Abstract
Aim: The present report is the first to show that, after ovariectomy, female mice with autotransplanted ovarian sections can maintain pregnancy after embryo transfer (ET) independent of the transplantation site.
Methods: Three‐month‐old ICR females were ovariectomized, and sections from their own ovaries were transplanted either under their kidney capsule (KC group) or into a subcutaneous space (SC group) just after ovariectomy. In vitro fertilized blastocysts were transferred into uterine horns of the pseudopregnant mice that had received the transplanted ovarian tissues. Cesarean sections were carried out 17 days after ET to deliver any live fetuses that were present, and the numbers of implantation sites and fetuses were noted. Transplanted ovarian sections were removed and fixed for histological analysis.
Results: Of the 10 mice in the KC group that received 107 blastocysts, two females (20%) became pregnant; they showed 12 implantation sites (11.2%) and produced four pups (3.7%). In the SC group, 101 blastocysts were transferred to eight females, and three females (37.5%) became pregnant; there were seven implantation sites (6.9%), and three pups (3.0%) were born. There were no statistically significant differences between the two groups in any of the parameters evaluated. On histological examination, luteinization and vascularization of the ovarian sections that were transplanted in the pregnant SC and KC females were noted.
Conclusion: The pregnancy and full‐term fetal development were obtained in ovariectomized mice using a combination of heterotopic ovarian tissue autotransplantation and transfer of embryos produced by in vitro fertilization.
Keywords: embryo transfer, heterotopic ovarian transplantation, kidney capsule, mouse, subcutaneous space.
INTRODUCTION
IATROGENIC INFERTILITY CAUSED by a decline in ovarian function after radiotherapy and/or chemotherapy is a serious problem for patients with cancer. 1 , 2 , 3 Furthermore, ovariectomy in a gynecological cancer patient obviously causes infertility. 4 In these cases, orthotopic or heterotopic autotransplantation of ovarian tissues can be carried out to recover fertility after cancer therapy or an ovariectomy. 5 , 6 , 7 , 8 , 9 , 10 Ovarian tissue autotransplantation could preserve fertility in patients who survive cancer. 11 , 12 Furthermore, it is possible to recover oocytes from the transplanted ovarian sections. 13 , 14 , 15
High levels of follicular stimulating hormone (FSH) and luteinizing hormone (LH) are seen during premature menopause in women who have had ovariectomy or radiotherapy/chemotherapy for cancer. 16 However, the internal hormonal environment can be restored with heterotopic ovarian transplantation in mice, 8 rats 17 and humans. 5 Steroid hormones, such as estrogen and progesterone produced by granulosa cells and corpora lutea, are essential for implantation and the maintenance of pregnancy. Although transplanted ovarian tissues can secrete these steroid hormones, 16 little is known about whether these hormones secreted from grafts could control all of the necessary reproductive endocrine functions, especially implantation and pregnancy. Therefore, we investigated whether follicles in heterotopically transplanted ovaries were able to transform into corpora lutea, and whether, after ovariectomy, mice autotransplanted with ovarian tissues could maintain pregnancy after embryo transfer. In addition, the site‐effect of the transplantation (kidney capsule and subcutaneous space) was compared in terms of the pregnancy and implantation rates of the transferred embryos.
MATERIALS AND METHODS
Animals
FOR OVARY AUTOTRANSPLANTATION, 3‐month‐old Jcl/ICR, closed colony with white coat color, strain female mice were used. Oocyte donors were BALB/c × C57BL/6 J F 1 female mice, whose offspring are distinguishable by their coat color. ICR male mice were used as sperm donors. All mice were housed at about 22–24°C and under 14‐h light/10‐h dark photocycle conditions.
Autotransplantation of ovaries
Under anesthesia, ICR female mice were ovariectomized. Each ovary was removed from bilateral bursae that were opened carefully using a scalpel; the ovaries were then dissected into small pieces (about 1 mm3) in CZB‐Hepes medium. 18 Just after ovariectomy, the ovary sections were autotransplanted underneath the kidney capsules (KC group) or into subcutaneous spaces (SC group). Ovariectomized mice subjected to a sham operation of tissue transplantation (C‐O group) and nonovariectomized mice subjected to a similar sham operation (C‐N group) served as control groups. At least 2 weeks later, estrous cycles of some mice in KC and SC groups were checked for 1 week.
