Abstract
Ovarian tissue banking is a feasible strategy for fertility preservation for young women after cancer treatments. Ovarian tissue, after thawing, is used for several options; orthotopic grafting (normal site), autologous heterotopic grafting and collection of ovarian follicles for culture. Recent reports of live birth encouraged clinicians and researchers to apply this technology to premature ovarian failure (POF) resulting from strong cancer therapy. Grafting, however, carries a risk of malignant cell recurrence. For safety, development of a culture method is necessary but optimum culturing conditions for less‐developed follicles abundant in the ovary are not well known. In the present article, the current status of ovarian tissue cryopreservation, and in vitro oocyte growth and maturation from the preserved ovaries are reviewed.
Keywords: cancer, fertilization, infertility, premature ovarian failure, vitrification.
INTRODUCTION
ADVANCED ASSISTED REPRODUCTIVE technology (ART) has provided useful therapy for patients suffering from infertility since 1978, when Edwards and Steptoe succeeded in achieving a full‐term birth after in vitro fertilization (IVF). 1 Effective treatments are now available for tube blockage, sperm factors, immunological factors and unknown factors. Cancer therapy has also advanced, and survival rates of young patients have significantly improved as a result of chemotherapy and/or radiotherapy. However, as a side‐effect, ovarian failure as a result of these aggressive treatments is often inevitable. Considering the quality of life (QOL) of young women, ovarian cryopreservation is a feasible method to restore fertility. 2 , 3 , 4 , 5 Theoretical options for the use of cryopreserved ovarian tissue are shown in Figure 1. This strategy could also help some patients with severe infertility, such as naturally‐occurring premature ovarian failure, if the ovarian tissues can be preserved before deteriorating further. In addition, ovarian tissue banking might be applicable to career women who would like to conceive at older ages.
Figure 1.
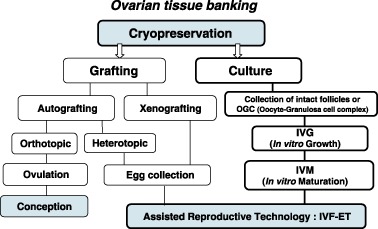
Theoretical options for the use of cryopreserved ovarian tissue. Two applications, grafting and in vitro culture, are available for cryopreserved ovarian tissue usage. Grafting has two further options, autografting and xenografting. Orthotopic autografting successfully produced two live births in clinical use and heterotopic autografting produced 4‐cell‐stage embryos. Xenografting provides useful information in research but would not be used for humans. In the culture system, oocyte‐granulosa cell complexes are collected from the cryopreserved ovaries and subjected to in vitro growth‐in vitro maturation as well as in vitro fertilization and embryo transfer. There is no risk of recurrent malignant cells in this option. (Modified from Oktay et al. Fertil Steril 1998, 69: 1–7)
OVARIAN TISSUE BANKING
THERE ARE SEVERAL options for ovarian tissue banking (Fig. 1). Cryopreserved ovaries are grafted or cultivated to restore fertility. Autografting is a method used to resume the menstrual cycle and achieve natural pregnancy. In fact, two successful births have been reported by this method. 6 , 7 Alternatively, heterotopic autografting has also been reported. 8 , 9 The ovarian follicles implanted in the patient's lower abdominal wall grew by hormonal stimulation and recovered eggs were fertilized, cleaved and transferred to the uterus. 8 Regardless of the method, orthotopic or heterotopic, autografting carries a risk of reintroduction of malignant cells to patients who have recovered from cancer. Xenografting is an alternative method in which human ovaries are grafted to immunodeficient animals. The animals are treated with follicle growth stimulant to recover fertilizable mature eggs. The mature eggs are recovered from the follicles. This method provides useful information on ovarian grafting, but would be applied for research purposes only because of potential unknown viral infection as well as ethical problems. 10 , 11 , 12 Hence, a culture system which covers in vitro growth (IVG) and maturation (IVM) of oocytes to yield fertilizable eggs is desirable. Practically, oocyte‐granulosa cell complexes are collected from the cryopreserved ovaries and cultured for IVG followed by IVM. The mature eggs with metaphase II are used for IVF‐ET.
