Abstract
Various factors such as gonadotrophins, growth factors, and steroid hormones play important roles in the regulation of oocyte/follicular growth in mammalian ovaries. In addition to these factors, there is a bidirectional interaction between oocytes and granulosa cells that is essential for achieving optimal oocyte developmental competence. Oocytes play a key role in this interaction by secreting paracrine factors that alter the activities of neighboring cumulus cells, such as the expression of a specific amino acid transporter, cholesterol biosynthesis, and levels of glycolysis in the cumulus cells. Among the known oocyte‐derived factors, growth differentiation factor 9 (GDF9) is the dominant factor mediating the regulation by oocytes leading to cumulus expansion and granulosa cell proliferation. GDF9 frequently interacts with other oocyte‐derived factors in a synergistic manner. It seems reasonable to speculate that oocytes growing in vitro require interactions similar to those in vivo. Some of the oocyte‐mediated regulations have been confirmed in vitro, providing evidence of the usefulness of culture systems as a strong tool for such studies. This review discusses in vitro culture of growing oocytes in terms of oocyte–granulosa cell interactions.
Keywords: BMP15, GDF9, In vitro growth, Oocyte–granulosa cell interaction, Ovarian follicle
Introduction
In the mammalian ovary, numerous oocytes degenerate either before growth or at various stages of growth [1]. Those redundant but “potential” oocytes can be rescued if they are provided with suitable culture conditions that allow them to escape degeneration and continue to grow. It is desirable for oocytes’ health and growth to be maintained in culture systems, and granulosa cells around the oocyte ought to proliferate to prevent spontaneous oocyte denudation. On that basis, both oocytes and granulosa cells should be functional. In addition to this, recent studies have indicated that a bidirectional interaction between oocytes and granulosa cells is essential for normal oocyte development. It seems reasonable to speculate that oocytes growing in vitro require similar interactions. In this review, the oocyte–granulosa cell interactions are considered in terms of culture conditions, which are presumed to be crucial for supporting optimum oocyte growth in vitro.
Fertility of oocytes grown in vitro
We already have evidence that oocytes can grow and mature into “normal” ova in vitro. Eppig and Schroeder [2] reported the production of the first mice derived from oocytes cultured for the latter half of their growth period. Several other studies have also reported viable mouse offspring produced from a similar growth stage of oocytes [3, 4, 5, 6]. A combination of preantral follicle culture following an 8‐day organ culture of newborn mouse ovaries produced the first live offspring, named Eggbert, from an oocyte grown in vitro for the entire growth period [7]. The culture system has since been remarkably improved [8]. Besides the mouse, the cow is the only mammal the offspring of which have been born from oocytes grown in vitro from the stage where they were approximately 75–80% of the maximum oocyte diameter after a 14‐day culture period [9, 10].
On the other hand, rat oocytes grown in vitro were able to complete meiosis, but did not undergo preimplantation development [11]. Similarly, pig oocytes cultured for 16 days from preantral follicles were able to mature and undergo fertilization, but were unable to develop the male pronucleus [12].
Basic culture conditions
Before discussing the oocyte–granulosa cell interactions, it is useful to summarize the culture systems developed to date and the basic conditions necessary to support the survival of oocytes and granulosa cells. This is because if culture conditions are suboptimal for either oocytes or granulosa cells, no further bidirectional communication would be expected.
Two major types of culture systems
Several different culture systems have been developed [13]. These can be divided into two types according to the structure of the follicles or oocyte–granulosa cell complexes [13] depending on whether follicles/complexes spread on a substratum (the substratum‐adhering type, Fig. 1a–d) or maintain their spherical shape (the sphere type, Fig. 1e, f). In the former type, preantral follicles or oocyte–granulosa cell complexes adhere to the substratum and proliferate outward, creating a gentle swelling around the oocyte [4, 5, 7, 14]. The simplicity of this type of system gives it an advantage in terms of narrowing down the basic conditions for oocyte growth regulation [15]. Alternatively, follicular cells on the substratum proliferate to form a dome‐like structure, as has been reported in the mouse [16, 17, 18], rat [19], cow [10], and pig [20]. In the sphere type of system, each preantral follicle or oocyte–granulosa cell complex maintains or grows into a spherical shape, developing an antral cavity if subjected to proper stimulation, as has been reported in the mouse [3, 21, 22, 23, 24], cow [25, 26, 27, 28], pig [12, 29], sheep [30, 31], goat [32, 33, 34], and human [35, 36]. To achieve a 3‐D culture, follicles/complexes are often embedded in collagen‐ [37] or alginate‐based matrices [36, 38, 39].
Figure 1.
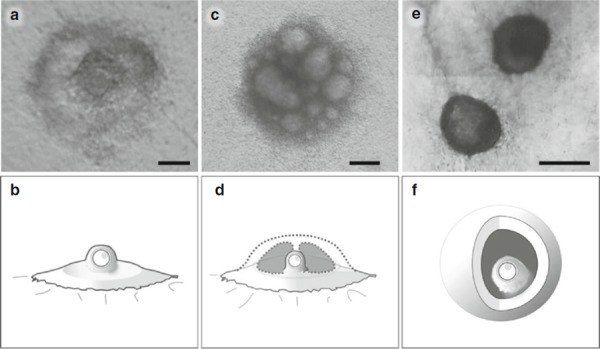
Typical morphologies of oocyte–granulosa cell complexes developed in vitro. a Mouse complexes after a 10‐day culture period on collagen‐coated substratum, and b a simplified illustration. c Bovine complexes after a 14‐day culture period on collagen‐coated substratum, and d a simplified illustration. e Bovine complexes after a 16‐day culture period within the collagen matrices, and f a simplified illustration. Scale bars 100 μm (a), 200 μm (c), and 500 μm (e)
Medium supplements affecting follicle/oocyte viability
Studies on the mitogenic effect of follicle‐stimulating hormone (FSH) and its second messenger cyclic adenosine monophosphate (cAMP) have been conducted. While FSH is not indispensable to mouse oocyte growth [2], intact preantral follicles (composed of the oocyte, granulosa cells, theca cells, and the basement membrane) survive and grow better in FSH‐supplemented medium than in a control medium [40, 41]. Similarly, porcine oocyte–granulosa cell complexes survived better in a medium containing FSH than in one without [42]. In contrast, a luteinizing hormone (LH)‐supplemented medium diminished the viability of follicles [43].