In vitro fertilization and embryo transfer
One to two weeks after autotransplantation of the ovaries, the female mice were mated by vasoligature ICR males to induce pseudopregnancy. In vitro fertilization was carried out according to a previous report 19 and the zygotes were cultured into the blastocyst stage for 96 h. About 10–20 blastocysts were transferred into each uterus of the 4‐day pseudopregnant female mice that had previously received autotransplantation of the ovaries or into the uteri of control group females. Cesarean sections were carried out to deliver live fetuses 17 days after embryo transfer; the numbers of implantation sites and fetuses were noted.
Histological preparations
Transplanted ovarian tissues were removed at the time of cesarean section and prepared for histology. Ovarian tissues were fixed for 4–7 days in 4% formaldehyde in phosphate buffer saline at pH 7.4, dehydrated in a graduated 70–100% ethanol series, and cleared in xylene. After embedding in paraffin, the tissues were cut into 7‐µm sections and stained with hematoxylin and eosin.
Statistics
Statistical evaluation was carried out using the χ2‐test to compare the rates of pregnancy, implantation of embryos and production of fetuses.
RESULTS
A TOTAL OF 34 female mice were used for the experiment; 13 underwent autotransplantation of ovarian sections underneath the kidney capsule (KC group), nine underwent autotransplantation of ovarian sections into the subcutaneous space (SC group), eight served as a nonovariectomy (C‐N group) control group and four underwent ovariectomy followed by sham tissue transplantation (C‐O group) as another control group. Their estrous cycles were checked for 14–21 days after ovarian transplantation. Their normalcy was ascertained by the cytological change in their vaginal smears.
After mating with vasoligature ICR males, females with vaginal plugs were regarded as being pseudopregnant. There were no pseudopregnant mice in the C‐O group, whereas ten females (76.9%) in the KC group, eight females (88.9%) in the SC group and seven females (87.5%) in the C‐N group were pseudopregnant (Table 1). There was no difference in the rates of pseudopregnancy among the three pseudopregnant groups.
Table 1.
Results of ovarian tissue autotransplantation and embryo transfer
| Groups | No. pseudopregnant mice/transplanted mice | No. embryo transferred mice | No. pregnant mice (%) | No. transferred blastocysts | No. implanted (%) | No. fetuses (%) |
|---|---|---|---|---|---|---|
| KC | 10/13 | 10 | 2 (20.0) | 107 | 12 (11.2) | 4 (3.7*) |
| SC | 8/9 | 8 | 3 (37.5) | 101 | 7 (6.9*) | 3 (3.0*) |
| C‐N | 7/8 | 7 | 4 (57.1) | 70 | 14 (20.0**) | 9 (12.9**) |
| C‐O | 0/4 | 0 | – | – | – | – |
C‐N, subjected to a similar sham operation but nonovariectomized; C‐O, ovariectomized and subjected to a sham tissue transplantation operation; KC, tissue transplantation under the kidney capsule; SC, tissue transplantation into the subcutaneous space.*,**Values with different superscripts in the same column are significantly different (P < 0.05).
Of the ten females in the KC group that received a total of 107 blastocysts, two females (20.0%) became pregnant; they showed 12 implantation sites (11.2%) and produced four pups (3.7%). In the SC group, eight pseudopregnant females received a total of 101 blastocysts; three (37.5%) became pregnant with seven implantation sites (6.9%) and produced three pups (3.0%). In the C‐N group, seven females received a total of 70 blastocysts; four (57.1%) became pregnant and showed 14 implantation sites (20.0%) and produced nine pups (12.9%). The coat colors of fetuses were to be colored and albino, which were expected in backcross progeny. There was no statistically significant difference between the KC and the SC groups in any of the evaluated parameters. However, there was a significant difference in the number of implanted embryos between the SC and the C‐N groups (P < 0.05), and the number of fetuses was significantly lower in the KC and SC groups than in the C‐N group (P < 0.05). In contrast, no pseudopregnant mice were observed in the C‐O group; therefore, embryo transfer could not be carried out in this group. The appearance of estrus on vaginal smears of the KC and SC groups was reflected by the presence of cornified epithelial cells; this suggests that all females who received transplanted ovarian tissues appeared to have an estrus cycle after autotransplantation of the ovaries.
