Abstract
Background and Aims
Although phenotypic plasticity has been shown to be beneficial for plant competitiveness for light, there is limited knowledge on how variation in these plastic responses plays a role in determining competitiveness.
Methods
A combination of detailed plant experiments and functional–structural plant (FSP) modelling was used that captures the complex dynamic feedback between the changing plant phenotype and the within-canopy light environment in time and 3-D space. Leaf angle increase (hyponasty) and changes in petiole elongation rates in response to changes in the ratio between red and far-red light, two important shade avoidance responses in Arabidopsis thaliana growing in dense population stands, were chosen as a case study for plant plasticity. Measuring and implementing these responses into an FSP model allowed simulation of plant phenotype as an emergent property of the underlying growth and response mechanisms.
Key Results
Both the experimental and model results showed that substantial differences in competitiveness may arise between genotypes with only marginally different hyponasty or petiole elongation responses, due to the amplification of plant growth differences by small changes in plant phenotype. In addition, this study illustrated that strong competitive responses do not necessarily have to result in a tragedy of the commons; success in competition at the expense of community performance.
Conclusions
Together, these findings indicate that selection pressure could probably have played a role in fine-tuning the sensitive shade avoidance responses found in plants. The model approach presented here provides a novel tool to analyse further how natural selection could have acted on the evolution of plastic responses.
Keywords: Arabidopsis, competition, functional–structural plant model, phenotypic plasticity, shade avoidance, tragedy of the commons
INTRODUCTION
Plants compete for resources with their neighbours, which influences species composition and vegetation dynamics in both natural (Kiaer et al., 2013; Kunstler et al., 2016) and managed plant communities (Olsen et al., 2005; Yu et al., 2015). Plants experience both above- and below-ground competition, and the relative importance of the degree of competition for plant performance depends on the availability of resources, e.g. nutrients or light (Kiaer et al. 2013). The degree of competition for resources and therefore plant functioning is influenced by differences in plant phenotype, created by the component traits and their values (Kunstler et al., 2016). These values can be genotype specific, but may also be modulated by environmental factors through phenotypic plasticity. Phenotypic plasticity is the ability of a genotype to express multiple phenotypes in various environments (Bradshaw, 1965; Sultan, 2000).
Here we emphasize that expression of different phenotypes in different environments is mediated by dynamic organ-level responses to environmental signals. From an evolutionary perspective, one can argue that plants have evolved to optimize plastic responses to maximize resource acquisition in different environments (Sultan, 2000). Plastic responses to changes in vegetation density and the associated light conditions constitute a well-known form of phenotypic plasticity in plants, called the shade avoidance syndrome (SAS; Casal, 2012; Ballaré and Pierik, 2017). An increase in the stem or petiole extension rate, reduction in branch production, increase in leaf inclination (hyponasty) and advanced flowering time are typical SAS responses that plants exhibit when encountering increased competition for light, though the combination of responses differ between species.
Relationships between species, component traits and their values, and their relationship with competitiveness have been studied intensively to understand ecosystem processes (Dybzinski et al., 2011; Farrior et al., 2013; Bardgett et al., 2014; Kunstler et al., 2016). For instance, game-theoretical studies suggest that because plants compete for resources, plants can evolve traits associated with a relatively large investment in resource harvesting (e.g. leaves, stems and roots) instead of reproduction. This means that under competition, natural selection can result in plant traits that will not optimize performance of the plant population, also referred to as a tragedy of the commons (Falster and Westoby, 2003; McNickle and Dybzinski, 2013). The existence of such a tragedy of the commons may have profound consequences for vegetation performance (Anten and Vermeulen, 2016). However, studies that evaluate the role of resource-harvesting traits for competition often do not take phenotypic plasticity into account (but see, for example, Dybzinski et al., 2013). Analysing how plastic responses affect competition is challenging because plastic responses affect trait values that influence the dynamic interaction between plant phenotype and environmental conditions and signals. Environmental signals elicit plastic responses that induce small trait changes which in turn change the light climate and thus modify the environmental signals. Furthermore, small changes early in plant development eventually can be amplified into substantial consequences for competitiveness. Although phenotypic plasticity is identified to be beneficial for plant performance, illustrated by adequate stem or petiole length matching to different environments (Schmitt et al., 1995; Dudley and Schmitt, 1996; Pierik et al., 2003; Weijschede et al., 2008), it is unknown to what extent subtle variation in the plastic response itself has consequences for plant performance in competitive settings. Large consequences of such subtle variation would probably result in strong selection for a fine-tuned detection and signal transduction system.
Our main objective was to determine to what extent differences in plastic responses between neighbouring plants affect the outcome of competition for light, considering the dynamic feedback between plant phenotype and environment. We use SAS responses in Arabidopsis thaliana (arabidopsis) as a case study for phenotypic plasticity. Arabidopsis rosettes show two major SAS responses: increased leaf angle (hyponasty) and petiole elongation (Pierik and de Wit, 2014). When arabidopsis plants are grown in dense stands, leaf angles will first increase due to physical touching among growing leaves (de Wit et al., 2012). This resulting vertical stand structure will change the ratio of red to far-red (R:FR) light scattered by the elevated leaves. This decrease of R:FR light is the most important signal for the subsequent induction of further leaf hyponasty and petiole elongation (Pierik and de Wit, 2014). To quantify the effect of differences in these SAS responses on plant competitiveness, we used a combination of detailed plant experiments and functional–structural plant (FSP) modelling (Bongers et al., 2014). FSP models can capture the dynamic feedback between the changing plant phenotype and the surrounding light environment by simulating plant phenotypic development and biomass growth over time in three dimensions at the organ level (Vos et al., 2010; Evers, 2016). We implemented phenotypic plasticity as the ability to express organ-level plastic responses: changes in the rate of petiole elongation and changes in the rate of hyponasty. These plastic responses were modelled using response curves that relate organ change to the R:FR (Gautier et al., 2000; Evers and Vos, 2013). In parallel with model analysis, variation in these plastic responses was explored in experiments using arabidopsis mutants. Ultimately, by simulating the R:FR distribution as a function of the dynamic 3-D plant phenotypes that are created by the interaction of resource acquisition and growth at the organ level, plastic responses at the organ level were quantitatively linked to whole-plant performance during competition.
