Abstract
Purpose
The purpose of this study was to evaluate the effect of transportation at prolonged low temperatures on the survival of pre‐antral follicles.
Methods
Ovarian tissue was removed from six women with gender identity disorder. Tissues were stored in an icebox at 4 °C for 6 or 18 h prior to vitrification. After warming, ovarian tissues were cultured for 24 h and follicle survival was assessed via a viability/cytotoxicity kit. Morphological features and oxygen consumption rate (OCR) were evaluated by scanning electrochemical microscopy (SECM).
Results
Survival rate of isolated primordial follicles was 95.7 and 100 %, and that of primary follicles was 91.7 and 81.8 % in the 6‐ and 18‐h groups respectively. There was no difference in morphology between the 6‐ and 18‐h storage groups. In comparison with OCR of vitrified‐warmed follicles and OCR of 24‐h culture after vitrified‐warmed follicles, OCR of 24‐h culture after vitrified‐warmed primordial follicles was significantly higher in both 6‐hour (0.02 ± 0.02 vs 0.07 ± 0.04, P < 0.05) and 18‐h groups (0.02 ± 0.02 vs 0.11 ± 0.10, P < 0.05).
Conclusions
This strongly suggests that prolonged transportation of ovarian tissue at low temperatures is useful when there are no available local systems for fertility preservation.
Keywords: Follicle, Human ovary, Oxygen consumption rate, Storage, Transport
Introduction
Improvements in oncologic treatment have resulted in an improved survival rate for many young cancer patients. Indeed, the 5‐year survival rate for all cancers combined is currently more than 64 % in women [1]. However, use of chemotherapy and/or radiation therapy in these patients can adversely affect ovarian function, leading to infertility and decreased quality of life. A number of strategies have been developed in recent years to enable these patients to have children using their own gametes. For example, when radiotherapy alone is administered, it is possible to keep the ovaries outside of the radiation field. Further, when chemotherapy can be postponed, it is possible to use ovarian stimulation to obtain oocytes, which can be frozen in either a fertilized or an unfertilized state [2, 3].
Cryopreservation of ovarian tissue is another very promising alternative to preserve fertility for prepubertal girls and women who undergo chemotherapy [4]. The tissue can be cryopreserved at centers specializing in reproductive medicine and can be transplanted into the pelvic cavity (or to a heterotopic site for oocyte retrieval and in vitro fertilization) if the patient experiences premature ovarian failure. Donnez et al. [2] reported the first live birth after autotransplantation of cryopreserved ovarian tissue in humans. Orthotopic reimplantation has so far led to the birth of 20 healthy babies [5, 6, 7, 8]. Compared to the slow freezing method, vitrification is a simple and rapid procedure that can cryopreserve various types of living cells in a high concentration of cryoprotectant without ice crystal formation. Vitrification may become a more widely‐used alternative to slow cooling. Current human ovary tissue vitrification is focused on novel analytical approaches and techniques to estimate ovarian tissue function and pre‐antral follicle viability. It has been reported that measuring the oxygen consumption rate (OCR) using a scanning electrochemical microscope (SECM) may be effective as a non‐invasive evaluation of oocyte/early embryo quality of various mammalian species, including human oocyte/early embryo [9, 10, 11, 12].
Many assisted reproductive technology (ART) clinics have no experience with cryopreservation of ovarian tissue, and if a patient wishes to preserve ovarian tissue prior to undergoing antineoplastic therapy, tissues are transferred to a special facility for implantation. Thus, the purpose of this study was to evaluate the effect of transportation at prolonged low temperatures on the survival of vitrified and warmed pre‐antral follicles using SECM and dual fluorescent vital staining.
Materials and methods
Patients
Ovaries were removed from 6 consenting women with gender identity disorder. The mean age of the women was 30.8 ± 9.8 years, ranging in age from 22 to 39 years. Written informed consent was obtained from all patients, and the research protocol was approved by the institutional review board (IRB) of Kyono ART clinic and the IRB of Osaka New ART clinic.
