Abstract
Background
A postovulatory mammalian oocyte decreases developmental potential with in vivo aging in the oviduct or in vitro aging in the culture dish. The mechanism underlying oocyte aging still largely remains an enigma. Accumulating data suggest that the epigenetic alterations such as histone acetylation are also associated with postovulatory aging.
Objective
To perform a review evaluating a new aspect of oocyte aging in terms of the epigenetic alterations focusing on lysine acetylation.
Methods
In addition to a search of the literature in Pubmed, we introduced our recent published data.
Results
Histone acetylation in the mouse oocyte increases during aging, potentially impacting gene regulation in the subsequent embryonic development. Oocyte aging results in increased acetylation of alpha‐tubulin, a non‐histone protein, and nicotinamide, an inhibitor of class III HDAC, partially prevents some of oocyte aging phenotypes.
Conclusion
Abnormal regulation of protein acetylation itself is suggested in oocyte aging and could contribute to the aging phenotypes.
Keywords: HDAC, Lysine acetylation, Mouse, Oocyte, Postovulatory in vitro aging
Introduction
After ovulation, mammalian oocytes are arrested at meiotic metaphase II (MII) until they are activated by penetrating spermatozoa or artificial stimuli. However, mammalian oocytes have a limited time for fertilization after ovulation. The window for optimal fertilization differs in different species, and it has been determined that mouse oocytes exhibit the most potential between 8 and 12 h after ovulation, the rat (12–14 h), and monkeys and humans (<24 h) [1]. After that time, unfertilized mature oocytes progressively undergo a time‐dependent process of aging, a so‐called “oocyte aging”, during which changes are observed in morphology and cell biology such as creation of a large perivitelline space, disruption of microfilament, elongation of spindle, chromosome scattering and so on [2]. These changes contribute to a decrease in the potential for fertilization and embryo development. Further, aged oocytes show the abnormalities of a high proportion of cellular fragmentation and cell death [3, 4]. In the mouse, following ovulation, the fragmentation of unfertilized mature oocytes has been viewed as a manifestation of apoptosis, or programmed cell death [5, 6, 7, 8].
Recently, however, it has been reported that the epigenetic alterations including DNA methylation and histone acetylation are also associated with postovulatory aging [9]. DNA methylation is involved in transcriptional repression, X chromosome inactivation and genomic imprinting [10]. It also plays pivotal roles in normal embryonic development. Acetylation, one of the most common modifications of histones, also serves as a key modulator of chromatin structure and gene transcription during normal development [11]. Therefore, it is also important to study how oocyte aging impacts their epigenetic status and the underlying mechanism, and to develop the methods to prevent their alterations, since they could be transferred to the cells of offspring and impact their phenotypes.
Further, lysine acetylation is found in not only histones but also in more than 80 transcription factors and various cytoplasmic proteins [12]. Does abnormal acetylation occur only in histones in aged oocytes? Recently, we found that α‐tubulin, a typical non‐histone protein, is more acetylated in aging mouse oocytes [13]. Considered together, we illustrate that oocyte aging may not impact only on acetylation status in chromatin but also in non‐histone proteins, which could alter the protein functions to lead to lower “oocyte quality”. Thus, protein acetylation may be a key to understanding the impaired development associated with oocyte aging.
Lysine acetylation and its regulation
Post translational modification (PTM) is crucial for regulating the functions of many eukaryotic proteins as represented by serine, threonine, and tyrosine phosphorylation. The lysine side chain is a target of different PTMs such as ubiquitination, methylation, and acetylation, which are mutually exclusive on the same lysine. The correlation between histone acetylation and increased transcription has been known for many years [14]. However, as mentioned above, lysine acetylation, one of the PTMs, is found in not only histones but also in more than 80 transcription factors and various cytoplasmic proteins [12]. Acetylation is a reversible lysine modification. Lysine is acetylated by histone acetyltransferases (HATs) (also referred as KATs: lysine acetyltransferases) and deacetylated by histone deacetylases (HDACs) (also referred as KDACs: lysine deacetylases). There are three major groups of HATs: Gcn5‐related N‐acetyltransferases, E1A‐associated protein of 300 kDa (p300)/CREB‐binding protein (CBP), and MYST proteins [15]. In contrast, HDACs are divided into five categories: class I (HDAC 1–3 and 8), class IIa (HDAC 4, 5, 7, and 9), class IIb (HDAC 6 and 10), class III (Sirt 1–7), and class IV (HDAC 11) [16]. These enzymes are expressed in a cell type‐specific manner to form a complex with other proteins in order to specifically regulate the targeted protein functions through acetylation.