MATERIALS AND METHODS
Plant experiments
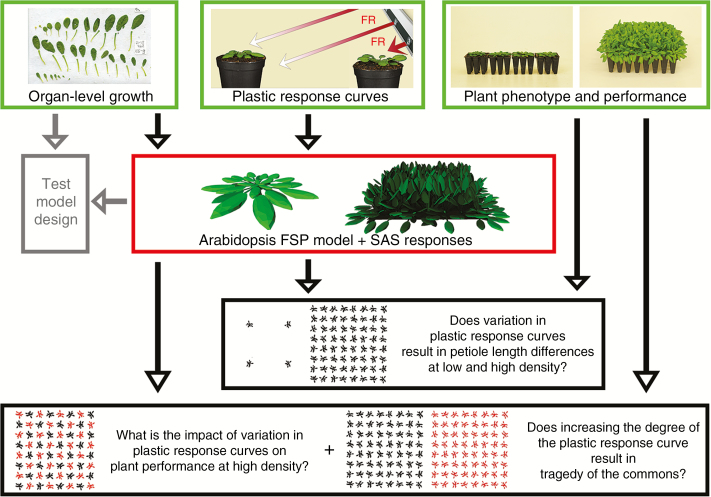
Three independent experiments were conducted to obtain organ-level growth data, petiole elongation response curves, and plant phenotype and performance of various genotypes of Arabidopsis thaliana, for model design and validation (outlined in Fig. 1). To obtain organ-level growth data, wild-type Col-0 plants were used. To explore the variation in SAS responses, we tested various arabidopsis mutants for their SAS responses (Fig. 2; Supplementary Data Fig. S1). For model validation, the genotypes hfr1-5 and rot3-1 were used because of their clear distinct levels of petiole elongation (Fig. 2). Arabidopsis seeds were sown on potting soil (mix Z2254, Primasta B.V., The Netherlands), stratified for 4 d at 4 °C in the dark, after which they germinated and grew in a growth chamber with a 9 h photoperiod of 200 µmol m–2 s–1 photosynthetically active radiation (PAR), an R:FR of 2.3, 20 °C and 70 % relative humidity. Ten days after germination, seedlings were transplanted to individual 19 mL pots (Ø 2.5 cm) and plants grew in the same growth chamber with bottom-up watering for soil water saturation.
Fig. 1.
Overview of the research design, in which three independent experiments (bordered in green) are combined with functional–structural plant (FSP) modelling (bordered in red) to address three questions (bordered in black). Data of organ growth and detailed plastic responses of arabidopsis were used to develop an FSP model that included two plastic responses of the shade avoidance syndrome (SAS); hyponasty and petiole elongation. The model design was tested by comparing phenotypic and performance data from plant experiments and model simulation (Scenario 1; bordered in grey). Additional model simulations and plant experiments were performed to validate model output (Scenarios 2 and 3) and answer the three research questions (Scenarios 2–6). See Supplementary Data Video for a visualization of arabidopsis plants growing in high and low population density.
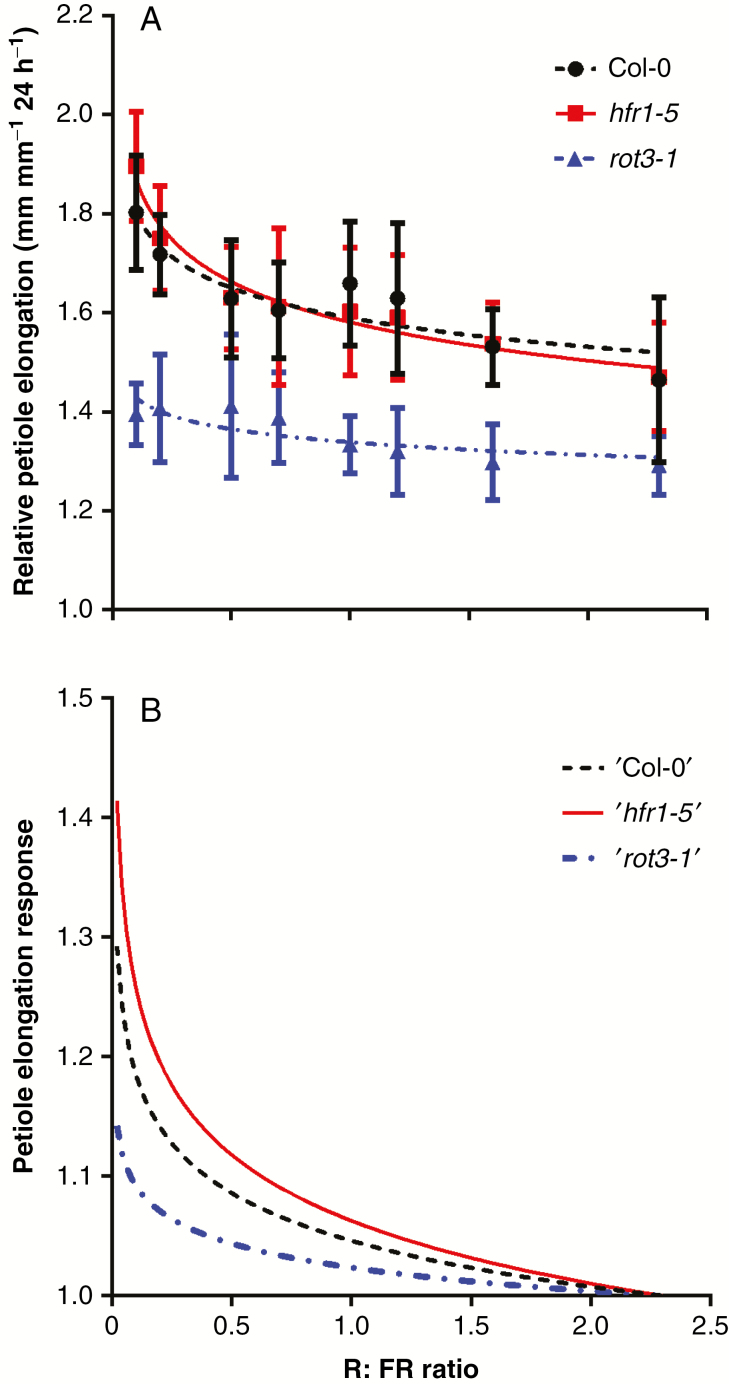
Fig. 2.
Petiole elongation response curves from three arabidopsis genotypes. (A) Measured relative petiole elongation at different R:FR values for Col-0, hfr1-5 and rot3-1 with genotype-specific fitted curves [Eqn (1)]. Experimental data represent the mean ± s.d. (n = 12). (B) Petiole elongation response curves for the corresponding arabidopsis genotypes that were used in the model.
Experiments for model design.
To obtain organ-level growth data, arabidopsis wild-type Col-0 was grown solitarily (referred to as ‘low density’ in the Results) or in high-density stands of 7 × 7 plants with interplant distance (IPD) of 2.5 cm, until bolting. During stand development, R:FR measurements were taken in the high-density stands at seven locations with a LI-COR1800 spectroradiometer (LiCor, Lincoln, NE, USA) using a glass fibre with cosine corrector (SKL 904, spectroSense2, Skye, UK). The R:FR was calculated from the irradiance within the wavelengths of 654–664 nm for R and 724–734 nm for FR light. Per location in the stand, readings in four horizontal directions were taken and