Abstract
Implantation is a process of the first feto‐maternal encounter in the uterus. A competent blastocyst and a receptive uterus are critical for successful implantation. For an acquisition of uterine receptivity, the following conditions need to be satisfied in the uterine environments: the endometrial preparation with stromal proliferation and epithelial differentiation in the pre‐receptive phase and proper interactions between the uterus and blastocyst later in the phase. Focusing on these points and primarily referring to the mouse in vivo evidence, this review article has shown detailed molecular mechanisms for successful implantation.
Keywords: Cytokines, Embryo implantation, Embryo–uterine interactions, Ovarian steroid hormones, Uterine receptivity
Introduction
Pregnancy is a complicated physiological phenomenon comprising a series of these processes: ovulation, fertilization, implantation, embryonic growth, decidualization, feto‐placental growth and parturition. Each process is strictly coordinated and essential for successful pregnancy. Implantation, a process of the first feto‐maternal encounter in the uterus, consists of the following three steps: apposition, adhesion, and invasion of the embryo. Successful implantation is the result of appropriate molecular communication between the uterus and the blastocyst during these steps. Animal studies, especially mouse studies, have taken the lead in implantation research [1]. Notably, it is no wonder that recent studies using different kinds of genetically‐altered mice have given us valuable information in this research field. Since the current major concepts in embryo implantation have primarily arisen from mouse studies, here we principally refer to the mouse studies.
What are mandatory components for successful implantations? There are two essentials. One is an implantation‐competent blastocyst because poor embryo quality is likely to be one of the major causes of implantation failure [2]. On the other hand, “uterine receptivity” is also a significant factor, defined as uterine capacity in order to accommodate the competent blastocyst [1]. This capacity allows an appropriate endometrial preparation with stromal proliferation and epithelial differentiation stimulated by ovarian steroids in advance before the phase of embryo–uterine interactions (Fig. 1). In this process, progesterone‐dependent morphological changes in endometrial stroma are observed, which we call “pre‐decidualization” (Fig. 1) [3]. The small spike of ovarian estrogen is followed by an acquisition of the endometrial status, and then, the endometrium provides the embryo with adhesion activity. Thus, the uterus enters into the receptive phase. This acquisition of adhesion activity in the dormant blastocyst by endometrium‐derived factors is called “blastocyst activation” (Fig. 1) [1]. Further, the blastocyst adhesion onto the uterus induces an endometrial attachment reaction, in which stromal cells surrounding the blastocyst start to differentiate concurrently with polyploid formation, which is called “decidualization” (Fig. 1) [3]. The receptive phase of the uterus is transient, and unless the blastocyst adhesion occurs, the endometrium enters into the refractory phase, when any functional blastocysts are incapable of adhesion to the endometrium. Thus, the endometrium can allow blastocyst adhesion in the restricted period, which is usually regarded as the “implantation window” (Fig. 1) [3]. This sequence of events is critical for starting implantation.
Figure 1.
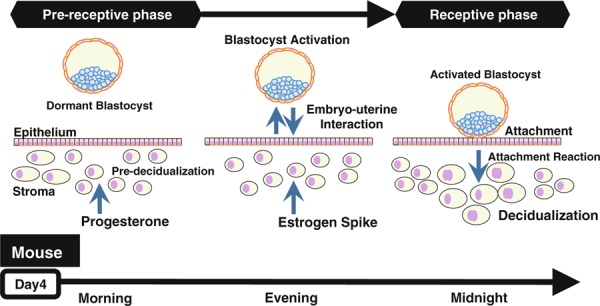
The process of embryo implantation
Ovarian steroid hormones produce and maintain uterine receptivity throughout implantation processes. Under the influences of progesterone and estrogen, there are important mediators for cell‐to‐cell communications in the uterine microenvironments during implantation: cytokines and growth factors such as leukemia inhibitory factor (LIF) and heparin‐binding epidermal growth factor‐like growth factor (HB‐EGF). For example, maternal LIF is essential for successful implantation [4], and HB‐EGF plays a key role in a two‐way communication between the embryo and the uterus [5]. These factors are considered to be crucial for uterine receptivity and blastocyst activation. In this review article, we describe the acquisition process of uterine receptivity and embryo–uterine molecular interactions through the major secreted mediators.
