Abstract
Background : Men presenting with non‐obstructive azoospermia (NOA) caused by germinal failure can now be treated in some cases using testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI). However, TESE is a blind procedure that does not identify the focal sperm‐producing areas until excision of the testicular tissue. Microdissection TESE, which is the only method available for obtaining excised dilated seminiferous tubules under the operating microscope, improves sperm yield with minimal tissue excision in NOA patients.
Methods and Results : We performed this procedure on 16 NOA patients. All subjects underwent a microdissection TESE on the right testis, and triple biopsy on the left testis in consecutive fashion in order to compare the efficacy of microdissection TESE with that of a standard biopsy. Although dilated seminiferous tubules were presented in all patients, spermatozoa were retrieved in only a single patient by microdissection TESE. Furthermore, spermatozoa could not be identified by standard biopsies.
Conclusion : In this series, microdissection TESE did not contribute to spermatozoa recovery in NOA patients. Further study is needed in order to arrive at a reliable assessment of microdissection TESE relative to a standard multiple biopsy in cases of NOA. (Reprod Med Biol 2002; 1: 31–34)
Keywords: microdissection, non‐obstructive azoospermia, testicular sperm extraction
Introduction
IN THE ERA of advanced assisted reproductive technology (ART), testicular sperm extraction (TESE) has been widely used as a means of obtaining spermatozoa for the patients with non‐obstructive azoospermia (NOA). Since the introduction of intracytoplasmic sperm injection (ICSI), testicular spermatozoa can be successfully used to alleviate infertility because of NOA. 1
Spermatogenesis is believed to be limited in NOA patients. Failure of TESE has been reported in NOA patients, and it may occur in over 50%. 1 , 2 , 3 Extensive multiple biopsies may be needed before spermatozoa are found in NOA patients. Some groups have used fine‐needle aspiration for sperm retrieval. Although this technique is minimally invasive, the number of retrieved spermatozoa is very limited. Most investigators accept that open biopsy is required to obtain enough spermatozoa for ICSI. 3 , 4 However, detrimental effects of open biopsy have been documented. Testicular biopsies could result in the loss of large amounts of tissue. Furthermore, these techniques can interrupt the testicular blood supply traveling under the tunica albuginea, creating the possibility of testicular devascularization and subsequently causing decreased testosterone secretion and testicular volume. 5 , 6
Testicular sperm extraction is a blind procedure that does not identify the focal sperm‐producing areas of the testicle until the completion of tissue excision from the patient. Microdissection TESE was initially reported by Schlegel and Li in 1998. 7 This technique indicates that the identification of spermatogenically active regions of the testicle is possible by direct examination of individual seminiferous tubules. 4 , 7 Microdissection TESE involves the concept that seminiferous tubules containing many developing germ cells may be larger and more opaque than tubules lacking sperm production. Schlegel and Li concluded that the availability of microdissection appears to improve the frequency of sperm retrieval; moreover, the removal of less testicular tissue was evident in NOA patients. We report the clinical experience and outcome of microdissection TESE in our institute (Aichi Medical University Hospital, Nagakute, Japan).
Materials and methods
Patients
THIRTY‐SEVEN NON‐OBSTRUCTIVE azoospermic men visited our male infertility clinic from June 1998 to August 2001. Absolute ejaculatory azoospermia was confirmed by several semen analyses. Sixteen individuals, displaying severe bilateral testicular atrophy (under 10 mL in volume) and elevated follicle stimulating hormone (FSH; greater than 20 mIU/mL) levels, were enrolled in the present study. These men demonstrated no evidence of major systemic illness or previous history of urogenital infection. Six patients received previous diagnostic biopsy at other institutes. In these cases, pathological diagnoses were Sertoli cell‐only syndrome. Luteinizing hormone (LH), FSH, testosterone, chromosome count, and testicular size were determined in all patients. To compare the efficacy of microdissection TESE with standard multiple biopsies, subjects underwent microdissection on the right testis and triple biopsies on the left side in consecutive fashion. Excised weight was also evaluated.