CRYOPRESERVATION
Preimplantation embryo and unfertilized eggs
TWO METHODS ARE available for cryopreservation, slow freezing using a computer‐aided programmed freezer and rapid freezing by vitrification. The vitrification method was significantly improved and the embryo survival rate after vitrification was almost 100%. Intensive studies have been carried out to exploit devices and protocols for vitrification, and unfertilized oocytes at the metaphase II have been able to be stored by vitrification. 13 , 14 , 15 , 16 In humans, successful live births have been reported from eggs vitrified and warmed before fertilization. 16 , 17 , 18 This was a surprise because the oocytes at this stage have a dynamic balance of polymerized microtubules attached to chromosomes, increased fluidity of membrane for sperm penetration and biologically active cytoplasm that is preparing for postfertilization events.
Ovarian tissue
Ovarian tissues are quite large compared with unfertilized eggs and pre‐implantation embryos. Longer exposure time is needed for permeation of cryoprotectant into the tissues. In contrast, prolonged exposure to the cryoprotectants could cause toxic damage in the cells. Optimum conditions regarding kinds and concentrations of cryoprotectant chemicals, incubation time and temperature should be determined in the ovarian tissues of different animals.
Figure 2 shows the effects of exposure time to cryoprotectant on mouse ovarian histology. Increasing incubation time gave better morphology. Incubation of 2 min showed good morphology in just a thin area of ovarian cortex. With longer incubation times, the morphology became better in the inner tissues. Incubation of 30 min seemed to be enough to maintain comparative morphology to fresh ovarian sections (Fig. 2a,e). These results indicated that cryoprotectants time‐dependently permeated and protected the tissues from cryodamage. Vitrification by 30‐min immersion in the cryoprotectants showed satisfactory results for mouse ovarian cryopreservation. When applied to large animals, it might be necessary to dissect the tissues into smaller pieces or thinner slices to allow cryoprotectants to permeate into the central portions of the tissue. Tissue dissection is advantageous not only to promote permeation of cryoprotectants, but also to increase occasions for IVG and IVM.
Figure 2.
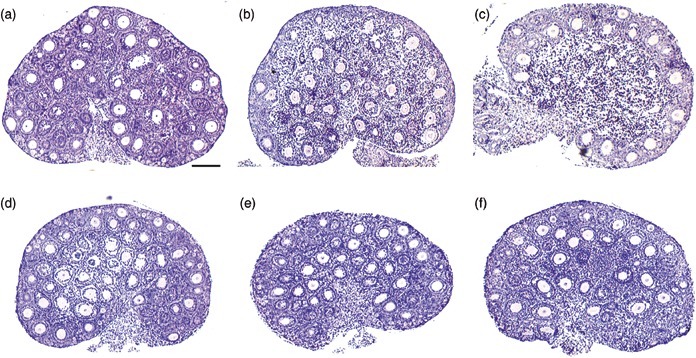
Histological examinations of cryopreserved mouse ovaries during incubation times in cryoprotectant solution before storage in liquid nitrogen. (a) fresh; (b) 2 min; (c) 10 min; (d) 20 min; (e) 30 min; (f) 60 min. (b, c) When the incubation time was short, ovarian tissues just survived in the peripheral regions. (d) The surviving region extended into the medulla in 20‐min incubation. (e) The morphology from cryopreserved ovary treated with cryoprotectant for 30 min (a) resembles fresh ovarian tissue. (f) Further incubation, did not change the ovarian morphology compared to 30‐min incubation. Bar is 100 µm.
In the literature, histological examinations of grafted ovarian tissues have shown promising results in animals 19 , 20 and humans. 10 , 21 In animal experiments, successful pup births from cryopreserved ovaries after grafting have been reported in mice and domestic animals. 22 , 23 , 24 These observations indicate that oocytes included in less‐developed follicles could grow, mature and develop into full‐term births after cryopreservation.
