Abstract
Purpose
The purpose was to establish a mild ovarian stimulation protocol that would help assisted reproductive technology (ART) units to avoid scheduling on weekends.
Methods
This protocol directed patients to take 50 mg/day of clomiphene citrate between days 3 and 7 of the menstrual cycle: 225 IU of recombinant follicle‐stimulating hormone (rec‐FSH) were administered on days 3, 5 and 7; human chorionic gonadotropin (hCG) was administered on day 9; and, oocyte pick‐up (OPU) was planned for day 11. From October 2008 through October 2009, 514 women underwent ART treatment with mild stimulation at the Sugiyama Clinic, and we evaluated whether OPU was accomplished on the planned day.
Results
Of all the treatment cycles, 419 (81.5%) underwent OPU on day 11 (scheduled group). Additional rec‐FSH administration was needed in 83 cycles, in which case OPU was performed on day 12 or later. In 12 cycles, OPU was canceled. The unscheduled group (n = 95) consisted of delayed OPU cycles and canceled cycles. Of all treatment cycles, 332 cycles in the scheduled group and 68 cycles in the unscheduled group underwent embryo transfer, with 81 and 16, respectively, resulting in pregnancies.
Conclusions
Using this protocol, OPU was performed on the scheduled day in about 80% of the cycles. Most weekend scheduling of OPU can be avoided using this mild stimulation.
Keywords: ART, Clomiphen citrate, Mild stimulation, Recombinant‐FSH, Scheduled OPU
Introduction
Ovarian stimulation is a key component of assisted reproductive technology (ART) treatment. Gonadotrophin‐releasing hormone (GnRH) agonists and gonadotrophins were first used in ART treatment for ovarian stimulation in 1984 [1]. GnRH agonist has since become a key component of successful ART treatment, and numerous studies have demonstrated the efficacy of this treatment protocol. It is common knowledge that the GnRH agonist long protocol is now the gold standard protocol for ART treatment, and is the most frequently used stimulation protocol [2]. With improving success rates, attention has shifted toward the adverse effects of ART treatment, which are mainly associated with profound ovarian stimulation and multiple pregnancies [2, 3].
Recently, a mild ovarian stimulation protocol, which consists of recombinant follicle‐stimulating hormone (rec‐FSH) with a GnRH antagonist or with clomiphene citrate (CC), has gradually become widespread in an effort to avoid ovarian hyperstimulation syndrome and to reduce the physical and economic burden on infertile couples. The mild ovarian stimulation protocol is gentler for patients, while reducing their burden. However, the day of oocyte pick‐up (OPU) cannot be determined in advance with mild ovarian stimulation because early luteinization is not prevented, as it is when a GnRH agonist is used. As a result, oocyte retrieval and embryo transfer (ET) must sometimes be performed on weekends, which can cause problems for the staff of an ART unit.
A paper published a decade ago showed how scheduled ovarian hyperstimulation with a GnRH agonist long protocol could make weekend OPU avoidable [4]. However, the mild ovarian stimulation protocol, which consists of CC and rec‐FSH, is now used routinely to reduce the physical and economic burden on patients. Use of this protocol sometimes forces staff of the ART unit to work on weekends because it does not use an agent that suppresses the luteinizing hormone (LH) surge. The purpose of the present study was to analyze the mild ovarian stimulation protocol and attempt to establish a method that would help the ART unit to avoid scheduling staff on weekends.
Materials and methods
Patients and the mild stimulation protocol
From October 2008 through October 2009, a total of 514 women who had undergone ART treatment with mild stimulation at the Sugiyama Clinic were recruited for the present cohort study. Informed consent was obtained from all patients, and the study was approved by the Institutional Review Board of the Sugiyama Clinic. The indications for ART treatment included tubal factor infertility (19.5%), unexplained infertility (51.5%), endometriosis (9.2%), and male factor infertility (19.8%). All male factor infertility cases were treated by intracytoplasmic sperm injection (ICSI).
All treatment cycles (n = 514) were stimulated using the mild stimulation protocol, CC and rec‐FSH. Briefly, patients took 50 mg of CC (Serophen®, Merck Serono, Tokyo, Japan) per day for 5 days between day 3 and 7 of the menstrual cycle, and 225 International Units (IU) of rec‐FSH (Follistim®, Shering‐Plau, Tokyo, Japan) were administered on days 3, 5 and 7 of the menstrual cycle. On day 9, when the dominant follicles reached ≥16 mm in diameter, 10,000 IU of human chorionic gonadotropin (hCG; Gonatropin, Mochida, Tokyo, Japan) was administered, and OPU was performed 35 h later (Fig. 1). Additional rec‐FSH (150 IU per a day) was administered as needed, based on follicular growth.