the average calculated. Between day 21 and day 46, plants were harvested every 2–4 d and, in each harvest, two high-density stands and ten individually grown plants were selected. In each stand, the outer two rows of plants were excluded from the harvest to diminish border effects. Before every harvest, leaf angle of rank numbers 8 and 10 were measured with a protractor. For every harvested plant, laminas and petioles were scanned (at 600 dpi). For all leaves with a rank higher than 6 and with a distinct petiole, all laminas and petioles were pooled separately and dried for 48 h to obtain lamina and petiole dry weight. The remaining above-ground plant material was pooled and dried to obtain total above-ground biomass. Root material was not harvested. Leaf scans were analysed with ImageJ (https://imagej.net) to collect petiole length and width, and lamina area, length, width and shape. Petiole length and lamina area were used to determine parameter values for the organ growth function (Supplementary Data Materials and Methods). Data of all harvested plants per developmental stage and density were used to calculate trait value averages. All parameter values used in the model and extracted from this experiment are given in Supplementary Data Table S1.
To obtain petiole elongation response curves for three Arabidopsis genotypes, 10-day-old seedlings were transplanted in 70 mL pots (Ø 5 cm) and grown for 28 d, at which time they were subjected to one of eight R:FRs (2.3, 1.6, 1.2, 1.0, 0.7, 0.5, 0.2 and 0.1) for 24 h, n = 12 per R:FR. These eight different R:FRs were created by supplementing normal light (R:FR 2.3) with FR light-emitting diodes (LEDs; 730 nm; Philips Green Power, The Netherlands). Two petioles per plant (start length 4–6 mm) were measured at the start and end of the experiment with a digital calliper. The relative elongation per petiole was calculated and the mean of the two petioles per plant was used for further analysis. Relative elongation of all genotypes was described with:
| (1) |
where P is the relative petiole elongation (mm mm–1 24 h–1), a is a slope coefficient and b is the elongation rate at R:FR 1. Parameters were fitted for each genotype separately.
Experiments for model validation.
Three different arabidopsis genotypes (Col-0, hfr1-5 and rot3-1) were grown solitarily (low density) or in high-density stands of 8 × 8 plants (IPD of 2.5 cm) composed of plants of the same genotype (monoculture) or plants of two genotypes grown in a checkerboard pattern (mixture; Keuskamp et al. 2010). After 46 d of growth, five solitary plants per genotype and five replicated plots per genotype-specific monocultures and mixture were harvested. For all solitary plants and three plants per genotype per plot, laminas and petioles were scanned, dried and measured similarly to the first experiment. The mean values of the middle 16 or eight plants per genotype per plot were calculated and used as independent values for further analysis. Paired Student’s t-test was used to test significant difference between genotypes within the mixture, and unpaired Student’s t-test was used to test significant difference between monocultures.