Histological samples of ovarian tissues transplanted underneath the kidney capsule showed growing follicles and corpora lutea, which were thought to be derived from ruptured follicles in the transplanted ovarian sections (Fig. 1). Various stages of folliculogenesis, such as the preantral and antral stages, were found in the histological samples obtained from the KC group (Fig. 2). In the KC group, theca‐luteal cells were observed around the corpus luteum, and vascularization into the transplanted ovarian tissues was also observed (Fig. 1). In the histological samples obtained from the SC group, corpora lutea, theca‐luteal cells, growing follicles and vascularization were also found (Fig. 3). These histological findings show that ovarian tissues were successfully transplanted, and that the transplanted ovarian tissues might be able to produce estrogen and progesterone needed for implantation of transferred embryos and the maintenance of the pregnancy. Because these samples were collected at the time of cesarian section, the next follicular growth was seen as growing follicles (Fig. 3). However, it remains to be determined whether matured oocytes ‘ovulated’ within the kidney capsule or subcutaneous space, and/or were retained within the unruptured follicles.
Figure 1.
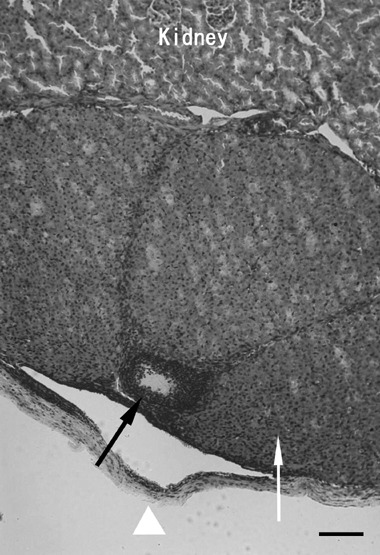
An ovarian tissue specimen autotransplanted underneath the kidney capsule. Corpora lutea (arrow) are seen underneath the kidney capsule (arrow head). Corpora lutea and theca‐luteal cells were located adjacent to kidney tissue; these corpora lutea might be derived from follicles that had ovulated. One small follicle (black arrow) is also observed in this ovarian tissue. Bar, 100 µm.
Figure 2.
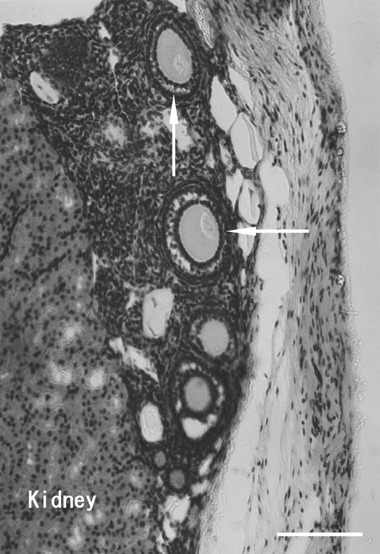
An ovarian tissue specimen autotransplanted underneath the kidney capsule. There are several preantral follicles (arrows) in the transplanted ovarian tissue. The integrity of the follicles was preserved after transplantation and these follicles would be expected to develop to the preovulatory stage. Bar, 100 µm.
Figure 3.
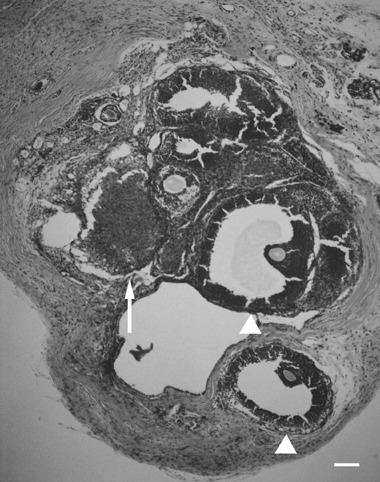
An ovarian tissue specimen autotransplanted into the subcutaneous space. Corpora lutea (arrow) and growing follicles (arrow heads) including oocytes are seen. This ovarian section shows normal ovarian function although it was surrounded by fatty tissue. Bar, 100 µm.