IN VITRO CULTURE
TO PREVENT MALIGNANT cell reintroduction, development of a culture system is necessary. As the varying stages in ovarian follicles (oocytes) have different requirements, several isolation options are proposed (Fig. 3). First, sufficiently‐grown follicles can mature in culture containing adequate supplements such as epithelial growth factor (EGF), follicle‐stimulating hormone (FSH) without additional IVG. For treatment of polycystic ovarian syndrome (PCOS), IVM of oocytes obtained by aspiration from small antral follicles is a feasible technique resulting in pregnancies, and many healthy babies are successfully born. 25 , 26 , 27 , 28 However, antral follicles in the ovary are small in number and susceptible to cytoskeletal and cell membrane damage by ice crystal formation caused by the surrounding fluid. In contrast, primordial and very small primary follicles are abundant in the ovary and resistant to cryopreservation, but the method to induce them to grow is unknown at the present time. Early preantral follicles are present in young ovaries in considerable numbers and more resistant to cryopreservation than antral follicles. Therefore, preantral follicles might serve as a good source for providing mature oocytes after cryopreservation. As a mouse model experiment, IVG‐IVM of this type of follicle is described in the next section.
Figure 3.
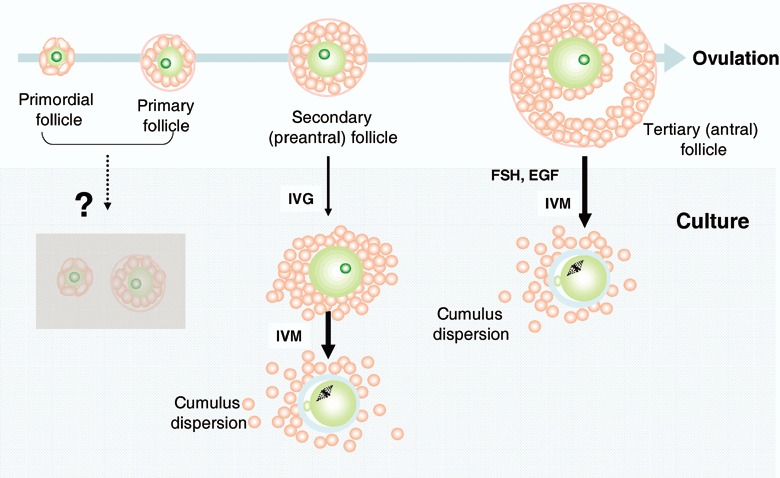
Schematic drawing of in vitro growth (IVG) and in vitro maturation (IVM). Sufficient information is not currently available on primordial and primary follicles, or gonadotropin‐independent phases to trigger growth. The oocytes from full‐grown follicles can mature into metaphase II (MII) in IVM culture. In the intermediate stage of secondary (preantral) follicles, the oocytes can grow in adequate IVG conditions and develop into fertilizable eggs.
Mouse
Practically, two methods have been used for IVG in mouse experiments. One is follicle‐intact culture, 29 , 30 and the other is oocyte‐granulosa cell complex (OGC) culture. 31 , 32 The intact follicles are collected by mechanical dissection and the OGC are collected by collagenase treatment in which the basement membrane and theca cell layers are removed. The appearance of intact follicles and OGC are not different under optical microscopic observation (Fig. 4a,b), but optimum conditions are different in the two methods. Higher FSH concentrations and serum factors are necessary in follicle‐intact IVG. After IVG, the intact follicle method showed an antral‐like structure, whereas the OGC method produced granulosa cell proliferation and spread on the substratum (Fig. 4c,d). The granulosa cells from both methods dispersed and mucified (Fig. 4e,f). The eggs from both methods produced fertilizable eggs and grew into the blastocyst stage and subsequently achieved full‐term development. 33
Figure 4.