Cyclic AMP is known to be involved in various aspects of ovarian regulation [44]. Dibutyryl cyclic AMP (dbcAMP), a cell‐permeable analogue of cAMP, has frequently been used to test its effect in culture. Mouse follicle growth in vitro was promoted with dbcAMP [22]. Similarly, 4 mM hypoxanthine, a natural inhibitor of phosphodiesterase, which degrades intracellular cAMP [45], was beneficial to the survival of bovine oocyte–granulosa cell complexes [25, 27]. The concentration of hypoxanthine in mouse follicular fluid was estimated to be 2–4 mM [46].
The effects of other factors on the viability of follicles and oocytes have been also examined. Epidermal growth factor (EGF)‐supplemented medium was twice as effective as the control medium in promoting the survival of mouse follicles [7]. A synergistic effect of EGF and insulin‐like growth factor I (IGF‐I) on oocyte survival has been observed in the goat [32] and pig [29]. In the pig, however, the same combination increased apoptosis in a serum‐free medium [29].
Medium supplements affecting oocyte growth
Mouse oocytes grown in a medium containing fetal bovine serum are more competent in embryogenesis than those grown in a serum‐free medium [47]. The addition of dbcAMP [22] or hypoxanthine [25, 27, 42] to the medium is also beneficial to the growth and acquisition of meiotic competence of oocytes. Similarly, a combination of FSH and LH can promote the acquisition of oocyte meiotic competence [43] and developmental competence [48]. However, under culture conditions optimized without the use of FSH, supplementary FSH can reduce oocyte developmental competence [49].
Epidermal growth factor [7] and IGF‐I [50] used in mouse oocyte growth cultures have improved the developmental competence of oocytes. In addition, the growth of caprine preantral follicles is improved by a synergistic effect of IGF‐I and EGF [32]. Activin A is also beneficial to bovine oocyte growth [28], but follicle survival was impaired after activin A‐treatment in a mouse study [51].
Androgens are other biological factors that promote the acquisition of meiotic competence in vitro [52, 53]. Conversely, oocytes grown in a medium containing anti‐androgenic compounds are not capable of maturation [18]. Therefore, it appears to be beneficial to add androgen to the medium for oocyte growth, particularly when theca cells are removed before the culture. On the other hand, exogenous estradiol may be unnecessary for the production of oocytes that are capable of maturation [41, 54]. Rather, excess exposure to estrogen during oocyte growth appears to decrease the probability of successful fertilization [48]. However, a recent mouse study has uncovered a role for estrogen and oocyte‐derived factors together in promoting the ability of cumulus cells to undergo expansion [55].
It is well established that c‐Kit and c‐Kit ligand (KL) are involved in oocyte–granulosa cell interactions [56]. The addition of the KL to the culture medium promotes the growth of mouse oocytes within the cultured follicles [57, 58] and even in those without associated granulosa cells [59]. Within the follicles, however, appropriately supplemented FSH is needed in the modulation by KL to promote oocyte growth [60].
Besides biological factors present in ovaries, high concentrations of polyvinylpyrrolidone (PVP; molecular weight: 360,000) improve the survival and growth of bovine oocyte–granulosa cell complexes [10]. A calf was produced from an oocyte grown in medium supplemented with 4% PVP [10]. Furthermore, a combined supplement of PVP and fibroblast growth factor‐7 improved the growth of bovine oocytes [61].
Medium supplements affecting granulosa cells
Carroll et al. [22] have reported a remarkable improvement in the growth of mouse preantral follicles after the addition of dbcAMP to the medium. Similarly, hypoxanthine promotes the survival of intact bovine early antral follicles [27] and helps to maintain the association between oocytes and the surrounding granulosa cells [62]. In another study, a specific phosphodiesterase present in granulosa cells was targeted with two phosphodiesterase type 3‐inhibitors (PDE3‐Is), org9935 and cilostamide, resulting in the promotion of growth, differentiation, and survival of mouse preantral follicles [63].
Intact mouse preantral follicles cultured in medium without FSH show reduced survival during long‐term culture [64]. In addition, FSH plays an important role in promoting granulosa cell differentiation in cells from preantral follicles, so that these cells respond to LH stimulation [65]. However, granulosa cells exposed to excess FSH during follicle growth in vitro, show unusual expression of LH receptors, which may impair the developmental competence of oocytes [49].
Besides the factors described above, estradiol [66] and androstenedione [67, 68] are potent stimulators of follicular growth in vitro. IGF‐I also enhances granulosa cell proliferation, maintenance of follicular integrity, and the survival of oocytes in vitro [29, 32].
Medium supplements affecting antrum formation
Intact mouse preantral follicles develop into morphologically normal antral follicles in FSH‐supplemented medium and retain their spherical shape [23]. On the other hand, in a substratum‐adhering system, the spherical shape collapses, instead transforming into a dome‐like structure [16, 19, 21, 40, 69]. Bovine and porcine oocyte–granulosa cell complexes develop a dome‐like structure in media containing high concentrations of PVP [10, 20]. However, FSH was not added to the culture medium in these studies. It is interesting that the “mural” granulosa cell masses grow in a rim pattern remotely from the oocyte at the center, even in the absence of the dome formation [14].
Besides FSH, antral formation of mouse preantral follicles is promoted by the addition of dbcAMP [69] and androstenedione [67, 68] to the medium. Activin A has also been shown to promote follicular antrum formation in the rat [70] and humans [35].
Oocyte–granulosa cell interactions
The discovery and molecular characterization of oocyte‐derived factors in the 1990s showed that oocytes secrete paracrine factors, thereby generating bidirectional interactions between oocytes and granulosa cells, which are essential for achieving optimal oocyte developmental competence [71, 72]. Until then, many lines of experimental evidence drew attention to the passive activity of the oocyte during growth, for example, a large part of the nutrition of growing oocytes is delivered by the associated granulosa cells [73]. However, a recent study still identified a role for granulosa cells in the regulation of intraoocyte pH via gap junctions [74]. Therefore, oocytes and granulosa cells are both important in the elaborate mechanisms controlling oogenesis and folliculogenesis.