Abnormal epigenetic status associated with postovulatory aging
As mentioned above, histone acetylation status at the lysine residues is disrupted during postovulatory aging. Immunofluorescence analysis with specific antibodies AcH3K9, AcH3K14, AcH4K5, AcH4K8, AcH4K12, and AcH4K16, with the exception of a weak signal for AcH4K8, shows a lack of signal in mouse oocytes at 14 h post‐hCG, suggesting a very low level of these modifications in fresh oocytes. However, with the progression of postovulatory time to 19 h after hCG injection, fluorescence signals for AcH4K8 and AcH4K12 increase gradually. When aging progresses to 24 h past hCG injection, AcH3K14 shows an increase of fluorescence signals in the oocytes (Table 1) [17]. This activity can be partially prevented by the addition of pyruvate [18] or caffeine [17] to the culture medium to improve fertilizability and developmental capacity of aged oocytes [18, 19]. Unlike in mouse oocytes, AcH4K12 is acetylated in mature porcine oocytes with its acetylation level increasing during postovulatory aging [20]. Thus, postovulatory oocyte aging is correlated with hyperacetylation at lysine residues of histones.
Table 1.
Profiles of lysine acetylation modification during postovulatory aging
Interestingly, 5 h treatment of fresh mouse oocytes with trichostatin A (TSA), an inhibitor of class I/IIb HDACs, induces hyperacetylation in H3K14 and H4K12 but not in H3K9 and H4K5 [17]. Further, the higher spontaneous activation rate in the TSA group compared to that of the control group shows that mouse oocyte aging is accelerated. Taken together, HDAC activity in fresh oocytes is crucial to maintain lysine acetylation of histone at a low level and their inhibition could accelerate oocyte aging.
Loss of some DNA methylation in oocytes is also associated with postovulatory aging. For example, Snrpn, a maternally imprinted gene, maintains the normal DNA methylation pattern in oocytes at 13 and 21 h, whereas lost methylation is observed in some oocytes at 29 h of hCG injection but not Peg1/Mest, another maternally imprinted gene [21]. The defects of DNA methylation induced by oocyte aging are suggested to be time‐dependent and locus‐specific. Together, histone acetylation modifications in oocytes as well as DNA methylation are changed with progression of oocyte aging, which may contribute to lower development.
Abnormal α‐tubulin acetylation associated with postovulatory aging
Aging oocytes have increased histone acetylation [17]. However, it is not known how protein acetylation is connected with oocyte aging. In addition to histones, other classes of nonhistone proteins such as transcription factors are also regulated by dynamic acetylation and deacetylation; α‐tubulin, a cytoskeletal protein, is the best characterized one. Acetylation status of α‐tubulin influences its stability and a variety of intracellular trafficking through the interaction with other proteins [22]. Therefore, we focused on the acetylation status of α‐tubulin during aging in oocytes. Compared with fresh collected oocytes (0 h), aging oocytes gradually increased the amount of both acetylated α‐tubulin (Ac α‐tubulin) and α‐tubulin until 24 h, with Ac α‐tubulin accumulating more than α‐tubulin (Fig. 1). It should be noted that at 36 h, aged oocytes showed astral microtubules which were not always associated with Ac α‐tubulin. Thus, these data indicate that in addition to the amount of α‐tubulin, its acetylation status changes along with oocyte aging, providing a new insight into the mechanism underlying abnormal acetylation during oocyte aging.
Figure 1.
