Abstract
Purpose
Our previous study demonstrated that vitrified–warmed ovarian tissue autotransplantation (VOAT) into estrus cycle‐ceased ovariectomized mice restored fertility to achieve full‐term fetal development for transferred embryos, while less steroidogenesis in the corpus luteum was observed in VOAT mice. It has been reported that the window of uterine receptivity for blastocyst implantation is extended at lower estrogen levels. Therefore, we hypothesized that duration of the window in VOAT mice could be extended.
Methods
Blastocysts were transferred into VOAT mice on day 5 of pseudopregnancy. Immunohistochemical analysis was performed to examine the potential in VOAT ovarian tissues.
Results
The rate of live birth pups from embryos transferred on day 5 of pseudopregnant VOAT mice was not different from that of embryos transferred on day 4 of pseudopregnancy in VOAT mice, while embryo transfer on day 5 into intact mice showed no pregnancy. Immunohistochemical analysis of the corpus luteum of day 8 pseudopregnant VOAT mice with uteri having decidualization induced on day 5 showed less steroidogenesis and blood vessel formation as compared to intact mice.
Conclusions
Uterine receptivity was extended in VOAT mice. Less steroidogenesis and blood vessel formation in the transferred ovarian tissues may be associated with the extended uterine receptivity.
Keywords: Embryo transfer, Implantation, Ovarian tissue autotransplantation, Pregnancy, Vitrification
Introduction
Ovarian cryopreservation appears to be a potentially valuable method for fertility preservation. Because iatrogenic infertility caused by a decline in ovarian function after chemotherapy and radiotherapy is a serious problem for cancer patients [1], ovarian cryopreservation followed by re‐implantation has been widely advocated [2, 3, 4, 5, 6]. However, the best technical approaches are still controversial [7].
The mouse is an important model for studies in ovarian transplantation and cryopreservation. For example, xenografts can survive in immunodeficient host animals, such as severe combined immunodeficiency (SCID) and nude mice, which have been used to test viability after transport or cryopreservation of ovarian tissue [8]. Allograft of cryopreserved ovarian tissue into the kidney capsule of SCID mice allows the recovery of oocytes for in vitro maturation, followed by in vitro fertilization (IVF), embryo transfer, and subsequent birth of pups [9, 10].
In contrast, autotransplantation of ovarian tissue has been demonstrated in mice. Our previous study demonstrated that fresh ovarian tissues that were autotransplanted underneath the kidney capsule supported pregnancy and full‐term fetal development for transferred embryos [11]. We also demonstrated that vitrified–warmed ovarian tissue autotransplantation (VOAT) into estrus cycle‐ceased ovariectomized mice restored fertility to achieve full‐term fetal development for transferred embryos [12]. Although VOAT mice using our methods possessed sufficient potential to support pregnancy and full‐term development, steroidogenesis and blood vessel formation in the corpus luteum in VOAT mice were lower than those in intact mice [12].
To successfully establish pregnancy in mice, the uterine receptivity for implantation lasts for a limited time period [13, 14, 15, 16]. At this stage, the uterine environment is able to support blastocyst growth, attachment, and the subsequent events of implantation. The major hormones that specify uterine receptivity are the ovarian steroids progesterone (P4) and estrogen (E2). The prereceptive uterus on day 3 of pregnancy (day 1 = vaginal plug) becomes receptive on day 4 under the influence of rising P4 and a small amount of ovarian E2 secretion on the morning of day 4 of pregnancy [17].
In contrast, it has been reported that the window of uterine receptivity for blastocyst implantation in mice remains open for an extended period at lower E2 levels [18]. Our previous study showed less steroidogenesis in the corpus luteum in VOAT mice as compared to that in intact mice [12]. Therefore, the implantation window could be prolonged in VOAT mice. To address this issue, we performed embryo transfer into VOAT mice on day 5 of pseudopregnancy to examine whether the VOAT mice support pregnancy and full‐term fetal development. We also examined uterine decidualization, ovarian steroidogenesis and blood vessel formation in the corpus luteum in VOAT mice.