Tissue transportation
For the purpose of estimating the impact of transportation, ovaries were placed in 50‐ml conical tubes (Becton–Dickinson, Bedford, MA, USA) containing 40 ml of Leibowitz's medium (L‐15; SIGMA, Saint Louis, MO, USA) supplemented with sodium pyruvate (2 mM), glutamine (2 mM), 10 % (v/v) synthetic serum substitute (SSS; Irvine Scientific, Santa Ana, CA, USA), penicillin G (75 μg/ml), streptomycin (50 μg/ml) and ascorbic acid (50 μg/ml). These preparations were placed on ice in a vacuum‐based cooling box for 6 h (6‐h transportation). Some of the samples were removed from the icebox and placed in a refrigerator for another 12 h (18‐h transportation).
Vitrification and warming
Ovarian cortical tissues were cut into 1 × 10 × 10 mm pieces and vitrified in accordance with the Cryotissue method described by Kagawa et al. [13]. Briefly, tissues were equilibrated at room temperature in handling medium [7.5 % ethylene glycol (EG) and 7.5 % dimethyl sulphoxide (DMSO) in HEPES‐buffered TCM‐199 solution supplemented with 20 % (v/v) SSS] for 25 min and then transferred to vitrification solution (20 % EG and 20 % DMSO in HEPES‐buffered TCM‐199 solution with 0.5 mol/l sucrose) for 15 min. Tissues were placed in a minimum volume of the vitrification solution on the Cryotissue metal grid and were immersed in liquid nitrogen [13]. The warming procedure was performed in four steps. The tissues were warmed in warming solution supplemented with 1.0 mol/l sucrose at 37 °C for 1 min and then transferred to a diluent solution supplemented with 0.5 mol/l sucrose for 3 min. Next, the tissues were twice placed in washing solution for 5 min [13].
Vitrified and warmed cortical tissue culture
The vitrified and warmed ovarian cortical fragments were cut with a scalpel into smaller pieces (1–0.5 mm3) in 2 ml of pre‐warmed Leibovitz's medium. These pieces were then individually placed in 24‐well cell culture plates (Corning B.V. Life Sciences Europe, Amsterdam, Netherlands) containing 300 μl of McCoy's 5a medium (SIGMA, Saint Louis, MO, USA) with bicarbonate supplemented with HEPES (20 mM), SSS (0.1 %), glutamine (3 mM), penicillin G (0.1 mg/ml), streptomycin (0.1 mg/ml), transferrin (2.5 μg/ml), selenium (4 ng/ml), insulin (10 ng/ml), and ascorbic acid (50 μg/ml). After they were incubated for 24 h at 37 °C in 6 % CO2, 5 % O2, and 89 % N2, pre‐antral follicles were isolated from the small pieces and OCR measurement was carried out.
Histological analysis
Cortical pieces before vitrification (non‐vitrified) and vitrified‐warmed pieces after either 6 or 18 h of low temperature storage were subjected to histological analysis. The pieces were fixed in 10 % formalin for 6 h to allow histological analysis of the follicles. The pieces were then dehydrated and embedded in paraffin wax. Subsequently, they were cut into 5‐μm‐thick sections and stained with hematoxylin‐eosin.
Isolation of pre‐antral follicles from vitrified and warmed cortical tissue
Vitrified and warmed cortical pieces were further cut into smaller cortical pieces using a scalpel. They were then rinsed in Dulbecco's PBS (Invitrogen, Carlsbad, CA, USA) twice and supplemented with 1 mg/ml collagenase type IV (Sigma‐Aldrich) and digested at 37 °C for 60–80 min. The digested cortical pieces were transferred to 60 mm culture dishes containing 2 ml of Leibowitz's medium to terminate enzymatic reaction. Subsequently, pre‐antral follicles were mechanically isolated from cortical pieces using 30 gauge needles (Dentronics, Tokyo, Japan) for the measurement of OCR.
Half of the small cortical pieces were subjected to follicular isolation immediately after warming. The other half were subjected to follicular isolation after 24 h of incubation in McCoy's‐5a medium.
