Abstract
Objective: The number of embryos transferred is very important to avoid multiple pregnancies without compromising pregnancy rates in in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI)–embryo transfer (ET). We established criteria for the elective transfer of two embryos (age <40, first treatment cycle, good‐quality embryos ≥3) to avoid high‐order multiple pregnancies, and reported their usefulness. In the current study, we compared the clinical outcome of day 2 versus day 3 elective transfer of two good‐quality embryos, in order to investigate the day of preferential transfer.
Methods: A total of 228 cycles were treated with IVF/ICSI–ET from August 1999 to August 2002. From this total, 114 patients who were less than 40 years old and also had a first treatment cycle were enrolled in the present study (50.0%). The elective transfer of two good‐quality embryos was carried out in 36 cycles (31.6%). Patients were randomized for transfer on either day 2 or day 3 after oocyte retrieval.
Results: The pregnancy rate of women who received two good‐quality embryos was 44.4% (16 out of 36). The multiple pregnancy rate was 12.5% (two out of 16) and all pregnancies outcomes were twins. There were no significant differences between day 2 and day 3 ET for the following criteria: the number of cycles (24, 12); age (32.8 ± 3.4 years, 32.5 ± 2.7 years); number of oocytes retrieved (10.0 ± 3.3, 9.0 ± 6.0); number of embryos developed (7.6 ± 3.5, 6.9 ± 3.7); and number of good‐quality embryos cryopreserved (3.5 ± 2.7, 3.6 ± 2.1). Higher pregnancy and implantation rates were obtained in day 3 ET than day 2 ET (37.5 and 20.8% in day 2 ET vs 58.3 and 33.3% in day 3 ET); however, there were no significant differences.
Conclusion: Day 3 ET appears to be preferable to achieve more viable embryos than day 2 ET. (Reprod Med Biol 2004; 3: 99–104)
Keywords: elective transfer of two embryos, implantation, in vitro fertilization/intracytoplasmic sperm injection–embryo transfer, pregnancy
INTRODUCTION
SINCE THE START of in vitro fertilization (IVF), embryos have been transferred 2 days after IVF at the two‐ to four‐cell stage. The timing of arrival of the embryo in the uterus, however, is earlier than the embryo produced in vivo, which enters the uterus on day 4 or 5 after ovulation. The transfer of embryos to the uterus on day 3 after oocyte retrieval may therefore be closer to the physiological time of uterine entry than transfer on day 2. Moreover, delaying embryo transfer (ET) allows the selection of the most viable embryos for transfer.
Several studies comparing ET on day 2 versus day 3 after oocyte retrieval have been performed but the conclusions are conflicting. One prospective, randomized study found no difference in the pregnancy rates between day 2 and day 3 ET, but a higher percentage of viable pregnancies was observed on day 3 ET. 1 Another study concluded from a retrospective study that pregnancy rates were similar between day 2 and day 3 transfers, but that the implantation rate in the day 3 group was higher. 2 Carrilo et al. reported that day 3 ET was associated with a significant increase in the implantation and pregnancy rates in a retrospective study. 3 Recent prospective studies found no difference in the implantation and pregnancy rates between transfers on day 2 versus day 3. 4 , 5 , 6 , 7 The topic of preferential transfer on day 2 or 3 remains controversial.
However, the pregnancy rate after IVF‐ET is clearly correlated with the number of embryos transferred. It rises steadily with the number of transferred embryos, and reaches a plateau at three replaced embryos. 8 , 9 The need to regulate the number of embryos transferred has been debated; 10 , 11 however, the elective transfer of two good‐quality embryos could solve the problem of triplet or high‐order pregnancies. 12 , 13 , 14 In our previous report, we established criteria for the elective transfer of two good‐quality embryos as follows: age <40 years, a first treatment cycle, and more than three good‐quality embryos available for transfer. 15 Although the pregnancy rate after application of the criteria was as high as that before application, the multiple pregnancy rate was lower. However, there were no significant differences of the pregnancy and multiple pregnancy rates between the groups. No high‐order multiple pregnancies occurred after application of the criteria.
In the present study, we compared the clinical outcome of day 2 versus day 3 elective transfer of two good‐quality embryos, in order to investigate the preferred day of transfer.