DISCUSSION
IN THE PRESENT study, successful pregnancy and the production of live pups were obtained by a combination of heterotopic auto‐transplantation of ovarian tissues and embryo transfer. The results suggest that the transplanted ovarian sections can secrete estrogen and progesterone, which are essential for the development of uterine endometrium and the maintenance of pregnancy. 20 , 21 Indeed, histological examination showed that follicular development, luteinization and vascularization were present in the transplanted ovarian sections of pregnant females in the KC and SC groups.
Heterotopic autotransplantation of ovarian tissues is an effective method for recovering the internal hormonal environment without the need for external hormonal supplementation in the mouse ovariectomy model. 17 A significant decrease of ovarian follicular reserve, and high serum FSH and LH levels have been reported in women rendered menopausal by radiotherapy and/or chemotherapy for cancer. 22 In contrast, a normal estrus cycle with lower FSH and LH levels occurred with heterotopic ovarian transplantation. 6 , 23 Most recently, it has been reported that a woman gave birth to a baby after orthotopic and heterotopic autografts of ovarian tissues. 24 After transplantation of ovarian cortical strips to the forearm, multiple antral follicles were present in the patients, therefore it was shown that follicles can grow in heterotopically transplanted ovarian tissues under a recovered hormonal environment. 25 In the present study, oocytes were certainly ovulated from grown follicles with the effect of LH surge because luteinization is certain evidence of ovulation and sequential copulation in rodents. Thus, ovarian transplantation appears to be a novel technique that preserves endocrine function and also oocyte growth in women treated with certain cancer therapies. 16
Though the values of the parameters studied differed between mice with ovarian sections transplanted underneath the kidney capsule and mice with ovarian sections transplanted into the subcutaneous space, none of the differences was statistically significant. However, the results of both of these groups were significantly lower than those of the C‐N group. It is thought that the transplantation site can affect the success rate of autotransplantation of ovarian tissues in terms of vascularization and hormone secretion. 26 , 27 Sufficient blood circulation has been found underneath the kidney capsule compared with the subcutaneous space. 26 A few days are needed for vascularization to occur into the transplanted ovarian sections 14 and some follicles, including an oocyte, can undergo necrosis during this time. 28 Thus, in the present study, delayed and insufficient vascularization might have disrupted the hormonal environment in the KC and SC groups, resulting in low rates of implantation and live fetus production. However, Demeestere et al. 24 have reported that, in women subjected to heterotopic ovarian transplantation, basal markers (FSH, inhibin B) of ovarian reserve returned to levels that were within normal premenopausal values. Similar results have been reported in rats, 29 rabbits 30 and sheep. 31 Furthermore, it has been reported that various stages of folliculogenesis were shown in young murine ovaries transplanted syngeneically into the testes. 32 Thus, the site of transplantation of ovarian tissues might be insignificant for the restoration of ovarian function after heterotopic ovarian transplantation.
It has been found that the size of ovarian sections should be reduced, because vascularization can occur earlier in smaller ovarian sections than in bigger ones 33 and gonadotropin administration might be effective in stimulating the follicles that survive within transplanted grafts. 34 Furthermore, transplantation of a whole ovary with vascular anastomosis has been investigated as a way to reduce ischemic necrosis and to increase the survival of grafted ovaries. 35 The cryopreservation of ovaries will also be useful for clinical ovarian tissue transplantation. 36
In conclusion, pregnancy and full‐term fetal development were obtained in ovariectomized mice using a combination of heterotopic ovarian tissue autotransplantation and transfer of embryos produced by in vitro fertilization. Functional corpora lutea were found in ovarian sections that were transplanted underneath the kidney capsule and in those transplanted into the subcutaneous space. These results suggest that this technique might be able to restore the fertility of women with iatrogenic infertility.