Figure 1.
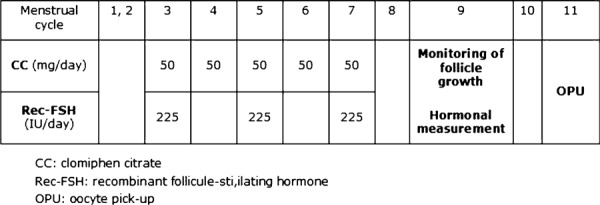
The mild stimulation protocol used in the present study. The mild stimulation protocol directed patients to take 50–100 mg/day of CC between days 3 and 7 of the menstrual cycle, and 225 IU of rec‐FSH was administered on days 5, 7 and 9 of the cycle. On day 9, when dominant follicles reached ≥18 mm in diameter, 10,000 IU of human chorionic gonadotropin was administered and OPU was performed 35 h later. CC clomiphene citrate, rec‐FSH recombinant follicle‐stimulating hormone, and OPU oocyte pick‐up
IVF/ICSI procedure and embryo transfer
The IVF procedure used in the present study has been previously described [5]. Oocytes were retrieved transvaginally using a needle‐guided technique, aided by ultrasonography. All follicles with a mean diameter >15 mm were aspirated individually, using a 17‐gauge needle connected to a tube and a machine for suction (Flush pump/oocyte Incubator; AIREY Co. Ltd, Fukushima, Japan). The needle was removed after aspiration of each follicle. The aspiration was interrupted and a new syringe was used if blood appeared in the tube connected to the syringe, thus avoiding contamination by blood. All follicles were washed with culture medium. Semen was produced by masturbation and, after washing, motile sperm were separated using a 30–60 min swim‐up period. In vitro insemination was performed by incubation of each oocyte with 50–100 × 103 motile sperm within 5–6 h of collection. When there was evidence of male‐factor infertility, intracytoplasmic sperm injection (ICSI) was performed instead of in vitro insemination, as previously described [6]. Oocytes were examined using a dissecting microscope 16–18 h after insemination or ICSI. The presence of two pronuclei with extrusion of the second polar body was taken as evidence of successful fertilization.
In all cases, one embryo was placed transcervically into the uterus of each patient 72 h after in vitro insemination or ICSI. The embryos that met the following criteria were defined as usable embryos: developed to at least the 7‐cell stage with less than 10% fragmentation on day 3 following oocyte retrieval. The residual embryos were cryopreserved using the vitrification method [7]. On days 1, 4 and 7 following ET, each patient received an injection of 3,000 IU hCG for luteal support. A combination of estrogen and progesterone was administered orally for 10 days after ET. Patients at elevated risk of ovarian hyperstimulation syndrome were administered hydroxyprogesterone caproate every 4 days, instead of hCG.
Hormonal assay, Kaufmann pretreatment and endpoint of this study
FSH and LH levels were checked on days 3 and 9 of the menstrual cycle using commercially available ELISA kits (IMMULIZE 2000; Diagnostic Products Corporation, Los Angeles, CA, USA). Estradiol (E2) and progesterone were also measured using commercially available ELISA kits (IMMULIZE 2000; Diagnostic Products Corporation, Los Angeles, CA, USA) on the same timetable.
Kaufmann therapy was used in pretreatment cycles due to patients’ oligomenorrhea. Kaufmann therapy used in this study was as follows: 1.3 mg of conjugated equine estrogens (CEE) was taken daily for 10 days, and 1.3 mg of CEE and 5 mg of medroxyprogesterone acetate (MPA) were taken daily for an additional 10 days. A pregnancy was recognized when the development of a gestational sac was detected by transvaginal ultrasound imaging on the 21st day after oocyte retrieval. A miscarriage was defined as a pregnancy loss before 22 weeks of gestation, and the fertilization rate was defined as the number of two pronuclear (2PN) embryos divided by the number of retrieved oocytes for each treatment cycle × 100 (%). Under this protocol, OPU was planned for day 11 (D11) of the menstrual cycle. Therefore, the primary endpoint was whether OPU was accomplished on the planned day, and the characteristics of the patients who achieved this were analyzed.