MATERIALS AND METHODS
Subjects
A TOTAL OF 228 cycles were treated with IVF/intracytoplasmic sperm injection (ICSI)‐ET from August 1999 to August 2002 at the Department of Obstetrics and Gynecology, Jichi Medical School, Japan. From this total, 114 patients who were less than 40 years old and also had a first treatment cycle were enrolled in the present study (50.0%). The elective transfer of two good‐quality embryos was carried out in 36 cycles (31.6%). This randomized study was carried out after obtaining consent to receive elective transfer of two good‐quality embryos, when the patient's age at the time of human chorionic gonadotropin (hCG) administration was less than 40 years old, and it was a first treatment cycle, and more than three good‐quality embryos were obtained on the day of ET. Patients were randomized at hCG injection for transfer on either day 2 or day 3 after oocyte retrieval.
In vitro fertilization/intracytoplasmic sperm injection–embryo transfer protocol and morphological assessment of embryos
Treatment of IVF/ICSI‐ET was performed as previously described. 16 , 17 , 18 , 19 , 20 , 21 , 22 In brief, following pituitary down‐regulation using a gonadotrophin‐releasing hormone agonist (GnRH‐a) (Nafarelin acetate; Yamanouchi Pharmaceutical, Tokyo, Japan) in the mid‐luteal phase of the previous cycle, ovarian stimulation was initiated after the onset of withdrawal bleeding with 300 IU/day of follicle stimulating hormone (FSH; Fertinom P, Serono, Tokyo, Japan) for 3 days followed by 150 IU/day of human menopausal gonadotrophins (hMG; Humegon, Nippon Organon K.K., Osaka, Japan). Ovarian stimulation was monitored by the measurement of serum estradiol (E2) concentration and by ultrasonographic assessment of the follicle diameters and endometrial thickness and pattern. 23 A total of 5000 IU of hCG (Mochida, Tokyo, Japan) was administered when at least one of the leading follicles reached 17 mm in diameter. Oocyte retrieval was performed through transvaginal ultrasonography‐guided aspiration approximately 36 h after hCG administration.
On the second or third day after oocyte retrieval, the embryos were assessed morphologically before transfer with an inverted microscope using the Veeck's classification. 24 Grade 1 embryos with regular blastomeres and no cytoplasmic fragments, and grade 2 embryos with regular blastomeres and minor cytoplasmic fragments were considered good‐quality embryos. Grade 3, 4 and 5 embryos were considered poor‐quality embryos. The elective transfer of only two good‐quality embryos was carried out to reduce high‐order multiple pregnancies. 15 Luteal support followed and clinical pregnancy was diagnosed 21 days after ET when a gestational sac was identified with transvaginal ultrasonography.
Statistical analysis
Statistical analysis of the data was carried out with χ2 analysis using STATVIEW 5.0 (Abacus Concepts, Barkeley, CA, USA) for Macintosh, and P < 0.05 was defined as representing a significant difference.
RESULTS
Patient profiles
A TOTAL OF 114 patients were randomized for the study. Because more than three good‐quality embryos were not obtained on the day of ET, 78 patients were excluded. Cancellation rates were 68.0% (51 out of 75) on day 2 ET and 69.2% (27 out of 39) on day 3 ET. There was no significant difference between the two groups. After randomization, no differences were found in age, duration of infertility, type of infertility or indication of IVF/ICSI between groups of patients who received their elective transfer on day 2 and day 3 after oocyte retrieval (Table 1).
Table 1.
Distribution of patients with day 2 versus day 3 elective transfer of two good‐quality embryos
| Day 2 (%) | Day 3 (%) | |
|---|---|---|
| Number of patients | 24 | 12 |
| Age (years, mean ± SD) | 32.8 ± 3.4 | 32.5 ± 2.7 |
| Duration of infertility (years, mean ± SD) | 3.8 ± 2.7 | 4.2 ± 2.3 |
| Type of infertility | ||
| Primary | 14 (58.3) | 9 (75.0) |
| Secondary | 10 (41.7) | 3 (25.0) |
| Indication | ||
| Unexplained | 10 (41.7) | 2 (16.7) |
| Tube | 8 (33.3) | 6 (50.0) |
| Male | 5 (20.8) | 3 (25.0) |
| Immunological | 1 (4.2) | 1 (8.3) |
The difference between the two groups were not significant for any of the variables. SD, standard deviation.