Implantation in mice and humans
Since a time‐line and hormonal conditions in the peri‐implantation period look comparable between mice and humans (Fig. 2), we have much to learn about embryo implantation from the previous mouse studies [1]. In mice, a vaginal plug is observed in the morning on the day after ovulation and mating, which we define as day 1 of pregnancy. Luminal epithelium strongly proliferates and the uterus looks swollen under the influence of an estrogen surge. On day 3 of pregnancy, corpora lutea are newly formed, and start to produce progesterone. Progesterone becomes completely dominant by the morning of day 4 when heightened progesterone makes endometrial stromal cells proliferate, called pre‐decidualization, and this phenomenon is similarly observed in humans. At the same time, the luminal epithelium ceases proliferation and differentiates for the blastocyst attachment reaction. Late on the morning of day 4, a small estrogen surge occurs as a starting signal of implantation. This estrogen spike leads to stromal edema and luminal closure placing the blastocyst in close apposition with the luminal epithelium, and makes the uterus produce some blastocyst activators like osteopontin (OPN) [6]. It is followed by an intimate adherence of the blastocyst trophectoderm to the luminal epithelium, marking the first discernible sign of implantation on the night of day 4 (2200–2400 h). Immediately after the implantation, stromal cells surrounding the blastocyst start differentiation, change their stromal morphology into epithelioid type with polyploidy, and form a new layer around the embryo. This process is known as decidualization. The attachment reaction coincides with an increased stromal vascular permeability at the site of the blastocyst. Embryo‐derived trophoblast cells invade the endometrium, and finally, embryo implantation is completed [1].
Figure 2.
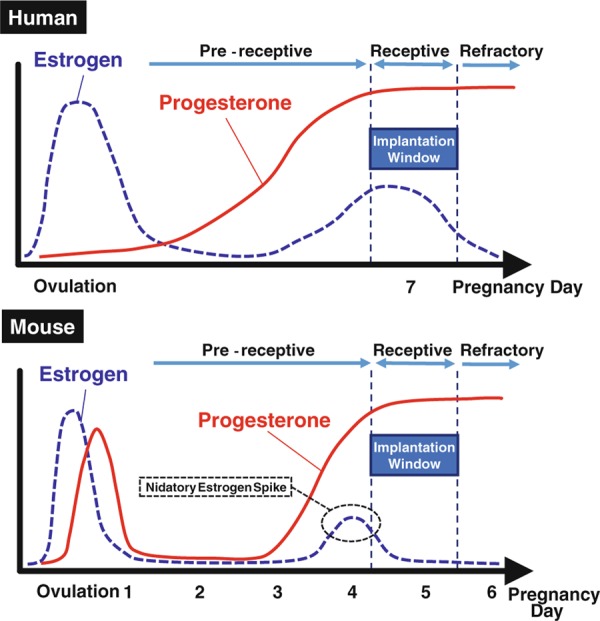
The hormonal status in early pregnancy
As described above, the present general concepts in the embryo implantation are gained from mouse studies. Since both in vivo and in vitro experiments, especially about blastocyst activation and decidualization (not pre‐decidualization), are technically and ethically difficult to perform in humans, mouse models are the most powerful approach to understand embryo implantation in vivo, and are globally applied in the current research of reproduction.
