Abstract
Several lines of evidence indicate that phospholipase A2 (PLA2) plays a crucial role in plant cellular responses through production of linolenic acid, the precursor of jasmonic acid, from membrane phospholipids. Here we report the purification and characterization of a 48-kD PLA2 from the membrane fractions of leaves of broad bean (Vicia faba). The plant PLA2 was purified to near homogeneity by sequential column chromatographies from the membrane extracts. The purified 48-kD protein migrated as a single band on a SDS-PAGE gel and its density correlated with the PLA2 activity. It was further confirmed that this 48-kD protein is the PLA2 enzyme based on immunoprecipitating the activity with a monoclonal antibody against it and purifying the enzyme to homogeneity with the antibody affinity column. The purified plant PLA2 preferred 2-linolenoyl-sn-glycerol-3-phosphocholine (GPC) to 2-linoleoyl-GPC, 2-palmitoyl-GPC and 2-arachidonyl-GPC as substrates with a pH optimum at pH 7.0 to 8.0. The plant PLA2 was activated by calmodulin and inhibited by pretreatment of 5,8,11,14-eicosatetraynoic acid known as an inhibitor of mammalian PLA2s. The enzyme was characterized as a Ca2+-independent PLA2 different from mammalian PLA2s. This membrane-associated and Ca2+-independent PLA2 is suggested to play an important role in the release of linolenic acid, the precursor of jasmonic acid, through a signal transduction pathway.
Phospholipase A2 (PLA2, EC 3.1.1.4) is a family of enzymes that catalyze the hydrolysis of the fatty acyl ester bond at the sn-2 position of glycerophospholipids. In animals, it is well known that arachidonic acid (AA) released from membrane phospholipids by PLA2 is subsequently metabolized to eicosanoids such as prostaglandins, thromboxanes, and leukotrienes (Samuelsson et al., 1987; Dennis et al., 1991). These lipid mediators play important roles in many pathophysiological mechanisms involved in inflammation and tissue injury (Bonventre, 1992; Kudo et al., 1993).
Mammalian cells have been known to contain secretory and cytosolic forms of Ca2+-dependent PLA2 based on their biochemical properties, localization, and primary structures (Dennis, 1994). Secretory PLA2 (sPLA2) is localized in secretory granules, and has optimal activity at millimolar concentrations of Ca2+. It exhibits no preference to AA at the sn-2 position of the phospholipids. On the other hand, cytosolic PLA2 (group IV cPLA2) is localized in cytosol, and is active at micromolar concentrations of Ca2+, which triggers it to translocate to membranes. It shows high preference for AA at the sn-2 position of the phospholipids and may play an important role in the signal-coupled release of AA (Serhan et al., 1996).
A Ca2+-independent form of PLA2 (iPLA2) having a molecular mass of 80 kD was purified from cytosol of mammalian tissues, and the cDNA has been cloned (Tang et al., 1997). Although iPLA2 preferentially hydrolyzes dipalmitoyl phosphatidylcholine (PC), the role of this enzyme in cellular function remains to be elucidated (Ackermann et al., 1994). Moreover, a novel membrane-bound form of iPLA2 was also identified, characterized, and designated as cPLA2-γ, which contains a domain with significant homology to the catalytic domain of the 85-kD cPLA2 (Underwood et al., 1998).
However, in plants little is known about the presence and characteristics of PLA2 although many lines of indirect evidence indicate that the enzyme is involved in various plant signal transduction mechanisms (Munnik et al., 1998).
Several studies propose an involvement of PLA2 in auxin signaling. For example, auxin is suggested to stimulate elongation of plant cells through the activation of a PLA2 in microsomes of zucchini, which is blocked by the treatment of polyclonal antibody raised against an auxin-binding protein (Andre and Scherer, 1991). Moreover, 5,8,11,14-eicosatetraynoic acid (ETYA), an inhibitor of PLA2, was found to block the auxin-dependent growth in hypocotyl segments of etiolated zucchini seedling (Scherer and Arnold, 1997).
PLA2 is also suggested to play an important role in elicitor-induced defense responses. Elicitor treatment rapidly elevated a cellular level of free linolenic acid, and the time course for the accumulation of linolenic acid and linoleic acid was correlated with those for the accumulation of jasmonic acid and expression of defense genes (Blechert et al., 1995; Conconi et al., 1996). Linolenic acid is known to serve as the precursor of jasmonic acid and other octadecanoid-derived chemical mediators that stimulate the expression of defense-related genes (Bergey et al., 1996; Creelman and Mullet, 1997). Thus, the release of linolenic acid by a plant PLA2 is thought to be the rate-limiting step in this signal transduction pathway as is in the case of AA-prostaglandins system in mammalians (Kudo et al., 1993).
In plants patatin, a 40-kD protein of potato tuber, was reported to have acyl transferase as well as PLA2 activity (Senda et al., 1996). Patatin is the major tuber protein that shows optimal activity at neutral pH, and requires millimolar concentrations of Ca2+ for the optimal PLA2 activity. A recent report also suggested that a 46-kD protein, homologous to patatin and designated as Hev b 7, could be a defense-related PLA2 (Kostyal et al., 1998). However, the role of patatin and its involvement in cellular signaling remain to be elucidated. A soluble PLA2 was purified and characterized from developing elm seeds (Stahl et al., 1998). This enzyme seems to be related to the secretory form of mammalian PLA2s, based on the similarity of biochemical properties and sequence homologies of catalytic motifs. These results suggest that plant PLA2s exist as multiple forms of enzyme as is in the case of mammalians.
We previously identified two types of PLA2 in the 100,000g supernatants and a membrane-associated PLA2 in the membrane fractions of leaves of broad bean (Vicia faba; Kim et al., 1994). The two types of the soluble PLA2 were partially purified and shown to be different from mammalian PLA2s.
In the present study we purified and characterized a membrane-associated iPLA2 as a homogeneous protein of a 48-kD enzyme. This enzyme was activated by calmodulin, but inhibited by ETYA, which was known as an inhibitor of mammalian PLA2s. The purified plant PLA2 preferred 2-linolenoyl sn-glycerol-3-phosphocholine (GPC) to 2-linoleoyl GPC by approximately 1.5-fold. These results suggest that this membrane-associated 48-kD PLA2 may be regulated by calmodulin to produce linolenic acid, the precursor of jasmonic acid.
RESULTS
Purification of the Membrane-Associated Plant PLA2
The plant PLA2 was purified to near homogeneity with a yield of 4.8% and 2,300-fold increase in the specific activity over the homogenate of leaves of broad bean by the sodium deoxycholate (SDC)-extraction and ammonium sulfate-precipitation steps followed by several chromatographies (Table I). The sequential purification steps of SDC-extraction and ammonium sulfate-precipitation resulted in a yield of 56.8% and 28-fold increase in the specific activity over the homogenate. The leaf-derived components of dark green color were removed during these purification steps. This clear PLA2 enzyme preparation was applied to the Phenyl-5PW hydrophobic HPLC column as the first chromatography, where we purified the PLA2 activity by 163-fold increase in the specific activity over the homogenate. The partially purified enzyme activity was unstable, but stable for several months at −70°C after the DEAE-5PW anion-exchange HPLC column as the next step.
Table I.