Comparison of the number of oocytes and embryos
A summary of the number of oocytes retrieved and embryos developed is shown in Table 2. Both groups had a comparable mean number of oocytes at oocyte retrieval, the same proportion of embryos developed and good‐quality embryos, and a comparable mean number of good‐quality embryos cryopreserved.
Table 2.
Comparison of in vitro fertilization/intracytoplasmic sperm injection outcome between patients treated by day 2 and day 3 embryo transfer
| Day 2 | Day 3 | P | |
|---|---|---|---|
| Number of patients | 24 | 12 | Not significant |
| Number of oocytes retrieved | 10.0 ± 3.3 | 9.0 ± 6.0 | Not significant |
| Number of embryos developed | 7.6 ± 3.5 | 6.9 ± 3.7 | Not significant |
| Number of good‐quality embryos | 5.5 ± 2.6 | 5.5 ± 2.5 | Not significant |
| Number of good‐quality embryos transferred | 2 | 2 | Not significant |
| Number of embryos cyropreserved | 5.1 ± 3.4 | 4.1 ± 2.2 | Not significant |
| Number of good‐quality embryos cryopreserved | 3.5 ± 2.7 | 3.6 ± 2.1 | Not significant |
| Clinical pregnancies | 9 | 7 | Not significant |
| Clinical pregnancies rate per embryo transfer cycle (%) | 37.5 | 58.3 | Not significant |
| Multiple pregnancies | 1 | 1 | Not significant |
| Multiple pregnancy rate per embryo transfer cycle (%) | 11.1 | 14.3 | Not significant |
| Implantation rate (%) | 20.8 | 33.3 | Not significant |
Comparison of the implantation and pregnancy rates
The pregnancy rate of women who received two good‐quality embryos was 44.4% (16 out of 36). The multiple pregnancy rate was 12.5% (two out of 16). All multiple pregnancies were twins.
As shown in Fig. 1, higher pregnancy and implantation rates were seen in the day 3 ET group (37.5 and 20.8% in day 2 ET vs 58.3 and 33.3% in day 3 ET); however, there were no significant differences between the two groups (P = 0.24, P = 0.25, respectively).
Figure 1.
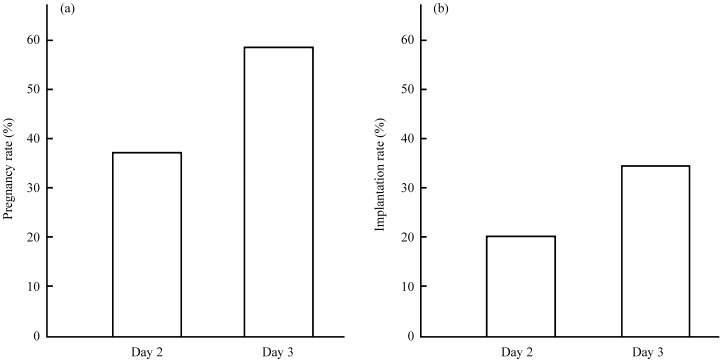
Comparison of the (a) pregnancy rates and (b) implantation rates between day 2 and day 3 transfer of two good‐quality embryos. Higher pregnancy and implantation rates were found in day 3 embryo transfer (ET) than day 2 ET (37.5 and 20.8% in day 2 ET vs 58.3 and 33.3% in day 3 ET); however, there were no significant differences between the two groups (a: P = 0.24, b: P = 0.25, respectively).
DISCUSSION
TO THE BEST of our knowledge, this is the first randomized study comparing the day 2 and day 3 elective transfer of two good‐quality embryos. The results suggest that ET on day 3 offers the selection of more viable embryos than day 2.
Delaying the ET to day 3 made it possible to observe the embryos for an additional 24 h. This allowed the separation of embryos that arrested or slowed down after day 2 from those that continued to develop. This latter group constituted the most viable group of embryos as shown by the resulting pregnancy rate, which was higher than the pregnancy rate obtained in the other embryo group. This is consistent with the finding that good‐quality embryos are more successful at developing beyond the four‐cell stage than poor‐quality embryos. On day 3, the majority of embryos developed eight cells or more. We are able to easily select the developing embryos when compared to day 2.
The placement of embryos in a more optimal uterine environment (day 3 after oocyte retrieval) may also be beneficial. During natural conception, the human embryo enters the uterus as morula 4 or 5 days after ovulation. The return of embryos to the uterus on day 3 may be closer to the physiologic time of uterine entry than day 2. This may have resulted in a significant improvement in the uterine environment for the embryos.