Materials and methods
Animals
Mice were purchased from CLEA Japan, Inc., (Tokyo, Japan) and bred in our animal care facility. All of the experiments in the present study were conducted in compliance with the guide for the care and use of laboratory animals published by Utsunomiya University. Three‐month‐old BALB/c × C57BL/6J F1 female mice were used for VOAT. Vasectomized 3‐ to 5‐month‐old ICR male mice were used for mating to induce pseudopregnancy. Spermatozoa for IVF were harvested from the cauda epididymidis of these mice.
Vitrification of ovarian tissues and autotransplantation
Vitrification of ovarian tissues and autotransplantation were performed as described previously with slight modifications [11, 12]. Briefly, ovaries were dissected into small pieces (approximately 1–2 mm wide) and autotransplanted underneath the kidney capsules. Vitrification of ovarian tissues was performed by immersion in an equilibration solution composed of 7.5% (v/v) ethylene glycol (EG) and 7.5% (v/v) dimethyl sulfoxide (DMSO) in HEPES‐buffered tissue culture medium 199 containing 20% (v/v) fetal bovine serum (mTCM‐199) for 15 min, followed by a vitrification solution composed of 15% EG, 15% DMSO, and 0.5 M sucrose in mTCM‐199 for 3 min at room temperature. Ovarian tissues placed on polyester sheets were submerged directly into liquid nitrogen. For warming, the polyester sheets were placed directly in a warming solution composed of 1.0 M sucrose in mTCM‐199 for 1 min at room temperature. The ovarian tissues detached from the polyester sheets were transferred into dilution solution composed of 0.5 M sucrose in mTCM‐199 for 3 min. After washing, the ovarian tissues were warmed to 37°C for 15 min and autotransplanted.
In vitro fertilization and embryo transfer
In vitro fertilization was performed as described previously with slight modifications [12, 19]. Donors of oocytes and sperm were BALB/c × C57BL/6J F1 females and ICR males, respectively. Female mice were treated with eCG (5 IU) followed by injections of hCG (5 IU) at 48 h post eCG. Collected oocytes were used for IVF. For VOAT mice, oocytes were recovered from the oviduct at the time of collection of ovaries for ovarian tissue vitrification. Vitrification and warming of oocytes were performed using Cryotip‐L (Kitazato Biopharma, Shizuoka, Japan), according to the manufacturer's instructions. Blastocysts at 96 h after IVF were transferred into uteri of mice in the morning (0900–1000 h) on day 4 or day 5 of pseudopregnancy. For embryo transfer into pseudopregnant VOAT mice, blastocysts from their own vitrified–warmed oocytes were transferred into the uteri. Recovery of estrus cycle was determined by vaginal smear, and then embryo transfer was performed at least 2 weeks after the VOAT. For pseudopregnant intact mice, blastocysts derived from fresh ovulated oocytes recovered from different mice were transferred into the uteri. Caesarean sections were performed to deliver live fetuses on day 19 of pregnancy (day 1 = vaginal plug).
Induction of decidualization
To examine uterine differentiation in mice treated by VOAT on the seventh day after ovariectomy, decidualization was induced as described previously [12, 20]. Briefly, mice were mated with vasectomized males to induce pseudopregnancy (day 1 = vaginal plug). The induction of decidualization was initiated by an intraluminal infusion of sesame oil (25 μl) into one uterine horn. The contralateral horn served as an intact control. The mice were killed on day 8 of pseudopregnancy, and the wet weights of their infused and noninfused (control) uterine horns were recorded. The fold induction in uterine wet weights was used as an index to compare decidualization in VOAT mice with that in intact mice. To perform the immunohistochemical analysis, transplanted ovarian tissues or intact ovaries were removed and fixed at the same time.