Summary of purification of the 48-kD membrane-associated PLA2 from leaves of broad bean
| Step | Protein | Total Activity | Specific Activity | Purification | Yield |
|---|---|---|---|---|---|
| mg | pmol min−1 | pmol min−1 mg−1 | fold | % | |
| Lysate | 4,770 | 14,310 | 3 | 1 | 100 |
| SDC extracts | 185 | 8,880 | 48 | 16 | 62.1 |
| (NH4)2SO4 fractionation | 98 | 8,134 | 83 | 28 | 56.8 |
| Phenyl-5PW | 2.70 | 1,323 | 490 | 163 | 9.2 |
| DEAE-5PW | 0.48 | 1,238 | 2,580 | 860 | 8.7 |
| G3000-PW | 0.31 | 1,190 | 3,840 | 1,280 | 8.3 |
| Mono Q | 0.13 | 750 | 5,770 | 1,923 | 5.2 |
| Superose 12 | 0.10 | 690 | 6,900 | 2,300 | 4.8 |
Sequential steps used in purification of the plant PLA2, resulting in a 2,300-fold purification with 4.8% recovery.
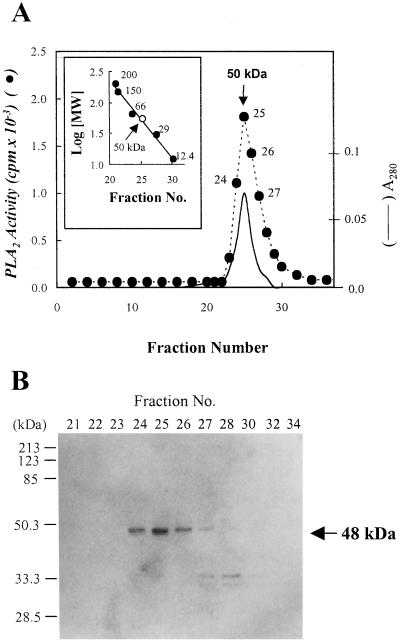
To further purify the enzyme and estimate the apparent molecular mass of the activity, the active fractions of the Mono Q FPLC were subjected to Superose 12 gel-filtration FPLC. The highest peak of the PLA2 activity was eluted at a molecular mass of approximately 50 kD as calibrated with molecular-mass standards. When these active fractions were subjected to 12% (w/v) SDS-PAGE, a single band migrated as a molecular mass of 48 kD and its relative intensity paralleled the elution profile of the PLA2 activity as shown in Figure 1.
Figure 1.
Gel filtration column chromatography on Superose 12 FPLC. The active fractions obtained from Mono Q FPLC column were applied to a Superose 12 gel filtration FPLC column as described in “Materials and Methods.” The inset shows the calibration curve for the estimation of the apparent molecular mass of the PLA2. The standard protein markers for the gel filtration chromatography were as follows: β-amylase (200 kD), alcohol dehydrogenase (150 kD), BSA (66 kD), carbonic anhydrase (29 kD), and cytochrome c (12.4 kD) (A). The active fractions from Superose 12 gel filtration FPLC columns were analyzed by 12% (w/v) SDS-PAGE and visualized by silver stain as described in “Materials and Methods.” The standard protein markers were as follows: myosin (200 kD), β-galactosidase (116 kD), phosphorylase b (97.4 kD), BSA (66.2 kD), ovalbumin (45 kD), carbonic anhydrase (31 kD), and trypsin inhibitor (21.5 kD). The molecular mass (arrow) of the plant PLA2 was extrapolated from Rf value (B). Essentially identical results were obtained in three independent experiments.
Immunoprecipitation of the Plant PLA2
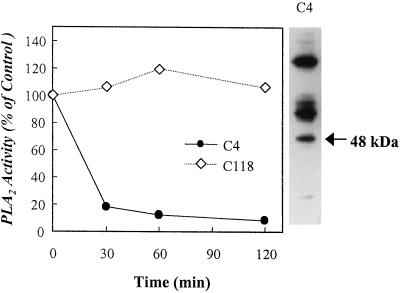
To further define the 48-kD protein as the PLA2 enzyme, we raised mouse monoclonal antibodies against the 48-kD protein and examined whether the antibodies immunoprecipitate the PLA2 activity. We screened hybridoma cells using the active fractions obtained from the Mono Q FPLC column, and identified two positive hybridoma clones, C4 and C118. As shown in Figure 2, only the hybridoma clone C4-derived antibody immunoprecipitated the PLA2 activity partially purified from the DEAE-5PW column in a time-dependent manner, but it did not react with the 48-kD protein in a western blot (data not shown). When each of immunoprecipitates obtained from the hybridoma media was subjected to SDS-PAGE and visualized by a silver-staining kit, the immunoprecipitate by only the hybridoma clone C4-derived antibody migrated as a single band of the 48-kD protein on a SDS-PAGE gel (Fig. 2).
Figure 2.
Immunoprecipitation of the plant PLA2 activity by a monoclonal antibody raised against the purified 48-kD protein. Culture media of mouse hybridoma clones raised against the plant 48-kD protein were mixed with packed Protein A-Sepharose beads and incubate with 25 μg of partially purified PLA2 as described in “Materials and Methods.” The resulting supernatants were assayed for the PLA2 activity. The immunoprecipitates were subjected to 12% (w/v) SDS-PAGE as described in “Materials and Methods.” C4 and C118 mean the mouse hybridoma clones raised against the plant 48-kD protein, which were positively reactive in ELISA. Essentially identical results were obtained in three independent experiments.
Purification of the 48-kD Plant PLA2 by Using Anti-48-kD PLA2 Protein Antibody Affinity Column
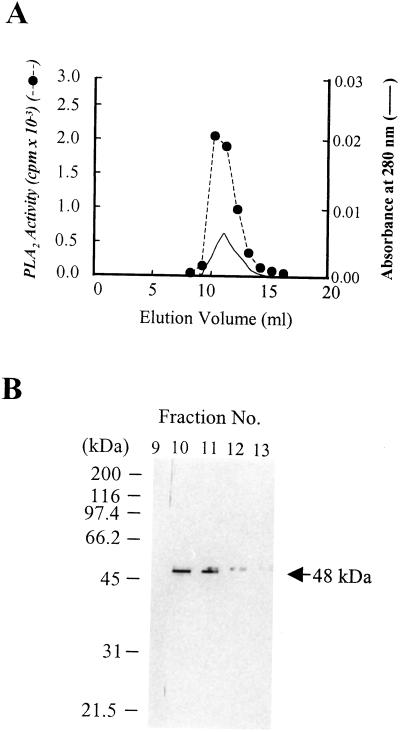
To further confirm the 48-kD protein as the PLA2 enzyme and purify the enzyme in a more efficient manner, we made an anti-48-kD protein antibody affinity column by coupling the antibody to the N-hydroxysuccinimide (NHS)-activated column. The partially purified PLA2 enzyme preparation obtained by the purification procedure yielded a single band of 48-kD protein on a SDS-PAGE gel, which correlated with the enzymatic activity (Fig. 3, A and B). The N-terminal sequencing of the 48-kD protein revealed sequence similarity to a cucumber patatin-like lipase (May et al., 1998; K.M. Jung and D.K. Kim, unpublished data) as well as a potato patatin. As these proteins were reported to act as lipid acyl hydrolases, these similarities also confirm our result that the 48-kD protein is a plant PLA2.
Figure 3.
Purification of the 48-kD plant PLA2 by using anti-48-kD PLA2 protein antibody affinity column. The plant PLA2 partially purified from the plant homogenates was applied to an anti-48-kD protein antibody affinity column (1-mL bed volume) as described in “Materials and Methods.” Aliquots of each fraction were assayed for the PLA2 activity (A) as described in “Materials and Methods,” analyzed by 12% (w/v) SDS-PAGE (B), and visualized by a silver-staining kit. Essentially identical results were obtained in three independent experiments.