Model description
An FSP model (Vos et al., 2010; Evers, 2016) of arabidopsis rosette growth and development was constructed using the simulation platform GroIMP v1.5 (https://sourceforge.net/projects/groimp). The rosettes were represented as a collection of leaves that were composed of petioles and laminas. An additional root compartment functioned only as a sink for carbon assimilates. The leaves were provided with values for reflectance, transmittance and absorbance of PAR, R and FR light, which were used by the radiation model to simulate the light environment and calculate the absorption of PAR and perception of the R:FR. The appearance rate and shape of the leaves were based on empirical data, and the leaves grew in time in three dimensions based on light interception, photosynthesis and carbon allocation mechanisms (explained in more detail in Supplementary Data Materials and Methods, and in Evers and Bastiaans, 2016). During each simulated time step (representing 24 h), individual leaves absorbed PAR that was converted to an amount of carbon through photosynthesis, and the perceived R:FR that determined the shade avoidance responses (see below). Therefore, simulated plant growth depended on the level of competition for light that individual plants experienced with neighbouring plants: plant phenotype, size and biomass were thus an emergent property of the simulated model scenarios. Parameter values for organ structure, physiological processes and environment signals were obtained from the experiments described above and from the literature (Supplementary Data Table S1). The complete model is available on request from the corresponding author.
Shade avoidance responses.
Two SAS responses were included: hyponasty (by touching and by the R:FR) and petiole elongation (by the R:FR). Hyponasty by leaf touching is induced upon mechanical interaction at the tips of two growing leaves before the R:FR in a canopy decreases significantly (de Wit et al., 2012). This touch-induced hyponasty was simulated to occur when the distance between lamina tips of neighbouring leaves was <2 mm. Hyponasty induced by R:FR perception was simulated to happen when the perception of the R:FR by the lamina was below a threshold value of 0.5. In every model time step (24 h), when touch or low R:FR threshold criteria were met, leaf angle increased by a fixed amount, for which either a default value of 16° (based on measurements on Col-0) was used or a scenario-dependent value (see below ‘Model scenarios’). The leaf angle over time was therefore a function of the number of time steps in which touch or low R:FR perception occurred, with a maximum leaf angle of 80° (see Supplementary Data Video for hyponastic response of arabidopsis plants in high density). Leaves with rank number up to six did not become hyponastic.
The second SAS response incorporated in the model was relative petiole elongation. R:FRs perceived at lamina level were used as input for the response curves (Kozuka et al., 2010). The petiole response curve based on arabidopsis type Col-0 was used as default setting (Fig. 2B); for other settings see ‘Model scenarios’. The fitted function for the relative petiole elongation obtained from the petiole elongation experiment was normalized for growth at control R:FR light (R:FR 2.3). In this way the relative petiole elongation rate could be simulated in addition to petiole growth by carbon allocation. Petiole elongation and related extra investment of substrates was modelled in two steps. First, the petiole elongated by multiplying the petiole length by the relative petiole elongation value (representing cell expansion without extra biomass demand; Sasidharan et al., 2010; Huber et al., 2014). Secondly, the longer elongated petiole increased its carbon demand to correct for the needed biomass corresponding to the length (representing increased biomass allocation to the petiole; Poorter et al., 2012; de Wit et al., 2015). Petioles could only show the elongation response during the actual growth phase. Petiole length over time was therefore a result of daily calculated carbon growth based on PAR absorption and petiole elongation based on R:FR perception.
Model scenarios.
In all scenarios, plants were simulated solitarily (representing low density) or in high-density monocultures or mixture (consisting of 8 × 8 plants and an IPD of 2.5 cm) for 46 d (Supplementary Data Video), and different plant types were created by adjusting relevant SAS response values. In Scenario 1, three plant types were simulated solitarily and in monocultures to test the extent to which the model could simulate arabidopsis phenotype and growth: the first plant type had default SAS response values as measured for arabidopsis wild-type Col-0 (referred to as ‘Col-0’) in the experiment; two additional plant types had either no hyponastic responses (‘noHypo’) or no petiole elongation response (‘noPE’). The R:FR in the vegetation stand was captured by placing virtual sensors at soil level that measured the R:FR from four directions, to mimic the measurements of the R:FR in the experimental arabidopsis stands. Dynamic changes of leaf angle, petiole length, lamina area and total above-ground biomass of these plant types were compared with data from experimentally grown Col-0 arabidopsis grown in low- or high-density stands. In Scenario 2 we simulated two plant types with different values for their petiole elongation curves as measured for the hfr1-5 and rot3-1 arabidopsis genotypes (0.073 for ‘hfr1-5’ and 0.028 for ‘rot3-1’) in low- and high-density stands to validate if variation in the petiole elongation response curve could result in distinct petiole length differences at low and high density. Of these simulated plant types, the petiole lengths per rank after 46 d of growth were compared with measured petiole lengths after 46 d of the two corresponding arabidopsis genotypes.