Ovarian hormones: estrogen and progesterone
Estrogen and progesterone play crucial roles throughout pregnancy. The following two processes under the control of ovarian steroids are needed for successful implantation: preparation of endometrial proliferation and differentiation, and appropriate embryo–uterine communication. In the pre‐receptive phase, the endometrium must keep specific differentiation status in which luminal epithelium ceases proliferation and subluminal stroma start to proliferate in a progesterone‐dominant condition. Next, a small spike of estrogen occurs just before the receptive phase. This nidatory estrogen with consistent influences of progesterone gives starting signals for embryo–uterine interactions to the uterus, the dormant blastocyst is activated, and the uterus turns to be receptive. Thus, the implantation‐competent blastocyst and the receptive uterus are prepared through the molecular communications between the embryo and uterus under the influence of ovarian hormones [1].
The endometrial proliferation and differentiation in the pre‐receptive phase is influenced by ovarian hormones, dominantly by progesterone which is known as a "hormone of pregnancy". Progesterone acts on the uterus via progesterone receptors (PR) throughout the pregnancy [7]. PR deficient mice show impaired reproductive phenotypes in ovulation, implantation and decidualization and a compromised status of endometrial proliferation and differentiation on day 4 of pregnancy [7]. In addition, mice with deletion of PR cochaperone FKBP52 have uterine progesterone resistance and impaired induction of progesterone‐responsive genes on the morning of day 4 [8]. The mutant females also have an impaired uterine status of proliferation and differentiation in the pre‐receptive phase, and further cause implantation failure [8]. Taken together, progesterone signaling is a major pathway regulating the endometrial preparation for appropriate differentiation and proliferation in the pre‐receptive phase.
Constant influences of progesterone are essential in the uterus during and after implantation, while transient estrogen influences are needed for the induction of implantation. Among the estrogen‐responsive genes, it is known that LIF and OPN are functionally important secreted proteins during implantation [4, 6]. Estrogen injection up‐regulates both genes in the glandular epithelium in the delayed implantation mouse model [6, 9]. These findings suggest that nidatory estrogen induces LIF and OPN in glandular epithelium, and these factors participate in embryo–uterine interactions to continue the subsequent implantation process. In addition, HB‐EGF is the most famous secretory factor for embryo–uterine interactions [5]. The detailed functions of these secreted factors are separately described in the following sections.
Leukemia inhibitory factor
The presence of various cytokines and their receptors in the uterus and the embryo during early pregnancy suggests their roles in implantation [1]. It is known that several cytokines such as LIF and M‐CSF are critical for normal female fertility by studies using gene‐altered mice [4, 10].
LIF, a cytokine in the interleukin‐6 family, is expressed in the uterus and plays critical roles in embryo implantation. LIF deficient mice reveal complete implantation failure [4]. In addition, the phenotype of implantation failure is reversed by recombinant LIF injection into the mutant females [4, 11]. LIF null embryos can develop normally and implant in the wild‐type uteri after blastocyst transfer to wild‐type recipients; however, wild‐type embryos do not implant in LIF deficient uteri after blastocyst transfer to the null females [4, 12, 13]. These findings indicate that maternal LIF is critical for successful implantation.
Then how does LIF work in the uterus during implantation? Since LIF expression rapidly increases after estrogen injection in the uteri of ovariectomized mice, LIF is considered to be an estrogen responsive gene in the mouse uterus. Therefore, estrogen might be a major regulator of uterine LIF expression during implantation [9, 14]. In fact, LIF is expressed at the highest level on day 1 of pregnancy when the uterus is under the influence of a preovulatory estrogen surge. Thereafter, it is expressed in uterine glands on the morning of day 4, and then in the stroma surrounding the blastocyst at the time of the attachment reaction on the night of day 4 and persists through the morning of day 5 [9]. Thus, LIF is expressed in day 4 pregnant uteri at two different times in two different cell types, and its expression is at low basal levels during the post‐implantation period [9]. These findings indicate that LIF is not required for pregnancy maintenance but for implantation. Although previous studies show such evidence, the precise effects of maternal LIF on implantation, especially on blastocyst activation, at the molecular level remain unclear.