Characterization of the Purified Plant PLA2
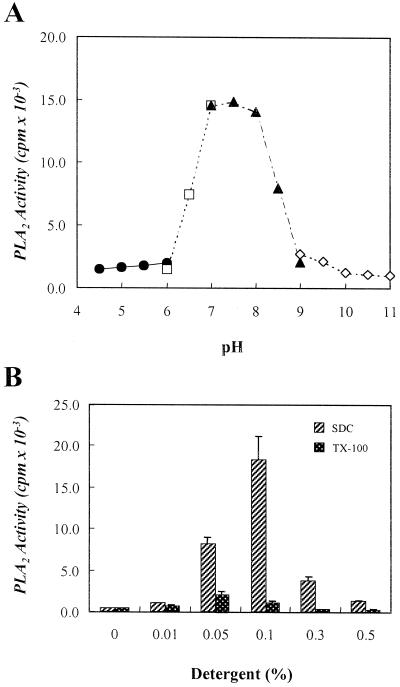
To determine pH dependency of the purified 48-kD plant PLA2, an aliquot of the active fractions obtained from the Superose 12 gel filtration column was assayed in the range of pH 4.5 to 11.0. The PLA2 showed optimal activity at a narrow range of neutral pH of 7.0 to 8.0 (Fig. 4A).
Figure 4.
Effects of pH and detergents on the purified PLA2 activity. The purified PLA2 activity from the Superose 12 gel filtration FPLC was assayed in the range of pH 4.5 to 11.0. The pH buffers were as follows: Gly-HCl, pH 4.5 to 6.0 (●); imidazole-HCl, pH 6.0 to 7.0 (□); Tris-HCl, pH 7.0 to 9.0 (▴); Gly-NaOH, pH 9.0 to 11.0 (◊) (A). The purified PLA2 activity was assayed as described in “Materials and Methods” in the presence of the indicated concentrations of SDC (w/v %) or Triton X-100 (TX-100, v/v %), respectively. Each data point represents the means ± se of three independent experiments.
The effects of detergents on the purified plant PLA2 activity were examined. When 0.1% (w/v) SDC and 0.05% (w/v) Triton X-100 was added to the assay mixture, the enzymatic activity was increased by 44- and 5-fold, respectively (Fig. 4B).
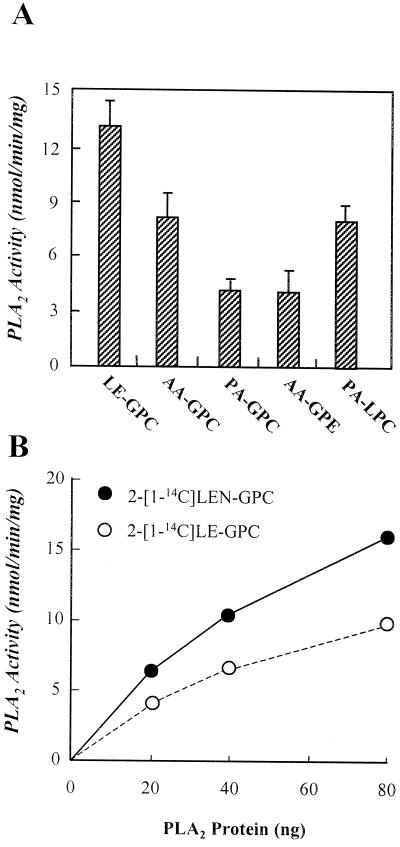
The substrate specificity for the plant PLA2 enzyme was also examined as shown in Figure 5A. The PLA2 showed the highest specific activity of 11.1 nmol min−1 mg−1 protein for 2-[1-14C]linoleoyl-GPC, which is higher by 1.6- and 3.3-fold than activity with 2-[1-14C]arachidonyl-GPC and 2-[1-14C]palmitoyl-GPC, respectively. The plant PLA2 preferred PC to phosphatidylethanolamine (PE), and unsaturated fatty acids to saturated fatty acids at sn-2 position of the phospholipids. No radioactive LPC was detected when the enzyme was incubated with sn-2-labeled GPC, indicating that the enzyme has no PLA1 activity (data not shown). However, when assayed with 1-[1-14C]palmitoyl-LPC as substrate, this enzyme also showed a considerable lysophospholipase activity. To compare the specific activity for 1-stearoyl-2-[1-14C]linoleoyl-sn-glycero-3-PC (2-[1-14C]LE-PC) with that for 1-stearoyl-2-[1-14C]linolenoyl-sn-glycero-3-PC (2-[1-14C]LEN-PC), the substrates were synthesized by enzymatically transferring [1-14C]linoleic acid and [1-14C]linolenic acid, respectively, to 1-stearoyl-2-hydroxy-sn-glycero-3-PC (Lyso PC) and isolated the [1-14C]PCs by a normal phase μ-porasil HPLC. This synthesis procedure produced 2-[1-14C]LE-PC and 2-[1-14C]LEN-PC with yield of 81.3% and 76.2%, respectively. As shown in Figure 5B, the purified 48-kD PLA2 preferred 2-[1-14C]LEN-PC to 2-[1-14C]LE-PC by approximately 1.5-fold. When human plasma as the positive control was assayed for triacylglycerol lipase activity as described (Shirai et al., 1981), the purified 48-kD PLA2 did not reveal any triacylglycerol lipase activity with glycerol tri[1-14C]oleate (Amersham Pharmacia Biotech, Buckinghamshire, UK) as substrate (data not shown).
Figure 5.
Substrate specificity for the purified 48-kD plant PLA2. A, An aliquot of the active pool obtained from the Superose 12 gel filtration HPLC column was assayed for the PLA2 activity with 25 μm of the indicated substrates as described in “Materials and Methods.” B, 2-[1-14C]LEN-PC and 2-[1-14C]LE-PC were synthesized as described in “Materials and Methods.” The indicated amounts of the active fractions from the Superose 12 gel filtration HPLC column was assayed for the PLA2 activity with approximately 11.2 μm substrates as described in “Materials and Methods.” LE-GPC, 1-Palmitoyl-2-[1-14C]linoleoyl-GPC; PA-GPC, 1-palmitoyl-2-[1-14C]palmitoyl-GPC; AA-GPE, 1-stearoyl-2-[1-14C]arachidonyl-sn-glycerol-3-PE; PA-LPC, 1-[1-14C]palmitoyl-2-hydroxy-sn-glycerol-3-PC.