Dominant roles for oocytes
In the bidirectional oocyte–granulosa cell interactions, oocytes play a key role [75]. Using the expression of LH receptor mRNA as a marker of the mural granulosa cell phenotype in the mouse, Eppig et al. [76] clearly demonstrated that paracrine factor(s) secreted by oocytes play a dominant role in the establishment of granulosa cell phenotypic heterogeneity. Later, by using cumulus marker mRNA transcripts such as Slc38a3 and Amh, Diaz et al. [77] demonstrated that oocytes induce cumulus cell differentiation through the SMAD2/3 signaling pathway. Bovine oocytes are capable of determining phenotypic differences between cumulus cells and mural granulosa cells [78].
Mouse oocytes alter the metabolic activity of neighboring cumulus cells to counterbalance oocyte‐specific metabolic deficits [79, 80, 81]. The paracrine signals secreted by oocytes promote the expression of a sodium‐coupled neutral amino acid transporter in cumulus cells, which then increases the oocytes’ uptake of amino acids via the cumulus cells [79]. Oocytes apply the same strategy to cholesterol biosynthesis [80] and the glycolytic enzymes of cumulus cells to increase metabolism cooperatively [81]. Furthermore, bone morphogenetic protein‐15 (BMP15) and fibroblast growth factors (FGFs) secreted by oocytes cooperate to promote glycolysis in cumulus cells [82].
Growth differentiation factor 9 and BMP15
Growth differentiation factor 9 and BMP15 represent two major oocyte‐derived factors essential for regulating folliculogenesis [83, 84, 85]. GDF9 and BMP15 are known to regulate the function of cumulus cells in a synergistic manner [86, 87, 88]. Both of these factors are expressed in mouse oocytes growing in organ‐cultured ovaries [89]. The characteristics of GDF9‐null mice are arrested folliculogenesis at the primary stage and aberrant expressions of various genes are involved in follicular functions [90].
One of most well‐known activities of oocyte‐derived factors is the promotion of granulosa cell proliferation [91, 92, 93]. In the culture of bovine oocyte–granulosa cell complexes isolated from early antral follicles, granulosa cell proliferation ceases after removing the oocyte (Fig. 2). GDF9 is the major contributor to growth‐promoting activity [94]. In fact, GDF9‐supplemented medium enhances the growth of preantral follicles [95, 96, 97]. However, GDF9 does not account for the entire mitogenic activity originating from oocytes [98]. For example, BMP15 can promote the growth of granulosa cells [99].
Figure 2.
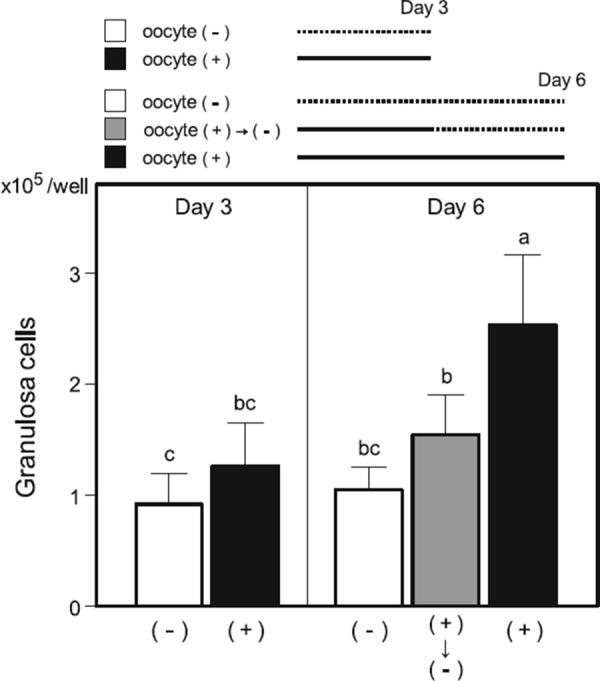
Number of granulosa cells cultured for 3 or 6 days as an oocyte–granulosa cell complex (+) or as a granulosa cell mass after the removal of the oocyte (−) in the wells of 96‐well culture plates. a–cValues with different superscripts are significantly different (p < 0.05, Tukey test)
A rat study suggested an anti‐apoptotic effect of GDF9 on the cultured preantral follicles, and also the involvement of PI3/Akt pathway in the activity [100]. Using bovine cumulus–oocyte complexes, however, Hussein et al. [101] found that bone morphogenic proteins prevent cumulus cell apoptosis but GDF9 does not.
Cumulus expansion‐enabling activity
Cumulus expansion‐enabling factors (CEEFs) were the first oocyte‐derived factors experimentally ascertained to play a regulatory role in cumulus cell differentiation [102, 103]. Later, studies utilizing GDF9 null mice [104] and an RNA interference approach [105] confirmed that GDF9 is a mediator in oocyte regulation of cumulus expansion. A recent study found that the SMAD 2/3 signaling pathway was involved in the oocytes’ cumulus expansion‐enabling process [106]. In fact, activins as well as GDF9, both SMAD 2/3 signaling pathway activators, can act as CEEFs [106].
Granulosa cells in mouse preantral follicles do not expand in response to hormonal induction. The reason for this has been identified as a lack of CEEFs from the growing oocytes and insufficient expression of necessary transcripts such as Tnfaip6 mRNA [107].
Oocyte–granulosa cell interactions reproduced in vitro
In the author's experiments with bovine oocyte–granulosa cell complexes, granulosa cells proliferate better in the presence of oocytes (Fig. 2), and, after a 14‐day culture period, cumulus cells acquire the competence to undergo expansion (Fig. 3). These observations suggest possible interactions between oocytes and granulosa cells as discussed above.
Figure 3.