Immunohistochemical analyses
To investigate the expressions and cellular distributions of CYP11A1 and CD34, immunohistochemical analysis was performed as previously described with slight modifications [12, 21]. CYP11A1 (also known as P450scc) is a steroidogenic enzyme [22, 23]; CD34 is a marker of vascular endothelial cells [24, 25]. Removed ovaries were fixed in Bouin solution, then embedded in paraffin wax and sectioned at 5 μm. Sections were deparaffinized in xylene, rehydrated in a graded ethanol series, immersed in 3% (v/v) hydrogen peroxide in methanol, treated with 10% (v/v) normal goat serum in Ca2+ and Mg2+‐free Dulbecco's phosphate‐buffered saline (PBS), and then incubated overnight at 4°C with primary antibodies. For the primary antibody reaction, sections were incubated with rabbit polyclonal antibody to CYP11A1 (Millipore, Billerica, MA, USA) or to rat monoclonal antibody CD34 (Hycult biotechnology, Uden, The Netherlands). After washing, the sections were incubated with secondary antibodies: biotinylated goat anti‐rabbit antibody (Zymed Laboratories, Inc., San Francisco, CA, USA) for CYP11A1 and biotinylated goat anti‐rat antibody (Zymed Laboratories) for CD34. After the incubation of horseradish peroxidase‐conjugated streptavidin (Zymed Laboratories), the reactions were visualized using 3‐amino‐9‐ethyl carbazole (AEC; Zymed Laboratories) as a chromogen, followed by counterstaining with hematoxylin. Reddish deposits indicate the sites of immunoreaction. Images shown are representative of at least 6 sections from more than 3 different animals at each stage. The experiments were repeated at least three times.
Statistical analysis
A Chi‐square test was used to evaluate differences in rates of decidualized mice, pregnant mice and birth of pups. Comparisons with expected values of less than 5 were analyzed using Fisher's exact probability test. An unpaired Student's t test was used to evaluate increases in uterine weight of decidualization. P < 0.05 was considered to be significant.
Results
Embryo transfer in VOAT mice on day 5 of pseudopregnancy showed full‐term fetal development
When blastocysts were transferred into intact mice on day 4 of pseudopregnancy, rates of pregnancy and live pups were 100% (4/4) and 45.0% (18/40), respectively (Table 1). In contrast, embryo transfer on day 5 into intact mice showed no pregnancy (Table 1). In VOAT mice, the pregnancy rates of embryo transferred VOAT mice on day 4 and day 5 were 80.0% (8/10) and 75.0% (3/4), respectively (Table 1). The rates of live birth pups per transferred embryo in VOAT mice on day 4 and day 5 were 25.3% (22/87) and 18.2% (4/22), respectively (Table 1). There were no significant differences in VOAT mice between on day 4 and day 5. Furthermore, weights of live pups and their placentas were similar on both days 4 and 5 (Fig. 1a, b). These results indicate that embryo transfer on day 5 into VOAT mice was available to support the pregnancy and production of offspring as well as embryo transfer on day 4 into VOAT mice, although fertility of VOAT mice was lower as compared to intact mice.
Table 1.
Pregnancy and birth of pups depend on pseudopregnancy dates in embryo transfer of blastocysts
| Treatment | Days of embryo transfer | No. of mice examined | No. (%) of pregnant mice A | No. of embryos transferred | No. (%) of pups |
|---|---|---|---|---|---|
| Intact | Day 4 | 4 | 4 (100) A | 40 | 18 (45.0) A |
| Intact | Day 5 | 4 | 0 (0)b | 40 | 0 (0)b |
| VOAT | Day 4 | 10 | 8 (80.0) A | 87 | 22 (25.3)c |
| VOAT | Day 5 | 4 | 3 (75.0)ab | 22 | 4 (18.2)c |
APregnancy was evaluated on the day of Caesarean section
a–cValues with different superscripts within each column differ significantly (P < 0.05)
Figure 1.