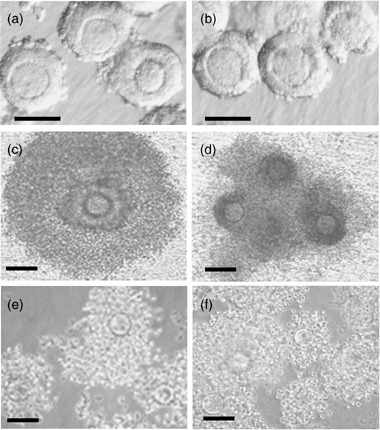
(a) Morphological change of intact follicles and (b) oocyte‐granulosa cell complexes (OGC) during in vitro growth (IVG). Intact follicles were collected by mechanical dissection and OGC were collected by collagenase‐treatment. (c) After 7 day‐IVG, intact follicles formed antral‐like structures. (d) In the same incubation time, the granulosa cells of OGC proliferated around oocytes. (e, f) After further incubation in in vitro maturation, granulosa cells from both cultures were dispersed and mucified with a similar morphology. Bars are 100 µm.
In the studies using domestic animals, live pup births through IVG‐IVM from less‐grown oocytes were reported. 34 , 35 , 36 , 37 Using cryopreserved ovaries, successful development into the blastocyst stage or pup birth from isolated less‐developed oocytes has been documented by many researchers. 38 , 39 , 40 In addition to ovaries, OGC cryopreservation has also achieved live pup birth. 41
Recently, our laboratory achieved full‐term development by implantation of two‐cell‐stage mouse embryos into host mothers from cryopreserved ovaries through IVG‐IVM. 42 Although the success rate (birth/implant) was low, it is similar to that from fresh ovary. This suggests that cryopreservation does not affect oocyte quality and live birth. The IVG conditions should be improved rather than the cryopreservation protocol. Physiological events such as genomic recombination, epigenic modification and cytoplasmic maturation might not adequately occur. Further improvements are necessary for optimum IVG conditions. Information on IVG‐IVM conditions from animal models is important for human application because it is essential to resolve ethical, moral and religious issues in the use of human ovaries.
Human
In humans, challenging trials have been reported by several researchers. 43 , 44 , 45 , 46 , 47 Hovatta et al. reported human ovarian tissue recovered from cryopreservation survived 4 weeks in culture conditions. 45 They also showed tissue culture gave a better result than isolated follicle culture. Insulin, insulin‐like growth factors and GDF‐9 might have promising effects on follicle survival and growth. 46 , 47 , 48 The information on signal–receptor interactions and growth control mechanism of human folliculogenesis is quite insufficient at this time.
Factors involved in folliculogenesis
Follicle growth is orchestrated by paracrine/autocrine factors in harmony. Various growth factors and oocyte‐specific gene expressions have been identified at specific stages of the follicular development, as reviewed in articles 49 , 50 , 51 but information is still insufficient to show the full mechanism of follicular growth. Recent molecular biology provides possible key molecules in early follicular development such as c‐kit/kitL, antimüllerian hormone and stem cell factor. In particular, oocyte‐specifically expressed TGF‐β superfamily including BDF‐9 and BMP‐15 is being intensively studied. The molecules promote follicle development beyond the primary follicle stage. Another interesting factor, FIGLA, which regulates zona pellucida protein expressions by binding upstream of their genes, is involved in primordial follicle formation from germline. The mechanisms of ovarian organization and folliculogenesis have gradually been made clear at the molecular level. These findings encourage us to continue the research of utilizing less‐developed oocytes for ovarian tissue banking.
CONCLUSION
VITRIFICATION OF OVARIAN tissues has provided morphologically satisfactory results in several animal species. In mice, growing oocytes collected from preantral follicles of vitrified‐warmed ovary can develop into live births. Further investigations using non‐human primates are needed for application to human use. Continuous efforts will benefit many young women facing premature ovarian failure.