REFERENCES
- 1. Aubard Y, Piver P, Pech JC, Galinat S, Teissier MP. Ovarian tissue cryopreservation and gynecologic oncology: a review. Obstet Gynecol 2001; 97: 5–14. [DOI] [PubMed] [Google Scholar]
- 2. Le Floch O, Dnaldson S, Kaplan HS. Pregnancy following oophoropexy and total nodal irradiation in women with Hodgkin's disease. Cancer 1976; 38: 2263–2268. [DOI] [PubMed] [Google Scholar]
- 3. Apperly JF, Reddy N. Mechanisms and management of treatment related gonadal failure in recipients of high dose chemotherapy. Blood Rev 1995; 9: 93–116. [DOI] [PubMed] [Google Scholar]
- 4. Husseinzadeh N, Nahhas WA, Welkley DE, Whittney CW, Mortel R. The preservation of ovarian function in young women undergoing pelvic radiation therapy. Gynecol Oncol 1984; 18: 373–379. [DOI] [PubMed] [Google Scholar]
- 5. Kim SS, Battaglia DE, Soules MR. The future of human ovarian cryopreservation and transplantation: fertility and beyond. Fertil Steril 2001; 75: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 6. Oktay KH, Yih M. Preliminary experience with orthotopic and heterotopic transplantation of ovarian cortical strips. Semin Reprod Med 2002; 20: 63–74. [DOI] [PubMed] [Google Scholar]
- 7. Shaw JM, Cox SL, Trounson AO, Jenkin G. Evaluation of the long‐term function of cryopreserved ovarian grafts in the mouse, implications for human applications. Mol Cell Endocrinol 2000; 161: 103–110. [DOI] [PubMed] [Google Scholar]
- 8. Callejo S, Salvador C, Miralles A, Vilaseca S, Lailla JM, Bulasch J. Long‐term ovarian function evaluation after autografting by implantation with fresh and frozed‐thawed human ovarian tissue. J Clin Endorinol Metab 2001; 86: 4489–4494. [DOI] [PubMed] [Google Scholar]
- 9. Aubard Y, Piver P, Cogni Y, Fermeaux V, Poulin N, Driancourt MA. Orthotopic and heterotopic autografts of frozen‐thawed ovarian cortex in sheep. Hum Reprod 1999; 14: 2149–2154. [DOI] [PubMed] [Google Scholar]
- 10. Smitz J, Cortvrindt R. First childbirth from transplanted cryopreserved ovarian tissue brings hope for cancer survivors. Lancet 2004; 364: 1379–1380. [DOI] [PubMed] [Google Scholar]
- 11. Fabbri R, Venturoli S, D’Errico A et al Ovarian tissue banking and fertility preservation in cancer patients: histolical and immunohistochemical evaluation. Gynecol Oncol 2003; 89: 259–266. [DOI] [PubMed] [Google Scholar]
- 12. Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril 2004; 82: 930–932. [DOI] [PubMed] [Google Scholar]
- 13. Candy CJ, Wood MJ, Whittingham DG. Follicular development in cryopreserved marmoset ovarian tissue after transplantation. Hum Reprod 1995; 10: 2334–2338. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Mooney S, Wen Y, Behr B, Polan ML. Follicle development in grafted mouse ovaries after cryopreservation and subcutaneous transplantation. Am J Obstet Gynecol 2002; 187: 370–374. [DOI] [PubMed] [Google Scholar]
- 15. Smitz J. Oocyte developmental competence after heterotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 363: 832–833. [DOI] [PubMed] [Google Scholar]
- 16. Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA 2001; 286: 1490–1493. [DOI] [PubMed] [Google Scholar]
- 17. Callejo J, Vilaseca S, Medina M, Salvador C, Valls C, Lailla JM. Inhibin and follicular development in heterotopical ovary transplants without vascular pedicle in syngeneic Lewis rats. Fertil Steril 2003; 79: 743–748. [DOI] [PubMed] [Google Scholar]
- 18. Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of rendome‐bred 1‐cell mouse embryos in vitro. J Reprod Fertil 1989; 86: 679–688. [DOI] [PubMed] [Google Scholar]
- 19. Yoshizawa M, Nakamoto S, Fukui E, Muramatsu T, Okamoto A. Chromosomal analysis of first‐cleavage mouse eggs fertilized in caffeine‐containing medium. J Reprod Dev 1992; 38: 107–113. [Google Scholar]