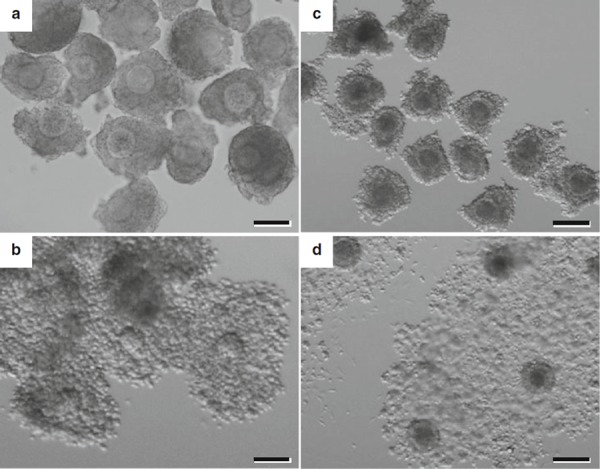
Expansion of mouse and bovine cumulus cells observed around maturing oocytes after growth in vitro. a Mouse complexes after a 10‐day culture period and b after maturation in vitro. c Bovine complexes after a 14‐day culture period and d after maturation in vitro. Scale bars 100 μm (a, b) and 200 μm (c, d)
In mouse studies, some of the oocyte‐mediated regulations have been realized specifically in vitro [18, 55, 89, 108]. Therefore, culture systems for oocyte growth can provide a strong tool for studies of the oocyte‐mediated regulation of granulosa cell differentiation, such as differentially expressed androgen receptor protein in mural granulosa cells and cumulus cells in follicles [18], the acquisition of the ability of cumulus cells to undergo expansion in response to EGF [108], and the coordinating activity of GDF9 and BMP15 [55, 89].
Conclusions
Some of the culture systems discussed in this review have already been used to address important questions with regard to the oocyte–granulosa cell interactions (summarized in Fig. 4). Although many other oocyte factors presumably mediate granulosa cell differentiation in vitro, the in vitro oocyte growth systems will provide a strong platform for the analysis of the activities of these factors. In the meantime, it is clear that further improvements to existing culture systems are necessary, because oocyte competence does not yet match its in vivo counterparts. A greater understanding of the oocyte–granulosa cell interactions will benefit us in our search for the optimal conditions to culture growing oocytes.
Figure 4.
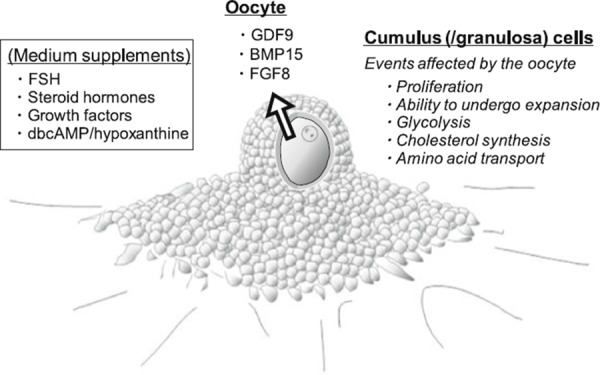
Oocyte‐derived paracrine factors and hormonal factors known to be involved, or presumably involved, in the regulation of the oocyte and neighboring granulosa cells during oocyte/follicle growth in vitro
Acknowledgments
This article is supported in part by a grant (22380151) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Baker TG Austin CR. Short RV. Oogenesis and ovulation. Reproduction in mammals, vol. 1, 1982. 2 Cambridge: Cambridge University Press; 17–45 [Google Scholar]
- 2. Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod, 1989, 41, 268–276 10.1095/biolreprod41.2.268 [DOI] [PubMed] [Google Scholar]
- 3. Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod, 1994, 9, 527–532 [DOI] [PubMed] [Google Scholar]
- 4. dela Pena EC, Takahashi Y, Katagiri S, Atabay EC, Nagano M. Birth of pups after transfer of mouse embryos derived from vitrified preantral follicles. Reproduction, 2002, 123, 593–600 10.1530/rep.0.1230593 [DOI] [PubMed] [Google Scholar]
- 5. Hasegawa A, Mochida N, Ogasawara T, Koyama K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil Steril, 2006, 86, 1182–1192 10.1016/j.fertnstert.2005.12.082 [DOI] [PubMed] [Google Scholar]
- 6. Smitz JE, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction, 2002, 123, 185–202 10.1530/rep.0.1230185 [DOI] [PubMed] [Google Scholar]
- 7. Eppig JJ, O'Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod, 1996, 54, 197–207 10.1095/biolreprod54.1.197 [DOI] [PubMed] [Google Scholar]
- 8. O'Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod, 2003, 68, 1682–1686 10.1095/biolreprod.102.013029 [DOI] [PubMed] [Google Scholar]
- 9. Yamamoto K, Otoi T, Koyama N, Horikita N, Tachikawa S, Miyano T. Development to live young from bovine small oocytes after growth, maturation and fertilization in vitro. Theriogenology., 1999, 52, 81–89 10.1016/S0093‐691X(99)00111‐9 [DOI] [PubMed] [Google Scholar]
- 10. Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte–granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod, 2004, 70, 83–91 10.1095/biolreprod.103.021238 [DOI] [PubMed] [Google Scholar]
- 11. Daniel SA, Armstrong DT, Gore‐Langton RE. Growth and development of rat oocytes in vitro. Gamete Res., 1989, 24, 109–121 10.1002/mrd.1120240113 [DOI] [PubMed] [Google Scholar]
- 12. Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. J Reprod Fertil, 1994, 100, 333–339 10.1530/jrf.0.1000333 [DOI] [PubMed] [Google Scholar]
- 13. Hirao Y. Conditions affecting growth and developmental competence of mammalian oocytes in vitro. Anim Sci J., 2011, 82, 187–197 10.1111/j.1740‐0929.2010.00870.x [DOI] [PubMed] [Google Scholar]
- 14. Gore‐Langton RE, Daniel SA. Follicle‐stimulating hormone and estradiol regulate antrum‐like reorganization of granulosa cells in rat preantral follicle cultures. Biol Reprod, 1990, 43, 65–72 10.1095/biolreprod43.1.65 [DOI] [PubMed] [Google Scholar]
- 15. Eppig JJ, Hosoe M, O'Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol, 2000, 163, 109–116 10.1016/S0303‐7207(99)00247‐6 [DOI] [PubMed] [Google Scholar]
- 16. Mitchell LM, Kennedy CR, Hartshorne GM. Effects of varying gonadotrophin dose and timing on antrum formation and ovulation efficiency of mouse follicles in vitro. Hum Reprod, 2002, 17, 1181–1188 10.1093/humrep/17.5.1181 [DOI] [PubMed] [Google Scholar]
- 17. Liu HC, He Z, Rosenwaks Z. In vitro culture and in vitro maturation of mouse preantral follicles with recombinant gonadotropins. Fertil Steril, 2002, 77, 373–383 10.1016/S0015‐0282(01)02977‐6 [DOI] [PubMed] [Google Scholar]