ACKNOWLEDGMENTS
THE PRESENT RESEARCH work was supported in part by the High‐Tech Research Center Project for Private Universities, matching fund subsidy from the Japanese Ministry of Education, Culture, Sports Science and Technology, 2004–2008 and a Grant‐in‐Aid for Science Research (No. 18390453) from the Japan Society for the Promotion of Science.
REFERENCES
- 1. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet 1978; 2: 366. [DOI] [PubMed] [Google Scholar]
- 2. Newton H. The cryopreservation of ovarian tissue as a strategy for preserving the fertility of cancer patients. Hum Reprod Update 1998; 3: 237–247. [DOI] [PubMed] [Google Scholar]
- 3. Oktay K, Sonmezer M. Ovarian tissue banking for cancer patients: fertility preservation, not just ovarian cryopreservation. Hum Reprod 2004; 19: 1924–1925. [DOI] [PubMed] [Google Scholar]
- 4. Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril 2006; 85: 1–11. [DOI] [PubMed] [Google Scholar]
- 5. Fabbri R. Cryopreservation of human oocytes and ovarian tissue. Cell Tissue Bank 2006; 7: 113–122. [DOI] [PubMed] [Google Scholar]
- 6. Donnez J, Dolmans MM, Demylle D et al Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 364: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 7. Meirow D, Levron J, Eldar‐Geva T et al Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 2005; 353: 318–321. [DOI] [PubMed] [Google Scholar]
- 8. Oktay K, Buyuk E, Veeck L et al Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet 2004; 363: 837–840. [DOI] [PubMed] [Google Scholar]
- 9. Newton H, Aubard Y, Rutherford A, Sharma V, Gosden R. Low temperature storage and grafting of human ovarian tissue. Hum Reprod 1996; 11: 1487–1491. [DOI] [PubMed] [Google Scholar]
- 10. Oktay K, Newton H, Gosden RG. Transplantation of cryopreserved human ovarian tissue results in follicle growth initiation in SCID mice. Fertil Steril 2000; 73: 599–603. [DOI] [PubMed] [Google Scholar]
- 11. Snow M, Cox SL, Jenkin G, Trounson A, Shaw J. Generation of live young from xenografted mouse ovaries. Science 2002; 297: 2227. [DOI] [PubMed] [Google Scholar]
- 12. Sato Y, Terada Y, Utsunomiya H et al Immunohistochemical localization of steroidogenic enzymes in human follicle following xenotransplantation of the human ovarian cortex into NOD‐SCID mice. Mol Reprod 2003; 65: 67–72. [DOI] [PubMed] [Google Scholar]
- 13. Begin I, Bhatia B, Baldassarre H, Dinnyes A, Keefer CL. Cryopreservation of goat oocytes and in‐vivo derived 2‐ to 4‐cell embryos using the cryoloop (CLV) and solid‐surface vitrification (SSV) methods. Theriogenology 2003; 59: 1839–1850. [DOI] [PubMed] [Google Scholar]
- 14. Abe Y, Hara K, Matsumoto H et al Feasibility of a nylon‐mesh holder for vitrification of bovine germinal vesicle oocytes in subsequent production of viable blastocysts. Biol Reprod 2005; 72: 1416–1420. [DOI] [PubMed] [Google Scholar]
- 15. Fujihira T, Kishida R, Fukui Y. Developmental capacity of vitrified immature porcine oocytes following ICSI: effects of cytochalasin B and cryoprotectants. Cryobiology 2004; 49: 286–290. [DOI] [PubMed] [Google Scholar]
- 16. Fuchinoue K, Fukunaga N, Chiba S, Nakajo Y, Yagi A, Kyono K. Freezing of human immature oocytes using cryoloops with Taxol in the vitrification solution. J Assist Reprod Genet 2004; 21: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon TK, Kim TJ, Park SE et al Live births after vitrification of oocytes in a stimulated in vitro fertilization‐embryo transfer program. Fertil Steril 2003; 79: 1323–1326. [DOI] [PubMed] [Google Scholar]