In our recent study, the mice with uterine specific p53 deletion show normal implantation in spite of the reduction of LIF expression on the morning of day 4 [15]. The stromal LIF expression pattern surrounding the blastocyst at the time of attachment at midnight on day 4 is normal in the mutant females [15], suggesting that the reduced LIF expression levels in p53‐deleted uteri on the morning of day 4 is not a limiting factor for implantation. In addition, the CD1 mice with deficiency of PR cochaperone FKBP52 show implantation failure because of progesterone resistance, and have the reduced LIF expression at glandular epithelium on the morning of day 4 and at stroma on the night of day 4 [16]. Progesterone supplementation to the mutant mice can reverse both the phenotype of defective implantation and the stromal LIF expression at midnight on day 4, although the LIF expression at glandular epithelium on the morning of day 4 is still reduced after progesterone treatment [16]. These findings also suggest that the stromal LIF at midnight on day 4 might be more important than the epithelial one on the morning of day 4. Nonetheless, it is controversial where and when uterine LIF is expressed more critically on day 4 of pregnancy, and further investigations are required to clarify this issue (Fig. 3).
Figure 3.
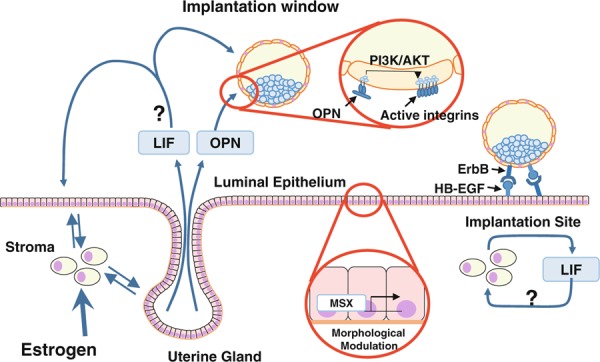
The secretory factors control embryo implantation
Recently, MSX homeobox genes are reported to be essential transcriptional regulators which morphologically modulate luminal epithelium and control normal implantation in mice [17]. Uterine deletion of both Msx1 and Msx2 completely inhibits blastocyst implantation [17]. LIF reduces uterine Msx1 expression and deficiency of Msx1/2 reduces LIF expression [17]. These findings suggest that MSXs are critical modulators in the system of uterine LIF expression in the peri‐implantation period.
LIF binds LIF receptor which dimerizes with glycoprotein gp130, the common signaling receptor for IL‐6 family cytokines, to activate several signaling pathways including the Jak–Stat pathway, the Ras–Raf–ERK pathway, and the PI3K–AKT pathway. In mouse embryonic stem (ES) cells, LIF up‐regulates Klf4 through Jak–Stat3 pathway and Tbx3 through PI3K–AKT pathway, and strongly stimulates the expressions of Sox2 and Nanog to maintain the Oct3/4 expression [18]. In contrast, LIF also activates the Ras–Raf–ERK pathway to inhibit Tbx3 activity, suggesting that these downstream pathways of LIF coordinately regulate the differentiation of ES cells [18]. Compared with this, LIF does not activate ERK but Stat3 in luminal epithelium on day 4 morning [19], suggesting the tissue‐selective activation of signaling pathways by LIF.
Osteopontin
Osteopontin, known as secreted phosphoprotein 1, is a glycoprotein and is involved in bone remodeling, leukocyte migration and endothelial cell attachment, and apoptosis [20]. OPN has an Arg‐Gly‐Asp (RGD) motif recognized by integrins, transmembrane heterodimeric cell‐adhesion proteins, to bridge cell‐to‐cell adhesion [20]. In pigs, trophoblast‐derived estrogen stimulates OPN secretion from the uterus [21], and OPN bridges trophoblast‐endometrial attachment through integrin αvβ6 of trophoblast and αvβ3 of luminal epithelium [22]. OPN is also expressed in rabbits, ewes, and humans in the peri‐implantation period [23, 24, 25]. In mice, OPN is expressed at uterine glands in an estrogen‐dependent manner immediately before implantation, stimulates integrins on the cell surface of trophoblast to activate an intracellular PI3K/AKT signaling pathway (Fig. 3) [6]. Since an activated blastocyst has more active integrins on the surface of trophectoderm than a dormant one [6], it is speculated that a major role of OPN in integrin is to activate a blastocyst. However, OPN null females do not show any reproductive defects and, therefore, other integrin activators must induce blastocyst activation corporately with OPN. In fact, both fibronectin and entactin, RGD motif‐containing proteins, promote mouse blastocyst adhesion [26, 27]. Although OPN is recently reported to be one of mediators in embryo–uterine interactions, HB‐EGF is the one which was initially identified and is the most well‐known [1], and is fully described in the section “Heparin‐binding epidermal growth factor‐like growth factor”.