Data were analyzed using a Statistics Package for the Social Sciences (SPSS, Surrey, UK). Statistical analysis was performed using an unpaired t test and square test, and statistical significance was set at P < 0.05.
Results
The background of patients and ART outcomes of all patients are summarized in Table 1. The mean age was 37.9 years of age (range: 29–44); the mean number of previous ART attempts was 1.5 times; and the percentage of nulliparous patients was 54%. The basal hormonal values on the 2nd to 4th days of their menstrual cycles were as follows; E2: 58.6 ng/ml; LH: 4.4 IU/L; FSH: 10.5 IU/L, respectively. The mean number of retrieved oocytes was 5.6 ± 0.2, fertilization and cleavage rates were 74.1 and 91.1%, respectively, and the mean number of transferred embryos was 1.5 ± 0.2.
Table 1.
The background of patients and ART outcomes of all patients
| Number of patients | 514 |
| Number of stimulated cycles | 514 |
| Age (years) a | 37.9 ± 0.3 |
| Previous ART attempts (times) a | 1.4 ± 0.1 |
| Percentage of nulliparous patients (%) | 54% |
| Basal hormone levels b | |
| Estradiol (pg/ml) | 58.6 |
| LH (IU/L) | 4.4 |
| FSH (IU/L) | 10.5 |
| Percentage of ICSI patients (%) | 43.5% |
| Number of retrieved oocytes a | 5.6 ± 0.2 |
| Fertilization rate (%) | 74.1% |
| Cleavage rate (%) | 91.2% |
| Number of usable embryos a | 2.7 ± 0.2 |
| Number of ET cycles | 398 |
| Number of transferred embryos a | 1.5 ± 0.2 |
aValues were mean ± SEM
bBasal hormonal values were measured between the 2nd and 4th days of their menstrual cycles
Of all the treatment cycles, 419 (81.5%) underwent OPU on D11 (scheduled group). The E2, LH and progesterone levels on D9 of the scheduled group were 1279.7 ± 58.7 pg/ml, 4.6 ± 0.2 IU/L and 0.40 ± 0.02 ng/ml, respectively (mean ± SEM). Additional rec‐FSH administration was needed in 83 cycles due to inadequate follicular growth, in which case OPU was performed on day 12 or later. In 12 cycles, OPU was canceled because of premature luteinization or ovulation. The unscheduled group (n = 95) consisted of delayed OPU cycles and canceled cycles. The ART outcomes in each group are summarized in Table 2. The E2, LH and progesterone levels on D9 for the unscheduled group were 1,146 ± 47.4 pg/ml, 4.0 ± 0.5 IU/L and 0.60 ± 0.02 ng/ml, respectively (mean ± SEM).
Table 2.
ART outcome in the scheduled and unscheduled groups
| Scheduled group | Unscheduled group | |
|---|---|---|
| Number of stimulated cycles | 419 | 95 |
| Number of ET cycles | 332 | 68 |
| Age (years) a | 37.9 ± 0.3 | 37.7 ± 0.3 |
| Days of injections (days) | 3 | 4 |
| Rec‐FSH dose (IU) | 675 | 767 |
| Number of retrieved oocytes a | 5.5 ± 0.5 | 5.3 ± 0.5 |
| Number of cleaved oocytes a | 3.6 ± 0.3 | 3.5 ± 0.3 |
| Number of usable embryos a | 2.8 ± 0.2 | 2.7 ± 0.2 |
| Number of transferred embryos a | 1.4 ± 0.2 | 1.6 ± 0.2 |
aValues were mean ± SEM
In the scheduled group, 84.7% of the cycles were regular menstrual cycles, and this was significantly higher than that for the unscheduled group (71.8%, P = 0.01).
The OPU success rate on the scheduled day was 81.7% (138/169) for patients who had a basal FSH level of less than 9 IU/L. The rates were 85.4% (158/185) in patients who had more than 9 IU/L and less than 12 IU/L of FSH, and 88.0% (81/92) in patients who had more than 12 IU/L and less than 15 IU/L of FSH. In patients who had a basal FSH level of more than 15 IU, the rate was 61.6% (42/68), and this was significantly lower than the other groups (P < 0.05; Table 3).
Table 3.