Operation
The operation was conducted under local anesthesia with a sedative. A spermatic cord block was achieved with 1% lidocaine. A scrotal vertical incision was effected. The right testis was exposed and then observed under an operating microscope. A stay suture (4–0 polypropylene) was placed into the tunica albuginea of the testis. A full transverse incision was made with careful attention to avoid subtunical vessels. The testicular tissue was dissected bluntly. Bleeding was controlled by bipolar cautery; however, its application was minimized so as to avoid local heat that may have proved harmful to spermatozoa. Upon isolation of larger seminiferous tubules, excision was performed. This procedure was continued until spermatozoa were located or an hour had elapsed. Each excised specimen was collected in center‐well organ culture dishes (Becton Dickinson Labware, Franklin Lakes, NJ, USA) filled with sperm‐washing medium (modified human tubal fluid; Irvine Scientific Co., Santa Ana, CA, USA). Specimens were subsequently cut into smaller portions in order to allow spermatozoa release from the inside of the seminiferous tubules. The tunica albuginea was sutured with 4–0 polypropylene via a running method.
Following the termination of this procedure, the left testis was exposed and triple open biopsies were performed. Excised specimens were also placed in sperm‐washing medium. Dispersal was effected via isolation and mincing of individual seminiferous tubules with glass slides. Tunica vaginalis was sutured and skin closure was achieved by using a stapler. A drainage tube was not maintained; however, scrotal support was provided. Differences in excised tissue weight between microdissection TESE and triple biopsies were analyzed statistically by using anova. Fisher’s test was used for post‐hoc comparisons if P < 0.05. All the data were expressed as mean ± SEM.
Results
THE 16 PATIENTS ranged in age from 25 to 50 years (mean 32.4 years). Patient spouses ranged in age from 24 to 36 years (mean 30.6 years). Testicular volume was 6.0 ± 0.8 mL (range; 2–10) in the right testis and 5.0 ± 0.7 mL (range; 2–10) in the left testis. Serum LH value was 9.69 ± 1.67 mIU/mL (range; 3.6–18.6). Follicle stimulating hormone and testosterone values were 38.11 ± 6.32 mIU/mL (range; 20.6–76.7), and 380 ± 55 ng/dL (range; 64–577), respectively. All subjects displayed a normal chromosome count with the exception of two patients presenting with Klinefelter’s syndrome (non‐mosaic, 47XXY).
Dilated seminiferous tubules were detected in excised tissue of all patients who underwent an operative microscopic exam (Fig. 1) Unfortunately, spermatozoa could only be retrieved in a single subject by using microdissection TESE. In contrast, spermatozoa were not recovered when multiple biopsy techniques were used. The right testis volume of this patient was 6 mL, and the FSH value was 76.7 mIU/mL. In this case, ICSI was not attempted because of the condition of the spouse. The excised weight was 66.9 ± 7.1 mg in the case of microdissection, and 172.8 ± 17.5 mg in the case of triple biopsies. Significant differences in excised weight were observed between the two groups (P < 0.0001).
Figure 1.
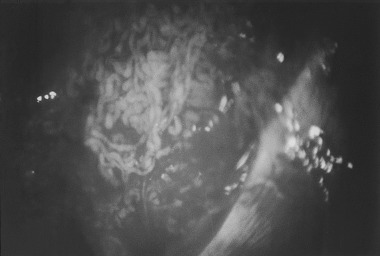
Dilated seminiferous tubules were observed under an operative microscope. In this case, spermatozoa could not be retrieved from dilated seminiferous tubules.
A single individual displayed postoperative lump hematoma; however, it subsided spontaneously within 2 weeks. All patients required analgesics on the day of operation. Testosterone was re‐tested in four patients 1 month later as part of a postoperative evaluation; however, decreased levels were not evident (prior operation: 375, 481, 437 and 328 ng/dL, after operation: 439, 605, 451 and 285 ng/dL, respectively). No apparent complications occurred because of the wide incision in the tunica albuginea.