A number of studies have already been published comparing day 2 to day 3 ET. Van Os et al. reported no difference in the pregnancy rate, but that a higher percentage of viable pregnancies was obtained on day 3 transfer in a prospective randomized study. 1 In a retrospective study, the pregnancy rates were similar between day 2 and day 3 transfers; however, the implantation rates were higher in the day 3 group. 2 Another retrospective study concluded that day 3 ET was associated with a significant increase in the implantation and pregnancy rates. 3 In contrast, four recent prospective studies found no difference in the implantation and pregnancy rates. 4 , 5 , 6 , 7 In our hypothesis, the delay of ET by 1 day allowed an increase in positive clinical outcome. However, no concurrence of results in these studies was obtained. The data from the present study suggest that ET on day 3 allows the selection of more viable embryos than day 2. However, as a result of the small number of patients, there were no significant differences between the two groups.
It can be considered that a delay of 1 day is too short to differentiate between the quality of embryos. In recent years therefore an extended delay of transfer, up to the blastocyst stage, has been studied. Although several investigators reported increased implantation rates following blastocyst transfer, others have failed to observe any benefit compared with cleavage‐stage ET. High implantation rates after blastocyst transfer were shown in comparative or retrospective studies, 25 , 26 , 27 , 28 , 29 , 30 , 31 whereas in a series of prospective randomized trials, either a benefit from blastocyst transfer 32 , 33 , 34 or a similar implantation rate was observed. 35 , 36 , 37 , 38 Recently, sequential media have been introduced for the culture of later stage embryo development. In three recently published, prospective studies, sequential media were used for both day 2 or day 3, and day 5 transferred embryos. 39 , 40 , 41 Van der Auwera et al. found a higher clinical pregnancy rate after blastocyst replacement, 39 while another two studies concluded that similar implantation and pregnancy rates between day 2 or day 3, and day 5 embryos. 40 , 41 Preferential transfer in the cleavage stage or blastocyst stage remains controversial.
In conclusion, day 3 ET appears to allow the selection of more viable embryos than day 2 ET. Furthermore, blastocyst transfer risks the loss of embryos during prolonged culture and a lower number of blastocysts available for freezing. Day 3 ET is also advantageous in this area.
REFERENCES
- 1. Van Os HC, Alberda AT, Janssen‐Caspers HA, Leerentveld RA, Scholtes MC, Zeilmaler GH. The influence of the interval between in vitro fertilization and embryo transfer and some other variables on treatment outcome. Fertil Steril 1989; 51: 360–362. [DOI] [PubMed] [Google Scholar]
- 2. Dawson KJ, Conaghan J, Ostera GR, Winston RM, Hardy K. Delaying transfer to the third day post‐insemination, to select non‐arrested embryos, increases development to the fetal heart stage. Hum Reprod 1995; 10: 177–182. [DOI] [PubMed] [Google Scholar]
- 3. Carrillo AJ, Lane B, Pridman DD et al. Improved clinical outcomes for in vitro fertilization with delay of embryo transfer from 48 to 72 hours after oocyte retrieval: use of glucose‐ and phosphate‐free media. Fertil Steril 1998; 69: 329–334. [DOI] [PubMed] [Google Scholar]
- 4. Ertzeld G, Dale PO, Tanbo T, Storeng R, Kjekshus E, Abyholm T. Clinical outcome of day 2 versus day 3 embryo transfer using serum‐free culture media: a prospective randomized study. J Assist Reprod Genet 1999; 16: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Laverge H, De Sutter P, Van der Elst J, Dhont M. A prospective, randomized study comparing day 2 and day 3 embryo transfer in human IVF. Hum Reprod 2001; 16: 476–480. [DOI] [PubMed] [Google Scholar]