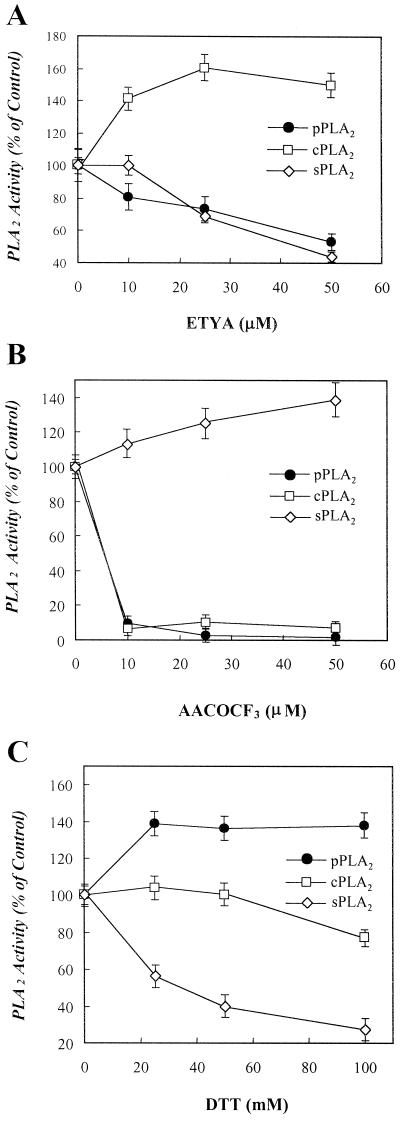
ETYA, an inhibitor of mammalian PLA2, has been shown to block auxin-induced growth (Scherer and Arnold, 1997). This finding prompted us to examine the effect of the inhibitors of mammalian PLA2s on the plant PLA2 activity. First, as shown in Figure 6A, ETYA inhibited in a dose-dependent manner the purified plant PLA2 and the pancreatic form of sPLA2, but not porcine spleen group IV cPLA2. Second, arachidonyl trifluoromethyl ketone (AACOCF3), a trifluoromethyl ketone analog of AA, is known to inhibit group IV cPLA2 (Street et al., 1993) and iPLA2 (Ackermann et al., 1995). This compound inhibited the purified plant PLA2 with potency similar to that for inhibition of group IV cPLA2, but not the pancreatic form of PLA2 (Fig. 6B). Third, dithiothreitol (DTT) has been used as an inhibitor to differentiate group IV cPLA2 from sPLA2, which contains seven disulfide bonds sensitive to DTT (Kudo et al., 1993). Figure 6C shows that the reducing agent inhibited the sPLA2, but it increased the plant PLA2 activity slightly.
Figure 6.
Effects of various mammalian PLA2 inhibitors on the purified PLA2 activity. The purified plant PLA2 (pPLA2) obtained from the Superose 12 gel filtration FPLC was pre-incubated with the indicated concentrations of mammalian PLA2 inhibitors, ETYA (A), AACOCF3 (B), or DTT (C), at 37°C for 10 min followed by assaying the PLA2 activity as described in “Materials and Methods.” The inhibitors-free activity of pPLA2, group IV cPLA2, and sPLA2 were 3,250, 3,010, and 3,670 cpm, respectively, under the assay condition. Ethanol (2.5%, v/v) and 5% (w/v) dimethyl sulfoxide as vehicles did not affect the PLA2 activity. Each data point represents the means ± se of three independent experiments.
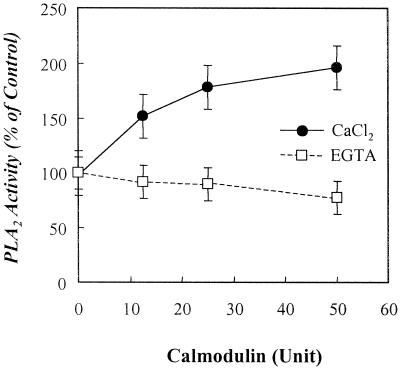
We examined the requirement of Ca2+ for the activity. The purified plant PLA2 obtained from the Superose 12 gel filtration FPLC column was assayed in the presence of 2 mm EGTA or various concentrations of CaCl2. The calcium concentrations were determined in EGTA/CaCl2 buffers at pH 7.4 described previously (Durham, 1983). The activity was not significantly changed in Ca2+ concentrations of 10−8 to 10−2 m. While 2 mm EGTA slightly inhibited the activity by 8%, 2 mm EDTA inhibited it by 28% (data not shown). Thus, it is found that the 48-kD plant PLA2 is of a Ca2+-independent form. To elucidate the biochemical mechanisms by which the 48-kD plant PLA2 is regulated in intact plant cells, the effects of calmodulin on the PLA2 activity were examined. When calmodulin was pre-incubated with the purified plant PLA2 in the presence of 3 mm CaCl2 or 2 mm EGTA, it enhanced the plant PLA2 activity by approximately 2-fold at 50 unit in a dose-dependent manner only when Ca2+ was present in the assay (Fig. 7).
Figure 7.
Effects of calcium and calmodulin on the purified plant PLA2 activity. The purified plant PLA2 obtained from the Superose 12 gel filtration FPLC column was pre-incubated with the indicated concentrations of calmodulin (40,000 units/mg protein) in the presence of 3 mm CaCl2 or 2 mm EGTA at 37°C for 10 min followed by assaying the PLA2 activity as described in “Materials and Methods.” Each data point represents the means ± se of three independent experiments.
DISCUSSION
PLA2 has been known to play a crucial role in signal transduction of plant cells. However, the biochemical characteristics of plant PLA2 have not been fully determined because the enzyme has not been purified to homogeneity. Accumulating evidence suggested that a membrane-associated form of PLA2 is implicated in a number of cellular responses in plant cells. It was reported that a PLA2 from the microsomal fractions of the plant cells was activated through agonist-coupled signal transduction (Andre and Scherer, 1991). It was also shown that a membrane-associated PLA2 was activated via auxin-induced signaling (Scherer and Andre, 1989; van der Hoeven et al., 1996). Plant PLA2 is known to liberate linolenic acid from the membrane phospholipids, which is subsequently metabolized to produce jasmonic acid as a second messenger. This process is known as octadecanoid pathway in plant similar to eicosanoid pathway in mammalian and has been suggested to occur in plasma membranes and plastids (Blechert et al., 1995; Conconi et al., 1996).
In this context the present study was focused on a membrane-associated form of plant PLA2. The specific activity of the purified plant PLA2 was 6.9 nmol min−1 mg−1 protein, which was relatively low by approximately 100-fold compared with that of group IV cPLA2 previously purified from porcine spleen (Kim and Bonventre, 1993). This may result from the assay condition that is different in the presence of SDC or the amount of substrate. Otherwise, this may be due to its localization in membranes: This membrane-associated enzyme may catalyze its membrane substrates efficiently in spite of low specific activity.
As shown in Figure 1, the highest peak of the enzyme activity migrated as an apparent molecular mass of 50 kD during the Superose 12 gel filtration chromatography. These data also indicate that the 48-kD protein is likely to be the plant PLA2 enzyme, and suggest that the enzyme exist as a monomer in the Superose 12 gel filtration column. As shown in Figure 2, the 48-kD protein was further confirmed to be the PLA2 enzyme by immunoprecipitating the activity using a monoclonal antibody raised against the 48-kD protein. Furthermore, only the immunoprecipitate obtained from the medium that immunoprecipitated the PLA2 activity revealed the 48-kD protein band on a SDS-PAGE gel, indicating that the 48-kD protein is responsible for the PLA2 activity. However, in western blot analysis, the 48-kD protein band was not shown, suggesting that the antibody does not recognize the denatured 48-kD protein.
To further confirm that the 48-kD protein is really the PLA2 enzyme, we established the anti-48-kD protein monoclonal antibody affinity column, by which the PLA2 protein was purified. As shown in Figure 3, the 48-kD protein was purified as a single band from the DEAE-5PW-purified enzyme preparation. Fortunately, despite exposure to an acidic condition of pH 2.6, the fractions from the antibody affinity column revealed a considerable activity correlating with their band density (Fig. 3, A and B).
The plant PLA2 activity is increased by approximately 44-fold under assay condition containing 0.1% (w/v) SDC (Fig. 4B), where the activities of group IV cPLA2 and sPLA2 were completely inhibited (data not shown). However, whether SDC directly stimulates the plant PLA2 or affects its availability for the substrate remains to be determined.
It is known that auxin-induced plant cell growth is inhibited by ETYA (Scherer and Arnold, 1997), further suggesting a crucial role of PLA2 in the plant cell growth. As shown in Figure 6A, ETYA inhibited both the plant and secretory forms of PLA2 with a similar potency. This suggests that the 48-kD membrane-associated PLA2 may be responsible for inhibition of auxin-induced plant cell growth by ETYA.