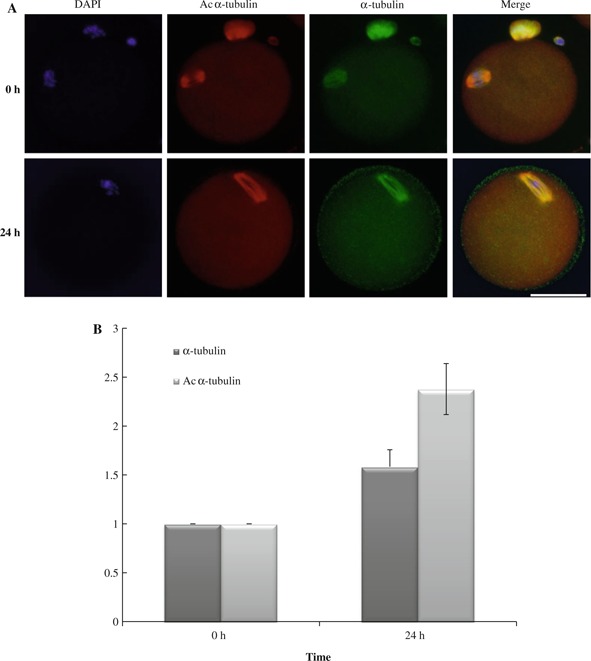
Increased acetylated α‐tubulin 24 h after oocyte collection. a Immunostaining of aging oocytes with anti‐Ac α‐tubulin and anti‐α‐tubulin antibodies revealed that during oocyte aging, the level of Ac α‐tubulin and α‐tubulin was increased until 24 h. b Summary of quantification. Data are given as mean % ± SEM. Ac α‐tubulin showed a greater increase than α‐tubulin itself
Dynamics of lysine acetylation in oocytes
As previously mentioned, lysine is acetylated by HATs and deacetylated by HDACs. How is lysine acetylation regulated in the oocyte? In general, fresh oocytes show extremely low levels of acetylation both for histone and α‐tubulin [23, 24]. However, once oocyte activation occurs, they show hyperacetylation. Consistently, we have showed that a pan‐acetyl‐K antibody, which recognizes acetylated lysine in a wide range of sequence contexts such as acetylated histones, p53, CBP, PCAF and so on, strongly labeled microtubules and nuclei after oocyte activation, which was even more enhanced under TSA [25]. We also found that HDAC activity was significantly reduced after oocyte activation. Interestingly, a 3 h TSA treatment of activated oocytes resulted in more accumulation of acetylated lysine than a 3 h TSA treatment of unactivated oocytes, suggesting that increased HAT activities may be induced after oocyte activation [25]. Thus, lysine acetylation status in oocytes is precisely regulated at a low level, which dynamically increases coupled to oocyte activation.
How can a lower level of lysine acetylation in fresh oocytes be achieved? Actually ovulated oocytes are rich in multiple HDACs including HDAC1, 2, 3, 4, 6, 8 and 9, for which expression is reduced before the 4‐cell stage [26]. Further, class III HDACs (Sirt1 ~7) are also abundantly expressed, which are also reduced at the 2‐cell stage [26, 27]. Actually, only 3 h of TSA treatment are required for unactivated oocytes to become hyperacetylated in the cytoplasm and spindle [25]. Thus, only fresh oocytes are especially rich in HDACs which guarantee a low acetylation status, suggesting the important roles in the maintenance of oocyte quality and reprogramming during zygotic stage.
