Abstract
Erythrodermic psoriasis (EP), 1 of the most rare and severe forms of psoriasis, is characterized by general erythema with silvery scales. Systemic vasodilatation in EP is potentially life-threatening, however, the degree and extent of inflammation in subcutaneous tissues are difficult to estimate accurately using standard skin inspections or ultrasound examinations. Computed tomography can be a useful modality in solving this problem. The authors report a case of EP. Sequential contrast-enhanced whole-body computed tomography before and after treatment with a tumor necrosis factor-α inhibitor (infliximab) visualized the inflammation and the effect of the treatment.
Keywords: Erythrodermic psoriasis, Inflammation, Vasodilatation, Computed tomography
Introduction
Erythrodermic psoriasis (EP), the most rare and severe form of psoriasis, is characterized by general redness of skin with silvery scales, and accounts for 1.5% to 2.25% of all psoriasis cases, with a reported mortality rate of up to 9% [1], [2]. Currently, psoriasis has become recognized as a systemic inflammatory disease and an independent cardiovascular risk [3], [4]. This is primarily based on evidence of increased serum inflammatory biomarkers and increased [18F]-fluorodeoxyglucose (FDG) uptake in multiple organs, including vascular systems, in patients with psoriasis [2], [3], [5], [6], [7]. Vascular inflammation in patients with EP is potentially life-threatening, and can increase the risk for atherosclerosis, myocardial infarction, and stroke. Systemic vasodilatation can develop into lethal hypothermia, high-output cardiac failure, and loss of essential proteins and fluids [2], [3], [4], [8]. Nevertheless, vascular inflammation is often subclinical because standard skin inspections cannot precisely assess the degree of inflammation in the subcutaneous tissues, and ultrasound examination is inadequate for assessing the entire body. Although a few authors have reported [18F]-FDG positron emission tomography-computed tomography (PET-CT) imaging findings in moderate-to-severe psoriasis [5], [6], [7], imaging findings in EP using computed tomography (CT) are largely unknown. We report a case of EP assessed using sequential contrast-enhanced whole-body CT follow-up before and after tumor necrosis factor (TNF)-α inhibitor (infliximab) treatment.
Case presentation
A 45-year-old man with a 6-year history of uncontrolled plaque psoriasis treated with a topical preparation of steroid and vitamin D3 presented with generalized erythema. Physical examination revealed erythematous plaques with silver scales covering more than 80% of his body surface area, with a Psoriasis Area and Severity Index (PASI) score of 43.0. Vital signs of the patient were all stable. Laboratory investigations revealed leukocytosis (11.0 × 109 cells/L; normal range 3.5-10.5 × 109 cells/L) and elevated C-reactive protein concentration (39.3 mg/L; normal range < 3 mg/L). Before starting the TNF-α inhibitor infliximab, contrast-enhanced whole-body CT was performed to exclude latent tuberculosis or malignancies.
On contrast-enhanced CT (Acquilion One; Toshiba, Tochigi, Japan) after injection of 100 mL iohexol (Omnipaque 300; GE Healthcare, Cork, Ireland), widespread thickening of the skin with fat stranding and subcutaneous vasodilatation were demonstrated, accompanied by lymphadenopathy of bilateral axillary and inguinal regions (Fig. 1). The patient did not exhibit any subjective nor objective signs suggestive of contrast media allergy.
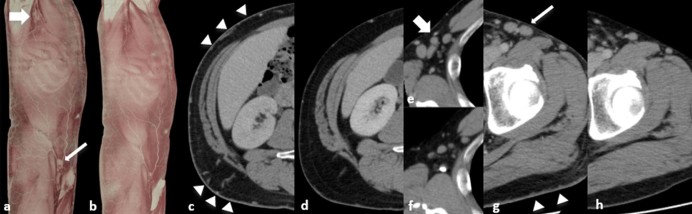
Fig. 1.