- 6. Aboulghar MM, Aboulghar MA, Mansour RT, Serour GI, Amin YM, Abou‐Setta AM. Pregnancy rate is not improved by delaying embryo transfer from days 2–3. Eur J Obstet Gynecol Reprod Biol 2003; 107: 176–179. [DOI] [PubMed] [Google Scholar]
- 7. De los Santos MJ, Mercader A, Galan A, Albert C, Romero JL, Pellicer A. Implantation rates after two, three, or five days of embryo culture. Placenta 2003; 24: S13–S19. [DOI] [PubMed] [Google Scholar]
- 8. Wood C, McMaster R, Rennie G, Trounson A, Leeton J. Factors influencing pregnancy rates following in vitro fertilization and embryo transfer. Fertil Steril 1985; 43: 245–250. [DOI] [PubMed] [Google Scholar]
- 9. Sharma V, Riddle A, Mason BA, Pampiglione J, Cambell S. An analysis of factors influencing the establishment of a clinical pregnancy in an ultrasound‐based ambulatory in vitro fertilization program. Fertil Steril 1988; 49: 468–478. [DOI] [PubMed] [Google Scholar]
- 10. Alikani M, Wiemer K. Embryo number for transfer should not be strictly regulated. Fertil Steril 1997; 68: 782–783. [DOI] [PubMed] [Google Scholar]
- 11. De Jonge CJ, Wolf DP. Embryo number for transfer should be regulated. Fertil Steril 1997; 68: 784–786. [DOI] [PubMed] [Google Scholar]
- 12. Nijs M, Geerts L, Van Roosendaal E, Segal‐Bertin G, Vanderzwalmen P, Schoysman R. Prevention of multiple pregnancies in an in vitro fertilization program. Fertil Steril 1993; 59: 1245–1250. [DOI] [PubMed] [Google Scholar]
- 13. Matson PL, Browne J, Deakin R, Bellinge B. The transfer of two embryos instead of three to reduce the risk of multiple pregnancy: a retrospective analysis. J Assist Reprod Genet 1999; 16: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devreker F, Emiliani S, Revelard P, Van den Bergh M, Govaerts I, Englert Y. Comparison of two elective transfer policies of two embryos to reduce multiple pregnancies without impairing pregnancy rates. Hum Reprod 1999; 14: 83–89. [DOI] [PubMed] [Google Scholar]
- 15. Shibahara H, Suzuki T, Tanaka Y et al. Establishment and application of criteria for the elective transfer of two good‐quality embryos to reduce high‐order multiple pregnancies. Reprod Med Biol 2002; 1: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shibahara H, Mitsuo M, Ikeda Y, Shigeta M, Koyama K. Effects of sperm immobilizing antibodies on pregnancy outcome in infertile women treated with IVF‐ET. Am J Reprod Immunol 1996; 36: 96–100. [DOI] [PubMed] [Google Scholar]
- 17. Shibahara H, Funabiki M, Shiotani T, Ikeda Y, Koyama K. A case of primary ovarian pregnancy after in‐vitro fertilization and embryo transfer. J Assist Reprod Genet 1997; 14: 63–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shibahara H, Naito S, Hasegawa A, Mitsuo M, Shigeta M, Koyama K. Evaluation of sperm fertilizing ability using the Sperm Quality Analyzer. Int J Androl 1997; 20: 112–117. [DOI] [PubMed] [Google Scholar]
- 19. Obara H, Shibahara H, Tsunoda H et al. Prediction of unexpectantly poor fertilization and pregnancy outcome using the strict criteria for sperm morphology before and after sperm separation in IVF‐ET. Int J Androl 2001; 24: 102–108. [DOI] [PubMed] [Google Scholar]
- 20. Hirano Y, Shibahara H, Obara H et al. Relationships between sperm motility characteristics assessed by the computer‐aided sperm analysis (CASA) and fertilization rates in vitro. J Assit Reprod Genet 2001; 18: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shibahara H, Shiraishi Y, Hirano Y, Suzuki T, Takamizawa S, Suzuki M. Diversity of the inhibitory effects on fertilization by anti‐sperm antibodies bound to the surface of ejaculated human sperm. Hum Reprod 2003; 18: 1469–1473. [DOI] [PubMed] [Google Scholar]
- 22. Shibahara H, Yamanaka H, Hirano Y et al. Analysis of factors associated with multinucleate formation in human IVF. Int J Androl 2003; 26: 211–214. [DOI] [PubMed] [Google Scholar]
- 23. Ayustawati, , Shibahara H, Obara H, Hirano Y et al. Influence of endometrial thickness and pattern on pregnancy rates in in vitro fertilization‐embryo transfer. Reprod Med Biol 2002; 1: 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Veeck LL. Oocyte assessment and biological performance. Ann NY Acad Sci 1998; 541: 259–274. [DOI] [PubMed] [Google Scholar]
