oil immersion (1000×). The viability and AR of the spermatozoa were evaluated from randomly selected fields of the triple‐stained spermatozoa until 400 spermatozoa had been examined. Based on the staining patterns, the spermatozoa were categorized according to the following characteristics: (a) dead and unreacted acrosome (Fig. 1a); (b) live and unreacted acrosome (Fig. 1b); (c) dead and reacted acrosome (Fig. 1c); and (d) live and acrosome reacted or lost (Fig. 1d).
Figure 1.
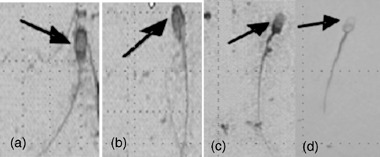
Triple‐stained porcine spermatozoa with different acrosome status. (a) Dead spermatozoa that had not undergone acrosome reaction (AR). (b) Live spermatozoa that had not undergone AR. (c) Dead spermatozoa that had undergone AR. (d) Live spermatozoa that had undergone AR.
Incorporation and oxidation of 14C(U)‐glucose
The metabolic activity of the spermatozoa was examined with or without ammonia by incorporation and oxidation of 14C(U)‐glucose semen samples, which were prepared in the same way as the samples for motility evaluation. A 100 µL aliquot of swim‐up spermatozoa (5 × 106/mL) from each treatment was incubated with 9.25 KBq/mL (specific activity 18.5 MBq/mmol) of 14C(U)‐glucose and prepared for scintillation counting according to the method described in our previous study. 14 The incorporation and oxidation of 14C(U)‐glucose by spermatozoa was determined using a liquid scintillation counter (LS‐6500; Beckman Instruments, Fullerton, CA, USA). The incorporation and oxidation values were expressed directly in c.p.m.
Ammonia determination
During the different incubation periods, the ammonia concentrations in the medium were assessed using the Bertholot–indophenol method as described in our previous study. 22 To determine the ammonia concentration in the medium, 100 µL of the culture medium was removed every 2–4 h and frozen at –40°C until measurement. The procedure was carried out five times for the analysis. A calibration curve in the range 0–0.30 mmol ammonia was run with each experiment. The mean coefficient for determination of the calibration curve of five experiments was 0.994.
Statistical analysis
Data were analyzed by anova using the General Linear Models procedure of the Statistical Analysis System (SAS Institute, Cary, NC, USA). All percentage data were subjected to arc‐sine transformation before statistical analysis. Data are expressed as mean ± standard error (n = 5). Duncan's multiple range tests were used to determine differences among sperm groups. A value of P < 0.05 was considered to be statistically significant.
RESULTS
THE EFFECT OF exogenous ammonia on the percent age of progressive motility, viability and AR in porcine spermatozoa over 0–4 h of incubation is shown in Figure 2. The percentage of progressive motility, viability and AR decreased significantly (P < 0.05) in medium containing 300–600 µmol/L ammonia. However, concentrations of ammonia ≤150 µmol/L did not show any significant adverse effects on progressive motility, viability and AR.
Figure 2.
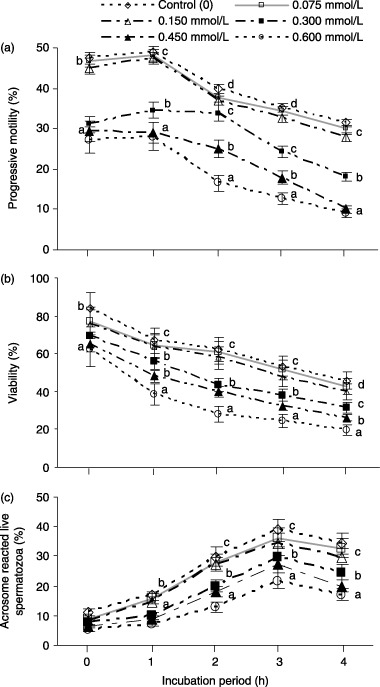
Effect of exogenous ammonia on the percentage of (a) progressive motility, (b) viability and (c) acrosome‐reacted live spermatozoa over a 0–4 h incubation of porcine spermatozoa. Each line with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the same incubation period.