Discussion
MEN WITH AZOOSPERMIA, markedly elevated FSH hormone levels and testicular atrophy were previously believed to be irreversibly infertile. This condition is considered to be NOA. Presently, men displaying NOA are able to initiate a pregnancy with ICSI‐provided sperm, which can be recovered in even relatively small numbers directly from testicular tissue. The NOA patient demonstrates a 17–90% probability of successful sperm retrieval by TESE. 8 , 9 , 10
In contrast, no precise definition exists regarding FSH values and testes volume of NOA patients. In our series, only patients exhibiting FSH values in excess of 20 mIU/mL and bilateral atrophic testes under 10 mL in volume were enrolled. A single patient (7.6%) achieved successful sperm retrieval. This fact may be related to patient selection where the candidate possesses both of the aforementioned criteria.
Some groups have used fine‐needle aspiration for sperm retrieval in cases of NOA. This technique is minimally invasive; however, the number of extracted spermatozoa is very limited. Friedler et al. compared the efficiency of testicular sperm retrieval by testicular fine‐needle aspiration (TEFNA) with that of open TESE in NOA patients. 3 They found that spermatozoa recovered by TEFNA, enabling performance of ICSI, were evident in only 11% of subjects, whereas open TESE yielded spermatozoa in 43%. Thus, most investigators now agree that an open biopsy is required to obtain spermatozoa from poorly functioning gonads. 3 , 4
Regarding the biopsy number, a single biopsy yielded spermatozoa in less than 30% of TESE procedures for NOA patients. 11 Among men experiencing successful TESE, the average total number of biopsies required initially to find sperm was 4.5. Furthermore, the maximum number of biopsies required initially to isolate spermatozoa was 14. Thus, Ostad et al. recommend the use of a multiple biopsy in NOA patinets. 11
In contrast, guided by diagnostic biopsies, testis sperm extraction procedures fail in 25–50% of NOA patients, largely caused by the clinical difficulty associated with sperm location. Scientific approaches to the lesion of small foci of sperm production in the testis have not been defined. In order to resolve this problem, Turek et al. made a precise systematic mapping of testis to confirm sperm production. Unfortunately, they concluded that differences in detection frequency of sperm were not statistically significant when using the map. 12
Microdissection TESE was originally described by Schlegel and Li. 7 The procedure for direct microscopic identification of functioning seminiferous tubules is referred to as microdissection. The concept of this procedure is as follows: seminiferous tubules containing many developing germ cells are larger and more opaque than tubules lacking sperm production. The larger tubules are excised exclusively under an operative microscope to prevent the removal of unnecessarily large volumes of testicular parenchyma. They reported that the ability to find spermatozoa increased from 45 to 63% following the use of microdissection TESE. Moreover, dissected samples displayed an average weight of 9.4 mg of tissue in microdissection, whereas sample weights averaged 720 mg in instances of standard biopsy. 4 Schlegel recommended that a standard biopsy be applied when dilated seminiferous tubules could not be identified during microdissection. 4
Six months following testicular biopsy, acute inflammatory changes resolved by leaving linear scars or calcifications. Transient adverse physiological effects are common in the testis for up to 6 months after TESE. Previously, Schlegel and Su recommended that the risk of devascularization related to TESE may be minimized with an open biopsy technique using optical magnification to directly identify testicular vessels. 5 The surgical procedure for sperm retrieval in NOA is frequently linked to multiple and extensive testicular biopsies in highly spermatogenically deficient testicles. Vascular injuries can occur as a result of testicular biopsy. The testicular blood supply is derived primarily from branches of the internal spermatic artery, with collateral branches of the cremasteric and vasal arteries. The decline in testosterone concentration following a testicular biopsy, caused by a large degree of excised tissue, was reversible to a certain extent within the first year of follow up; however, this effect was not completely reversible.