Requirements of HDACs for normal oocyte maturation and embryonic development
Accumulating data suggest that HDACs play pivotal roles during oocyte meiosis and preimplantation development. Inhibition of meiotic HDACs with TSA induces aneuploidy in fertilized mouse oocytes resulting in embryonic death in utero at an early stage of development [28]. Even after fertilization, inhibition of HDACs with TSA during the one‐cell stage leads to reducing embryonic developmental rates [29, 30, 31]. It is noted that inhibition of HDACs during the one‐cell stage show a surprising improvement in embryonic development after somatic‐cell nuclear transfer (SCNT) [32]. Referring to each HDAC, HDAC2 regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in the mouse [33]. HDAC6 is a HDAC detected in the cytoplasm of germinal vesicle (GV) stage oocytes and 1‐cell embryos, of which ectopic expression alters the nuclear structure and causes premature compaction of the chromatin through its ubiquitin‐binding activity but not deacetylase activity [34]. In contrast, HDAC1 is expressed at low levels in MII oocytes and 1‐cell embryos, but at high levels in 2‐cell and 4‐cell embryos. Thus, HDAC1 is the major zygotically activated HDAC, with the most number of interactions with other genes in a gene network for mouse 2‐cell embryos. This activity suggests that HDAC1 is the key regulator of the correct patterns of gene expression required for further development [35]. Further, sirtuins, which are a class III HDAC, belonging to NAD+‐dependent protein deacetylase family, are also abundant in MII oocytes [27]. Interestingly, inhibition of sirtuins by nicotinamide prevents some abnormalities of oocyte aging such as the elongation of the spindle (Fig. 2), suggesting the involvement of sirtuins in oocyte aging [13]. Furthermore, Sirt3 protects mouse preimplantation embryos against oxidative stress‐induced p53‐mediated developmental arrest [27]. Thus, multiple HDACs are involved in meiosis and embryonic development, of which correct regulation is essential for normal development.
Figure 2.
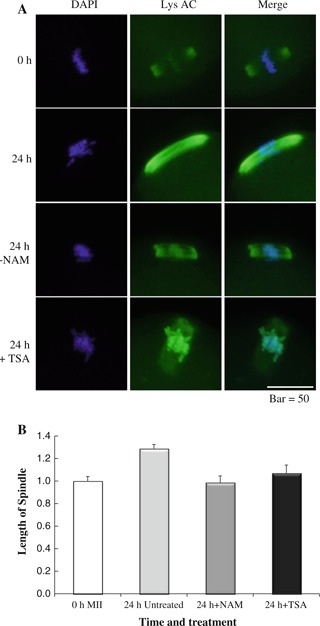
Effect of HDAC inhibitors on spindle shape 24 h after oocyte collection. These were visualized with DAPI and anti‐acetylated lysine antibody. a Immunostaining of aging oocytes with anti‐Ac K antibody at 24 h revealed that NAM treatment maintained compact spindle formation at MII but that TSA treatment partially prevented spindle elongation associated with microtubule loss in spindles. b Summary of quantification of the length of spindles compared with freshly collected oocytes (0 h)
Concluding remarks
In this review, we introduced ideas about abnormal lysine acetylation associated with postovulatory oocyte aging and the roles of HDACs in oocyte and embryos. Abnormal histone acetylation and DNA methylation of oocytes are also associated with advanced maternal age [9]. Thus, the epigenetic status of oocytes is not static, but rather dynamic, and easily changes with aging, possibly affecting the developmental potential of embryos. As mentioned above, HDACs are abundant in oocytes and 1‐cell stage, suggesting the importance of their roles during this time period. The current view demonstrates that multiple HDACs correctly regulate the acetylation status of histone and nonhistone proteins in fresh oocytes. A lack of regulation then leads to an imbalance of acetylation, and essentially, hyperacetylation in aged oocytes. Future studies should address the mechanism underlying lysine acetylation activity and develop methods to control it in order to increase the efficiency of reproduction from aged oocytes.
Acknowledgments
We acknowledge discussions with T. Castranio. We are grateful to all the laboratory members for their comments and help. Part of the work cited in this paper was supported by the PRESTO (Precursory Research for Embryonic Science and Technology) program of the Japan Science and Technology Agency and a Grant‐in‐Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (grant number 23580416, to SK).
Conflict of interest
The author declares that they have no conflict of interest.