To quantify the impact of variation in plastic response curves on plant performance in competitive settings, and to determine if stronger response curves would result in high plant competitiveness but sub-optimal population performance (tragedy of the commons), four additional scenarios were simulated (Scenarios 3–6). In these scenarios, mixtures of two plant types, placed in a checkerboard design, and the associated monocultures, were simulated for 46 d. Organ growth, light absorption and total above-ground biomass during the development of the stands were recorded as model output. In Scenario 3, two plant types were only different in their petiole elongation response curve; ‘Col-0’ having a slope of 0.054 and ‘hfr1-5’ of 0.073 (respectively matching the measured Col-0 and hfr1-5 arabidopsis genotypes). Simulated total above-ground biomass was compared with total above-ground biomass measured from the validation experiment with these same genotypes. In Scenario 4, two plant types had different hyponastic responses but similar petiole elongation response curves; plants increased their angle by 10° (‘10deg’) or 15° (‘15deg’) per hyponastic event. These hyponasty values were chosen based on observed variation in hyponastic values of different arabidopsis genotypes (data not shown). To analyse if competitiveness depends on the difference in plastic responses between two competing plant types, we simulated mixtures with distinct differences between the plastic response values of the two plant types. In all mixtures, a ‘wild-type’ plant type competed with a ‘competitor’ plant type that had a different value for the petiole elongation response (Scenario 5) or the hyponastic response (Scenario 6). The ‘wild-type’ plant type had a petiole elongation response value of 0.054 and a hyponastic response value of 20°. The absolute difference in above-ground biomass of the ‘competitor’ compared with the ‘wild-type’ was a measure of the degree of competitiveness. In addition, over the same range of petiole elongation and hyponastic response values, monoculture stands were simulated. All model simulations were replicated ten times to capture the variation in plant growth created by the stochastic nature of the light model and the random plant rotation angle. The mean values of the middle 16 (monocultures) or eight (mixtures) plants per genotype per plot were calculated and used as independent values for further analysis.
RESULTS
Variation in the petiole elongation response curve
Arabidopsis genotypes showed a gradually increasing relative petiole elongation with a decreasing R:FR (Fig. 2A; Supplementary Data Fig. S1). Col-0 and hfr1-5 showed only a marginally different elongation response, where rot3-1 clearly had a lower relative petiole elongation rate under the same R:FR conditions compared with the other two. However, all the fitted curves had distinct slope values for their response curves: 0.054 for Col-0, 0.073 for hfr1-5 and 0.028 for rot3-1. The normalization procedure resulted in three response curves with distinct slopes that all increased with decreasing R:FR ratio (Fig. 2B).
Test model design (Scenario 1)
During the development of a dense arabidopsis stand, the leaf area index (LAI) increased and the R:FR decreased in time (Supplementary Data Fig. S2). This decrease in the R:FR is primarily created by increased leaf angles through the touching of leaves (de Wit et al., 2012). Consequently, the R:FR decrease induced hyponastic and petiole elongation responses that further change plant phenotype. The dynamic change of leaf angle and petiole length of experimentally grown plants in low- and high-density stands were best simulated by the plant type that included both SAS responses (referred to as ‘Col-0’) (Fig. 3). When the hyponastic responses were set to zero (‘noHypo’), plants did not become hyponastic in high density compared with the ‘Col-0’ type. The simulated ‘Col-0’ plants increased the leaf angles slightly later during stand development than the experimentally measured leaf angles. Plants that had no petiole elongation response (‘noPE’) could not grow longer petioles in high density compared with low density, illustrating that the petiole elongation response curve included in the ‘Col-0’ plant type is needed to simulate long petiole lengths in high-density population stands. Overall, when including the SAS response values based on wild-type Col-0 (‘Col-0’), the model predictions were in good agreement with the experimental above-ground biomass accumulated during stand development in low- and high-density stands (Fig. 3C).
Fig. 3.
Experimentally and simulated obtained data of plant phenotype and performance. (A) Leaf angle change of plant growing in a high-density stand obtained from experimental data and simulated for plant types that did (‘Col-0’) or did not (‘NoHypo’) exhibit hyponastic responses. (B) Petiole length change of plants growing in low- and high-density stands, from experimental data and simulated for plant types that did not show petiole elongation (‘noPE’) or did show petiole elongation (‘Col-0’). Petiole rank number 12 was used as it was representative for other leaf ranks. (C) Total above-ground biomass of a plant growing in low- and high-density stands, from experimental data and simulated by the default plant type ‘Col-0’ that included both hyponastic and petiole elongation responses. Experimental data represent the mean ± s.d., with (n = 10 for low and n = 18 for high density). Simulated data represent the mean (n = 10).
Validation of the petiole elongation response curve (Scenario 2)
Validation of the petiole elongation response curve (Scenario 2) revealed that the magnitude of the experimentally observed petiole length difference between hfr1-5 plants grown in low- or high-density stands was predicted by the model that used the ‘hfr1-5’ response curve, although petiole lengths of leaves with high ranks were underestimated (Fig. 4A). In addition, the model predicted no petiole length difference when using the ‘rot3-1’ response curve, which is in agreement with the experimentally observed petiole lengths of rot3-1 plants grown in low- or high-density stands (Fig. 4B). In absolute terms, the model overestimated petiole lengths due to the higher constitutive growth of the simulated Arabidopsis plants compared with the natural rot3-1 plants.
Fig. 4.
Petiole lengths of all leaf ranks per plant after 46 d of growth of two arabidopsis genotypes. (A) Petiole lengths of hfr1-5 and (B) rot3-1 plants from experimental data or simulated by the model in low- and high-density stands. Experimental data represent the mean ± s.d. (with n = 10 for low and n = 18 for high density). Simulated data represent the mean (n = 10).