Scanning electrochemical microscopy
The OCR of follicles was measured on each sample by SECM using CRAS‐1.0 (Clino Ltd., Miyagi, Japan). For the measurement of OCR, modified human tubal fluid medium (m‐HTF; Irvine Scientific, Santa Ana, CA, USA) supplemented with 10 % SSS was placed into a cone‐shaped microwell plate. The Pt‐microdisk electrode was scanned according to the z‐direction from the side point of pre‐antral follicle that was located at the bottom of a microwell. The motor‐driven XYZ stage, which was controlled by a computer, was located on the microscope stage for electrode tip scanning. The oxygen consumption rate of pre‐antral follicles was calculated according to the spherical diffusion theory using custom software [14]. Measurement of OCR in each follicle was conducted over 30 s for each measurement. Thus, approximately 2 min were required for three serial measurements, from which the average respiration activity of each follicle was calculated.
Viability analysis
The cellular viability of vitrified‐warmed follicles was also evaluated in fluorescent vital stains for both groups (6‐ and 18‐h groups) using a LIVE/DEAD Viability/Cytotoxicity kit (Molecular Probes, Leiden, Netherlands), according to the method described by Van den Hurk et al. [15]. This vital staining kit provides a two‐color fluorescence cell viability assay that is based on simultaneous determination of live and dead cells. Briefly, in live cells, the virtually non‐fluorescent calcein produces an intense green fluorescence by intracellular esterase activity (excitation: 495 nm; emission: 517 nm). In dead cells, the damaged plasma membrane permits ethidium homodimer I to enter; upon binding to nucleic acid, it undergoes a 40‐fold enhancement of fluorescence, producing a bright red fluorescence (excitation: 495 nm; emission: 635 nm).
Statistical analysis
Statistical analyses were performed using the Statcel 3 programs for Excel. For comparisons of the OCRs of pre‐antral follicles in the 6‐ and 18‐h groups, analyses were performed using the repeated measures analysis of variance method. The average OCRs of primordial and primary follicles were compared with Welch's t test. P < 0.05 was considered statistically significant.
Results
We used non‐vitrified cortical tissues in order to evaluate follicular morphology in ovarian cortical tissue after prolonged low temperature. Non‐vitrified ovarian tissues after 6 or 18 h of low temperature storage had a similar morphology of their ovarian follicles. In a comparison of non‐vitrified and vitrified‐warmed cortical tissue, no alterations in the morphology of pre‐antral follicles were observed (Fig. 1). Pre‐antral follicles were isolated from vitrified cortical fragments of two patients in the 6‐h transportation group and four patients in the 18‐h transportation group. The fluorescent vital staining using the LIVE/DEAD viability cytotoxicity kit revealed the presence of ubiquitous intracellular esterase activity was in all 83 vitrified isolated follicles (36 primordial follicles and 47 primary follicles). The survival rate of isolated primordial follicles was more than 95 %, and that of primary follicles was more than 80 % (Table 1; Fig. 2). Furthermore, there were no significant differences in the average OCR (mean ± SD) of primordial and primary follicles between the two groups (6 and 18 h). In comparison with OCR of vitrified‐warmed follicles and OCR of 24‐h culture after vitrified‐warmed follicles, OCR of 24‐h culture after vitrified‐warmed primordial follicles was significantly higher, and that of 24‐h culture after vitrified‐warmed primary follicles was higher in each group (Table 2).
Figure 1.