The effects of exogenous ammonia on the rates of incorporation and oxidation of 14C(U)‐glucose by spermatozoa is shown in Figure 3. The incorporation rate of 14C(U)‐glucose was lower (P < 0.05) in ammonia‐treated (300–600 µmol/L) spermatozoa compared with the control over incubation times of 0–4 h. Concentrations of ammonia ≤150 µmol/L did not show any significant differences in the rates of incorporation. The trend of 14C(U)‐glucose oxidation was the same as 14C(U)‐glucose incorporation.
Figure 3.
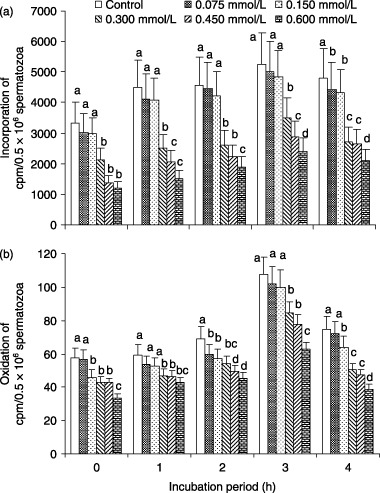
Effect of exogenous ammonia on the rates of (a) incorporation and (b) oxidation of 14C(U)‐glucose in porcine spermatozoa over a 0–4 h incubation. Each line with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the same incubation period.
The effects of each amino acid, dipeptide and their combinations on the percentage of progressive motility, viability and acrosome‐reacted live spermatozoa over 0–4 h of incubation is shown in Figure 4. The progressive motility and viability were significantly (P < 0.05) increased compared with the control after the addition of Ala and Gly, but there were no significant differences in the other amino acids compared with the control. The percentage of acrosome‐reacted live spermatozoa increased after 2–4 h of incubation. After almost 3 h of incubation acrosome‐reacted live spermatozoa were observed to have improved in the AlaGln, GlyGln and AlaGln + GlyGln treated sperm compared with the other amino acids and the control.
Figure 4.
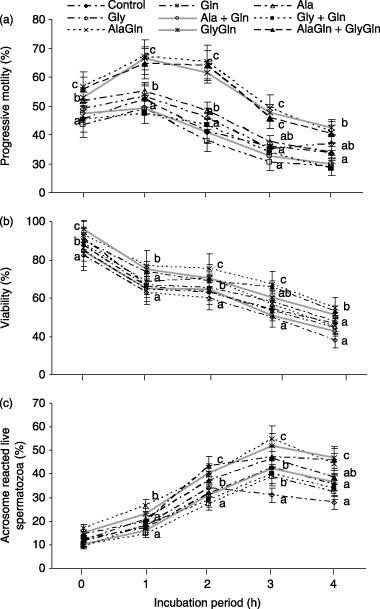
Effect of each amino acid (Gln, glutamine; Ala, alanine; Gly, glycine) at 1.0 mmol and their combinations (1.0 mmol + 1.0 mmol) and each dipeptide (AlaGln, l‐alanyl‐l‐glutamine; GlyGln, l‐glycyl‐l‐glutamine) at 2.0 mmol and their combinations (1.0 mmol + 1.0 mmol) supplemented in mTALP medium of porcine spermatozoa on the percentage of (a) progressive motility, (b) viability and (c) acrosome‐reacted live spermatozoa over a 0–4 h incubation. Each line with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the same incubation period.
The effects of each amino acid, dipeptide and their combinations on the rates of incorporation and oxidation of 14C(U)‐glucose in spermatozoa over 0–4 h of incubation is shown in Figure 5. The rates of incorporation and oxidation were higher after the addition of Ala and Gly compared with AlaGln and GlyGln, and AlaGln + GlyGln treated spermatozoa were observed to be significantly higher after 2–4 h of incubation.
Figure 5.