Contrast-enhanced computed tomography (CT) of a patient with erythrodermic psoriasis. (A) Three-dimensional (3D)-CT image before infliximab treatment revealing systemic vasodilatation and bilateral axillary (wide arrow) and inguinal (narrow arrow) lymphadenopathy. (B) 3D-CT image after infliximab treatment. Improvement of vasodilatation and lymphadenopathy is apparent. (C) Axial CT image before treatment revealing vasodilatation, diffuse skin thickening, and subcutaneous fat stranding (arrowheads). (D) Follow-up axial CT after treatment revealing improvement of these imaging findings. (E, G) Contrast-enhanced CT before treatment revealing lymphadenopathy of axillary (wide arrow) and inguinal regions (narrow arrow). Note the thickened skin and subcutaneous fat stranding (arrowheads). (F, H) Follow-up CT after treatment revealing improvement of these findings.
Until the skin biopsy results were returned 2 weeks later, treatment consisted of etretinate (vitamin A) and empirical antibiotics. Improvement of subjective symptoms was limited, with only a minor PASI score change from 43.0 to 41.6 (Fig. 2 A-C).
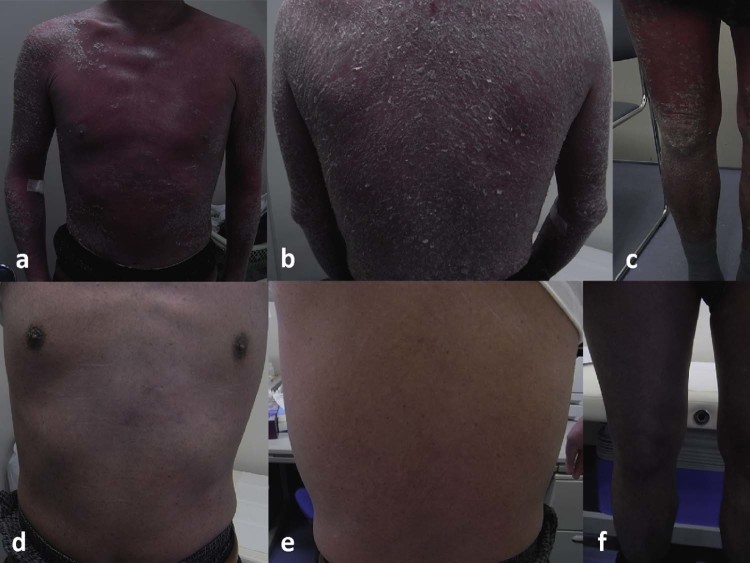
Fig. 2.
Photographs of a patient with erythrodermic psoriasis treated with infliximab. (A–C) Photograph at baseline photograph revealing general erythema with silvery scales covering more than 80% of body surface area. Psoriasis Area and Severity Index (PASI) score was 41.6. (D–F) After 4 courses of infliximab treatment, these skin findings improved, with a PASI score reduction to 5.6.
Skin biopsy results were consistent with psoriasis (Fig. 3), without any findings suggestive of neoplasm. Treatment with infliximab was started, and after 4 courses, the patient exhibited improvement of dermal symptoms, with a PASI score reduction from 41.6 to 5.6 (Fig. 2 D-F). On follow-up CT performed 15 weeks after the previous whole-body CT, thickening of the skin, systemic subcutaneous vasodilatation, and lymphadenopathy improved significantly (Fig. 1). As of February 15, 2017, the patient has maintained good condition with infliximab administration every 2 months.
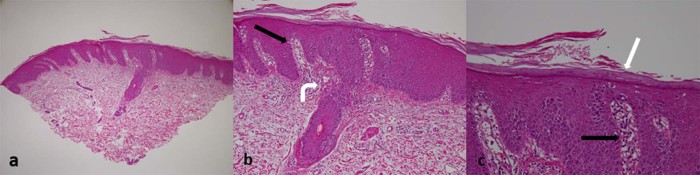
Fig. 3.
Pathological findings of the biopsied skin specimen. (A–C) microscopic examination (hematoxylin and eosin staining in 3 different power fields) demonstrating irregular elongation of rete ridges (black arrows), parakeratosis with neutrophil infiltration (white arrow), and vasodilatation in the papillary dermis (curved white arrow), all compatible with psoriasis.