- 25. Alves da Motta EL, Alegretti JR, Baracat EC, Olive D, Serafini PC. High implantation and pregnancy rates with transfer of human blastocysts developed in preimplantation stage one and blastocyst media. Fertil Steril 1998; 70: 659–663. [DOI] [PubMed] [Google Scholar]
- 26. Gardner DK, Vella P, Lane M, Wagley L, Sclenker T, Schoolcraft WB. Culture and transfer of human blastocysts increases implantation rates and reduces the need for multiple embryo transfers. Fertil Steril 1998; 69: 84–88. [DOI] [PubMed] [Google Scholar]
- 27. DelMarek D, Langley M, Gardner DK, Confer N, Doody K. Introduction of blastocyst culture and transfer for all patients in an in vitro fertilization program. Fertil Steril 1998; 72: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 28. Milki AA, Fisch JD, Behr B. Two‐blastocyst transfer has similar pregnancy rates and a decreased multiple gestation rate compared with three‐blastocyst transfer. Fertil Steril 1999; 72: 225–228. [DOI] [PubMed] [Google Scholar]
- 29. Milki AA, Hinckley MD, Fisch JD, Dasig D, Behr B. Comparison of blastocyst transfer with day 3 embryo transfer in similar patient population. Fertil Steril 2000; 73: 126–128. [DOI] [PubMed] [Google Scholar]
- 30. Balaban B, Urman B, Alatas C, Mercan R, Aksoy S, Isiklar A. Blastocyst‐stage transfer of poor‐quality cleavage‐stage embryos results in higher implantation rates. Fertil Steril 2001; 75: 514–518. [DOI] [PubMed] [Google Scholar]
- 31. Yoon HG, Yoon SH, Son WY, Kim JG, Im KS, Lim JH. Alternative embryo transfer on day 3 or day 5 for reducing the risk of multiple gestations. J Assit Reprod Genet 2001; 18: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gardner DK, Schoolcraft WB, Wagley L, Sclenker T, Stevens J, Hesla J. A prospective randomized trial of blastocyst culture and transfer in in‐vitro fertilization. Hum Reprod 1998; 13: 3434–3440. [DOI] [PubMed] [Google Scholar]
- 33. Hsieh YY, Tsai HD, Chang FCC. Routine blastocyst culture and transfer: 201 patients’ experience. J Assist Reprod Genet 2000; 17: 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karaki RZ, Samarraie SS, Youmis NA, Lahloubt M, Ibrahim MH. Blastocyst culture and transfer: a step toward improved in vitro fertilization outcome. Fertil Steril 2002; 77: 114–188. [DOI] [PubMed] [Google Scholar]
- 35. Scholtes MCW, Zeilmaker GH. A prospective randomized study of embryo transfer results after 3 or 5 days of embryo culture in in vitro fertilization. Fertil Steril 1996; 65: 1245–1248. [DOI] [PubMed] [Google Scholar]
- 36. Coskun S, Hollanders J, Al‐Hassan S, Al‐Sufyan H, Al‐Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: a controlled randomized trial. Hum Reprod 2000; 15: 1947–1952. [DOI] [PubMed] [Google Scholar]
- 37. Plachot M, Belaisch‐Allart J, Mayenga JM, Chouraqui A, Serkine AM, Tesquier L. Blastocyst stage transfer: the real benefit compared with early embryo transfer. Hum Reprod 2000; 15: 24–30. [PubMed] [Google Scholar]
- 38. Utsunomiya T, Naitou T, Nagaki M. A prospective trial of blastocyst culture and transfer. Hum Reprod 2002; 17: 1846–1851. [DOI] [PubMed] [Google Scholar]
- 39. Van der Auwera I, Debrock S, Spiessens C et al. A prospective randomized study: day 2 versus day 5 embryo transfer. Hum Reprod 2002; 17: 1507–1512. [DOI] [PubMed] [Google Scholar]
- 40. Rienzi L, Ubaldi F, Iacobelli M et al. Day 3 embryo transfer with combined evaluation at the pronuclear and cleavage stages compares favourably with day 5 blastocyst transfer. Hum Reprod 2002; 17: 1852–1855. [DOI] [PubMed] [Google Scholar]
- 41. Emiliani S, Delbaere A, Vannin AS et al. Similar delivery rates in a selected group of patients, for day 2 and day 5 embryos both cultured in sequential medium: a randomized study. Hum Reprod 2003; 18: 2145–2150. [DOI] [PubMed] [Google Scholar]