The sensitivity of the plant PLA2 to other inhibitors of mammalian PLA2s, AACOCF3 and DTT, was also examined. AACOCF3 is known to inhibit both group IV cPLA2 (Street et al., 1993) and 80-kD iPLA2 by competing with fatty acyl chains at the sn-2 position of phospholipids (Ackermann et al., 1995), but not the low Mr forms of sPLA2. In contrast, DTT inhibits the low Mr forms of sPLA2 through the reduction of the disulfide bonds, but not the group IV cPLA2 (Winkler et al., 1994). In this context, the 48-kD plant PLA2 seems to recognize crucially the fatty acyl chains at the sn-2 position of phospholipids since the activity was inhibited by AACOCF3 with a similar potency to group IV cPLA2 (Fig. 6B). Moreover, a disulfide bond seems neither to exist within the PLA2 protein nor to be important for the activity because the activity was not sensitive to DTT (Fig. 6C).
It was reported that unsaturated fatty acids serve as activators of H+-ATPase (Nasyrova et al., 1996) and protein kinases (Klucis and Polya, 1987; Minichiello et al., 1989; Polya et al., 1990; van der Hoeven et al., 1996) to trigger plant cell responses. In particular linolenic acid is implicated in such responses since it could be metabolized to jasmonic acid through the octadecanoid pathway. It was also reported that PC and PE were the major phospholipids hydrolyzed by elicitor-induced PLA2 activation of plant cells (Roy et al., 1995). In our experiments the purified plant PLA2 preferred 2-[1-14C]linoleoyl-GPC to 2-[1-14C]palmitoyl-GPC and 2-[1-14C]arachidonoyl-GPC by 3.3- and 1.6-folds, respectively. Although the PLA2 activity should be tested for the PC and PE containing various species of fatty acids at the sn-2 position, PC seems to be the better substrate than PE for the PLA2 activity. These results suggest the possibility that this 48-kD PLA2 may be involved in the plant defense responses. On the other hand, to compare the PLA2 activity for 2-[1-14C]LEN-PC with that for 2-[1-14C]LE-PC, these substrates were synthesized by enzymatically transferring [1-14C]linolenic acid and [1-14C]linoleic acid to sn-2 position of Lyso PC, respectively. As shown in Figure 5B, the purified plant PLA2 preferred 2-[1-14C]LEN-PC to 2-[1-14C]LE-PC by approximately 1.5-fold. This suggests that the 48-kD PLA2 may not selectively hydrolyze phospholipids containing linolenoyl moiety at their sn-2 positions, but it does not mean that this PLA2 will not play a role in the production of linolenic acid as the precursor of jasmonic acid. Whether this PLA2 will be able to selectively release linolenoyl moiety from intact plant cell membranes, whose physical state is much different from the PC vesicles, remains to be studied.
Calcium ion is known to be an important factor for the activation of mammalian PLA2s. It serves as a cofactor mediating catalysis of the low Mr forms of PLA2 or triggering to translocate group IV cPLA2 to membranes (Yoshihara and Watanabe, 1990). The purified plant PLA2 showed no requirement for calcium ions. Thus, the plant PLA2 will be categorized into a form of iPLA2. The pH profile is also important for examining biochemical properties of the PLA2. Mammalian PLA2s reveal a relatively broad range of pH optimum and was fully activated at alkaline pH of 8.0 to 9.0 (Rordorf et al., 1991). However, the plant 48-kD PLA2 showed a pH profile optimally activated at a narrow range of neutral pH of 7.0 to 8.0 (Fig. 4A).
Calmodulin has been known as an activator of plant PLA2: It increased the PLA2 activity by 2-fold when added to the soluble fractions of potato leaves (Moreau, 1986) and potato tubers (Kawakita et al., 1993). We found that calmodulin increased the PLA2 activity of the crude enzyme extracts from the membrane fractions of leaves of broad bean (data not shown). Importantly, as shown in Figure 7, the purified plant PLA2 activity was markedly enhanced by calmodulin in the presence of Ca2+, but not in the presence of EGTA, a calcium chelator. In fact, recent report showed that calmodulin regulated a mammalian iPLA2 activity by unknown mechanism (Wolf and Gross, 1996). The fact that calmodulin-induced plant PLA2 activation is attributable to calcium ions suggests a role of intracellular calcium ions in a direct interaction between the proteins.
Recently, it was reported that a patatin-like PLA2 is transiently synthesized during seed germination and involved in the initiation of lipid body mobilization (May et al., 1998). The amino acid sequence of this 45-kD protein showed 47.9% identity with that of potato patatin. It is interesting that the N-terminal sequence of our 48-kD PLA2 also showed significant identities with these proteins, suggesting the possibility that patatin family proteins actually act as phospholipases involved in various plant cell responses.
In summary, the present study demonstrates that leaves of broad bean contain a membrane-associated 48-kD PLA2 activated by calmodulin and inhibited by pretreatment of ETYA. This is the first finding suggesting that this membrane-associated 48-kD PLA2 may be regulated by calmodulin to play a role in plant cell responses through production of linolenic acid.
MATERIALS AND METHODS
Materials
1-Stearoyl-2-[1-14C]arachidonyl-GPC (55.3 mCi/mmol), 1-palmitoyl-2-[1-14C]linoleoyl-GPC (55.0 mCi/mmol), 1-palmitoyl-2-[1-14C]palmitoyl-GPC (55.6 mCi/mmol), 1-stearoyl-2-[1-14C]arachidonoyl-sn-glycerol-3-PE (55.1 mCi/mmol), and 1-[1-14C]palmitoyl-Lyso PC (54.0 mCi/mmol) were purchased from the radio-chemical center, Amersham Life Science (Buckinghamshire, UK). [1-14C]Linoleic acid (52.8 mCi/mmol) and [1-14C]linolenic acid (55.0 mCi/mmol) were purchased from NEN Life Science Products (Boston). 1-Stearoyl-2-arachidonyl-sn-glycerol, AA, 1-stearoyl-2-arachidonoyl-GPC, porcine pancreatic group I PLA2 (sPLA2), ETYA, SDC, calmodulin from bovine brain, and DTT were purchased from Sigma (St. Louis). Lyso PC was purchased from AvantiPolar Lipids (Alabaster, AL). Mammalian 100-kD cPLA2 was purified from porcine spleen as described previously (Kim et al., 1993). Arachidonyl trifluoromethyl ketone (AACOCF3) was obtained from Biomol (Plymouth Meeting, PA). Protein G and Hitrap NHS-activated columns were purchased from Gibco-BRL/Life Technologies (Grand Island, NY) and Pharmacia LKB (Uppsala), respectively. All other chemicals were of the highest purity available from commercial sources.
Assay for PLA2 Activity
PLA2 activity was assayed using 2-[1-14C]AA-GPC as substrate unless specified otherwise. Each substrate was dried under a nitrogen stream and resuspended in the same volume of ethanol. The standard incubation system (100 μL) for assay of PLA2 activity contained 75 mm Tris [tris(hydroxymethyl)aminomethane]-HCl (pH 7.0), 3 mm CaCl2, 0.1% (w/v) SDC, and 4.5 nmol of radioactive phospholipids (approximately 55,000 cpm). Reactions were carried out at 37°C for 30 min and stopped by adding 320 μL of chloroform:methanol (1:1, by volume) and 30 μL of 2 N-HCl into the reaction mixture (Kim et al., 1997). The samples were then centrifuged, and an aliquot of the lower lipid phase was removed. Solvents were removed with a stream of nitrogen gas and the lipids were resuspended in a small aliquot of chloroform:methanol (1:1, by volume). Free fatty acid was separated by thin layer chromatography on Silica gel G plate (Merck, Darmstadt, Germany) using a solvent system, n-hexane:ethyl ether:acetic acid (160:40:3, v/v). After drying, the plates were subjected to iodine vapor and the spots were identified with comigrated authentic standards. Free fatty acid was quantified by scraping its corresponding spot into counting vial containing 2.5 mL of scintillation solution (Insta gel-XF, Packard Instrument, Meriden, CT), and counted for radioactivity in a Packard Tri-Carb liquid β-scintillation counter.