References
- 1. Austin CR Whittaher JR. Concepts of development, 1974. Sunderland: Sinauer Associates Inc., Publisher; [Google Scholar]
- 2. Miao YL, Kikuchi K, Sun QY, Schatten H. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum Reprod Update., 2009, 15, 573–585 10.1093/humupd/dmp014 [DOI] [PubMed] [Google Scholar]
- 3. Lanman JT. Delays during reproduction and their effects on the embryo and fetus. 2. Aging of eggs. New Engl J Med, 1968, 278, 1047–1054 10.1056/NEJM196805092781906 [DOI] [PubMed] [Google Scholar]
- 4. Sakai N, Endo A. Effects of delayed mating on preimplantation embryos in spontaneously ovulated mice. Gamete Res, 1988, 19, 381–385 10.1002/mrd.1120190409 [DOI] [PubMed] [Google Scholar]
- 5. Takase K, Ishikawa M, Hoshiai H. Apoptosis in the degeneration process of unfertilized mouse ova. Tohoku J Exp Med, 1995, 175, 69–76 10.1620/tjem.175.69 [DOI] [PubMed] [Google Scholar]
- 6. Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol., 1999, 213, 1–17 10.1006/dbio.1999.9344 [DOI] [PubMed] [Google Scholar]
- 7. Perez GI, Tao XJ, Tilly JL. Fragmentation and death (a.k.a. apoptosis) of ovulated oocytes. Mol Hum Reprod, 1999, 5, 414–420 10.1093/molehr/5.5.414 [DOI] [PubMed] [Google Scholar]
- 8. Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. Intracellular calcium oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod, 2002, 66, 1828–1837 10.1095/biolreprod66.6.1828 [DOI] [PubMed] [Google Scholar]
- 9. Liang X, Ma J, Schatten H, Sun Q. Epigenetic changes associated with oocyte aging. Sci China Life Sci., 2012, 55, 670–676 10.1007/s11427‐012‐4354‐3 [DOI] [PubMed] [Google Scholar]
- 10. Ma JY, Liang XW, Schatten H, Sun QY. Active DNA demethylation in mammalian preimplantation embryos: new insights and new perspectives. Mol Hum Reprod, 2012, 18, 333–340 10.1093/molehr/gas014 [DOI] [PubMed] [Google Scholar]
- 11. Clayton AL, Hazzalin CA, Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell, 2006, 23, 289–296 10.1016/j.molcel.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 12. Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non‐histone proteins. Gene, 2005, 363, 15–23 10.1016/j.gene.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 13. Lee AR, Kishigami S, Amano T, Matsumoto K, Wakayama T, Hosoi Y. Nicotinamide: a class III HDACi delays in vitro aging of mouse oocytes. J Reprod Dev., 2013, 59, 238–244 10.1262/jrd.2012‐1713934134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci USA, 1964, 61, 786–794 10.1073/pnas.51.5.786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol, 2007, 8, 284–295 10.1038/nrm2145 [DOI] [PubMed] [Google Scholar]
- 16. Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci, 2009, 32, 591–601 10.1016/j.tins.2009.06.0022771446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang JC, Yan LY, Lei ZL, Miao YL, Shi LH, Yang JW, Wang Q, Ouyang YC, Sun QY, Chen DY. Change in histone acetylation during postovulatory aging of mouse oocyte. Biol Reprod, 2007, 77, 666–670 10.1095/biolreprod.107.062703 [DOI] [PubMed] [Google Scholar]
- 18. Liu N, Wu YG, Lan GC, Sui HS, Ge L, Wang JZ, Liu Y, Qiao TW, Tan JH. Pyruvate prevents aging of mouse oocytes. Reproduction, 2009, 138, 223–234 10.1530/REP‐09‐0122 [DOI] [PubMed] [Google Scholar]
- 19. Ono T, Mizutani E, Li C, Yamagata K, Wakayama T. Offspring from intracytoplasmic sperm injection of aged mouse oocytes treated with caffeine or MG132. Genesis., 2011, 49, 460–471 10.1002/dvg.20756 [DOI] [PubMed] [Google Scholar]
- 20. Cui MS, Wang XL, Tang DW, Zhang J, Liu Y, Zeng SM. Acetylation of H4K12 in porcine oocytes during in vitro aging: potential role of ooplasmic reactive oxygen species. Theriogenology, 2011, 75, 638–646 10.1016/j.theriogenology.2010.09.031 [DOI] [PubMed] [Google Scholar]