Impact of variation in plastic response values on plant performance (Scenarios 3 and 4)
‘Col-0’ and ‘hfr1-5’ plant types had different simulated above-ground biomass after they were grown for 46 d together in a mixture but not when simulated separately in monocultures (Scenario 3; Fig. 5A). This difference in plant performance in monocultures compared with mixtures was also observed in the experimental data with Col-0 and hfr1-5 arabidopsis genotypes (Fig. 5B). In this scenario, the ‘hfr1-5’ type had slightly longer petioles than ‘Col-0’ both in the monocultures and in the mixtures, but the laminas of ‘hfr1-5’ absorbed more PAR than ‘Col-0’ only in the mixture (Fig. 6A, B). The higher PAR absorption at the individual lamina level resulted in higher simulated whole-plant PAR absorption for ‘hfr1-5’ compared with ‘Col-0’ in the mixtures, whereas in the monocultures there was no difference between the two plant types for lamina or whole-plant PAR absorption (Fig. 6C). Thus, in direct mixed competition, the plant type with the slightly stronger petiole elongation response (as reflected in a higher slope in the petiole elongation–R:FR curve) had a higher performance because it created slightly longer petioles that could put laminas in a better lit part of the canopy.
Fig. 5.
Total above-ground biomass of an individual arabidopsis plant grown in a monoculture or mixture for 46 d. Plant biomass simulated by the model (A, Scenario 3) or obtained from experimental data (B). Simulated plant types ‘Col-0’ and ‘hfr1-5’ had 0.054 and 0.073 for their response curves, respectively. Simulated data represent the mean ± s.d. (n = 10). Experimental data represent mean ± s.d. (n = 5). ns, not significant; *P < 0.05.
Fig. 6.
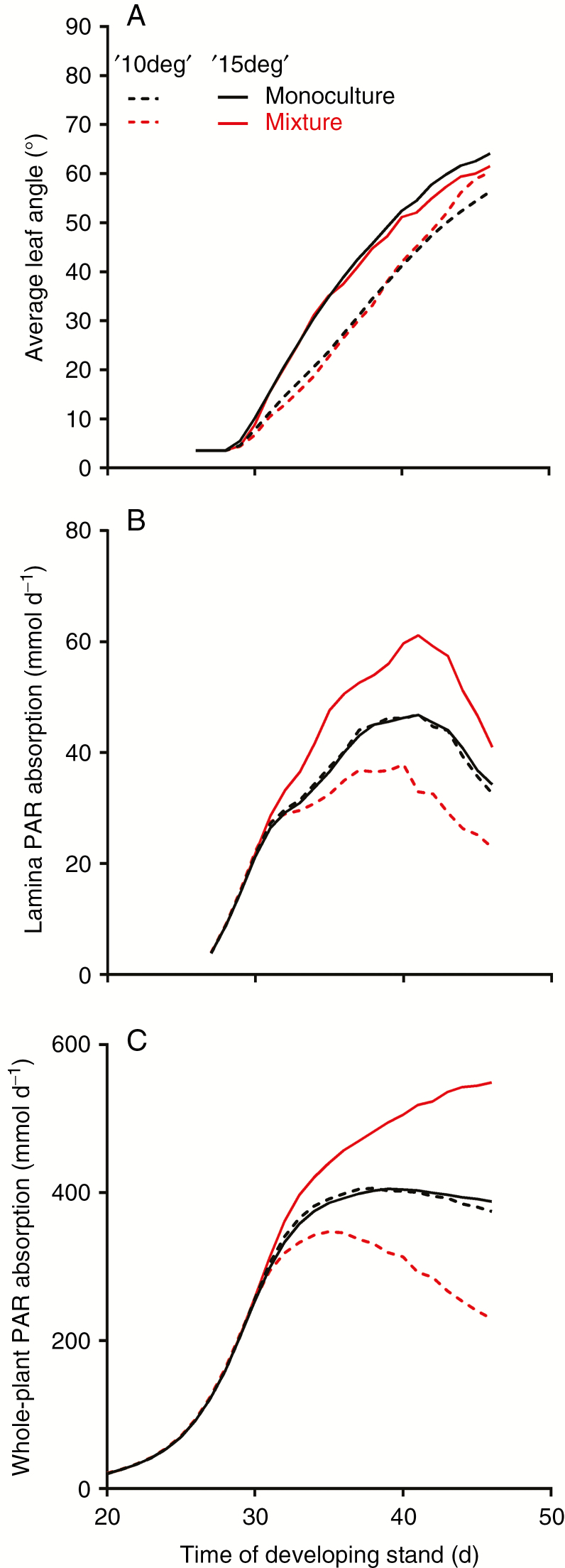
Simulated leaf and plant characteristics during the development of arabidopsis monocultures or mixtures consisting of two genotypes with distinct petiole elongation response curves (Scenario 3). The ‘hfr1-5’ type had a stronger petiole elongation response curve than the ‘Col-0’ type, shown in Fig. 2B. (A) Petiole length, (B) lamina-absorbed PAR and (C) whole-plant absorbed PAR during stand development. Leaf rank number 12 was used to visualize petiole length and lamina PAR absorption, and was representative for other leaf ranks.
In the monocultures and the mixture of Scenario 4, in which the strength of the hyponastic response was tested, both plant types showed increased leaf angles at the same developmental stage during stand development, but the ‘15deg’ plant type increased its leaf angle faster (Fig. 7A). In the mixture, this faster increase resulted in higher lamina PAR absorption that also resulted in higher whole-plant PAR absorption, compared with the weaker ‘10deg’ plant type (Fig. 7B, C). In the monocultures, the slightly higher leaf angle of the stronger ‘15deg’ type did not result in higher lamina or whole-plant PAR absorption compared with the ‘10deg’ type. These model simulations could not be validated due to the lack of appropriate arabidopsis mutants that have distinct hyponastic responses but overall similar growth forms.