In purification steps, the concentration of SDC in the incubation system was adjusted to 0.025%, and [1-14C]AA from 2-[1-14C]AA-GPC were extracted by modified Dole's method (Dole and Meimertz, 1960). Reactions were carried out at 37°C for 30 min and were stopped by adding 560 μL of Dole's reagent (n-heptane:isopropyl alcohol:1 N-H2SO4; 400:390:10, v/v) and 110 μL of water, vortex-mixed, and centrifuged. Then, 150 μL of upper phase was transferred to a new microtube, to which n-heptane (800 μL) and silica gel (approximately 6 mg) were added. The sample was vortex-mixed and centrifuged again and an aliquot (750 μL) of the supernatant was removed into 2.5 mL of the scintillation solution and counted for radioactivity in a Packard Tri-carb liquid β-scintillation counter.
Synthesis of 2-[1-14C]LE-PC and 2-[1-14C]LEN-PC
First, to prepare an acyltransferase enzyme, liver tissue (4.0 g) was dissected from 4-week-old Wistar rat anesthetized with ethyl ether and homogenized with 40 mL of 0.25 m Suc. The homogenate was centrifuged at 20,000g at 4°C for 20 min and the resulting supernatant was ultracentifuged at 100,000g at 4°C for 1 h. The resulting pellet was resuspended in 4 mL of 0.25 m Suc and used as a source of acyltransferase enzyme. This microsomal fraction contained approximately 1.0 μmol PC 3.6 mg−1 protein. Second, Lyso PC (1.0 μmol) and 2-[1-14C]LE-PC (approximately 1.0 μmol) or 2-[1-14C]LEN-PC (approximately 1.0 μmol) were incubated at 37°C for 2 h in a reaction system (2.0 mL) containing 10 mm MgCl2, 10 mm ATP, 300 μm coenzyme A and rat liver microsomal fractions (1.02 mg of protein and 0.3 μmol of PC). The amounts of PC in the microsomal fractions was determined by purifying the PC with a HPLC column as below and determined from a calibration curve of standard PC with evaporating light scattering detector. To extract total lipids, the reaction was stopped by adding 1.0 mL of CHCl3:MeOH:1 n HCl (100:50:3, v/v) and the lower phase was removed and transferred to a new glass tube. The extracted lipids were re-extracted by adding 6.7 mL of CHCl3:MeOH (9:1, v/v). Third, to purify 2- [1-14C]LE-PC or 2-[1-14C]LEN-PC, the extracted lipids were applied to a normal phase HPLC column (μ-porasil, 7.8 × 300 mm, Waters, Milford, MA) pre-equilibrated with an elution solvent (CH3CN:MeOH:H2O [50:45:6.5, v/v]) and isocratically eluted by monitoring by measuring UV A205 at a flow rate of 1 mL/min. The [1-14C]PCs eluted at approximately 22 min after injection were assayed for the PLA2 activity with 11.2 μm substrate as described in “Materials and Methods.” The amounts of the substrates were calculated based on that the radiolabeled phospholipids include 0.3 μmol PC mg−1 protein from the microsomal fraction as a source of acyltransferase. Each of the purified [1-14C]PCs was dried under nitrogen and resuspended in ethanol for the assay of the PLA2 activity as described as above.
Purification of a Membrane-Associated PLA2 from Leaves of Broad Bean
Broad bean (Vicia faba L. cv Long Pod; W. Atlee Burpee, Warminster, PA) seeds were planted in vermiculite mixed with humus soil. The plants were grown in a growth chamber at 23°C with light/dark cycles of 16 h/8 h. The light intensity of 180 to 200 μmol m−2 s−1 was provided. Leaves (500 g) of broad bean were cut and washed several times with buffer K (50 mm Tris-HCl, pH 9.0, 3 mm EDTA, 0.12 m NaCl, and 2 mm DTT). The leaves were homogenized with 1 L of buffer K using a polytron homogenizer (model Polytron PT 6000, Kinematica AG, Littau, Switzerland). The debris and unlysed tissues were removed by centrifuging the homogenates at 2,000g at 4°C for 20 min. The supernatants (lysates) were then centrifuged at 100,000g at 4°C for 60 min. The 100,000g pellets were resuspended with 500 mL of buffer K containing 2 mm SDC. After gentle stirring at 4°C for 2 h, the SDC-solubilized membrane fractions were centrifuged at 100,000g at 4°C for 1 h. The resulting 100,000g supernatants were adjusted to 1.5 m (NH4)2SO4, stirred at 4°C for 1 h, and centrifuged at 10,000g at 4°C for 40 min. The resulting supernatants were used as enzyme sources for next purification steps.
These enzyme preparations were loaded onto a preparative Phenyl-5PW hydrophobic column (21.5 mm × 15 cm, Tosoh, Tokyo) pre-equilibrated with buffer B [50 mm Tris-HCl, pH 7.5, containing 1 mm EDTA, and 0.5 m (NH4)2SO4] at a flow rate of 5.0 mL/min with a fraction/minute. After washing with buffer B, the column-binding proteins were eluted with a 100-mL linear gradient of 0.5 to 0.0 m (NH4)2SO4. This resulting active pool (10 mL) was loaded onto a DEAE-5PW column (7.5 mm × 7.5 cm, Tosoh) pre-equilibrated with buffer A (50 mm Tris-HCl, pH 7.5, and 1 mm EDTA). The active fractions (4 mL) were obtained with a 20-mL linear gradient elution of 0.0 to 1.0 m of NaCl at a flow rate of 1.0 mL/min. The active pool was then directly injected onto a G3000-PW gel filtration column (21.5 mm × 60 cm, Tosoh) pre-equilibrated with a buffer containing 50 mm Tris-HCl, pH 7.5, 0.3 m NaCl, and 1 mm EDTA. The active fractions were eluted with the same buffer at a flow rate of 5 mL/min with a fraction/minute. Next, this enzyme preparation (20 mL) was loaded onto a Mono Q anionic FPLC column (5.0 mm × 5.0 cm, Pharmacia LKB) pre-equilibrated with buffer A (50 mm Tris-HCl, pH 7.5, containing 1 mm EDTA) at a flow rate of 1.0 mL/min. The active fractions (3 mL) were eluted with a 20-mL linear gradient of 0.0 to 1.0 m of NaCl and concentrated into approximately 250 μL using a Centricon 10 (Amicon, Beverly, MA). As a final step, the concentrate was injected onto a Superose 12 gel filtration FPLC column (10 mm × 30 cm; Pharmacia LKB) pre-equilibrated with a buffer containing 50 mm Tris-HCl, pH 7.5, 0.3 m NaCl, and 1 mm EDTA. The column was eluted with the same buffer at a flow rate of 0.5 mL/min. Fractions (0.5 mL) were collected.
Protein Assay
To monitor the amount of protein during purifying the PLA2, the A280 was measured by a UV detector. Protein concentration of each sample was measured with Bradford reagents (Bio-Rad, Hercules, CA) using bovine serum albumin (BSA) as a standard.