- 18. Lenie S, Smitz J. Functional AR signaling is evident in an in vitro mouse follicle culture bioassay that encompasses most stages of folliculogenesis. Biol Reprod, 2009, 80, 685–695 10.1095/biolreprod.107.067280 [DOI] [PubMed] [Google Scholar]
- 19. Zhao J, Dorland M, Taverne MA, Weijden GC, Bevers MM, Hurk R. In vitro culture of rat pre‐antral follicles with emphasis on follicular interactions. Mol Reprod Dev, 2000, 55, 65–74 10.1002/(SICI)1098‐2795(200001)55:1<65::AID‐MRD9>3.0.CO;2‐H [DOI] [PubMed] [Google Scholar]
- 20. Hashimoto S, Ohsumi K, Tsuji Y, Harauma N, Miyata Y, Fukuda A, Hosoi Y, Iritani A, Morimoto Y. Growing porcine oocyte–granulosa cell complexes acquired meiotic competence during in vitro culture. J Reprod Dev., 2007, 53, 379–384 10.1262/jrd.18132 [DOI] [PubMed] [Google Scholar]
- 21. Cortvrindt R, Smitz J, Steirteghem AC. Ovary and ovulation: in vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod, 1996, 11, 2656–2666 [DOI] [PubMed] [Google Scholar]
- 22. Carroll J, Whittingham DG, Wood MJ. Effect of dibutyryl cyclic adenosine monophosphate on granulosa cell proliferation, oocyte growth and meiotic maturation in isolated mouse primary ovarian follicles cultured in collagen gels. J Reprod Fertil, 1991, 92, 197–207 10.1530/jrf.0.0920197 [DOI] [PubMed] [Google Scholar]
- 23. Nayudu PL, Osborn SM. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil, 1992, 95, 349–362 10.1530/jrf.0.0950349 [DOI] [PubMed] [Google Scholar]
- 24. Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle‐stimulating hormone in a three‐dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod, 2005, 73, 942–950 10.1095/biolreprod.105.042390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harada M, Miyano T, Matsumura K, Osaki S, Miyake M, Kato S. Bovine oocytes from early antral follicles grow to meiotic competence in vitro: effect of FSH and hypoxanthine. Theriogenology., 1997, 48, 743–755 10.1016/S0093‐691X(97)00298‐7 [DOI] [PubMed] [Google Scholar]
- 26. Itoh T, Kacchi M, Abe H, Sendai Y, Hoshi H. Growth, antrum formation, and estradiol production of bovine preantral follicles cultured in a serum‐free medium. Biol Reprod, 2002, 67, 1099–1105 10.1095/biolreprod67.4.1099 [DOI] [PubMed] [Google Scholar]
- 27. Senbon S, Miyano T. Bovine oocytes in early antral follicles grow in serum‐free media: effect of hypoxanthine on follicular morphology and oocyte growth. Zygote., 2002, 10, 301–309 10.1017/S0967199402004033 [DOI] [PubMed] [Google Scholar]
- 28. McLaughlin M, Telfer EE. Oocyte development in bovine primordial follicles is promoted by activin and FSH within a two‐step serum‐free culture system. Reproduction, 2010, 139, 971–978 10.1530/REP‐10‐0025 [DOI] [PubMed] [Google Scholar]
- 29. Mao J, Smith MF, Rucker EB, Wu GM, McCauley TC, Cantley TC, Prather RS, Didion BA, Day BN. Effect of epidermal growth factor and insulin‐like growth factor I on porcine preantral follicular growth, antrum formation, and stimulation of granulosal cell proliferation and suppression of apoptosis in vitro. J Anim Sci, 2004, 82, 1967–1975 [DOI] [PubMed] [Google Scholar]
- 30. Newton H, Picton H, Gosden RG. In vitro growth of oocyte–granulosa cell complexes isolated from cryopreserved ovine tissue. J Reprod Fertil, 1999, 115, 141–150 10.1530/jrf.0.1150141 [DOI] [PubMed] [Google Scholar]
- 31. Cecconi S, Capacchietti G, Russo V, Berardinelli P, Mattioli M, Barboni B. In vitro growth of preantral follicles isolated from cryopreserved ovine ovarian tissue. Biol Reprod, 2004, 70, 12–17 10.1095/biolreprod.103.016774 [DOI] [PubMed] [Google Scholar]
- 32. Zhou H, Zhang Y. Effect of growth factors on in vitro development of caprine preantral follicle oocytes. Anim Reprod Sci., 2005, 90, 265–272 10.1016/j.anireprosci.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 33. Silva CMG, Matos MHT, Rodrigues GQ, Faustino LR, Pinto LC, Chaves RN, Araujo VR, Campello CC, Figueiredo JR. In vitro survival and development of goat preantral follicles in two different oxygen tensions. Anim Reprod Sci., 2010, 117, 83–89 10.1016/j.anireprosci.2009.03.015 [DOI] [PubMed] [Google Scholar]
- 34. Magalhaes DM, Duarte ABG, Araujo VR, Brito IR, Soares TG, Lima IMT, Lopes CAP, Campello CC, Rodrigues APR, Figueiredo JR. In vitro production of a caprine embryo from a preantral follicle cultured in media supplemented with growth hormone. Theriogenology., 2011, 75, 182–188 10.1016/j.theriogenology.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 35. Telfer EE, McLaughlin M, Ding C, Thong KJ. A two‐step serum‐free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum Reprod, 2008, 23, 1151–1158 10.1093/humrep/den070 [DOI] [PubMed] [Google Scholar]
- 36. Xu M, Barrett SL, West‐Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod, 2009, 24, 2531–2540 10.1093/humrep/dep228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carroll J, Whittingham DG, Wood MJ. Growth in vitro and acquisition of meiotic competence after the cryopreservation of isolated mouse primary ovarian follicles. Reprod Fertil Dev, 1991, 3, 593–599 10.1071/RD9910593 [DOI] [PubMed] [Google Scholar]