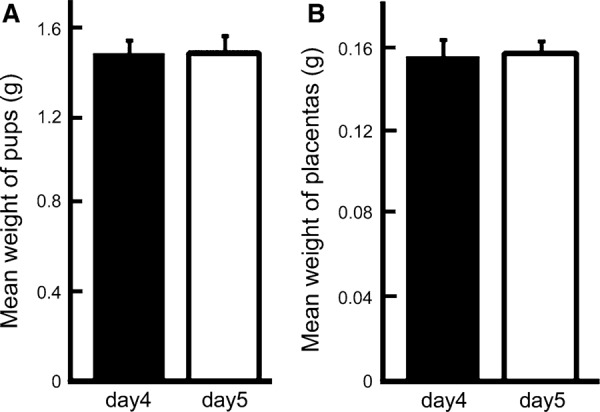
Live pups and placentas from VOAT recipients after embryo transfer on day 4 or 5 of pseudopregnancy. Transferred blastocysts were derived from their own vitrified–warmed oocytes. Weights of pups (a) and placentas (b). The results are mean ± SEM. Neither pups nor placentas showed significant differences in weights between days 4 and 5
Endometrial decidualization induced on day 5 of pseudopregnancy in VOAT mice
The above results showed that embryo transfer on day 5 into VOAT mice was available to support the pregnancy and production of offspring. In contrast, embryo transfer on day 5 into intact mice showed no pregnancy. Our previous study showed that decidual response induced by intraluminal oil infusion on day 5 of pseudopregnancy was similar to that on day 4 [26]. Decidual response is a critical component of successful implantation. To address the uterine potential of decidualization in VOAT mice on day 5, we examined oil infusion into the uterus on day 5 of pseudopregnancy followed by uterine decidualization on day 8. In the VOAT mice, decidualization occurred in 68.8% (11/16) of mice, while the rate was 50.0% (4/8) in the intact mice (Fig. 2). The fold increases (mean ± SEM) in decidualized uterine weights of intact and VOAT mice were 12.0 ± 0.8 and 7.9 ± 1.7, respectively (Fig. 2c); the difference between them was not significant. These results indicate that uterine potential of decidualization in both VOAT and intact mice was similar during the early postimplantation period.
Figure 2.
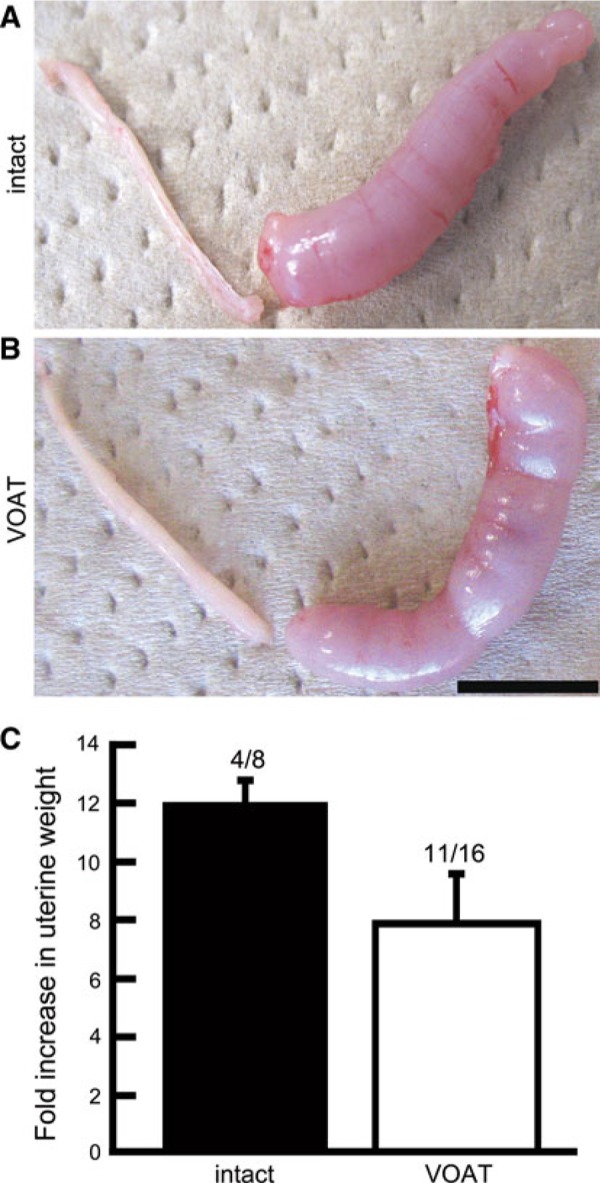
Induced uterine decidualization on day 5 of pseudopregnancy by oil infusion into one uterine horn of intact or VOAT mice. Decidualization was determined on day 8. Decidualization in uteri of intact (a) and VOAT (b) mice. Fold increases indicate comparison of weights between infused and non‐infused uterine horns (c, mean ± SEM). Numbers above bars indicate reacted/examined mice. Neither the appearance of decidualized mice nor the uterine fold increase was significantly different between intact and VOAT mice
Distributions of CYP11A1 and CD34 in the corpus luteum of mice with a uterus showing decidualization
For uterine potential of decidualization on day 5 of pseudopregnancy, no difference was observed between VOAT and intact mice. In contrast, VOAT recipients receiving blastocyst transfers on day 5 supported the pregnancy and production of offspring, while intact recipients receiving blastocyst transfers on day 5 showed no pregnancy. These results suggest that pregnancy failure is caused during the postimplantation