The OPU success rate on the scheduled day according to the basal FSH values
| Basal FSH values (IU/L) | The OPU success rate on scheduled day (%) |
|---|---|
| FSH < 9 | 81.7 |
| 9 ≤ FSH < 12 | 85.4 |
| 12 ≤ FSH < 15 | 88.0 |
| FSH ≥ 15 | 61.6 a |
aThis was significantly lower than the other groups (P < 0.05)
The OPU success rate on the scheduled day when patients had received pretreatment with Kauffmann therapy was 83.3% (354/425), and this rate was significantly higher than that without pretreatment with Kauffmann therapy [P < 0.05; 73.0% (65/89)].
Of all treatment cycles, 332 cycles in the scheduled group and 68 cycles in the unscheduled group underwent embryo transfer on day 3. Embryo scoring was indicated as a percentage of usable embryos of all the transferred embryos. The embryos that met the following criteria were defined as usable: developed to at least the 7‐cell stage with less than 10% fragmentation on day 3 following OPU. The percentages of usable embryos in the scheduled and unscheduled groups were 74.4 and 70.6%, respectively. Pregnancy was accomplished in 81 cycles of the scheduled group and in 16 cycles of the unscheduled group. The pregnancy rates in the scheduled and unscheduled groups were 24.4 and 23.5%, respectively, and there was no significant difference in the pregnancy rate between the groups. The miscarriage rates in the scheduled and unscheduled groups were 22.2 and 25.0%, respectively, and this difference was not significant. Of all embryo‐transferred cycles (n = 400), 75 babies were born, and the take home baby rate per embryo‐transferred cycle was 18.8%.
Discussion
The mild stimulation protocol using rec‐FSH and GnRH‐antagonist reduces the physical and economic burden of patients compared with the conventional GnRH‐agonist long protocol. However, with this mild protocol, patients still need daily rec‐FSH administration and GnRH‐antagonist injection. Because of the need for daily gonadotropin injections, this protocol actually should not be considered mild ovarian stimulation. Because the GnRH antagonist protocol dose not really help patients, the mild ovarian stimulation protocol with CC and rec‐FSH was created to lessen the physical, mental and economic burden on patients. This mild stimulation protocol using CC with rec‐FSH meant that most patients did not require follicle growth monitoring and hormonal measurement twice, and the cost of ovarian stimulation and monitoring was half that of the GnRH‐antagonist protocol (data not shown).
Using this stimulation protocol, the pregnancy rate per number of cycles which started ovarian stimulation (n = 512) was inadequate because embryo transfer could not be performed in 114 cycles. Actually, it was posible to perform embryo transfer in 400 cycles, the pregnancy rate per embryo transfer was 24.3% (97/400), and the baby‐take‐home rate (BTHR) was 18.8%. Indeed, patients might be disappointed with this outcome. This protocol used CC for 5 days and CC sometimes causes reduced endometrial thickness; therefore, in this present study, the number of started cycles was 514, and 12 cycles of OPU were cancelled due to poor follicular growth or premature luteinization; embryo transfer was not performed in 102 cycles due to inadequate endometrial thickness. However, these patients (n = 102) had a chance to achieve pregnancy using cryopreserved embryos that had been thawed.
In our preliminary study, more than 80% of the patients who used our mild stimulation protocol were able to have OPU performed on the scheduled day with only one follicular monitoring on day 9 (data not shown), and we looked at whether or not OPU could be fixed on the scheduled day. Using this protocol, follicular monitoring and hormonal measurement were required only once on the 9th day of the menstrual cycle, and 419 out of 514 (81.5%) of the patients were able to have oocyte retrieval performed on the scheduled day (the 11th day of the menstrual cycle). Recently, Al‐Inany et al. [8] reported that CC could suppress the early rise of LH. Indeed, in the present study, the number of cancelation cycles was 12 out of 514 (cancellation rate of 0.19%) due to premature LH elevations. Under the present protocol, hCG administration for final follicular maturation was performed 2 days after the last day of CC administration, and, therefore, the effect of CC might have suppressed the early rise of LH.
It was previously demonstrated that basal FSH values before the stimulation began had no effect on the outcomes of ART [9]. However, elevated basal FSH values might have a positive relationship with poor ovarian response. In the present study, 84.5% of patients who had basal FSH values of less than 15 IU/L [(138 + 158 + 81 = 377)/(169 + 185 + 92 = 446)] were able to have oocyte retrieval performed on the scheduled day. However, among patients who had a basal FSH value of more than 15 IU/L, the OPU success rate on the scheduled day was significantly lower (61.6%) than that of the patients who had FSH values of less than 15 U/L, and patients showing high FSH levels required more gonadotropins due to their poor ovarian response.
