Abstract
Background: Patients with malignancy (n = 130) participated in the sperm cryopreservation program.
Methods: After washing and concentrating, sperm was cryopreserved using KS‐VIm cryoprotectant medium. Participant background factors such as age, marital status, underlying disease, presence or absence of previous treatment and semen findings (concentration, motility and morphology) were analyzed to determine parameters associated with the program.
Results: Patients in their 20s were most common (64 cases) and 94 cases were unmarried at the first visit. The main underlying diseases were testicular tumor (53 cases), leukemia (43 cases) and malignant lymphoma (13 cases). The program was completed for 118 cases. For leukemia, all semen parameters were closer to normal in patients without previous treatment (untreated group, UG) compared with the treated group (TG). When semen findings in the UG were classified according to underlying disease, sperm concentration was lower in patients with testicular tumor compared with those who had leukemia or malignant lymphoma. Four couples underwent reproductive therapies with the cryopreserved sperm through assisted reproductive technology, and three babies were born to two couples.
Conclusion: Sperm cryopreservation liberates patients with malignancy from iatrogenic infertility as a consequence of intensive therapy, allowing them to retain reproductive ability.
Keywords: infertility, malignancy, sperm cryopreservation
INTRODUCTION
TESTICULAR TUMOR, LEUKEMIA and malignant lymphoma occur frequently in young men. Remarkable progress in high‐dose chemotherapy and radiation therapy predicts a high chance of complete recovery in such patients. These intensive treatments generally cause the loss of testicular function, especially spermatogenesis, aiming to cure cancer at the expense of sacrificing fertility. High inguinal orchiectomy and castration are often used to treat testicular tumors and prostate cancer, respectively. Given these circumstances, the sperm cryopreservation program is aimed at patients with malignancy as a method to avoid iatrogenic infertility as a consequence of intensive therapies. 1 , 2 It is thus valuable from the viewpoint of quality of life that successfully treated patients can accomplish the dream of marriage and having children.
Sperm cryopreservation was first reported by Polge and Rowson. 3 Cryopreservation of sperm has been applied to both livestock and humans since the 1950s. To date, we have actively promoted the sperm cryopreservation program for patients with malignancy, and our program has already resulted in three babies being conceived with the cryopreserved sperm. The present study classifies semen findings according to patients’ medical histories (underlying diseases and presence or absence of certain previous treatments), and discusses issues associated with the program.
MATERIALS AND METHODS
Subjects
FROM OCTOBER 2002 to April 2005, 130 patients with malignancy visited the Reproduction Center (Ichikawa General Hospital, Tokyo Dental College, Ichikawa, Japan) to participate in the sperm cryopreservation program before chemotherapy and radiation therapy, which were expected to destroy spermatogenesis, were carried out,. The patients were confirmed to be negative for HIV, HCV, HBV and syphilis. All patients with testicular tumor had already undergone orchiectomy before the first visit.
Semen analyses and assessment of semen quality
Ejaculate was obtained by masturbation in the semen collection room at the outpatient clinic. After 30 min liquefaction at room temperature, semen findings (sperm concentration, motility and head morphology) were measured according to the WHO manual. 4 Patients with azoospermia, severe oligozoospermia (less than 1 × 106/mL) and teratozoospermia (normal morphology less than 1.0%) were excluded from the program.
Sperm cryopreservation
Human sperm cryopreservation was carried out as described previously. 5 , 6 Raw Percoll (1.0 L, Amersham, Uppsala, Sweden) was made isotonic using 10.0 mL of 2.0 mol/L HEPES‐NaOH, pH 7.4, and powdered ingredients (7.20 g NaCl, 0.32 g KCl, 0.045 g Na2HPO4, 0.054 g KH2PO4, 0.32 g NaHCO3, 0.84 g glucose, 0.12 g CaCl2, 0.045 g MgCl2, 0.045 g MgSO4, 0.05 g fosfomycin and 0.05 g cepharotin) and 10 mL of human serum albumin (25% w/v). The resulting isotonic 98% Percoll solution was sterilized with a Millipore filter (0.45 µm pore size). Then, 5 mL of 98% Percoll was placed in a conical tip test tube and 1.0 mL of Hank's solution was layered on top. The test tube was rotated 10 revolutions at an angle of 30° to make a density gradient. To remove fibers, microcalculi and micinous debris, the ejaculate was diluted twice with Hank's solution, filtered through nylon mesh (ART filter, 20 µm clearance, Nipro, Osaka, Japan), then allowed to stand in a test tube for 10 min to precipitate filterable microcalculi. The resulting suspension was placed on a density gradient, centrifuged at 400 × g for 30 min in a swing‐out rotor, and the sediment (0.2 mL) was mixed with an equal volume of KS‐VIm cryoprotective medium (20 mmol HEPES‐NaOH, pH 7.4, 12% glycerin, 10% egg yolk water soluble fraction, fosfomycin [0.05 g/L] and cepharotin [0.05 g/L] in Hank's solution). The mixture was frozen in liquid nitrogen vapor, stored at –196°C, and thawed in a tap water at 37°C.