Heparin‐binding epidermal growth factor‐like growth factor
Hormonal preparation for the receptive status with appropriate endometrial differentiation enables the uterine environment to proceed to the next phase of bidirectional molecular communications between the embryo and receptive uterus.
HB‐EGF, one of the EGF family members, is known as a key player in the embryo–uterine interactions with the subsequent uterine attachment reaction [5]. It is expressed in the luminal epithelium located around active blastocysts some hours before the attachment [28]. HB‐EGF is produced in soluble and transmembrane forms, and both forms affect blastocyst functions in an autocrine, paracrine, and/or juxtacrine manner [28, 29] via the EGF family of receptors which is expressed on the cell surface of trophectoderm [30, 31]. The soluble forms help to grow blastocysts [28], and the cell with transmembrane ones can adhere to the activated blastocyst [31] (Fig. 3). In addition, a recent mouse study shows that systemic deletion of HB‐EGF leads to perinatal lethality [32], and its uterine deletion defers implantation and reduces litter size [32], emphasizing its importance in implantation.
HB‐EGF and other EGF family members such as EGF, TGFα, betacellulin, epiregulin, neuregulin, and amphiregulin interact with the receptor subtypes of the ErbB family, ErbB1, ErbB2, ErbB3, and ErbB4, which have a tyrosine kinase domain for signal transduction. ErbBs form primarily homodimers or heterodimers to be activated by the ligands. Among these ErbB family members, ErbB1 and ErbB4 on the cell surface of trophectoderm can interact with uterine HB‐EGF in embryo implantation in mice [30, 31]. The expression of both ErbB1 and ErbB4 is down‐regulated in dormant blastocyst but is markedly up‐regulated in the activated blastocyst [30, 33]. The activated blastocyst also expresses HB‐EGF, which can induce HB‐EGF transcripts in the uterus. These findings suggest the presence of a molecular feed‐forward loop between the embryo and the uterus for the attachment reaction. Moreover, many studies also show significant roles of HB‐EGF in human implantation. For example, the endometrial expression level of HB‐EGF is the highest in the receptive epithelium [34, 35]. The cells expressing the transmembrane form of HB‐EGF can adhere to human blastocyst displaying cell surface ErbB4 [36]. Taken together, HB‐EGF is critical for embryo–uterine interactions during embryo implantation.
Conclusion
This review article described uterine receptivity and embryo–uterine interactions through key players such as ovarian hormones, HB‐EGF, LIF and OPN. Since their detailed mechanisms remain vague as described above, many further investigations are required to clarify them. New future findings are expected to be applied in a clinical setting for infertility treatment and contraception.