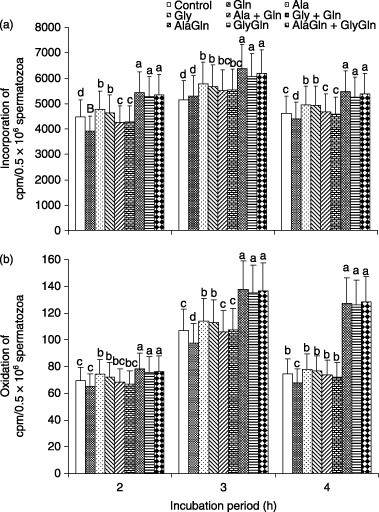
Effect of each amino acid (Gln, glutamine; Ala, alanine; Gly, glycine) at 1.0 mmol and their combinations (1.0 mmol + 1.0 mmol) and each dipeptide (AlaGln, l‐alanyl‐l‐glutamine; GlyGln, l‐glycyl‐l‐glutamine) at 2.0 mmol and their combinations (1.0 mmol + 1.0 mmol) supplemented in mTALP medium of porcine spermatozoa on the percentage of (a) incorporation and (b) oxidation of 14C(U)‐glucose over 2–4 h of incubation. Each line with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the same incubation period.
In the presence of 300 µmol/L ammonia in the medium, the effects of individual amino acids, dipeptides and their combinations on the percentages of progressive motility, viability and AR of porcine spermatozoa over different incubation periods is shown in Figure 6. All of the amino acids, dipeptides and their combinations used in the present study showed individually higher (P < 0.05) percentages of progressive motility, viability and AR compared with the ammonia‐supplemented control. Moreover, AlaGln, GlyGln and AlaGln + GlyGln showed significantly higher (P < 0.05) percentages of spermatozoa progressive motility, viability and AR compared with the other amino acids.
Figure 6.
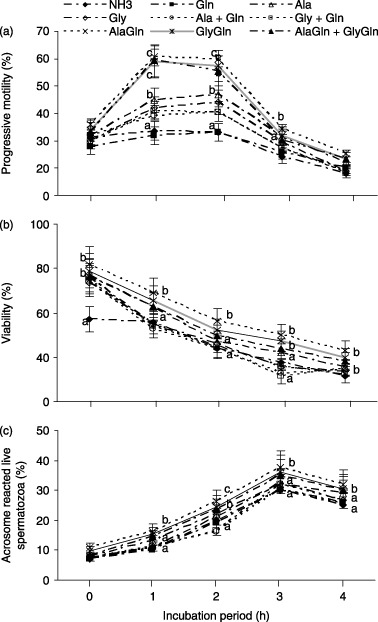
In the presence of 300 mmol/L ammonia (NH3) containing medium (mTALP), the effect of individual amino acids (Gln, glutamine; Ala, alanine; Gly, glycine) at 1.0 mmol and their combinations (1.0 mmol + 1.0 mmol) and each dipeptide (AlaGln, l‐alanyl‐l‐glutamine; GlyGln, l‐glycyl‐l‐glutamine) at 2.0 mmol and their combinations (1.0 mmol + 1.0 mmol) on the percentage of (a) progressive motility, (b) viability and (c) acrosome‐reacted live spermatozoa over a 0–4 h incubation. Each line with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the same incubation period.
The rates of incorporation and oxidation of 14C(U)‐glucose and the accumulation of ammonia in spermatozoa treated by amino acids, dipeptides and their combinations in the presence of 300 µmol/L ammonia is shown in Figure 7. The incorporation rate of 14C(U)‐glucose was higher (P < 0.05) in AlaGln, GlyGln and AlaGln + GlyGln treated spermatozoa than in the ammonia‐supplemented control after 3–4 h of incubation. The trend for 14C(U)‐glucose oxidation was the same as the incorporation. The present study examined whether amino acids or dipeptides reduced the amount of ammonia produced over incubation periods of 2–4 h of spermatozoa with 300 µmol/L ammonia in the medium. The accumulation of ammonia in the medium over different incubation periods was significantly reduced (P < 0.05) after the addition of almost all of the amino acids, dipeptides and their combinations, with the exception of Gln, Ala + Gln and Gly + Gln. Among them, AlaGln, GlyGln and AlaGln + GlyGln played the most important role in reducing the accumulation of ammonia in the culture medium compared with the other amino acids, dipeptides or their combinations.