- 18. Katayama KP, Stehlik J, Kuwayama M, Kato O, Stehlik E. High survival rate of vitrified human oocytes results in clinical pregnancy. Fertil Steril 2003; 80: 223–224. [DOI] [PubMed] [Google Scholar]
- 19. Sugimoto M, Maeda S, Manabe N, Miyamoto H. Development of infantile rat ovaries autotransplanted after cryopreservation by vitrification. Theriogenology 2000; 53: 1093–1103. [DOI] [PubMed] [Google Scholar]
- 20. Kagabu S, Umezu M. Transplantation of cryopreserved mouse, Chinese hamster, rabbit, Japanese monkey and rat ovaries into rat recipients. Exp Anim 2000; 49: 17–21. [DOI] [PubMed] [Google Scholar]
- 21. Gook DA, McCully BA, Edgar DH, McBain JC. Development of antral follicles in human cryopreserved ovarian tissue following xenografting. Hum Reprod 2001; 16: 417–422. [DOI] [PubMed] [Google Scholar]
- 22. Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at –196 degrees C. Hum Reprod 1994; 9: 597–603. [DOI] [PubMed] [Google Scholar]
- 23. Salle B, Demirci B, Franck M, Rudigoz RC, Guerin JF, Lornage J. Normal pregnancies and live births after autograft of frozen‐thawed hemi‐ovaries into ewes. Fertil Steril 2002; 7: 403–408. [DOI] [PubMed] [Google Scholar]
- 24. Migishima F, Suzuki‐Migishima R, Song SY et al Successful cryopreservation of mouse ovaries by vitrification. Biol Reprod 2003; 68: 881–887. [DOI] [PubMed] [Google Scholar]
- 25. Cha KY, Han SY, Chung HM et al Pregnancies and deliveries after in vitro maturation culture followed by in vitro fertilization and embryo transfer without stimulation in women with polycystic ovary syndrome. Fertil Steril 2000; 73: 978–983. [DOI] [PubMed] [Google Scholar]
- 26. Child TJ, Phillips SJ, Abdul‐Jalil AK, Gulekli B, Tan SL. A comparison of in vitro maturation and in vitro fertilization for women with polycystic ovaries. Obstet Gynecol 2002; 100: 665–670. [DOI] [PubMed] [Google Scholar]
- 27. Chian RC, Buckett WM, Abdul Jalil AK et al Natural‐cycle in vitro fertilization combined with in vitro maturation of immature oocytes is a potential approach in infertility treatment. Fertil Steril 2004; 82: 1675–1678. [DOI] [PubMed] [Google Scholar]
- 28. Le Du A, Kadoch IJ, Bourcigaux N et al In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Hum Reprod 2005; 20: 420–424. [DOI] [PubMed] [Google Scholar]
- 29. Spears N, Boland NI, Murray AA, Gosden RG. Mouse oocytes derived from in vitro grown primary ovarian follicles are fertile. Hum Reprod 1994; 9: 527–532. [DOI] [PubMed] [Google Scholar]
- 30. Cortvrindt R, Smitz J, Van Steirteghem AC. In‐vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod 1996; 11: 2656–2666. [DOI] [PubMed] [Google Scholar]
- 31. Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989; 4: 268–276. [DOI] [PubMed] [Google Scholar]
- 32. Hasegawa A, Hamada Y, Mehandjiev T, Koyama K. In‐vitro growth and maturation as well as fertilization of mouse preantral oocytes from vitrified ovaries. Fertil Steril 2004; 81: 824–830. [DOI] [PubMed] [Google Scholar]
- 33. Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54: 197–207. [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto K, Otoi T, Koyama N, Horikita N, Tachikawa S, Miyano T. Development to live young from bovine small oocytes after growth, maturation and fertilization in vitro. Theriogenology 1999; 52: 81–89. [DOI] [PubMed] [Google Scholar]