Discussion
EP often develops in patients with longstanding, poorly controlled psoriasis and some triggers are known—both administration and abrupt withdrawal of corticosteroid and methotrexate; drug reactions; sunburn; underlying systemic infection; and emotional stress [1], [2]. The whole-body CT findings in patients with EP are largely known. In the present case, the patient had 6-year history of uncontrolled plaque psoriasis before developing EP. EP is often challenging to treat and a mortality rate of up to 9% has been reported [2]. EP causes systemic vasodilatation, which may lead to severe hypothermia, high-output cardiac failure, and loss of proteins and fluids [8], [9]. Additionally, patients with EP are susceptible to infections [9]. Evidence has suggested that severe psoriasis is a systemic inflammatory disease and an independent risk factor for atherosclerosis, myocardial infarction, and stroke [2], [4]. In the present case, laboratory data revealed leukocytosis and increased C-reactive protein in the acute phase; however, the patient had no history of cardiovascular disease.
Several biological drugs targeting key pathological factors have been used for severe psoriasis including EP. The TNF-α inhibitors infliximab, etanercept and adalimumab, and the interleukin-12/23 p40 monoclonal antibody ustekinumab, are effective for moderate-to-severe psoriasis [2]. Viguier et al reported on the efficacy of these biological drugs for EP [2]. In the present case, after 4 courses of infliximab treatment, the PASI score of the patient fell from 41.6 to 5.6 in 13 weeks, consistent with the findings of a previous report [2].
The pathogenesis of potentially life-threatening systemic vasodilatation in patients with EP is not fully understood. Elevated blood flow in skin with plaque psoriasis has been confirmed, and vasodilatation in the papillary dermis is 1 of the characteristic histopathological hallmarks of psoriasis [10]. One of the key pathogenic factors in these vessel changes is TNF-α. Patel et al [11] reported that increased TNF-α levels lead to increased blood flow and endothelin-I release. Although endothelin-I spillover impairs endothelium-dependent vasodilatation, TNF-α still induces endothelium-independent vasodilatation, resulting in increased blood flow [12], [13]. Serum TNF-α levels in patients with psoriasis are elevated [14], [15]. Similarly, increased serum TNF-α levels may have been associated with EP in our patient, although it was not measured. The follow-up CT after TNF-α inhibitor (infliximab) treatment revealed improvement in systemic vasodilatation, which strongly suggests that TNF-α was a key factor in the development of these vessel changes.
Diagnostic imaging of EP remains under development. Mehta et al demonstrated [18F]-FDG uptake in foci of inflammation, including the skin, liver, joints, tendons, and aorta, in patients with moderate-to-severe psoriasis on FDG-PET-CT [6]. Naik et al reported on the correlation between vascular [18F]-FDG uptake and the severity of psoriasis [7]. However, although many authors have described CT imaging findings in bones and joints in patients with psoriatic arthritis, CT imaging findings in other tissues in psoriasis including EP are not well-known [13]. In many hospitals, PET-CT is not always a readily accessible modality compared with CT, and neither normal skin inspections nor ultrasound examinations are suitable for detailed whole-body screening to assess the degree and extent of inflammation in the skin and vessels. Therefore, understanding and appropriately interpreting CT imaging findings in EP is important to both dermatologists and radiologists.
Conclusion
We reported a case of EP assessed using sequential contrast-enhanced whole-body CT and follow-up. CT before infliximab treatment revealed general skin thickening, systemic subcutaneous vasodilatation, and lymphadenopathy of the bilateral axillary and inguinal regions. After 4 courses of infliximab, follow-up CT confirmed improvement of these findings.
Footnotes
Acknowledgments: We thank Dr Yasuro Miura for helping us to prepare for the figures.
Funding: This case report was not funded by any grant or company.
Availability of data and material: The dataset supporting the conclusions of this article is included within the article.
Authors' contribution: RK performed the background literature review, drafted the manuscript, and performed manuscript revisions. AH performed histopathological specimen analysis, presentation, and manuscript preparation. YN performed the skin examinations and manuscript revisions. KK assisted with image selection, image presentation, manuscript preparation, and manuscript revisions. All the authors read the final paper version and approved it.