Manning et al. emphasized the importance of regular checks of testosterone levels following TESE. 6 We performed TESE after obtaining informed consent, including a postoperative endocrine evaluation. Unfortunately, only four patients underwent endocrine testing after the operation. Decreased levels of testosterone were not observed in the patients; however, the small sample of patients later receiving endocrine evaluation might be related to the poor results with respect to sperm retrieval.
In the present investigation, excised tissue was obviously reduced using microdissection; however, spermatozoa were located in only one patient. Decreased testosterone values were not detected postoperatively in this instance. Further study is needed in order to arrive at a reliable assessment of microdissection TESE relative to standard multiple biopsy in cases of NOA.
References
- 1. Devroey P, Liu J, Nagy Z et al. Pregnancies after testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI) in non‐obstructive azoospermia. Hum Reprod 1995; 1: 1457–1460. [DOI] [PubMed] [Google Scholar]
- 2. Schlegel PN, Palermo GD, Goldstein M et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia. Urology 1997; 49: 435–440.DOI: 10.1016/s0090-4295(97)00032-0 [DOI] [PubMed] [Google Scholar]
- 3. Friedler S, Raziel A, Strassburger D, Soffer Y, Komarovsky D, Ron‐El R. Testicular sperm retrieval by percutaneous fine needle sperm aspiration compared with testicular sperm extraction by open biopsy in men with non‐obstructive azoospermia. Hum Reprod 1997; 12: 1488–1493. [DOI] [PubMed] [Google Scholar]
- 4. Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999; 14: 131–135. [DOI] [PubMed] [Google Scholar]
- 5. Schlegel PN, Su LM. Physiological consequences of testicular sperm extraction. Hum Reprod 1997; 12: 1688–1692. [DOI] [PubMed] [Google Scholar]
- 6. Manning M, Jünemann KP, Alken P. Decrease in testosterone blood concentrations after testicular sperm extraction for intracytoplasmic sperm injection in azoospermic men. Lancet 1998; 352: 37. [DOI] [PubMed] [Google Scholar]
- 7. Schlegel PN, Li PS. Microdissection TESE: sperm retrieval in non‐obstructive azoospermia. Hum Reprod Update 1998; 4: 439. [DOI] [PubMed] [Google Scholar]
- 8. Chen CS, Chu SH, Lai YM, Wang ML, Chan PR. Reconsideration of testicular biopsy and follicle‐stimulating hormone measurement in the era of intracytoplasmic sperm injection for non‐obstructive azoospermia? Hum Reprod 1996; 11: 2176–2179. [DOI] [PubMed] [Google Scholar]
- 9. Tournaye H, Liu J, Nagy PZ et al. Correlation between testicular histology and outcome after intracytoplasmic sperm injection using testicular spermatozoa. Hum Reprod 1996; 11: 127–132. [DOI] [PubMed] [Google Scholar]
- 10. Kim ED, Gilbaugh 3Rd JH, Patel VR, Turek PJ, Lipschultz LI. Testis biopsies frequently demonstrate sperm in men with azoospermia and significantly elevated follicle‐stimulating hormone levels. J Urol 1997; 157: 144–146. [PubMed] [Google Scholar]
- 11. Ostad M, Liotta D, Ye Z, Schlegel PN. Testicular sperm extraction for nonobstructive azoospermia: results of a multibiopsy approach with optimized tissue dispersion. Urology 1998; 52: 692–696.DOI: 10.1016/s0090-4295(98)00322-7 [DOI] [PubMed] [Google Scholar]
- 12. Turek PJ, Ljung BM, Cha I, Conaghan J. Diagnostic findings from testis fine needle aspiration mapping in obstructive and nonobstructed azoospermic men. J Urol 2000; 163: 1709–1716. [PubMed] [Google Scholar]