- 35. Brown BW, Radziewic T. Production of sheep embryos in vitro and development of progeny following single and twin embryo transfers. Theriogenology 1998; 49: 1525–1536. [DOI] [PubMed] [Google Scholar]
- 36. Hirao Y, Itoh T, Shimizu M et al In vitro growth and development of bovine oocyte‐granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod 2004; 70: 83–91. [DOI] [PubMed] [Google Scholar]
- 37. Kaneko H, Kikuchi K, Noguchi J, Hosoe M, Akita T. Maturation and fertilization of porcine oocytes from primordial follicles by a combination of xenografting and in vitro culture. Biol Reprod 2003; 69: 1488–1493. [DOI] [PubMed] [Google Scholar]
- 38. Segino M, Ikeda M, Aoki S, Tokieda Y, Hirahara F, Sato K. In vitro culture of mouse GV oocytes and preantral follicles isolated from ovarian tissues cryopreserved by vitrification. Hum Cell 2003; 16: 109–116. [DOI] [PubMed] [Google Scholar]
- 39. Cecconi S, Capacchietti G, Russo V, Berardinelli P, Mattioli M, Barboni B. In vitro growth of preantral follicles isolated from cryopreserved ovine ovarian tissue. Biol Reprod 2004; 70: 12–17. [DOI] [PubMed] [Google Scholar]
- 40. Newton H, Illingworth P. In vitro growth of murine pre‐antral follicles after isolation from cryopreserved ovarian tissue. Hum Reprod 2001; 16: 423–429. [DOI] [PubMed] [Google Scholar]
- 41. Dela Pena EC, Takahashi Y, Katagiri S, Atabay EC, Nagano M. Birth of pups after transfer of mouse embryos derived from vitrified preantral follicles. Reproduction 2002; 123: 593–600. [DOI] [PubMed] [Google Scholar]
- 42. Hasegawa A, Mochida N, Ogasawara T, Koyama K. Pup birth from mouse oocytes in preantral follicles derived from vitrified and warmed ovaries followed by in vitro growth, in vitro maturation, and in vitro fertilization. Fertil Steril 2006; 86: 1182–1192. [DOI] [PubMed] [Google Scholar]
- 43. Abir R, Franks S, Mobberley MA, Moore PA, Margara RA, Winston RM. Mechanical isolation and in vitro growth of preantral and small antral human follicles. Fertil Steril 1997; 68: 682–688. [DOI] [PubMed] [Google Scholar]
- 44. Liu J, Van der Elst J, Van den Broecke R, Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in‐vivo transplantation and in vitro maturation. Biol Reprod 2001; 64: 171–178. [DOI] [PubMed] [Google Scholar]
- 45. Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary and secondary ovarian follicles in long‐term culture: effect of partial isolation. Hum Reprod 1999; 14: 2519–2524. [DOI] [PubMed] [Google Scholar]
- 46. Louhio H, Hovatta O, Sjoberg J, Tuuri T. The effects of insulin, and insulin‐like growth factors I and II on human ovarian follicles in long‐term culture. Mol Hum Reprod 2000; 6: 694–698. [DOI] [PubMed] [Google Scholar]
- 47. Rahimi G, Isachenko E, Sauer H et al Measurement of apoptosis in long‐term cultures of human ovarian tissue. Reproduction 2001; 122: 657–663. [DOI] [PubMed] [Google Scholar]
- 48. Hreinsson JG, Scott JE, Rasmussen C, Swahn ML, Hsueh AJ, Hovatta O. Growth differentiation factor‐9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocrinol Metab 2002; 87: 316–321. [DOI] [PubMed] [Google Scholar]
- 49. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001; 122: 829–838. [DOI] [PubMed] [Google Scholar]
- 50. Epifano O, Dean J. Genetic control of early folliculogenesis in mice. Trends Endocrinol Metab 2002; 13: 169–173. [DOI] [PubMed] [Google Scholar]
- 51. Choi Y, Rajkovic A. Genetics of early mammalian folliculogenesis. Cell Mol Life Sci 2006; 63: 579–590. [DOI] [PMC free article] [PubMed] [Google Scholar]