- 38. Pangas SA, Saudye H, Shea LD, Woodruff TK. Novel approach for the three‐dimensional culture of granulosa cell–oocyte complexes. Tissue Eng., 2003, 9, 1013–1021 10.1089/107632703322495655 [DOI] [PubMed] [Google Scholar]
- 39. Xu M, West E, Shea LD, Woodruff TK. Identification of a stage‐specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod, 2006, 75, 916–923 10.1095/biolreprod.106.054833 [DOI] [PubMed] [Google Scholar]
- 40. Cortvrindt R, Smitz J, Steirteghem A. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Hum Reprod, 1997, 12, 759–768 10.1093/humrep/12.4.759 [DOI] [PubMed] [Google Scholar]
- 41. Spears N, Murray AA, Allison V, Boland NI, Gosden RG. Role of gonadotrophins and ovarian steroids in the development of mouse follicles in vitro. J Reprod Fertil, 1998, 113, 19–26 10.1530/jrf.0.1130019 [DOI] [PubMed] [Google Scholar]
- 42. Moritake S, Hirao Y, Miyano T. Hypoxanthine promotes the acquisition of meiotic competence in pig oocytes from early antral follicles during growth culture. J Mamm Ova Res., 2002, 19, 39–45 10.1274/jmor.19.39 [Google Scholar]
- 43. Cortvrindt R, Hu Y, Smitz J. Recombinant luteinizing hormone as a survival and differentiation factor increases oocyte maturation in recombinant follicle stimulating hormone‐supplemented mouse preantral follicle culture. Hum Reprod, 1998, 13, 1292–1302 10.1093/humrep/13.5.1292 [DOI] [PubMed] [Google Scholar]
- 44. Conti M, Andersen CB, Richard F, Mehats C, Chun S‐Y, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol, 2002, 187, 153–159 10.1016/S0303‐7207(01)00686‐4 [DOI] [PubMed] [Google Scholar]
- 45. Downs SM, Daniel SAJ, Bornslaeger EA, Hoppe PC, Eppig JJ. Maintenance of meiotic arrest in mouse oocytes by purines: Modulation of cAMP levels and cAMP phosphodiesterase activity. Gamete Res., 1989, 23, 323–334 10.1002/mrd.1120230309 [DOI] [PubMed] [Google Scholar]
- 46. Eppig JJ, Ward‐Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod, 1985, 33, 1041–1049 10.1095/biolreprod33.5.1041 [DOI] [PubMed] [Google Scholar]
- 47. Eppig JJ, O'Brien MJ. Comparison of preimplantation developmental competence after mouse oocyte growth and development in vitro and in vivo. Theriogenology., 1998, 49, 415–422 10.1016/S0093‐691X(97)00413‐5 [DOI] [PubMed] [Google Scholar]
- 48. Murray AA, Swales AKE, Smith RE, Molinek MD, Hillier SG, Spears N. Follicular growth and oocyte competence in the in vitro cultured mouse follicle: effects of gonadotrophins and steroids. Mol Hum Reprod., 2008, 14, 75–83 10.1093/molehr/gam092 [DOI] [PubMed] [Google Scholar]
- 49. Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle‐stimulating hormone and insulin. Biol Reprod, 1998, 59, 1445–1453 10.1095/biolreprod59.6.1445 [DOI] [PubMed] [Google Scholar]
- 50. Demeestere I, Gervy C, Centner J, Devreker F, Englert Y, Delbaere A. Effect of insulin‐like growth factor‐I during preantral follicular culture on steroidogenesis, in vitro oocyte maturation, and embryo development in mice. Biol Reprod, 2004, 70, 1664–1669 10.1095/biolreprod.103.023317 [DOI] [PubMed] [Google Scholar]
- 51. Smitz J, Cortvrindt R, Hu Y, Vanderstichele H. Effects of recombinant activin A on in vitro culture of mouse preantral follicles. Mol Reprod Dev, 1998, 50, 294–304 10.1002/(SICI)1098‐2795(199807)50:3<294::AID‐MRD5>3.0.CO;2‐E [DOI] [PubMed] [Google Scholar]
- 52. Gill A, Jamnongjit M, Hammes SR. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol, 2004, 18, 97–104 10.1210/me.2003‐0326 [DOI] [PubMed] [Google Scholar]
- 53. Taketsuru H, Hirao Y, Takenouchi N, Iga K, Miyano T. Effect of androstenedione on the growth of bovine oocytes from early antral follicles. Zygote., 2011, 1, 743–755 [DOI] [PubMed] [Google Scholar]
- 54. Huynh K, Jones G, Thouas G, Britt KL, Simpson ER, Jones MEE. Estrogen is not directly required for oocyte developmental competence. Biol Reprod, 2004, 70, 1263–1269 10.1095/biolreprod.103.022111 [DOI] [PubMed] [Google Scholar]
- 55. Sugiura K, Su Y‐Q, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte‐derived GDF9 and BMP15. Mol Endocrinol, 2010, 24, 2303–2314 10.1210/me.2010‐0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thomas FH, Vanderhyden BC. Oocyte–granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol., 2006, 4, 19 10.1186/1477‐7827‐4‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c‐kit receptor promotes oocyte growth. Dev Biol, 1994, 161, 194–205 10.1006/dbio.1994.1020 [DOI] [PubMed] [Google Scholar]
- 58. Thomas FH, Ismail RS, Jiang J‐Y, Vanderhyden BC. Kit ligand 2 promotes murine oocyte growth in vitro. Biol Reprod, 2008, 78, 167–175 10.1095/biolreprod.106.058529 [DOI] [PubMed] [Google Scholar]
- 59. Honda A, Hirose M, Inoue K, Hiura H, Miki H, Ogonuki N, Sugimoto M, Abe K, Kanatsu‐Shinohara M, Kono T, Shinohara T, Ogura A. Large‐scale production of growing oocytes in vitro from neonatal mouse ovaries. Int J Dev Biol, 2009, 53, 605–613 10.1387/ijdb.082607ah [DOI] [PubMed] [Google Scholar]