Competing interests: The authors declare that they have no competing interests.
Consent for publication: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Ethics approval and consent to participate: Patient presented in this report was investigated within a retrospective study approved by the ethical review board of Toshiba hospital.
References
- 1.Raychaudhuri S.K., Maverakis E., Raychaudhuri S.P. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13(4–5):490–495. doi: 10.1016/j.autrev.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Viguier M., Pages C., Aubin F., Delaporte E., Descamps V., Lok C. Efficacy and safety of biologics in erythrodermic psoriasis: a multicentre, retrospective study. Br J Dermatol. 2012;167(2):417–423. doi: 10.1111/j.1365-2133.2012.10940.x. [DOI] [PubMed] [Google Scholar]
- 3.Di Meglio P., Villanova F., Nestle F.O. Psoriasis. Cold Spring Harb Perspect Med. 2014;4(8) doi: 10.1101/cshperspect.a015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta N.N., Azfar R.S., Shin D.B., Neimann A.L., Troxel A.B., Gelfand J.M. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hern S., Stanton A.W., Mellor R., Levick J.R., Mortimer P.S. Control of cutaneous blood vessels in psoriatic plaques. J Invest Dermatol. 1999;113(1):127–132. doi: 10.1046/j.1523-1747.1999.00638.x. [DOI] [PubMed] [Google Scholar]
- 6.Mehta N.N., Yu Y., Saboury B., Foroughi N., Krishnamoorthy P., Raper A. Systemic and vascular inflammation in patients with moderate to severe psoriasis as measured by [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT): a pilot study. Arch Dermatol. 2011;147(9):1031–1039. doi: 10.1001/archdermatol.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naik H.B., Natarajan B., Stansky E., Ahlman M.A., Teague H., Salahuddin T. The severity of psoriasis associates with aortic vascular inflammation detected by FDG PET/CT and neutrophil activation in a prospective observational study. Arterioscler Thromb Vasc Biol. 2015;35(12):2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reuler J.B., Jones S.R., Girard D.E. Hypothermia in the erythroderma syndrome. West J Med. 1977;127:243–244. [PMC free article] [PubMed] [Google Scholar]
- 9.Jha P.K., Das S.R., Musleh G.S., Lakshmanan S., Kingston T.P., Deiraniya AK. Psoriasis-induced postoperative cardiac failure. Ann Thorac Surg. 2005;79(4):1390–1391. doi: 10.1016/j.athoracsur.2003.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson E.H., Epstein W.L. 1961. Clearance of I131 injected intralesionally in patients with psoriasis11from the division of Dermatology, Department of Medicine, University of California School of Medicine, San Francisco, California. Presented at the Twenty-second Annual Meeting of The Society for Investigative Dermatology, Inc., New York, N.Y., June 28, 1961. Journal of Investigative Dermatology, 37 (5) pp. 441-445. [Google Scholar]
- 11.Patel J.N., Jager A., Schalkwijk C., Corder R., Douthwaite J.A., Yudkin J.S. Effects of tumour necrosis factor-alpha in the human forearm: blood flow and endothelin-1 release. Clin Sci. 2002;103(4):409–415. doi: 10.1042/cs1030409. [DOI] [PubMed] [Google Scholar]
- 12.Johns D.G., Webb R.C. TNF-α-induced endothelium-independent vasodilation: a role for phospholipase A2-dependent ceramide signaling. Am J Physiol. 1998;275:1592–1598. doi: 10.1152/ajpheart.1998.275.5.H1592. [DOI] [PubMed] [Google Scholar]
- 13.Aleo E., Migone S., Prono V., Barbieri F., Garlaschi G., Cimmino M.A. Imaging techniques in psoriatic arthritis: update 2012-2014 on current status and future prospects. J Rheumatol Suppl. 2015;93:53–56. doi: 10.3899/jrheum.150637. [DOI] [PubMed] [Google Scholar]
- 14.Arican O., Aral M., Sasmaz S., Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005(5):273–279. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagami S., Rizzo H.L., Lee J.J., Koguchi Y., Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130(5):1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]