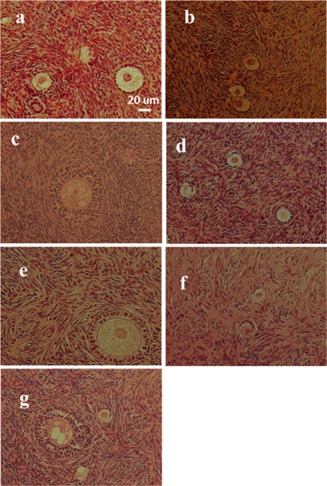
The histological analysis of fresh, non‐vitrified and vitrified‐warmed pre‐antral follicles. a Primordial and primary follicles in fresh ovary cortical tissue. b Primordial and primary follicles in non‐vitrified ovary cortical tissue after 6 h transportation. c Secondary follicle in non‐vitrified ovary cortical tissue after 6 h transportation. d Primordial and primary follicles in non‐vitrified ovary cortical tissue after 18 h transportation. e Secondary follicle in non‐vitrified ovary cortical tissue after 18 h transportation. f Primary follicles in vitrified‐warmed ovary cortical tissue after 18 h transportation. g Primordial to secondary follicles in vitrified‐warmed ovary cortical tissue after 18 h transportation. (200× : hematoxylin‐eosin staining). Scale bar = 20 μm
Table 1.
Survival rate of isolated vitrified and warmed pre‐antral follicles
| Transport time | Follicle stage | No. of surviving follicles (%) |
|---|---|---|
| 6 h | Primordial | 22/23 (95.7) |
| Primary | 33/36 (91.7) | |
| 18 h | Primordial | 13/13 (100.0) |
| Primary | 9/11 (81.8) |
Figure 2.
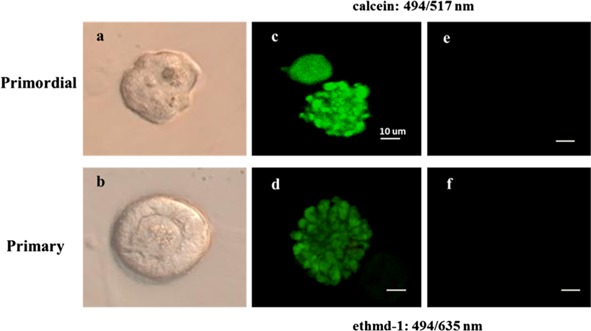
The dual fluorescent vital stain of vitrified and warmed pre‐antral follicles. a Primordial follicles were isolated from vitrified‐warmed cortical tissue digested with collagenase IV and trimmed using a 30‐G needle. b Primary follicles were isolated from vitrified‐warmed cortical tissue digested with collagenase IV and trimmed using a 30‐G needle. c The intense green fluorescence was detected in primordial follicles. d The intense green fluorescence was detected in primary follicles. e No red light fluorescence was detected in primordial follicles of follicular cells. f No red light fluorescence was detected in primary follicles of follicular cells. Scale bar = 10 μm. a, c and e are images of the same follicle. b, d and f are images of the same follicle
Table 2.
Oxygen consumption rate (OCR) in vitrified and warmed pre‐antral follicles
| Transport time | Follicle stage | Tissue condition | |
|---|---|---|---|
| Vitrified‐warmed (n) a | Vitrified‐warmed 24‐h culture (n) a | ||
| 6 h | Primordial | 0.02 ± 0.02 (10) b | 0.07 ± 0.04 (23) b |
| Primary | 0.03 ± 0.03 (10) | 0.12 ± 0.09 (22) | |
| 18 h | Primordial | 0.02 ± 0.02 (14) c | 0.11 ± 0.10 (13) c |
| Primary | 0.04 ± 0.05 (19) | 0.28 ± 0.22 (19) | |
a (n) number of sample
b P < 0.05
c P < 0.05
Discussion
The purpose of the present study was to determine the impact of prolonged low temperature storage prior to cryopreservation on the viability of vitrified and warmed human pre‐antral follicles using an electrochemical and enzymatic approach in vitro. Despite the long period of low temperature storage, the survival rate of primordial and primary follicles after warming was more than 80 %, and they were morphologically normal and exhibited high potential respiratory activity after incubation.
There are many ways to evaluate cell viability. One of them, intracytoplasmic mitochondrial respiration is a suitable marker of cell activity. More than 80 % of the total adenosine triphosphate (ATP) produced in mammalian blastocysts is produced via mitochondrial oxidative phosphorylation, which requires oxygen [16, 17].