Fig. 7.
Simulated leaf-specific and whole-plant characteristics during the development of arabidopsis monocultures or mixtures consisting of two genotypes with distinct hyponastic responses (Scenario 4). The ‘15deg’ plant type had a stronger hyponastic response than the ‘10deg’ plant type. (A) Leaf angle, (B) lamina-absorbed PAR and (C) whole-plant absorbed PAR during stand development. Leaf rank number 12 was used to visualize petiole length and lamina PAR absorption, and was representative for other leaf ranks.
Competitiveness depends on the difference in plastic responses (Scenario 5 and 6)
To determine how subtle variation in plastic responses can affect plant competitiveness, we simulated multiple mixtures in which a ‘wild-type’ competed with a ‘competitor’ with a different value for the petiole elongation response (Fig. 8A, Scenario 5) or with a different value for the hyponastic response (Fig. 8B, Scenario 6). The plant type with the stronger petiole elongation response always had a higher above-ground biomass, but when the difference in response was very large, the difference in above-ground biomass increased only marginally (Fig. 8A). The plant type with the stronger hyponastic response had only a higher above-ground biomass with absolute hyponastic values up to 30° (Fig. 8B). Increasing the difference in plastic responses when the absolute hyponastic response was >40° had no effect or a negative effect on competitiveness. When plant types with increased SAS response values grew in monocultures, the above-ground biomass of the plants decreased slightly (Supplementary Data Fig. S3), indicating that performance at population level is sub-optimal when plants increase their plastic response strength.
Fig. 8.
Simulated performance difference related to the difference in plastic response values of ‘wild-type’ and ‘competitor’ plant types in high-density mixtures (Scenario 5 and 6). Performance difference was calculated by the above-ground biomass of the ‘competitor’ minus the above-ground biomass of the ‘wild-type’ plant type. Performance difference related to the difference in (A) the petiole elongation response curve value (Scenario 5) or (B) the hyponastic response value (Scenario 6). The absolute petiole elongation and hyponastic response values for the two plant types are also expressed. Data represent the mean ± s.d. (n = 10).
DISCUSSION
In this study we showed that small differences in petiole elongation or hyponastic responses to changes in R:FR conditions can strongly affect plant phenotype and competitiveness. Model simulations illustrated that subtle variation in SAS response curves could influence competitiveness for light because a small change in a structural trait (petiole length or leaf angle) affected the interaction between plant phenotype and light environment, which had direct consequences for simulated PAR absorption and subsequently growth (Figs 6 and 7). Part of the model simulations were validated with a plant competition experiment that resulted in similar biomass accumulation in monocultures and mixtures for two arabidopsis genotypes with similar petiole elongation response curves to those used in the model simulations.
Model assumptions
Before going on to the implications of our work, we briefly reflect on the model assumptions, such that our findings can be properly interpreted. For model simplicity, only touch and the R:FR were the environmental cues that induced the studied SAS responses. It is, however, known that additional canopy-related light cues, notably a decrease in blue and PAR light intensity, are involved in shade avoidance (e.g. Casal, 2012; Pierik and de Wit, 2014) and can strengthen low R:FR responses (de Wit et al., 2016). In all scenarios, parameters related to leaf optical properties and photosynthesis were set to be independent of light conditions or leaf developmental stage. A decrease in potential photosynthesis with canopy depth (Anten et al., 1995) was not considered, as we assumed that such acclimations of photosynthetic parameters would be negligible in relatively young and quickly developing arabidopsis leaves compared with the role of phenotypic change due to the SAS responses studied. In addition, we assumed that chloroplasts in the petioles contributed to PAR absorption and photosynthesis, in contrast to other light competition models which make a clear distinction between height growth through investments in stems and branches that were considered not to contribute directly to CO2 fixation and ligh-harvesting organs (leaves) that do fix carbon (Anten, 2005; Dybzinski et al., 2011). We checked the photosynthetic contribution of petioles, and concluded that even without petiole photosynthesis, plants with a slightly different plastic response curve have different performances in mixtures but equal performances in monocultures (Supplementary Data Fig. S4).
Regarding plasticity costs, only two direct consequences of phenotypic changes were considered: (1) substrates invested in petiole length were consequently not available for lamina growth and (2) inclined leaf angles could potentially absorb less light than leaves with a horizontal position. Other indirect costs, such as vulnerability of strongly hyponastic leaves and long petioles to mechanical damage or hydraulic limitations, were not taken into account. Overall, the model predicted the observed relative differences in biomass production between genotypes with different petiole elongation responses well qualitatively (Fig. 5), suggesting that costs and benefits of the petiole elongation response were reasonably well captured in the current model regarding arabidopsis responses. Modelling the induction of both SAS responses was based on R:FR perception at the lamina (Kozuka et al., 2010). However, details on site of perception vs. site of response may differ between species, organs and responses (Casal and Smith, 1988a, b; Maddonni et al., 2002). The kind of organ-level plant modelling presented in this study makes it possible to explore the environmental context of R:FR distributions and functional implications of localized signalling.