Acknowledgments
We thank Chie Minoda and Ryoko Shimizu‐Hirota for editing the manuscript. This study was funded by grants from JST PRESTO program, the Grant‐in‐Aid for Scientific Research from Japan Society for the Promotion of Science, the Takeda Science Foundation, the Kowa Life Science Foundation and the Yamaguchi Endocrine Research Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T et al. Molecular cues to implantation. Endocr Rev, 2004, 25, 341–373 10.1210/er.2003-0020 [DOI] [PubMed] [Google Scholar]
- 2. Urman B, Yakin K, Balaban B. Recurrent implantation failure in assisted reproduction: how to counsel and manage. A. General considerations and treatment options that may benefit the couple. Reprod Biomed Online, 2005, 11, 371–381 10.1016/S1472-6483(10)60846-2 [DOI] [PubMed] [Google Scholar]
- 3. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med, 2012, 18, 1754–1767 10.1038/nm.3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature, 1992, 359, 76–79 10.1038/359076a0 [DOI] [PubMed] [Google Scholar]
- 5. Lim HJ, Dey SK. HB‐EGF: a unique mediator of embryo‐uterine interactions during implantation. Exp Cell Res, 2009, 315, 619–626 10.1016/j.yexcr.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaen T, Konno T, Egashira M, Bai R, Nomura N, Nomura S et al. Estrogen‐dependent uterine secretion of osteopontin activates blastocyst adhesion competence. PLoS ONE, 2012, 7, e48933 10.1371/journal.pone.0048933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev, 1995, 9, 2266–2278 10.1101/gad.9.18.2266 [DOI] [PubMed] [Google Scholar]
- 8. Tranguch S, Cheung‐Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA, 2005, 102, 14326–14331 10.1073/pnas.0505775102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX‐2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF‐deficient mice. Mol Endocrinol, 2000, 14, 1147–1161 10.1210/me.14.8.1147 [DOI] [PubMed] [Google Scholar]
- 10. Pollard JW, Hunt JS, Wiktor‐Jedrzejczak W, Stanley ER. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF‐1 in female fertility. Dev Biol, 1991, 148, 273–283 10.1016/0012-1606(91)90336-2 [DOI] [PubMed] [Google Scholar]
- 11. Cheng JG, Rodriguez CI, Stewart CL. Control of uterine receptivity and embryo implantation by steroid hormone regulation of LIF production and LIF receptor activity: towards a molecular understanding of “the window of implantation”. Rev Endocr Metab Disord, 2002, 3, 119–126 10.1023/A:1015402811650 [DOI] [PubMed] [Google Scholar]
- 12. Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology, 2000, 141, 4365–4372 10.1210/en.141.12.4365 [DOI] [PubMed] [Google Scholar]
- 13. Sherwin JR, Freeman TC, Stephens RJ, Kimber S, Smith AG, Chambers I et al. Identification of genes regulated by leukemia‐inhibitory factor in the mouse uterus at the time of implantation. Mol Endocrinol, 2004, 18, 2185–2195 10.1210/me.2004-0110 [DOI] [PubMed] [Google Scholar]
- 14. Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci USA, 1991, 88, 11408–11412 10.1073/pnas.88.24.11408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine‐specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest., 2010, 120, 803–815 10.1172/JCI40051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency‐conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest, 2007, 117, 1824–1834 10.1172/JCI31622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell, 2011, 21, 1014–1025 10.1016/j.devcel.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature, 2009, 460, 118–122 10.1038/nature08113 [DOI] [PubMed] [Google Scholar]
- 19. Cheng JG, Chen JR, Hernandez L, Alvord WG, Stewart CL. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci USA, 2001, 98, 8680–8685 10.1073/pnas.151180898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post‐translational phosphorylation and protein folding. J Cell Biochem, 2007, 102, 912–924 10.1002/jcb.21558 [DOI] [PubMed] [Google Scholar]