Figure 7.
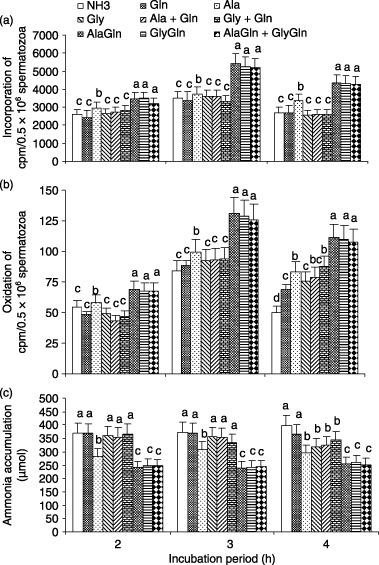
In the presence of 300 mmol/L ammonia (NH3) containing medium (mTALP), the effect of individual amino acids (Gln, glutamine; Ala, alanine; Gly, glycine) at 1.0 mmol and their combinations (1.0 mmol + 1.0 mmol) and each dipeptide (AlaGln, l‐alanyl‐l‐glutamine; GlyGln, l‐glycyl‐l‐glutamine) at 2.0 mmol and their combinations (1.0 mmol + 1.0 mmol) on the percentage of (a) incorporation and (b) oxidation of 14C(U)‐glucose and on the (c) accumulation of ammonia in porcine spermatozoa over a 2–4 h incubation. Each line with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the same incubation period.
DISCUSSION
IN THE PRESENT study, we have shown that motility, viability and AR were adversely affected when more than 300 µmol/L exogenous ammonia was supplemented into the mTALP medium. The observation that ammonia concentration reduces the rate of motility and viability in porcine sperm agrees with the study of Kim et al., 7 who reported that increased levels of ammonia in the culture medium led to progressive loss of human sperm motility. Our previous study 13 reported that the addition of exogenous ammonia to the culture medium for porcine oocytes resulted in a significant decrease in fertilization rate.
In our present study, supplementation of Ala and Gly in the mTALP medium improved the motility and AR of porcine spermatozoa. The optimum concentration of single amino acids or dipeptides or their combinations used in the present study are similar to the concentrations used by hamster sperm 23 and porcine oocytes. 12 Melendrez et al. 24 reported that the addition of Gly initiates the AR of human and porcine spermatozoa. Medium containing Ala, which is commonly used for bovine spermatozoa, has been found to have positive effects on motility and AR. 16 The amino acids Ala and Gly showed the highest rate of motility and AR compared with the other amino acids; thus, the present results are in agreement with the reports of Melendrez et al. 24 and Parrish et al. 16 Ammonium levels increase significantly during the culture period because of the deamination and spontaneous breakdown of amino acids, especially glutamine, in the medium. 5 , 6 In the absence of glutamine, non‐essential amino acids significantly stimulated the rate of sperm motility and AR. It is not known how glutamine confers its benefit to the oocytes, but it is most likely to be utilized as an energy source for the developing conceptus. 6 Although amino acids are present in the fluids of the female reproductive tract, any ammonium produced by the embryos could be absorbed or neutralized by the epithelial cells of the oviduct and uterus, thereby negating any possible toxic effects. 5 Tosic and Walton 25 reported that dead sperm released an enzyme called ‘amino acid oxidase’, which dehydrogenates and deaminates aromatic amino acids and produces hydrogen peroxides and ammonia. Ammonium in the culture medium may affect the developing embryo in several ways: ammonium could decrease the concentration of α‐ketoglutarate by its conversion to glutamate. 5
In the present study, we suggest that Ala and Gly have a positive effect on porcine sperm function; these amino acids induced AR without altering sperm motility and viability. Other amino acids used in this medium play a role in inhibiting sperm function. However, supplementation with dipeptides may recover the loss of sperm motility and AR. The amino acids AlaGln and GlyGln showed higher percentages of motility and AR than almost all of the individual amino acids. Eagle 8 reported that in in vitro culture medium, AlaGln and GlyGln are more stable and effective than the individual amino acids in improving mammalian cells. The results from the present study strongly support the idea that AlaGln and GlyGln can improve spermatozoa motility and AR. Our previous study 12 reported that the addition of AlaGln and GlyGln can play an important role in fertilization of porcine oocytes. An alternative is the use of medium containing glutamine dipeptides (e.g. AlaGln and GlyGln), which are stable in solution and are consumed by cells efficiently as glutamine. 26 Both glutamine‐containing dipeptides and glutamate have been used as substitutes for glutamine. AlaGln and GlyGln can replace glutamine in animal cell cuture. 27 However, hydrolysis by a cellular peptidase can result in the release of high levels of glutamine in the medium. 27