Patients’ informed consent
Prior to the procedure, patients who satisfied the collateral terms of the contract signed the informed consent form, approved by the Ethical Committee of Tokyo Dental College. In summary, the informed consent form stated that the cryopreserved sperm would be stored only while the patient was alive. The duration of cryopreservation is contracted on a 1‐year basis and consent needs to be renewed annually for continuance. The contract is cancelled automatically when a renewal is not made within 3 months after expiration of the agreement, or when the patient is 1 year overdue for the cryopreservation fee. Data can be used anonymously under strict confidentiality. Participants were informed that unavoidable accidents, such as natural calamities, might put the cryopreserved sperm beyond use.
Practice of assisted reproductive technology
After thawing, progressively motile sperm was separated using the swim‐up method, then inseminated through intracytoplasmic sperm injection (ICSI) or intrauterine insemination (IUI). ICSI was carried out in four cases (twice in two cases, once in two cases) and IUI was used in one case.
Statistical analyses
The patients’ data were analyzed by non‐parametric analysis of Mann–Whitney's U‐test by Stat‐View version 5.0 (SAS Institute, Cary, NC, USA). Values are expressed as mean ± standard deviation.
RESULTS
PATIENT AGES WERE widely distributed from 16 to 60 years‐of‐age (30.1 ± 17.7 years). At the first visit, 94 cases were unmarried (seven of these were engaged) and 33 cases had already received some anticancer treatments (treated group: TG), whereas 97 cases had not (untreated group: UG). In the TG, 76% of cases had leukemia, and all of them had undergone remission induction and post‐remission therapy. A short period was usually allowed until the start of the chemotherapy. The ejaculates for cryopreservation in each case were obtained from a maximum of three samples. In the following results, samples having the highest sperm concentration were used in the analyses. Patients’ underlying diseases and semen findings are summarized in Table 1. Testicular tumor was the most frequent diagnosis, followed by leukemia and malignant lymphoma, accounting for 84% of the patients. Comparing semen findings between the UG and TG, previous treatments significantly decreased sperm motility only (P < 0.05). Because the semen quality in 12 cases was below the exclusion criteria, only 118 cases were actually enrolled in the program. In the UG, 6.2% of the patients were excluded, whereas 18.2% of TG patients were excluded from the program.
Table 1.
Classification of underlying diseases and comparison of semen findings between untreated and treated groups
| Case | Treated (n = 33) | Untreated (n = 97) | Total | ||
|---|---|---|---|---|---|
| Exclude | Preserve | Exclude | Preserve | ||
| Testicular tumor | 0 | 2 | 4 | 47 | 53 |
| Leukemia | 3 | 22 | 2 | 16 | 43 |
| Malignant lymphoma | 3 | 0 | 0 | 10 | 13 |
| Aplastic anemia | 0 | 2 | 0 | 1 | 3 |
| Prostate cancer | 0 | 0 | 0 | 3 | 3 |
| Bladder tumor | 0 | 0 | 0 | 2 | 2 |
| Pharyngeal cancer | 0 | 0 | 0 | 2 | 2 |
| Liposarcoma | 0 | 1 | 0 | 0 | 1 |
| Hepatocellular carcinoma, retroperitoneal tumor, multiple myeloma, thymic tumor, intrapelvic tumor, small intestinal cancer, lingual cancer, colon cancer, rectum cancer, brain tumor | 0 | 0 | 0 | Each 1 (10) | 10 |
| 6 | 27 | 6 | 91 | 130 | |
| Sperm concentration (×106/mL) | 96 ± 133 | 87 ± 71 | 90 ± 90 | ||
| Motility (%) | 29.3 ± 24.4 | 40.7 ± 23.4 | 38.0 ± 24.0 | ||
| Normal morphology (%) | 6.5 ± 6.5 | 9.5 ± 8.9 | 8.8 ± 8.5 | ||
We compared semen findings among the three major diseases (Fig. 1). For testicular tumor, we found no significant difference between UG and TG in any parameter. However, because this subgroup of the TG comprised only two cases, it remains unclear whether previous treatments had an influence. For leukemia, all parameters were significantly suppressed (P < 0.05) by previous treatments. For malignant lymphoma, the percentage of normal morphology in the TG was significantly lower (P < 0.05) than in the UG.