Abe et al. [18] reported that measuring the OCR using SECM was an effective protocol in relation to OCR and mitochondrial activity. This system has given useful reports as a non‐invasive evaluation of mammalian oocyte/early embryos including human [18]. It has been reported that a high OCR in bovine embryos signifies a high developmental competence and a high conception rate after embryo transfer [19].
The correlation between OCR and the number of live cells in vitrified‐warmed porcine embryos has been reported [20]. The results of this study revealed that isolated follicles from vitrified cortical tissues exhibited attenuated respiratory activity immediately after warming; however, they resumed normal activity after 24 h of incubation.
Yamanaka et al. [21] showed that in regard to human blastocysts, the OCR of a vitrified blastocyst after warming was significantly lower than that of a non‐vitrified blastocyst. Furthermore, after 6 h of incubation it had recovered to the level exhibited by non‐vitrified blastocysts. Though mitochondrial cytochrome c oxidase activity was not observed immediately after warming, it was detected 24 h after warming
We attempted to investigate the OCR of human pre‐antral follicles isolated from vitrified and warmed cortical tissue after 6 or 18 h of transportation. In comparison with OCR of vitrified‐warmed follicles and OCR of 24‐h culture after vitrified‐warmed follicles, OCR of 24‐h culture after vitrified‐warmed primordial follicles was significantly higher, and that of 24‐h culture after vitrified‐warmed primary follicles was higher in each group. The reason why primordial OCR was significantly higher in spite of the high estimation in the primary follicles group may be the large difference in standard deviation.
Ovarian tissue cryopreservation is the most promising alternative to preserve fertility for prepubertal girls and women who undergo chemotherapy. Auto‐transplantation of slow‐freezing cortical tissue has facilitated the birth of healthy babies since 2004 [2, 22, 23, 24].
On the other hand, vitrification is a simple and rapid procedure that preserves tissues without ice crystal formation, and this strategy has yielded better results in animal models than slow freezing [25, 26].
However, cryopreservation and the transplantation of ovarian tissue require advanced techniques, and most ART clinics have no experience with these strategies. Thus, it is often necessary to transfer ovarian tissue to a specialty center.
The viability of fresh ovarian cortical tissue after 4–5 h of transportation prior to cryopreservation has previously been validated in the clinical setting, and five children have been born from transplanted warmed tissue that had been transported for 4–5 h in Denmark [27, 28]. Schmidt et al. [27] reported that they transported the ovarian tissues on ice, so we transported them by the same protocol to preserve the viability of ovarian cortical tissues.
Dittrich et al. [29] reported that one child has been born after 20 h transportation of ovarian tissue before cryopreservation with subsequent transplantation. In addition, Rosendahl et al. [30] reported on the ovarian cortex from a 6‐year‐old girl that was kept at 4 °C for 20 h prior to cryopreservation and was subsequently transplanted into a mouse for 4 weeks; at histological examination, the transplant showed morphologically healthy primordial follicles.
Isachenko et al. [31] estimated the impact of transportation time on the viability of pre‐antral follicles in transplants using an in vitro culture system. In their protocol, ovarian cortical fragments from 5 patients were transferred to a special modified medium for transport of ovarian tissue. The ovarian cortical fragments were cultured in culture medium for 15 days at 37 °C in 5 % CO2. They reported that exposure of ovarian tissue to suprazero temperatures for 0–26 h did not inhibit the development of follicles in subsequent in vitro culture.
The present study directly investigated the effect of prolonged low temperature storage on the viability of vitrified and warmed human pre‐antral follicles. We observed good survival of primordial and primary follicles after prolonged low temperature storage (up to 18 h) as demonstrated by assessment of OCR. This strongly suggests that prolonged transportation of ovarian tissue at low temperatures is feasible and useful in situations in which there are no available local systems for fertility preservation.