In mammalian embryos fertilization is characterized by a marked increase in the incorporation of amino acids. 28 This increase in amino acid incorporation reflects a more specific synthetic change than a general increase in metabolic activity. 29 The present study showed that Ala, Gly, AlaGln and GlyGln stimulated the rate of incorporation and oxidation of 14C(U)‐glucose in porcine spermatozoa in almost all incubation periods. Although, under normal physiological circumstances, oxidation of glucose is correlated with oxidation of Ala, Gly, AlaGln and GlyGln. Among them, AlaGln and GlyGln showed the highest rate of incorporation and oxidation. The trend of incorporation and oxidation of glucose is similar to AR and is supported by the findings of our previous study, 14 which also suggested that the incorporation and oxidation of glucose increased for up to 3 h of incubation for the production of more adenosine triphospate (ATP) during AR at that time. Mukai and Okuno 30 reported that glycolysis plays a major role in providing ATP for the flagellar movement of mouse sperm. In general, porcine sperm are effective in supporting sperm motility when glucose is provided as an energy source in the culture medium. Fructose 31 and glucose, 32 as exogenous energy sources, have various effects on spermatozoa and are required for stimulation of the AR in some species. Wongsrikeao et al. 33 reported that supplementation of glucose and fructose to the maturation medium showed similar percentages of total fertilization rates of pig oocytes. However, the inclusion of amino acids and dipeptides in the medium may act to stimulate sperm motility and AR because ATP, cyclic adenosine 3′,5′‐monophoshate (cAMP), adenylate cyclase and phosphodiesterase are known to be intricately involved in the motility, AR and survival of spermatozoa. 34 The G‐proteins activate adenylate cyclase to increase the intracellular cAMP and prevent cAMP breakdown by inhibiting the activity of cAMP phosphodiesterase. 35
In the present study, although Aln and Gly individually had no positive effect on reducing the accumulation of ammonia in the culture medium, their dipeptides AlaGln and GlyGln played a significant role in reducing the accumulation of ammonia in the medium during the incubation of porcine spermatozoa. Our previous study 12 reported that AlaGln and GlyGln in the culture medium reduced the accumulation of ammonia following IVF in porcine oocytes. The present study supports their results and confirms that AlaGln and GlyGln are able to overcome the accumulation of ammonia in the culture medium. A routine supplementation of Gln with the more stable forms of AlaGln or GlyGln in the culture medium markedly reduced the accumulation of ammonia in the mouse. 11 From this result, the dipeptides were able to increase the rate of motility, viability and AR by reducing the toxicity of ammonia in the medium.
In conclusion, the addition of Ala, Gly, AlaGln and GlyGln in mTALP medium has a significant effect on reducing the accumulation of ammonia and increases the rate of motility, viability and AR in porcine spermatozoa in vitro. Therefore, the present study suggests that the accumulation of ammonia can be reduced in the medium by supplementation with the dipeptides AlaGln and GlyGln, which can play an important role in the AR of porcine spermatozoa.