In the present study, the relationship between pretreatment with Kauffmann therapy and the OPU success rate on the scheduled day was evaluated, but it was found that pretreatment with Kauffmann therapy was not related to OPU success rate on the scheduled day. This is very important for clinicians because it is possible to avoid oocyte retrieval on the weekends through the use of a combination of the mild stimulation protocol and pretreatment with the Kauffmann therapy. For instance, the starting day of the patient's menstrual flow could be fixed on Sunday using the Kauffmann therapy, the stimulation could be started on Tuesday of the same week, the monitoring of follicular growth and hormones could be performed the following Monday, the maturation HCG trigger would be performed on the same Monday night, and then the OPU could be performed on Wednesday. If the embryo transfer was scheduled for day 3, the ET would be performed on Saturday. But if blastocyst transfer were possible on day 5, patients would not visit the hospital on the weekend for ART treatment, and the staff concerned with ART treatment would not need to work on the weekend. This protocol benefits the medical staff involved in ART treatment, such as medical doctors, nurses and embryologists, as they are able to avoid work on the weekend. The patients also benefit from the use of this protocol, due to a reduction in cost and fewer visits to the clinic. Moreover, patients can more easily arrange their schedule if they have advance notice of the day of oocyte retrieval.
In conclusion, using this mild stimulation protocol, OPU was performed on the scheduled day (day 11) in about 80% of the cycles. This possibility increased when patients had a regular menstrual cycle, but was not affected by the pretreatment. Therefore, most weekend scheduling of OPU can be avoided through the use of mild stimulation in ART treatment.
References
- 1.Poter RN, Smith W, Craft IL, Abdulwahid NA, Jacobs HS. Induction of ovulation for in vitro fertilization using buserelin and gonadotrophins. Lancet. 1984;2:1284–5. [DOI] [PubMed]
- 2. Fauser BC, Devroy P, Macklon NS. Multiple birth resulting from ovarian stimulation for subfertility treatment. Lancet, 2005, 365, 1807–1816 10.1016/S0140‐6736(05)66478‐1 [DOI] [PubMed] [Google Scholar]
- 3. Macklon NS, Ctouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev, 2006, 27, 170–207 10.1210/er.2005‐0015 [DOI] [PubMed] [Google Scholar]
- 4. Nakagawa K, Yamano S, Senuma M, Myogo K, Yamazaki J, Aono T. Avoidance of oocyte retrieval on the weekend thorough the use of scheduled ovarian hyperstimulation for in vitro fertilization and embryo transfer. Fertil Steril, 1997, 68, 787–790 10.1016/S0015‐0282(97)00327‐0 [DOI] [PubMed] [Google Scholar]
- 5. Nakagawa K, Ohgi S, Kojima R, Ito M, Horikawa T, Irahara M, Saito H. Reduction of perifollicular arterial blood flow resistance after hCG administration is a good indicator of the recovery of mature oocytes in ART treatment. J Assist Reprod Genet, 2006, 23, 433–438 10.1007/s10815‐006‐9087‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakagawa K, Yamano S, Moride N, Yamashita M, Yoshizawa M, Aono T. Effect of activation with Ca ionophore A23187 and puromycin on the development of human oocytes that failed to fertilize after intracytoplasmic sperm injection. Fertil Steril, 2001, 76, 148–152 10.1016/S0015‐0282(01)01839‐8 [DOI] [PubMed] [Google Scholar]
- 7. Sugiyama R, Nakagawa K, Shirai A, Sugiyama R, Nishi Y, Kuribayashi Y, Inoue M. Clinical outcomes resulting from the transfer of vitrified human embryos using a new device for cryopreservation (plastic blade). J Assist Reprod Genet, 2010, 27, 161–167 10.1007/s10815‐010‐9390‐y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Inany HG, Abou‐Setta AM, Aboulghar M. Gonadotropin‐releasing antagonists for assisted conception: a Cochran review. Reprod Biomed Online, 2007, 14, 640–649 10.1016/S1472‐6483(10)61059‐0 [DOI] [PubMed] [Google Scholar]
- 9. Kojima R, Nakagawa K, Nakashima A, Horikawa T, Ohgi S, Saito H. Elevated basal FSH levels, if it is under 15 IU/L, will not reflect poor ART outcomes. J Assist Reprod Genet, 2008, 25, 73–77 10.1007/s10815‐007‐9195‐9 [DOI] [PMC free article] [PubMed] [Google Scholar]