- 21. Liang XW, Zhu JQ, Miao YL, Liu JH, Wei L, Lu SS, Hou Y, Schatten H, Lu KH, Sun QY. Loss of methylation imprint of SNRPN in postovulatory aging mouse oocyte. Biochem Biophys Res Commun, 2008, 371, 16–21 10.1016/j.bbrc.2008.03.105 [DOI] [PubMed] [Google Scholar]
- 22. Verhey KJ, Gaertig J. The tubulin code. Cell Cycle, 2007, 6, 2152–2160 10.4161/cc.6.17.4633 [DOI] [PubMed] [Google Scholar]
- 23. Wang F, Kou Z, Zhang Y, Gao S. Dynamic reprogramming of histone acetylation and methylation in the first cell cycle of cloned mouse embryos. Biol Reprod, 2007, 77, 1007–1016 10.1095/biolreprod.107.063149 [DOI] [PubMed] [Google Scholar]
- 24. Schatten G, Simerly C, Asai DJ, Szöke E, Cooke P, Schatten H. Acetylated alpha‐tubulin in microtubules during mouse fertilization and early development. Dev Biol., 1988, 130, 74–86 10.1016/0012‐1606(88)90415‐0 [DOI] [PubMed] [Google Scholar]
- 25. Matsubara K, Lee AR, Kishigami S, Ito A, Matsumoto K, Chi H, Nishino N, Yoshida M, Hosoi Y. Dynamics and regulation of lysine‐acetylation during one‐cell stage mouse embryos. Biochem Biophys Res Commun, 2013, 434, 1–7 10.1016/j.bbrc.2013.03.083 [DOI] [PubMed] [Google Scholar]
- 26. Yoshida N, Brahmajosyula M, Shoji S, Amanai M, Perry AC. Epigenetic discrimination by mouse metaphase II oocytes mediates asymmetric chromatin remodeling independently of meiotic exit. Dev Biol, 2007, 301, 464–477 10.1016/j.ydbio.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 27. Kawamura Y, Uchijima Y, Horike N, Tonami K, Nishiyama K, Amano T, Asano T, Kurihara Y, Kurihara H. Sirt3 protects in vitro‐fertilized mouse preimplantation embryos against oxidative stress‐induced p53‐mediated developmental arrest. J Clin Invest., 2010, 120, 2817–2828 10.1172/JCI420202912189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akiyama T, Nagata M, Aoki F. Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. Proc Natl Acad Sci USA, 2006, 103, 7339–7344 10.1073/pnas.05109461031464342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito M, Sakai S, Nagata M, Aoki F. Effect of histone deacetylases inhibitors on early preimplantation development in mouse embryo. J Mammal Ova Res., 2000, 17, 90–99 10.1274/jmor.17.90 [Google Scholar]
- 30. Ma J, Svoboda P, Schultz RM, Stein P. Regulation of zygotic gene activation in the preimplantation mouse embryo: global activation and repression of gene expression. Biol Reprod, 2001, 64, 1713–1721 10.1095/biolreprod64.6.1713 [DOI] [PubMed] [Google Scholar]
- 31. Kishigami S, Ohta H, Mizutani E, Wakayama S, Bui HT, Thuan NV, Hikichi T, Suetsugu R, Wakayama T. Harmful or not: trichostatin A treatment of embryos generated by ICSI or ROSI. Cent Euro J Biol, 2006, 1, 376–385 10.2478/s11535‐006‐0023‐5 [Google Scholar]
- 32. Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun, 2006, 340, 183–189 10.1016/j.bbrc.2005.11.164 [DOI] [PubMed] [Google Scholar]
- 33. Ma P, Schultz RM. Histone deacetylase 2 (HDAC2) regulates chromosome segregation and kinetochore function via H4K16 deacetylation during oocyte maturation in mouse. PLoS Genet, 2013, 9, e1003377 10.1371/journal.pgen.10033773597510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verdel A, Seigneurin‐Berny D, Faure AK, Eddahbi M, Khochbin S, Nonchev S. HDAC6‐induced premature chromatin compaction in mouse oocytes and fertilised eggs. Zygote., 2003, 11, 323–328 10.1017/S0967199403002387 [DOI] [PubMed] [Google Scholar]
- 35. Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol., 2005, 283, 40–57 10.1016/j.ydbio.2005.03.038 [DOI] [PubMed] [Google Scholar]