- 60. Thomas FH, Ethier J‐F, Shimasaki S, Vanderhyden BC. Follicle‐stimulating hormone regulates oocyte growth by modulation of expression of oocyte and granulosa cell factors. Endocrinology., 2005, 146, 941–949 10.1210/en.2004‐0826 [DOI] [PubMed] [Google Scholar]
- 61. Cho JH, Itoh T, Sendai Y, Hoshi H. Fibroblast growth factor 7 stimulates in vitro growth of oocytes originating from bovine early antral follicles. Mol Reprod Dev, 2008, 75, 1736–1743 10.1002/mrd.20912 [DOI] [PubMed] [Google Scholar]
- 62. Eppig JJ, Downs SM. The effect of hypoxanthine on mouse oocyte growth and development in vitro: maintenance of meiotic arrest and gonadotropin‐induced oocyte maturation. Dev Biol, 1987, 119, 313–321 10.1016/0012‐1606(87)90037‐6 [DOI] [PubMed] [Google Scholar]
- 63. Nogueira D, Cortvrindt R, Everaerdt B, Smitz J. Effects of long‐term in vitro exposure to phosphodiesterase type‐3 inhibitors on follicle and oocyte development. Reproduction, 2005, 130, 177–186 10.1530/rep.1.00652 [DOI] [PubMed] [Google Scholar]
- 64. Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod, 2004, 19, 398–408 10.1093/humrep/deh074 [DOI] [PubMed] [Google Scholar]
- 65. Eppig JJ. Maintenance of meiotic arrest and the induction of oocyte maturation in mouse oocyte–granulosa cell complexes developed in vitro from preantral follicles. Biol Reprod, 1991, 45, 824–830 10.1095/biolreprod45.6.824 [DOI] [PubMed] [Google Scholar]
- 66. Palter SF, Tavares AB, Hourvitz A, Veldhuis JD, Adashi EY. Are estrogens of import to primate/human ovarian folliculogenesis?. Endocr Rev, 2001, 22, 389–424 10.1210/er.22.3.389 [DOI] [PubMed] [Google Scholar]
- 67. Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil, 1998, 113, 27–33 10.1530/jrf.0.1130027 [DOI] [PubMed] [Google Scholar]
- 68. Ikeda Y, Hirao Y, Miyano T. Effects of androgens on early development of mouse follicles in organ‐cultured ovaries. J Mamm Ova Res., 1999, 16, 148–153 10.1274/jmor.16.148 [Google Scholar]
- 69. Cecconi S, Rossi G, Coticchio G, Macchiarelli G, Borini A, Canipari R. Influence of thyroid hormone on mouse preantral follicle development in vitro. Fertil Steril, 2004, 81 (Suppl 1) 919–924 10.1016/j.fertnstert.2003.11.014 [DOI] [PubMed] [Google Scholar]
- 70. Zhao J, Taverne MA, Weijden GC, Bevers MM, Hurk R. Effect of activin A on in vitro development of rat preantral follicles and localization of activin A and activin receptor II. Biol Reprod, 2001, 65, 967–977 10.1095/biolreprod65.3.967 [DOI] [PubMed] [Google Scholar]
- 71. Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science., 2002, 296, 2178–2180 10.1126/science.1071965 [DOI] [PubMed] [Google Scholar]
- 72. Gilchrist RB, Lane M, Thompson JG. Oocyte‐secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update., 2008, 14, 159–177 10.1093/humupd/dmm040 [DOI] [PubMed] [Google Scholar]
- 73. Herlands RL, Schultz RM. Regulation of mouse oocyte growth: probable nutritional role for intercellular communication between follicle cells and oocytes in oocyte growth. J Exp Zool, 1984, 229, 317–325 10.1002/jez.1402290217 [DOI] [PubMed] [Google Scholar]
- 74. FitzHarris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development., 2006, 133, 591–599 10.1242/dev.02246 [DOI] [PubMed] [Google Scholar]
- 75. Eppig JJ, Wigglesworth K, Pendola FL. The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA, 2002, 99, 2890–2894 10.1073/pnas.052658699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod, 1997, 56, 976–984 10.1095/biolreprod56.4.976 [DOI] [PubMed] [Google Scholar]
- 77. Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci, 2007, 120, 1330–1340 10.1242/jcs.000968 [DOI] [PubMed] [Google Scholar]
- 78. Li R, Norman RJ, Armstrong DT, Gilchrist RB. Oocyte‐secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod, 2000, 63, 839–845 10.1095/biolreprod63.3.839 [DOI] [PubMed] [Google Scholar]
- 79. Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod, 2005, 73, 351–357 10.1095/biolreprod.105.041798 [DOI] [PubMed] [Google Scholar]
- 80. Su Y‐Q, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development., 2008, 135, 111–121 10.1242/dev.009068 [DOI] [PubMed] [Google Scholar]
- 81. Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol, 2005, 279, 20–30 10.1016/j.ydbio.2004.11.027 [DOI] [PubMed] [Google Scholar]
- 82. Sugiura K, Su Y‐Q, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte‐derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development., 2007, 134, 2593–2603 10.1242/dev.006882 [DOI] [PubMed] [Google Scholar]
- 83. Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor‐9 in the mammalian ovary. Mol Endocrinol, 1999, 13, 1035–1048 10.1210/me.13.6.1035 [DOI] [PubMed] [Google Scholar]
- 84. Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev, 2004, 25, 72–101 10.1210/er.2003‐0007 [DOI] [PubMed] [Google Scholar]
- 85. Juengel JL, Bodensteiner KJ, Heath DA, Hudson NL, Moeller CL, Smith P, Galloway SM, Davis GH, Sawyer HR, McNatty KP. Physiology of GDF9 and BMP15 signalling molecules. Anim Reprod Sci., 2004, 82–83, 447–460 10.1016/j.anireprosci.2004.04.021 [DOI] [PubMed] [Google Scholar]
- 86. Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol, 2001, 15, 854–866 10.1210/me.15.6.854 [DOI] [PubMed] [Google Scholar]