SDS-PAGE
Each aliquot (20 μL) of active fractions from the Superose 12 FPLC columns was mixed with an aliquot of Laemmli's sample buffer to make 0.125 m Tris-HCl, pH 6.8, 4% (w/v) SDS, 20% (w/v) glycerol, and 0.002% (w/v) bromphenol blue. After boiling for 5 min, the samples were cooled to room temperature and subjected to 12% (w/v) PAGE according to Laemmli's procedure (Laemmli, 1970). The separated proteins were stained with a PlusOne silver staining kit (Pharmacia LKB).
Preparation of a Monoclonal Antibody against the 48-kD PLA2 Protein
An aliquot (50 μg of protein in 0.5 mL) of active fractions obtained from the Superose 12 FPLC column was mixed with the same volume of Freund's complete adjuvant (Gibco-BRL/Life Technologies) and injected into a BALB/c mouse via intraperitoneal route. After boosting three times at a 3-week interval, the immunized mouse was sacrificed. The spleen cells were taken and fused with mouse myeloma cells V653 by PEG 50 (Sigma), and the produced hybridomas were screened by ELISA using the active fractions obtained from the Mono-Q FPLC column. In this way, two hybridoma clones were established. The culture media were used as a monoclonal antibody for the immunoprecipitation study.
Immnoprecipitation of the Plant 48-kD PLA2
Culture media (1.0 mL) of mouse hybridoma raised against the purified 48-kD protein were mixed with packed Protein A-Sepharose CL-4B beads (approximately 50-μL bed volume) pre-equilibrated with a buffer containing 20 mm Tris-HCl, pH 7.5, 1 mm EDTA, and 1.0% (w/v) BSA and incubated for 12 h at 4°C with constant shaking. The beads were washed six times with 1.0 mL of a buffer containing 20 mm Tris-HCl, pH 7.5, 1 mm EDTA, and 2.0% (w/v) BSA. An aliquot (100 μL) of Protein A-Sepharose CL-4B beads was incubated with a pool (25 μg of protein) of the active fractions obtained from the DEAE-5PW HPLC column for the indicated time at 4°C with constant shaking. The beads were then pelleted by centrifuging at 13,000g at 4°C for 30 s, and each aliquot of the resulting supernatants was assayed for the PLA2 activity. The pellets were washed six times with 1 mL of a buffer containing 20 mm Tris-HCl, pH 7.5, 0.5 m NaCl, 1 mm CaCl2, 1 mm MgCl2, and 0.1% (w/v) Tween 20. The washed beads, designed as immunoprecipitate, were mixed with Laemmli's sample buffer, boiled for 5 min, and centrifuged. The proteins in the resulting supernatants were separated on a SDS-PAGE gel and visualized by a silver staining kit.
Purification of the 48-kD Plant PLA2 by Using an Anti-48-kD PLA2 Protein Antibody Affinity Column
First, to prepare anti-48-kD protein antibody, ascitic fluid was developed by injecting of hybridoma C4 cells (5 × 106) into a BALB/c mouse via intraperitoneal route. After 14 d, the monoclonal antibody was purified from the ascitic fluid using a 1-mL prepacked protein G column (Gibco-BRL/Life Technologies). In brief, the collected ascite was centrifuged at 3,000g at 4°C for 15 min, the supernatant was diluted 2-fold with a loading buffer (10 mm sodium phosphate, pH 7.0, and 0.15 m NaCl), and loaded onto the Protein G column pre-equilibrated with the loading buffer. After washing with approximately 10 mL of the loading buffer, the antibody was eluted with 100 mm Gly HCl (pH 2.6). The eluate was immediately neutralized by adding 100 μL of 2 m Tris-HCl (pH 8.0).
The anti-48-kD protein antibody affinity column was then prepared using a Hitrap NHS-activated column (1-mL bed volume, Pharmacia LKB) as described by the manufacturer. Briefly, the affinity-purified antibody was dialyzed against 500 volumes of a coupling buffer (0.2 m NaHCO3 and 0.5 m NaCl, pH 8.3) and adjusted to the concentration of 1.2 mg protein/mL by concentrating the dialysate with a Centricon 10 concentrator (Amicon). The antibody sample was loaded onto the NHS-activated column (1-mL bed volume) followed by standing at 4°C for 4 h. The coupling was deactivated by washing three times with 2 mL of buffer C (0.5 m ethanolamine, pH 8.3, and 0.5 m NaCl) and three times with 2 mL of buffer D (0.1 m sodium acetate, pH 4.0, and 0.5 m NaCl) according to the manufacturer's instruction. To purify the 48-kD PLA2 protein with the antibody affinity column, the PLA2 enzyme preparation was obtained from the DEAE-5PW HPLC column as described above. The active pool from the DEAE-5PW column was loaded onto the anti-48-kD protein antibody affinity column pre-equilibrated with buffer E (50 mm Tris-HCl, pH 7.0, 1 mm EDTA, 100 μm phenylmehtylsulfonyl fluoride, 20 μm pepstatin, and 20 μm leupeptin) at a flow rate of 1.0 mL/min. Proteins bound to the columns were eluted with a 20-mL linear gradient with buffer F (100 mm Gly-HCl, pH 2.6).
Characterization of the Purified Plant 48-kD PLA2
To characterize the purified plant PLA2, the active fractions from the Superose 12 gel filtration column were pooled and desalted using a PD-10 desalting column (Sephadex G-25 m, Pharmacia LKB) pre-equilibrated with a buffer (10 mm Tris-HCl, pH 7.5). In some experiments, the enzyme sources and appropriate amounts of inhibitors or calmodulin (Sigma) were mixed and pre-incubated for 10 min at 37°C, followed by the addition of SDC and radiolabeled substrate for the PLA2 assay.
ACKNOWLEDGMENTS
We would like to thank Dr. Y. Lee for her invaluable advice for the initiation of this work, and Drs. P.G. Suh and S.H. Ryu for generating monoclonal antibodies against the 48-kD PLA2 protein.
Footnotes
This work was supported by grants from Genetic Engineering Research Fund from the Ministry of Education of Korea and the Korean Science and Engineering Foundation (grant no. 961–0719–118–2 to D.K.K.).