Figure 1.
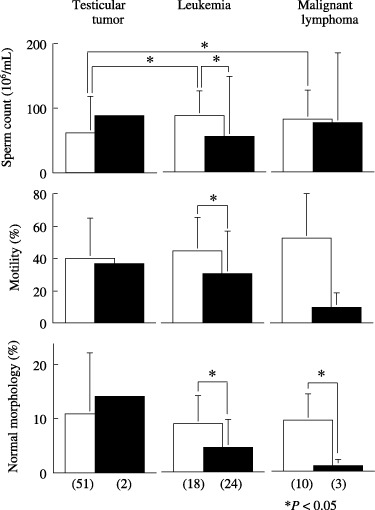
Semen findings in patients with or without previous anticancer treatment. Values in parentheses indicate the number of cases. (□) Untreated group; (▪) treated group.
For UG, sperm concentrations in the testicular tumor subgroup were significant lower (P < 0.05) than in the other diseases. In contrast, no significant difference was found between leukemia and malignant lymphoma. For TG, no parameter was significantly different among the three groups.
To date, cryopreservation has been terminated as a result of patient death in five cases, and four couples underwent assisted reproductive technology (ART) with the cryopreserved sperm, with two couples successfully obtaining three babies with the aid of ICSI (Table 2). Patient I (leukemia) cryopreserved his sperm (75 × 106/mL, 40% motility, in four tubes), then underwent bone marrow transplantation. The couple delivered their first child with the aid of ICSI. Furthermore, this couple delivered a second child in the same manner. After orchiectomy, patient II (testicular tumor) cryopreserved his sperm (110 × 106/mL, 47% motility, in three tubes). After his complete cure, the couple also delivered a baby through ICSI. Patients III and IV underwent ART, without resulting pregnancy.
Table 2.
Summary of assisted reproductive techniques with cryopreserved sperm
| Case | Age | Underlying disease | Anticancer treatment | Semen findings | Method of ART therapeutic result | |||
|---|---|---|---|---|---|---|---|---|
| concentration (×106/mL)/motility (%)/normal morphology (%) | ||||||||
| Ejaculate | ART | |||||||
| 1st | 2nd | 1st | 2nd | |||||
| 1 | 24 | Leukemia | Radiation chemotherapy bone marrow transplantation | 75/40/10.8 | 10/30/12.6 | 32/28/10.6 | ICSI delivery | ICSI delivery |
| 2 | 33 | Testicular tumor | Radiation | 110/46.7/8.9 | 170/41/20.3 | – | ICSI delivery | – |
| 3 | 50 | Malignant lymphoma | Radiation chemotherapy | 120/9.4/16.3 | 81/8.2/19.3 | 94/16/32.3 | ICSI not pregnant | ICSI not pregnant |
| 4 | 49 | Malignant lymphoma | Chemotherapy | 70/24/5.7 | 77/65/19.4 | 57/40/15.6 | AIH not pregnant | ICSI not pregnant |
AIH, artificial insemination with husband's semen; ART, assisted reproductive technology; ICSI, intracytoplasmic sperm injection.
DISCUSSION
INTENSIVE ANTICANCER TREATMENTS such as chemotherapy, bone marrow transplantation and some adjuvant therapies improve the survival rate in young patients with testicular tumor or leukemia. However, various complications, particularly iatrogenic infertility, are associated with these therapies. Although semen findings normalize after treatment, the possibility of genetic disturbances in sperm cannot be ruled out. 1 , 2 The ethical committee of our institution recommended that unmarried patients should be excluded from the program. As described in the results, patients in their 20s or younger (72 cases) and unmarried patients (94 cases) were in the majority. Considering overall patient quality of life, we decided to apply the program to unmarried patients as well.