period. Ovarian P4 is required for pregnancy maintenance. Therefore, we hypothesized that ovarian function in VOAT mice could be different from that in intact mice. To address this issue, we examined distributions of CYP11A1 and CD34 on day 8 of pseudopregnant mice with uterine decidualization induced on day 5. Immunohistochemical analysis in ovarian tissues showed that the expression of the steroidogenic enzyme CYP11A1 in luteal cells of VOAT mice was slightly less than that in intact mice (Fig. 3a, c). Furthermore, VOAT mice showed less CD34‐positive blood vessel density in the corpus luteum as compared to that in intact mice (Fig. 3b, d). These results indicate that the steroidogenesis and blood vessel density in VOAT mice were less than those of intact mice during decidualization.
Figure 3.
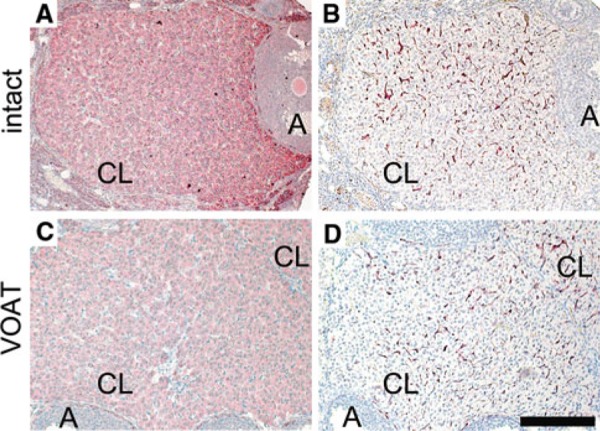
Immunohistochemical analysis for ovarian tissues in intact or VOAT mice. Expressions of CYP11A1 (a, c) and CD34 (b, d) in the corpus luteum of mice with uteri showing decidualization on day 8 of pseudopregnancy. a, b Intact mice, c, d VOAT mice. A Antral follicle, CL corpus luteum. Bar 200 μm
Discussion
In mice, the uterus is receptive on day 4 of pregnancy. The normal window of uterine receptivity for implantation lasts for a limited time period [13, 14, 15, 16]. The present study demonstrated that embryo transfer into VOAT mice on day 5 of pseudopregnancy was available to support the pregnancy and full term fetal development. Less steroidogenesis and blood vessel formation in the transferred ovarian tissues may be associated with the extended uterine receptivity.
The ovarian hormones enable uterine receptivity to accept blastocysts for implantation and pregnancy maintenance [13, 14, 15, 16]. In the present study, ovarian tissues on day 8 of pseudopregnant mice with uterine decidualization induced on day 5 showed that steroidogenesis and blood density in the corpus luteum in VOAT mice were lower as compared to those in intact mice. The window of uterine receptivity for blastocyst implantation in mice remains open for an extended period at lower E2 levels [18]. Furthermore, blastocysts can initiate implantation beyond the normal window of uterine receptivity when the P4 level is appropriate [27]. Therefore, different ovarian function in VOAT mice receiving blastocyst transfer on day 5 as compared to intact mice may be associated with extended uterine receptivity for blastocyst implantation and fetal development, although the potential of VOAT ovarian tissues was less than that in intact mice at the postimplantation period during early pregnancy.