References
- 1. Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer, 2004, 101, 3–27 10.1002/cncr.20288 [DOI] [PubMed] [Google Scholar]
- 2. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet, 2004, 364, 1405–1410 10.1016/S0140‐6736(04)17222‐X [DOI] [PubMed] [Google Scholar]
- 3. Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med, 2009, 360, 902–911292721710.1056/NEJMra0801454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kolp LA, Hubayter Z. Autotransplantation of cryopreserved ovarian tissue: a procedure with promise, risks, and a need for a registry. Fertil Steril., 2011, 95, 1879–1886 10.1016/j.fertnstert.2011.02.049 [DOI] [PubMed] [Google Scholar]
- 5. Donnez J, Dolmans MM. Preservation of fertility in females with haematological malignancy. Br J Haematol, 2011, 154, 75–184 10.1111/j.1365‐2141.2011.08723.x [DOI] [PubMed] [Google Scholar]
- 6. Donnez J, Jadoul P, Pirard C, Hutchings G, Demylle D, Squifflet J, Smitz J, Dolmans MM. Live birth after transplantation of frozen‐thawed ovarian tissue after bilateral oophorectomy for benign disease. Fertil Steril, 2012, 98, 720–725 10.1016/j.fertnstert.2012.05.017 [DOI] [PubMed] [Google Scholar]
- 7. Revel A, Laufer N, Ben Meir A, Lebovich M, Mitran E. Micro‐organ ovarian transplantation enables pregnancy: a case report. Hum Reprod, 2011, 26, 1097–1103 10.1093/humrep/der063 [DOI] [PubMed] [Google Scholar]
- 8. Silbers S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril, 2010, 94, 2191–2196 10.1016/j.fertnstert.2009.12.073 [DOI] [PubMed] [Google Scholar]
- 9. Ferguson EM, Leese HJ. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol Reprod Dev, 2006, 73, 1195–1201 10.1002/mrd.20494 [DOI] [PubMed] [Google Scholar]
- 10. Magnusson C, Hillensjö T, Hamberger L, Nilsson L. Oxygen consumption by human oocytes and blastocysts grown in vitro. Hum Reprod, 1986, 1, 183–184 [DOI] [PubMed] [Google Scholar]
- 11. Preis KA, Seidel GE Jr, Gardner DK. Reduced oxygen concentration improves the developmental competence of mouse oocytes following in vitro maturation. Mol Reprod Dev, 2007, 74, 893–903 10.1002/mrd.20655 [DOI] [PubMed] [Google Scholar]
- 12. Tejera A, Herrero J, Los Santos MJ, Garrido N, Ramsing N, Meseguer M. Oxygen consumption is a quality marker for human oocyte competence conditioned by ovarian stimulation regimens. Fertil Steril., 2011, 96, 618–623 10.1016/j.fertnstert.2011.06.059 [DOI] [PubMed] [Google Scholar]
- 13. Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. RBM Online., 2009, 18, 568–577 [DOI] [PubMed] [Google Scholar]
- 14. Shiku H, Shiraishi T, Aoyagi S, Utsumi Y, Matsudaira M, Abe H et al. Respiration activity of single bovine embryos entrapped in a cone‐shaped microwell monitored by scanning electrochemical microscopy. Anal Chim Acta., 2004, 522, 51–58 10.1016/j.aca.2004.06.054 [Google Scholar]
- 15. Hurk R, Spek ER, Hage WJ, Fair T, Ralph JH, Schotanus K. Ultrastructure and viability of isolated bovine preantral follicles. Hum Reprod Update., 1998, 4, 833–841 10.1093/humupd/4.6.833 [DOI] [PubMed] [Google Scholar]
- 16. Benos DJ, Baladan RS. Energy metabolism of preimplantation mammalian blastocysts. Am J Physiol, 1983, 245, 40–45 [DOI] [PubMed] [Google Scholar]
- 17. Leese HJ. What does an embryo need?. Hum Fertil., 2003, 6, 180–185 10.1080/1464770312331369463 [DOI] [PubMed] [Google Scholar]