Thirty‐three patients had already received anticancer treatments before their first visit. Even in the UG, the patients were allowed a short period to store the sperm. We have to make efforts to publicize the significance of the sperm cryopreservation program to oncologists.
When comparing semen findings between the UG and TG, the percentage of normal morphology decreased for malignant lymphoma patients, and all parameters decreased in leukemia patients. Serious adverse effects on testicular function might occur with the use of alkylating agents (i.e. cyclophosphamide and procarbozine). 7 , 8 Systemic radiotherapy with subsequent bone marrow transplantation 9 , 10 is frequently combined with these agents. It is therefore essential to cryopreserve sperm prior to anticancer treatments.
For the UG, sperm concentration was significantly lower for testicular tumor patients compared with leukemia and malignant lymphoma patients. Elevation in intrascrotal temperature 11 and production of antisperm antibodies 12 might suppress spermatogenesis in the contralateral testis. Just after orchiectomy, trauma to the contralateral testis often induced azoospermia, which normalized after one month. 13 If tumor progression were not so rapid, it might be useful to cryopreserve sperm after a certain period following orchiectomy.
It should be emphasized that patients, oncologists and the public should recognize the significance of a sperm cryopreservation program from an aspect of overall quality of life. However, a number of social and ethical problems need to be dealt with, for example, the legal status of cryopreserved sperm after the death of a patient. Recently in Japan, two widows conceived babies using cryopreserved sperm; however, the courts recognized neither of children as fathered. In the current contract used for this study, cryopreservation would be terminated upon patient death and ART should not be accepted. However, guidelines for administering this program require further debate that includes multiple points of view.
REFERENCES
- 1. Agarwal A. Semen banking in patients with cancer: 20‐years experience. Int J Androl 2000; 23 (Suppl 2): 16–19. [DOI] [PubMed] [Google Scholar]
- 2. Puscheck E, Philip PA, Jeyendran RS. Male fertility preservation and cancer treatment. Cancer Treatment Rev 2004; 30: 173–180. [DOI] [PubMed] [Google Scholar]
- 3. Polge C, Rowson LEA. Fertilizing capacity of bull spermatozoa after freezing at –79°C. Nature Lond 1952; 169: 626. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Laboratory Manual for the Examination of Human Semen and Semen–Cervical Mucus Interaction, 4th edn. Cambridge: Cambridge University Press, 1999. [Google Scholar]
- 5. Kobayashi T, Kaneko S, Hara I et al A simplified technique for freezing of human sperm for AIH; Cryosyringe/Floating platform on liquid nitrogen vapor. Arch Androl 1991; 27: 55–60. [DOI] [PubMed] [Google Scholar]
- 6. Kobayashi T, Kaneko S, Hara I et al Concentrating human sperm before cryopreservation. Andrologia 1991; 23: 25–28. [DOI] [PubMed] [Google Scholar]
- 7. Thachil JV, Jewett MA, Rinder WD. The effects of cancer and cancer therapy on male fertility. J Urol 1981; 126: 141–145. [DOI] [PubMed] [Google Scholar]
- 8. Costabile RA. The effects of cancer and cancer therapy on male reproductive function. J Urol 1993; 149: 1327–1330. [DOI] [PubMed] [Google Scholar]
- 9. Sanders JE, Hawley J, Levy W et al Pregnancies following high‐dose cyclophosphamide with or without high‐dose busulfan or total‐body irradiation and bone marrow transplantation. Blood 1996; 87: 3045–3052. [PubMed] [Google Scholar]
- 10. Jacob A, Barker H, Goodman A, Holmes J. Recovery of spermatogenesis following bone marrow transplantation. Bone Marrow Transplant 1998; 22: 277–279. [DOI] [PubMed] [Google Scholar]
- 11. Weissbach L, Vahlensieck W, Figge M. Diagnostik bei hodentumren. Urologe B 1980; 20: 106. [Google Scholar]
- 12. Guazzieri S, Lembo A, Ferro G et al Sperm anti‐bodies and infertility in patients with testicular cancer. Urology 1985; 26: 139–142. [DOI] [PubMed] [Google Scholar]
- 13. Tomomasa H, Oshio S, Amamiya R et al Testicular injury: late results of semen analyses after uniorchiectomy. Acrh Androl 1992; 29: 59–63. [DOI] [PubMed] [Google Scholar]