REFERENCES
- 1. Ka HH, Sawai K, Wang WH, Im KS, Niwa K. Amino acids in maturation medium and presence of cumulus cells at fertilization promote male pronuclear formation in porcine oocytes matured and penetrated in vitro . Biol Reprod 1997; 57: 1483–1487 [DOI] [PubMed] [Google Scholar]
- 2. Gassner FX, Hopwood ML. Seminal amino acid and carbohydrate pattern of bulls with normal and abnormal testes function. Proc Soc Exp Biol Medical 1952; 81: 37–43. [DOI] [PubMed] [Google Scholar]
- 3. Prior RL, Visek WJ. Effects of urea hydrolysis on tissue metabolite concentrations in rats. Am J Physiol 1972; 223: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 4. Visek WJ, Kolodny GM, Gross PR. Ammonia effects in cultures of normal transformed 3T3 cells. J Cell Physiol 1972; 80: 373–381. [DOI] [PubMed] [Google Scholar]
- 5. Gardner DK, Lane M. Amino acids and ammonium regulate mouse embryo development in culture. Biol Reprod 1993; 48: 377–385. [DOI] [PubMed] [Google Scholar]
- 6. Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod 2003; 69: 1109–1117. [DOI] [PubMed] [Google Scholar]
- 7. Kim SC, Kim HW. Effects of nitrogenous components of urine on sperm motility: an in vitro study. Int J Androl 1998; 21: 29–33. [DOI] [PubMed] [Google Scholar]
- 8. Eagle H. Utilization of dipeptides by mammalian cells in tissue culture. Proc Soc Exp Biol Med 1955; 89: 96–99. [DOI] [PubMed] [Google Scholar]
- 9. Adibi SA, Lochs H, Abumrad NN, Daniel H, Vazquez JA. Removal of glycyl glutamine by individual tissues: mechanism and impact on amino acid fluxes in postabsorption and starvation. J Nutr 1993; 123: 325–331. [DOI] [PubMed] [Google Scholar]
- 10. Roth E, Ollenschlager G, Hamilton G et al Influence of two glutamine‐containing dipeptides on growth of mammalian cells. In Vitro Cell. Dev Biol 1988; 24: 696–698. [DOI] [PubMed] [Google Scholar]
- 11. Biggers JD, McGinnis LK, Lawitts JA. Enhanced effect of glycyl‐L‐glutamine on mouse preimplantation embryos in vitro . Reprod Biomed Online 2004; 9: 59–69. [DOI] [PubMed] [Google Scholar]
- 12. Tareq KMA, Miah AG, Salma U, Yoshida M, Tsujii H. Effect of amino acids and dipeptides on accumulation of ammonia in the medium during in vitro maturation and fertilization of porcine oocytes. Rep Med Biol 2007; 6: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hossain MS, Hyeong LJ, Miah AG, Tsujii H. Effect of fatty acids bound to bovine serum albumin‐V on acrosome reaction and utilization of glucose in boar spermatozoa. Reprod Med Biol 2007; 6: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miah AG, Tareq KMA, Hamano K, Kohsaka T, Tsujii H. Effect of relaxin on acrosome reaction and utilization of glucose in boar spermatozoa. J Reprod Dev 2006; 52: 773–779. [DOI] [PubMed] [Google Scholar]
- 15. Hossain MS, Tareq KMA, Hamano K, Tsujii H. Effect of fatty acid on boar sperm motility, viability and acrosome reaction. Reprod Med Biol 2007; 6: 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parrish JJ, Krogenaes A, Susko‐Parrish JL. Effect of bovine sperm separation by either swim‐up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 1995; 15: 859–869. [DOI] [PubMed] [Google Scholar]
- 17. Flaherty CO, Rodriguez P, Srivastava S. L‐Arginine promotes capacitation and acrosome reaction in cryopreseved bovine spermatozoa. Bio Biop Act 2004; 1674: 215–221. [DOI] [PubMed] [Google Scholar]