- 87. Su Y‐Q, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte–cumulus cell complex in mice: genetic evidence for an oocyte–granulosa cell regulatory loop. Dev Biol, 2004, 276, 64–73 10.1016/j.ydbio.2004.08.020 [DOI] [PubMed] [Google Scholar]
- 88. Edwards SJ, Reader KL, Lun S, Western A, Lawrence S, McNatty KP, Juengel JL. The cooperative effect of growth and differentiation factor‐9 and bone morphogenetic protein (BMP)‐15 on granulosa cell function is modulated primarily through BMP receptor II. Endocrinology., 2008, 149, 1026–1030 10.1210/en.2007‐1328 [DOI] [PubMed] [Google Scholar]
- 89. Sadeu JC, Adriaenssens T, Smitz J. Expression of growth differentiation factor 9, bone morphogenetic protein 15, and anti‐Mullerian hormone in cultured mouse primary follicles. Reproduction, 2008, 136, 195–203 10.1530/REP‐08‐0065 [DOI] [PubMed] [Google Scholar]
- 90. Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9‐deficient ovary. Mol Endocrinol, 1999, 13, 1018–1034 10.1210/me.13.6.1018 [DOI] [PubMed] [Google Scholar]
- 91. Buccione R, Schroeder AC, Eppig JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod, 1990, 43, 543–547 10.1095/biolreprod43.4.543 [DOI] [PubMed] [Google Scholar]
- 92. Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays., 1991, 13, 569–574 10.1002/bies.950131105 [DOI] [PubMed] [Google Scholar]
- 93. Vanderhyden BC, Telfer EE, Eppig JJ. Mouse oocytes promote proliferation of granulosa cells from preantral and antral follicles in vitro. Biol Reprod, 1992, 46, 1196–1204 10.1095/biolreprod46.6.1196 [DOI] [PubMed] [Google Scholar]
- 94. Gilchrist RB, Ritter LJ, Myllymaa S, Kaivo‐Oja N, Dragovic RA, Hickey TE, Ritvos O, Mottershead DG. Molecular basis of oocyte‐paracrine signalling that promotes granulosa cell proliferation. J Cell Sci, 2006, 119, 3811–3821 10.1242/jcs.03105 [DOI] [PubMed] [Google Scholar]
- 95. Joyce IM, Clark AT, Pendola FL, Eppig JJ. Comparison of recombinant growth differentiation factor‐9 and oocyte regulation of KIT ligand messenger ribonucleic acid expression in mouse ovarian follicles. Biol Reprod, 2000, 63, 1669–1675 10.1095/biolreprod63.6.1669 [DOI] [PubMed] [Google Scholar]
- 96. Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor‐9 stimulates proliferation but suppresses the follicle‐stimulating hormone‐induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod, 2000, 62, 370–377 10.1095/biolreprod62.2.370 [DOI] [PubMed] [Google Scholar]
- 97. Hayashi M, McGee EA, Min G, Klein C, Rose UM, Duin M, Hsueh AJ. Recombinant growth differentiation factor‐9 (GDF‐9) enhances growth and differentiation of cultured early ovarian follicles. Endocrinology., 1999, 140, 1236–1244 10.1210/en.140.3.1236 [DOI] [PubMed] [Google Scholar]
- 98. Gilchrist RB, Ritter LJ, Cranfield M, Jeffery LA, Amato F, Scott SJ, Myllymaa S, Kaivo‐Oja N, Lankinen H, Mottershead DG, Groome NP, Ritvos O. Immunoneutralization of growth differentiation factor 9 reveals it partially accounts for mouse oocyte mitogenic activity. Biol Reprod, 2004, 71, 732–739 10.1095/biolreprod.104.028852 [DOI] [PubMed] [Google Scholar]
- 99. Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein‐15. Identification of target cells and biological functions. J Biol Chem., 2000, 275, 39523–39528 10.1074/jbc.M007428200 [DOI] [PubMed] [Google Scholar]
- 100. Orisaka M, Orisaka S, Jiang J‐Y, Craig J, Wang Y, Kotsuji F, Tsang BK. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol Endocrinol, 2006, 20, 2456–2468 10.1210/me.2005‐0357 [DOI] [PubMed] [Google Scholar]
- 101. Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci, 2005, 118, 5257–5268 10.1242/jcs.02644 [DOI] [PubMed] [Google Scholar]
- 102. Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH‐induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol, 1990, 138, 16–25 10.1016/0012‐1606(90)90172‐F [DOI] [PubMed] [Google Scholar]
- 103. Salustri A, Yanagishita M, Hascall VC. Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH‐stimulated cumulus cells. Dev Biol, 1990, 138, 26–32 10.1016/0012‐1606(90)90173‐G [DOI] [PubMed] [Google Scholar]
- 104. Vanderhyden BC, Macdonald EA, Nagyova E, Dhawan A. Evaluation of members of the TGFbeta superfamily as candidates for the oocyte factors that control mouse cumulus expansion and steroidogenesis. Reprod Suppl., 2003, 61, 55–70 [PubMed] [Google Scholar]
- 105. Gui L‐M, Joyce IM. RNA Interference evidence that growth differentiation factor‐9 mediates oocyte regulation of cumulus expansion in mice. Biol Reprod, 2005, 72, 195–199 10.1095/biolreprod.104.033357 [DOI] [PubMed] [Google Scholar]
- 106. Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB. Oocyte‐secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod, 2007, 76, 848–857 10.1095/biolreprod.106.057471 [DOI] [PubMed] [Google Scholar]
- 107. Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: Development of competence to undergo expansion. Dev Biol, 2006, 299, 91–104 10.1016/j.ydbio.2006.07.012 [DOI] [PubMed] [Google Scholar]
- 108. Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol, 2007, 305, 300–311 10.1016/j.ydbio.2007.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]