- 20. Scharl A, Vierbuchen M, Granupner J, Fischer R, Bolte A. Immunohistochemical study of distribution of estrogen receptors in corpus and cervix uteri. Arch Gynecol Obstet 1988; 241: 221–233. [DOI] [PubMed] [Google Scholar]
- 21. Kagabu S, Umezu M. Survival of mouse ovarian tissue transplanted into the uterine horn of post‐partum rats nursing pups of various numbers and sizes. Reprod Med Biol 2005; 4: 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid‐life: implications for forecasting menopause. Hum Reprod 1992; 7: 1342–1346. [DOI] [PubMed] [Google Scholar]
- 23. Callejo J, Jauregui MT, Valls C, Fernandez ME, Cabre S, Lailla JM. Heterotopic ovarian transplantation without vascular pedicle in syngeneic Lewis rats: six‐month control of estradiol and follicle‐stimulating hormone concentrations after intraperitoneal and subcutaneous implants. Fertil Steril 1999; 72: 513–517. [DOI] [PubMed] [Google Scholar]
- 24. Demeestere I, Simon P, Buxant F et al Ovarian function and spontaneous pregnancy after combined heterotopic and orthotopic cryopreserved ovarian tissue transplantation in a patient previously treated with bone marrow transplantation: case report. Hum Reprod 2006; 21: 2010–2014. [DOI] [PubMed] [Google Scholar]
- 25. Oktay K, Buyuk E, Rosenwaks Z, Rucinski J. A technique for transplantation of ovarian cortical strips to the forearm. Fertil Steril 2003; 80: 193–198. [DOI] [PubMed] [Google Scholar]
- 26. Hemandez‐Fonseca H, Bosch P, Sirisathien S, Wininger JD, Massey JB, Brackett BG. Effect of site transplantation on follicular development in human ovarian tissue transplanted into intact or castrated immunodeficient mice. Fertil Steril 2004; 81: 888–892. [DOI] [PubMed] [Google Scholar]
- 27. Kim SS, Kang HG, Kim NH, Lee HC, Lee HH. Assessment of the integrity of human oocytes retrieved from cryopreserved ovarian tissue after xenotransplantation. Hum Reprod 2005; 20: 2502–2508. [DOI] [PubMed] [Google Scholar]
- 28. Salehnia M. Autograft of vitrified mouse ovaries using ethylene glycol as cryoprotectant. Exp Anim 2002; 51: 509–512. [DOI] [PubMed] [Google Scholar]
- 29. Chiu DT, Hu G. Evaluation of the hormonal function and histological features of heterotopic isogenic ovarian transplantation in rats. Plast Reconstr Surg 2003; 15: 1646–1652. [DOI] [PubMed] [Google Scholar]
- 30. Meraz MM, Gracida CJ, Melchor JL, Revilla CM, De Buen N, Aburto EM. Restoration of endocrine function and ovulation after a heterotopic ovarian transplant in the inguinal region of rabbits using a vascular microsurgical technique. Transplant Proc 2006; 38: 952–957. [DOI] [PubMed] [Google Scholar]
- 31. Denschlag D, Knobloch C, Kockrow A et al Autologous heterotopic transplantation of ovarian tissue in sheep. Fertil Steril 2005; 83: 501–503. [DOI] [PubMed] [Google Scholar]
- 32. Sato M, Sakurai T, Kiryu K, Takeda M, Yasuoka Y. Folliculogenesis following syngeneic transplantation of young murine ovaries into the testes. Reprod Med Biol 2006; 5: 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petroianu A, Vasconcellos LS, Alberti LR, Nunes MB. The influence of venous drainage on autologous ovarian transplantation. J Surgi Res 2005; 124: 175–179. [DOI] [PubMed] [Google Scholar]
- 34. Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gnadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Moler Cell Endocr 2000; 163: 141–146. [DOI] [PubMed] [Google Scholar]
- 35. Arav A, Revel A, Nathan Y et al Oocyte recovery, embryo development and ovarian function after cryopreservation and transplantation of whole sheep ovary. Hum Reprod 2005; 20: 3554–3559. [DOI] [PubMed] [Google Scholar]
- 36. Donnez J, Dolmans MM, Martinez‐Madrid B, Demylle D, Van Langendonckt A. The role cryopreservation for women prior to treatment of malignancy. Curr Opin Obstet Gynecol 2005; 17: 333–338. [DOI] [PubMed] [Google Scholar]