Although the rate of pups from VOAT recipients receiving blastocyst transfers on day 5 of pseudopregnancy was higher than that of intact mice receiving blastocyst transfers on day 5, the uterine decidual response induced by intraluminal oil infusion on day 5 showed similar results between VOAT and intact mice. Since decidual response is a critical component of successful implantation, these results suggest that the uterine sensitivity on day 5 of pseudopregnancy in both VOAT and intact mice is similar during the periimplantation period. Therefore, development of transferred embryos at postimplantation could be supported in VOAT mice receiving blastocyst transfers on day 5 of pseudopregnancy, presumably due to altered endocrine functions.
Ovarian cryopreservation followed by re‐implantation appears to be a potentially valuable method for fertility preservation, e.g. in case of iatrogenic infertility caused by a decline in ovarian function after chemotherapy and radiotherapy which result in a serious problem for cancer patients [1]. In devising an animal model to address this issue, both cryopreservation of ovarian tissues and oocytes were be required. Therefore, we transferred the blastocysts derived from the mouseˈs own vitrified–warmed oocytes. The present study demonstrated the production of offspring derived from IVF of their own cryopreserved oocytes after embryo transfer into VOAT mice. Development to blastocyst stage in vitro takes 4 days after IVF. Therefore, we had to warm oocytes and perform IVF on the same day on which we mated females with vasectomized males for embryo transfer on day 4 of pseudopregnancy. If plug a positive pseudopregnant recipient is not obtained, the in vitro‐produced blastocysts can be cryopreserved. Repeated vitrification and warming decrease the quality of embryos in some cases. In contrast, for embryo transfer on day 5 of pseudopregnancy, oocyte warming and IVF can be performed on the next day after mating a female with a vasectomized male. If a plug positive female is not obtained, oocyte warming and IVF can be postponed. Therefore, embryo transfer on day 5 into VOAT mice could be helpful to obtain their own pups.
Acknowledgments
We thank Noriko Numata for expert technical assistance.
References
- 1. Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update, 2001, 7, 535–543 10.1093/humupd/7.6.535 [DOI] [PubMed] [Google Scholar]
- 2. Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod, 2008, 23, 2266–2272 10.1093/humrep/den244 [DOI] [PubMed] [Google Scholar]
- 3. Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Fertility preservation: successful transplantation of cryopreserved ovarian tissue in a young patient previously treated for Hodgkin's disease. Oncologist, 2007, 12, 1437–1442 10.1634/theoncologist.12‐12‐1437 [DOI] [PubMed] [Google Scholar]
- 4. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez‐Madrid B, Langendonckt A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet, 2004, 364, 1405–1410 10.1016/S0140‐6736(04)17222‐X [DOI] [PubMed] [Google Scholar]
- 5. Meirow D, Levron J, Eldar‐Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med, 2005, 353, 318–321 10.1056/NEJMc055237 [DOI] [PubMed] [Google Scholar]
- 6. Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, Ottosen C, Andersen CY. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod, 2006, 21, 2006–2009 10.1093/humrep/del140 [DOI] [PubMed] [Google Scholar]
- 7. Anderson RA, Wallace WH, Baird DT. Ovarian cryopreservation for fertility preservation: indications and outcomes. Reproduction, 2008, 136, 681–689 10.1530/REP‐08‐0097 [DOI] [PubMed] [Google Scholar]
- 8. Kim SS, Radford J, Harris M, Varley J, Rutherford AJ, Lieberman B, Shalet S, Gosden R. Ovarian tissue harvested from lymphoma patients to preserve fertility may be safe for autotransplantation. Hum Reprod, 2001, 16, 2056–2060 10.1093/humrep/16.10.2056 [DOI] [PubMed] [Google Scholar]
- 9. Kagawa N, Kuwayama M, Nakata K, Vajta G, Silber S, Manabe N, Kato O. Production of the first offspring from oocytes derived from fresh and cryopreserved pre‐antral follicles of adult mice. Reprod Biomed Online, 2007, 14, 693–699 10.1016/S1472‐6483(10)60670‐0 [DOI] [PubMed] [Google Scholar]