- 18. Vandevoort CA, Overstreet JW. Effect of glucose and other energy substrates on the hyperactivated motility of macaque sperm and the zona pellucida induced acrosome reaction. J Androl 1995; 16: 327–333. [PubMed] [Google Scholar]
- 19. Johnson LA, Aalbers JG, Grooten HJG. Artificial insemination of swine: fecundity of boar semen stored in Beltsville TS (BTS), modified Modena (MM), or MR‐A and inseminated on one, three and four days after collection. Zuchthyg 1988; 23: 49–55. [Google Scholar]
- 20. Parrish JJ, Susko‐Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod 1988; 38: 1171–1180. [DOI] [PubMed] [Google Scholar]
- 21. Ooba T, Sricharoen P, Areekijsree M, Kitiyanat Y, Pavasuthipaisit K. Evaluation of acrosome reaction in bovine sperm by a triple staining technique. J Physiol Sci 1990; 3: 91–104. [Google Scholar]
- 22. Tareq KMA, Obata R, Miah AG, Hamano K, Tsujii H. Ammonia concentration in porcine ovarian developing follicles. J Mamm Ova Res 2005; 22: 185–189. [Google Scholar]
- 23. Llanos MN, Ronco AM, Aguirre MC, Meizel S. Hamster sperm glycine receptor: evidence for its presence and involvement in the acrosome reaction. Mol Reprod Dev 2001; 58: 205–215. [DOI] [PubMed] [Google Scholar]
- 24. Melendrez CS, Meizel S. Studies of porcine and human sperm suggesting a role for a sperm glycine receptor/Cl‐ channel in the zona pellucida‐initiated acrosome reaction. Biol Reprod 1995; 53: 676–683. [DOI] [PubMed] [Google Scholar]
- 25. Tosic J, Walton A. Metabolism of spermatozoa. The formation and elimination of hydrogen peroxide by spermatozoa and effects on motility and survival. Biochem J 1950; 47: 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brand K, Fekl W, Hintzenstern J, Langer K, Luppa P, Schroener C. Glutamine metabolism in lymphocytes of the rat. Metabolism 1989; 38: 29. [DOI] [PubMed] [Google Scholar]
- 27. Christie A, Butler M. Glutamine‐based dipeptides are utilized in mammalian cell culture by extracellular hydrolysis catalyzed by a specific peptidase. J Biotechnol 1994; 37: 277–290. [DOI] [PubMed] [Google Scholar]
- 28. Hultin T. Incorporation of N15‐labeled glycine and alanine into the proteins of developing sea urchin eggs. Exp Cell Res 1952; 3: 494. [Google Scholar]
- 29. Brinster RL. Uptake and incorporation of amino acids by the preimplantation mouse embryo. J Reprod Fert 1971; 27: 329–338. [DOI] [PubMed] [Google Scholar]
- 30. Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 2004; 71: 540–547. [DOI] [PubMed] [Google Scholar]
- 31. Tsujii H, Ohta E, Miah AG, Hossain MS, Slma U. Effect of fructose on motility, acrosome reaction and in vitro fertilization capability of boar spermatozoa. Reprod Med Biol 2006; 5: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vandevoort CA, Overstreet JW. Effects of glucose and other energy substrates on the hyperactivated motility of macaque sperm and the zona pellucida‐induced acrosome reaction. J Androl 1995; 16: 327–333. [PubMed] [Google Scholar]
- 33. Wongsrikeao P, Otoi T, Taniguchi M et al Effects of hexoses on in vitro oocyte maturation and embryo development in pigs. Theriogenology 2006; 65: 332–343. [DOI] [PubMed] [Google Scholar]
- 34. Garbers DL, Kopf GS. The regulation of spermatozoa by calcium cyclic nucleotides. Adv Cyclic Nucleotide Res 1980; 13: 251–306. [PubMed] [Google Scholar]
- 35. Bartsch O, Bartlick B, Ivell R. Relaxin signalling links tyrosine phosphorylation to phosphodiesterase and adenylyl cyclase activity. Mol Hum Reprod 2001; 7: 799–809. [DOI] [PubMed] [Google Scholar]