LITERATURE CITED
- Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of macrophage Ca2+-dependent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J Biol Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- Ackermann EJ, Kempner ES, Dennis EA. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells: isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- Andre B, Scherer GFE. Stimulation by auxin of phospholipase A in membrane vesicles from an auxin-sensitive tissue is mediated by an auxin receptor. Planta. 1991;185:209–214. doi: 10.1007/BF00194062. [DOI] [PubMed] [Google Scholar]
- Bergey DR, Howe GA, Ryan CA. Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA. 1996;93:12053–12058. doi: 10.1073/pnas.93.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert S, Brodschelm W, Holder S, Kammerer L, Kutchan TM, Mueller MJ, Xia ZQ, Zenk MH. The octadecanoic pathway: signal molecules for the regulation of secondary pathways. Proc Natl Acad Sci USA. 1995;92:4099–4105. doi: 10.1073/pnas.92.10.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre JV. Phospholipase A2 and signal transduction. J Am Soc Nephrol. 1992;3:128–150. doi: 10.1681/ASN.V32128. [DOI] [PubMed] [Google Scholar]
- Conconi A, Miquel M, Browse JA, Ryan CA. Intracellular levels of free linolenic and linoleic acids increase in tomato leaves in response to wounding. Plant Physiol. 1996;111:797–803. doi: 10.1104/pp.111.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- Dennis EA, Rhee SG, Billah MM, Hannun YA. Role of phospholipases in generating lipid second messengers in signal transduction. FASEB J. 1991;5:2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Dole VP, Meimertz H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960;235:2595–2599. [PubMed] [Google Scholar]
- Durham AC. A survey of readily available chelators for buffering calcium ion concentrations in physiological solutions. Cell Calcium. 1983;4:33–46. doi: 10.1016/0143-4160(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Kawakita K, Senda K, Doke N. Factors affecting in vitro activation of potato phospholipase A2. Plant Sci. 1993;92:183–190. [Google Scholar]
- Kim DK, Bonventre JV. Purification of a 100-kD phospholipase A2 from spleen, lung, and kidney: antiserum raised to pig spleen phospholipase A2 recognizes a similar form in bovine lung, kidney and platelets, and immunoprecipitates phospholipase A2 activity. Biochem J. 1993;294:261–270. doi: 10.1042/bj2940261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK, Lee HJ, Lee Y. Detection of two phospholipase A2 (PLA2) activities in leaves of higher plant Vicia faba and comparison with mammalian PLA2's. FEBS Lett. 1994;343:213–218. doi: 10.1016/0014-5793(94)80558-x. [DOI] [PubMed] [Google Scholar]
- Kim SS, Kang MS, Choi YM, Suh YH, Kim DK. Sphingomyelinase activity is enhanced in cerebral cortex of senescence-accelerated mouse-P/10 with advancing age. Biochem Biophys Res Commun. 1997;28:583–587. doi: 10.1006/bbrc.1997.7133. [DOI] [PubMed] [Google Scholar]
- Klucis E, Polya GM. Calcium-independent activation of two plant leaf calcium-regulated protein kinases by unsaturated fatty acids. Biochem Biophys Res Commun. 1987;147:1041–1047. doi: 10.1016/s0006-291x(87)80175-4. [DOI] [PubMed] [Google Scholar]
- Kostyal DA, Hickey VL, Noti JD, Sussman GL, Beezhold DH. Cloning and characterization of a latex allergen (Hev b 7): homology to patatin, a plant PLA2. Clin Exp Immunol. 1998;112:355–362. doi: 10.1046/j.1365-2249.1998.00596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo I, Murakami M, Hara S, Inoue K. Mammalian non-pancreatic phospholipases A2. Biochim Biophys Acta. 1993;1170:217–231. doi: 10.1016/0005-2760(93)90003-r. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- May C, Preisig-Muller R, Hohne M, Gnau P, Kindl H. A phospholipase A2 is transiently synthesized during seed germination and localized to lipid bodies. Biochim Biophys Acta. 1998;1393:267–76. doi: 10.1016/s0005-2760(98)00081-2. [DOI] [PubMed] [Google Scholar]
- Minichiello J, Polya GM, Keane PJ. Inhibition and activation of oat leaf calcium-dependent protein kinase by fatty acids. Plant Sci. 1989;65:143–152. [Google Scholar]
- Moreau RA. Regulation of phospholipase activity in potato leaves by calmodulin and protein phosphorylation-dephosphorylation. Plant Sci. 1986;47:1–9. [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signaling in plants. Biochim Biophys Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Nasyrova GF, Beliaeva NV, Simchuk EE, Palladina TA. Effect of phospholipase A2 on H+-ATPase in the plasma membrane of H+-ATPase in maize root cells. Ukr Biokhim Zh. 1996;68:38–44. [PubMed] [Google Scholar]
- Polya GM, Nott R, Klucis E, Minichiello J, Chandra S. Inhibition of plant calcium-dependent protein kinases by basic polypeptides. Biochim Biophys Acta. 1990;1037:259–262. doi: 10.1016/0167-4838(90)90177-h. [DOI] [PubMed] [Google Scholar]
- Rordorf G, Uemura Y, Bonventre JV. Characterization of phospholipase A2 (PLA2) activity in gerbil brain: enhanced activities of cytosolic, mitochondrial, and microsomal forms after ischemia and reperfusion. J Neurosci. 1991;11:1829–1836. doi: 10.1523/JNEUROSCI.11-06-01829.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Pouenat M, Caumont C, Cariven C, Prevost M, Esquerre-Tugaye M. Phospholipase activity and phospholipid patterns in tobacco cells treated with fungal elicitor. Plant Sci. 1995;107:17–25. [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotriens and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Scherer GFE, Andre B. A rapid response to a plant hormone: auxin stimulates phospholipase A2 in vivo and in vitro. Biochem Biophys Res Commun. 1989;163:111–117. doi: 10.1016/0006-291x(89)92106-2. [DOI] [PubMed] [Google Scholar]
- Scherer GFE, Arnold B. Inhibitors of animal phospholipase A2 enzymes are selective inhibitors of auxin-dependent growth: implications for auxin-induced signal transduction. Planta. 1997;202:462–469. [Google Scholar]
- Senda K, Yoshioka H, Doke N, Kawakita K. A cytosolic phospholipase A2 from potato tissues appears to be patatin. Plant Cell Physiol. 1996;37:347–353. doi: 10.1093/oxfordjournals.pcp.a028952. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. FASEB J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- Shirai K, Barnhart RL, Jackson RL. Hydrolysis of human plasma high density lipoprotein 2-phospholipids and triglycerides by hepatic lipase. Biochem Biophys Res Commun. 1981;100:591–599. doi: 10.1016/s0006-291x(81)80217-3. [DOI] [PubMed] [Google Scholar]
- Stahl U, Ek B, Stymne S. Purification and characterization of a low-molecular-weight phospholipase A2 from developing seeds of elm. Plant Physiol. 1998;117:197–205. doi: 10.1104/pp.117.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street IP, Lin HK, Laliberte F, Ghomashchi F, Wang Z, Perrier H, Tremblay NM, Huang Z, Weech PK, Gelb MH. Slow- and tight-binding inhibitors of the 85-kDa human phospholipase A2. Biochemistry. 1993;32:5935–5940. doi: 10.1021/bi00074a003. [DOI] [PubMed] [Google Scholar]
- Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. J Biol Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- Underwood KW, Song C, Kriz RW, Chang XJ, Knopf JL, Lin LL. A novel calcium-independent phospholipase A2, cPLA2-γ, that is prenylated and contains homology to cPLA2. J Biol Chem. 1998;273:21926–21932. doi: 10.1074/jbc.273.34.21926. [DOI] [PubMed] [Google Scholar]
- van der Hoeven PC, Siderius M, Korthout HA, Drabkin AV, de Boer AH. A calcium and free fatty acid-modulated protein kinase as putative effector of the fusicoccin 14-3-3 receptor. Plant Physiol. 1996;111:857–865. doi: 10.1104/pp.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler JD, McCarte-Roshak A, Huang L, Sung CM, Bolognese B, Marshall LA. Biochemical and pharmacological comparison of a cytosolic, high molecular weight phospholipase A2, human synovial fluid phospholipase A2 and CoA-independent transacylase. J Lipid Mediat Cell Signal. 1994;10:315–330. [PubMed] [Google Scholar]
- Wolf MJ, Gross RW. The calcium-dependent association and functional coupling of calmodulin with myocardial phospholipase A2: implications for cardiac cycle-dependent alterations in phospholipolysis. J Biol Chem. 1996;271:20989–20922. doi: 10.1074/jbc.271.35.20989. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Watanabe Y. Translocation of phospholipase A2 from cytosol to membranes in rat brain induced by calcium ions. Biochem Biophys Res Commun. 1990;170:484–490. doi: 10.1016/0006-291x(90)92117-i. [DOI] [PubMed] [Google Scholar]