- 10. Wang X, Catt S, Pangestu M, Temple‐Smith P. Live offspring from vitrified blastocysts derived from fresh and cryopreserved ovarian tissue grafts of adult mice. Reproduction, 2009, 138, 527–535 10.1530/REP‐09‐0148 [DOI] [PubMed] [Google Scholar]
- 11. Mitsui A, Yoshizawa M. Successful pregnancy in ovariectomized mice using a combination of heterotopic autotransplantation of ovarian tissues and embryo transfer. Reprod Med Biol, 2007, 6, 85–90 10.1111/j.1447‐0578.2007.00170.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matsumoto H, Ezoe K, Mitsui A, Fukui E, Ochi M, Yoshizawa M. Vitrified–warmed ovarian tissue autotransplantation into ovariectomized mice restores sufficient ovarian function to support full‐term pregnancy. Reprod Med Biol, 2011, 10, 185–191 10.1007/s12522‐011‐0090‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet, 2006, 7, 185–199 10.1038/nrg1808 [DOI] [PubMed] [Google Scholar]
- 14. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev, 2004, 25, 341–373 10.1210/er.2003‐0020 [DOI] [PubMed] [Google Scholar]
- 15. Paria BC, Reese J, Das SK, Dey SK. Deciphering the cross‐talk of implantation: advances and challenges. Science, 2002, 296, 2185–2188 10.1126/science.1071601 [DOI] [PubMed] [Google Scholar]
- 16. Red‐Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal–fetal interface. J Clin Invest, 2004, 114, 744–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paria BC, Huet‐Hudson YM, Dey SK. Blastocyst's state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci USA, 1993, 90, 10159–10162 10.1073/pnas.90.21.10159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA, 2003, 100, 2963–2968 10.1073/pnas.0530162100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoshizawa M, Nakamoto S, Fukui E, Muramatsu T, Okamoto A. Chromosomal analysis of first‐cleavage mouse eggs fertilized in caffeine‐containing medium. J Reprod Dev, 1992, 38, 107–113 10.1262/jrd.38.107 [Google Scholar]
- 20. Matsumoto H, Ma W, Smalley W, Trzaskos J, Breyer RM, Dey SK. Diversification of cyclooxygenase‐2‐derived prostaglandins in ovulation and implantation. Biol Reprod, 2001, 64, 1557–1565 10.1095/biolreprod64.5.1557 [DOI] [PubMed] [Google Scholar]
- 21. Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase‐2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem, 2002, 277, 29260–29267 10.1074/jbc.M203996200 [DOI] [PubMed] [Google Scholar]
- 22. Agarwal P, Peluso JJ, White BA. Steroidogenic factor‐1 expression is transiently repressed and c‐myc expression and deoxyribonucleic acid synthesis are induced in rat granulosa cells during the periovulatory period. Biol Reprod, 1996, 55, 1271–1275 10.1095/biolreprod55.6.1271 [DOI] [PubMed] [Google Scholar]
- 23. Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS. Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod, 2005, 73, 1135–1146 10.1095/biolreprod.105.042739 [DOI] [PubMed] [Google Scholar]
- 24. Sakurai T, Tamura K, Okamoto S, Hara T, Kogo H. Possible role of cyclooxygenase II in the acquisition of ovarian luteal function in rodents. Biol Reprod, 2003, 69, 835–842 10.1095/biolreprod.102.010710 [DOI] [PubMed] [Google Scholar]
- 25. Sugino N, Suzuki T, Sakata A, Miwa I, Asada H, Taketani T, Yamagata Y, Tamura H. Angiogenesis in the human corpus luteum: changes in expression of angiopoietins in the corpus luteum throughout the menstrual cycle and in early pregnancy. J Clin Endocrinol Metab, 2005, 90, 6141–6148 10.1210/jc.2005‐0643 [DOI] [PubMed] [Google Scholar]
- 26. Song H, Lim H, Paria BC, Matsumoto H, Swift LL, Morrow J, Bonventre JV, Dey SK. Cytosolic phospholipase A2alpha is crucial [correction of A2alpha deficiency is crucial] for ‘on‐time’ embryo implantation that directs subsequent development. Development, 2002, 129, 2879–2889 [DOI] [PubMed] [Google Scholar]
- 27. Song H, Han K, Lim H. Progesterone supplementation extends uterine receptivity for blastocyst implantation in mice. Reproduction, 2007, 133, 487–493 10.1530/REP‐06‐0330 [DOI] [PubMed] [Google Scholar]