- 18. Abe H, Shiku H, Yokoo M et al. Evaluating the quality of individual embryos with a non‐invasive and highly sensitive measurement of oxygen consumption by scanning electrochemical microscopy. J Reprod Dev., 2006, 52 (Suppl) 55–64 [Google Scholar]
- 19. Lopes AS, Madsen SE, Ramsing NB, Løvendahl P, Greve T, Callesen H. Investigation of respiration of individual bovine embryos produced in vivo and in vitro and correlation with viability following transfer. Hum Reprod, 2007, 22, 558–566 10.1093/humrep/del404 [DOI] [PubMed] [Google Scholar]
- 20. Sakagami N, Yamamoto T, Akiyama K, Nakazawa Y, Kojima N, Nishida K, Yokomizo S, Takagi Y, Abe H, Suzuki C et al. Viability of porcine embryos after vitrification using water‐soluble pullulan films. J Reprod Dev., 2010, 56, 279–284 10.1262/jrd.09‐101H [DOI] [PubMed] [Google Scholar]
- 21. Yamanaka M, Hashimoto S, Amo A, Ito‐Sasaki T, Abe H, Morimoto Y. Developmental assessment of human vitrified‐warmed blastocysts based on oxygen consumption. Hum Reprod, 2011, 26, 3366–3371 10.1093/humrep/der324 [DOI] [PubMed] [Google Scholar]
- 22. Meirow D, Levron J, Eldar‐Geva T, Hardan I, Fridman E, Zalel Y, Schiff E, Dor J. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med, 2005, 353, 318–321 10.1056/NEJMc055237 [DOI] [PubMed] [Google Scholar]
- 23. Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, Schmidt KL, Andersen AN, Ernst E. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod, 2008, 23, 2266–2272 10.1093/humrep/den244 [DOI] [PubMed] [Google Scholar]
- 24. Silber SJ, DeRosa M, Pineda J et al. A series of monozygotic twins discordant for ovarian failure: ovary transplantation (cortical versus microvascular) and cryopreservation. Hum Reprod, 2008, 23, 1531–1537 10.1093/humrep/den032 [DOI] [PubMed] [Google Scholar]
- 25. Kuleshova LL, Lopata A. Vitrification can be more favourable than slow cooling. Fertil Steril., 2002, 78, 449–454 10.1016/S0015‐0282(02)03305‐8 [DOI] [PubMed] [Google Scholar]
- 26. Courbiere B, Odagescu V, Baudot A, Massardier J, Mazoyer C, Salle B, Lornage J. Cryopreservation of the ovary by vitrification as an alternative to slow‐cooling protocols. Fertil Steril., 2006, 86 ((Suppl 3)) 1243–1251 10.1016/j.fertnstert.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 27. Schmidt KL, Ernst E, Byskov AG, Andersen AN, Andersen CY. Survival of primordial follicles following prolonged transportation of ovarian tissue prior to cryopreservation. Hum Reprod, 2003, 18, 2654–2659 10.1093/humrep/deg500 [DOI] [PubMed] [Google Scholar]
- 28. Schmidt KT, Rosendahl M, Ernst E, Loft A, Andersen AN, Dueholm M, Ottosen C, Andersen CY. Autotransplantation of cryopreserved ovarian tissue in 12 women with chemotherapy‐induced premature ovarian failure: the Danish experience. Fertil Steril, 2011, 95, 695–701 10.1016/j.fertnstert.2010.07.1080 [DOI] [PubMed] [Google Scholar]
- 29. Dittrich R, Lotz L, Kech G, Hoffmann I, Mueller A, Bechmann MW, Ven H, Montag M. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril, 2012, 97, 387–390 10.1016/j.fertnstert.2011.11.047 [DOI] [PubMed] [Google Scholar]
- 30. Rosendahl M, Schmidt KT, Ernst E, Rasmussen PE, Loft A, Byskov AG et al. Cryopreservation of ovarian tissue for a decade in Denmark: a view of the technique. RBM Online., 2011, 22, 162–171 [DOI] [PubMed] [Google Scholar]
- 31. Isachenko E, Isachenko V, Nawroth F, Rahimi G, Weiss JM. Effect of long‐term exposure at suprazero temperatures on activity and viability of human ovarian cortex. Fertil Steril, 2009, 91, 1556–1559 10.1016/j.fertnstert.2008.09.068 [DOI] [PubMed] [Google Scholar]