- 21. White FJ, Ross JW, Joyce MM, Geisert RD, Burghardt RC, Johnson GA. Steroid regulation of cell specific secreted phosphoprotein 1 (osteopontin) expression in the pregnant porcine uterus. Biol Reprod, 2005, 73, 1294–1301 10.1095/biolreprod.105.045153 [DOI] [PubMed] [Google Scholar]
- 22. Erikson DW, Burghardt RC, Bayless KJ, Johnson GA. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol Reprod, 2009, 81, 814–825 10.1095/biolreprod.109.078600 [DOI] [PubMed] [Google Scholar]
- 23. Apparao KB, Illera MJ, Beyler SA, Olson GE, Osteen KG, Corjay MH et al. Regulated expression of osteopontin in the peri‐implantation rabbit uterus. Biol Reprod, 2003, 68, 1484–1490 10.1095/biolreprod.101.001347 [DOI] [PubMed] [Google Scholar]
- 24. Johnson GA, Burghardt RC, Joyce MM, Spencer TE, Bazer FW, Pfarrer C et al. Osteopontin expression in uterine stroma indicates a decidualization‐like differentiation during ovine pregnancy. Biol Reprod, 2003, 68, 1951–1958 10.1095/biolreprod.102.012948 [DOI] [PubMed] [Google Scholar]
- 25. Wolff M, Strowitzki T, Becker V, Zepf C, Tabibzadeh S, Thaler CJ. Endometrial osteopontin, a ligand of beta3‐integrin, is maximally expressed around the time of the “implantation window”. Fertil Steril, 2001, 76, 775–781 10.1016/S0015-0282(01)02015-5 [DOI] [PubMed] [Google Scholar]
- 26. Armant DR, Kaplan HA, Lennarz WJ. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev Biol., 1986, 116, 519–523 10.1016/0012-1606(86)90152-1 [DOI] [PubMed] [Google Scholar]
- 27. Yelian FD, Edgeworth NA, Dong LJ, Chung AE, Armant DR. Recombinant entactin promotes mouse primary trophoblast cell adhesion and migration through the Arg‐Gly‐Asp (RGD) recognition sequence. J Cell Biol, 1993, 121, 923–929 10.1083/jcb.121.4.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M et al. Heparin‐binding EGF‐like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF‐receptor in implantation. Development, 1994, 120, 1071–1083 [DOI] [PubMed] [Google Scholar]
- 29. Das SK, Tsukamura H, Paria BC, Andrews GK, Dey SK. Differential expression of epidermal growth factor receptor (EGF‐R) gene and regulation of EGF‐R bioactivity by progesterone and estrogen in the adult mouse uterus. Endocrinology, 1994, 134, 971–981 10.1210/en.134.2.971 [DOI] [PubMed] [Google Scholar]
- 30. Paria BC, Elenius K, Klagsbrun M, Dey SK. Heparin‐binding EGF‐like growth factor interacts with mouse blastocysts independently of ErbB1: a possible role for heparan sulfate proteoglycans and ErbB4 in blastocyst implantation. Development, 1999, 126, 1997–2005 [DOI] [PubMed] [Google Scholar]
- 31. Raab G, Kover K, Paria BC, Dey SK, Ezzell RM, Klagsbrun M. Mouse preimplantation blastocysts adhere to cells expressing the transmembrane form of heparin‐binding EGF‐like growth factor. Development, 1996, 122, 637–645 [DOI] [PubMed] [Google Scholar]
- 32. Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ et al. Maternal heparin‐binding‐EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci USA, 2007, 104, 18315–18320 10.1073/pnas.0707909104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paria BC, Das SK, Andrews GK, Dey SK. Expression of the epidermal growth factor receptor gene is regulated in mouse blastocysts during delayed implantation. Proc Natl Acad Sci USA, 1993, 90, 55–59 10.1073/pnas.90.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin‐binding epidermal growth factor‐like growth factor in the human endometrium: a possible role in blastocyst implantation. Dev Genet, 1997, 21, 102–108 [DOI] [PubMed] [Google Scholar]
- 35. Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK et al. Multiple roles for heparin‐binding epidermal growth factor‐like growth factor are suggested by its cell‐specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab, 1999, 84, 3355–3363 10.1210/jc.84.9.3355 [DOI] [PubMed] [Google Scholar]
- 36. Chobotova K, Spyropoulou I, Carver J, Manek S, Heath JK, Gullick WJ et al. Heparin‐binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech Dev, 2002, 119, 137–144 10.1016/S0925-4773(02)00342-8 [DOI] [PubMed] [Google Scholar]