Tragedy of the commons
Tragedy of the commons in light competition assumes that plants investing relatively more in light harvesting compared with neighbour plants are the most successful competitors, but because of the costs associated with this investment, such plants will perform less when growing as monocultures (Falster and Westoby, 2003; McNickle and Dybzinski, 2013). This conflict between individual-based selection and population performance has been proposed to have major consequences for vegetation functioning, and knowledge of this phenomenon may provide input for crop management and breeding systems (Anten and Vermeulen, 2016). Our experimental results showed that the plant type with the stronger petiole response, and thus a higher petiole investment, outcompeted the individual with the weaker response in the mixtures but had equal performance in monoculture (Fig. 5). This is in contrast to (mostly theoretical) studies that evaluate tragedy of the commons in competition for light. Additional model simulations also illustrated that although the competitiveness increased with stronger plastic responses, the population-level performance decreased only marginally (Fig. 8; Supplementary Data Fig. S3). These results suggest that selection on shade avoidance responses that favour light competition does not necessarily result in a strong decrease of population-level performance. The extent to which these results can be extrapolated to other plant types, such as forest trees or crops that often have different growth forms and associated SAS responses than arabidopsis, still needs to be explored. However, if the pattern that small difference in SAS responses affect competitive ability with limited or no impact on monoculture performance extends to crops, it could provide useful breeding targets.
Promising avenues
In this study, we described plasticity as trait responses to a range of changing environmental conditions during the lifetime of the individual plant. Differences in degree of plasticity were described by different shapes of the response curves (Fig. 2), and these differences in response curves allowed quantification of how variation in trait responses would affect plant competitiveness. The sensitivity of plant competiveness to small differences in plastic responses due to mutations (i.e. use of arabidopsis mutants such as hfr1-5 and rot3-1) suggest that selection on finely tuned signal transduction pathways is likely. Quantifying more contributors to the signal transduction pathway that influence plastic responses could be a next step in breeding programmes that search for optimal plastic genotypes to deal with changing environments.
A next step with this model approach could be to analyse how natural selection could have acted on plastic responses in plants. Analysing how natural selection could have acted on trait values has often been approached by using game-theoretical models (Falster and Westoby, 2003; McNickle and Dybzinski, 2013). However, analysing selection for plastic responses is challenging because a model system needs to consider (1) the possibility of a single genotype to express multiple phenotypes; (2) the dynamic interaction between phenotypic changes and changes in environmental conditions; and (3) variation in plasticity that is incorporated by a single parameter. The model system presented here complies with these three requirements, because genotypes varied in their plastic responses due to different values of a single parameter. In that manner, it extends previous game-theoretical studies (e.g. Dybzinski et al., 2013; Vermeulen, 2015) by explicitly considering dynamic environmental trait responses rather than environment-dependent trait values. We thus argue that our approach provides a novel way to analyse natural selection for plasticity (Bongers et al., 2014).
Conclusions
Here we illustrated that substantial difference in competitiveness may arise between phenotypes with slightly different SAS response levels, due to the amplification of plant growth differences by small changes in plant phenotype. These findings indicate that selection pressure could have played a role in fine-tuning the sensitive shade avoidance responses found in plants.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Materials and Methods: detailed information of model description. Video: visualization of arabidopsis plants growing in low- and high-density vegetation stands, simulated by the functional–structural plant model. Table S1: overview of all used parameters in the FSP model of arabidopsis, with parameter description, unit, value and source of parameter value. Figure S1: experimentally obtained petiole elongation response curves from five arabidopsis genotypes. Figure S2: dynamically changing R:FR and lamina area index (LAI) during the development of a high-density arabidopsis stand (1600 plants m–2). Figure S3: simulated above-ground biomass of an individual plant related to the plastic response value of the plants in the monoculture. Figure S4: simulated total above-ground biomass of an individual plant growing in monoculture or mixture.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. de Vries for help with model development, and M. F. Fouce Fernández and members of the UU Plant Ecophysiology team for help with harvesting plant experiments. We also thank two anonymous reviewers for their constructive comments on an earlier version of this manuscript. This work was supported by the Netherlands Organisation for Scientific Research [ALW grant no. 821.01.014 to N.P.R.A, VIDI grant no. 86412.003 to R.P]. All authors designed the research; F.J.B. performed experiments, model simulations and data analysis; all authors interpreted the data; F.J.B. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
LITERATURE CITED
- Anten NPR. 2005. Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Annals of Botany 95: 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anten NPR, Vermeulen PJ. 2016. Tragedies and crops: understanding natural selection to improve cropping systems. Trends in Ecology and Evolution 31: 429–439. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Schieving F, Werger MJA. 1995. Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonous species. Oecologia 101: 504–513. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Pierik R. 2017. The shade-avoidance syndrome: multiple signals and ecological outputs. Plant, Cell and Environment 40: 2530–2543. [DOI] [PubMed] [Google Scholar]
- Bardgett RD, Mommer L, De Vries FT. 2014. Going underground: root traits as drivers of ecosystem processes. Trends in Ecology and Evolution 29: 692–699. [DOI] [PubMed] [Google Scholar]
- Bongers FJ, Evers JB, Anten NPR, Pierik R. 2014. From shade avoidance responses to plant performance at vegetation level: using virtual plant modelling as a tool. New Phytologist 204: 268–272. [DOI] [PubMed] [Google Scholar]
- Bradshaw AD. 1965. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13: 115–155. [Google Scholar]
- Casal JJ. 2012. Shade avoidance. The Arabidopsis Book 10: e0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Smith H. 1988a Persistent effects of changes in phytochrome status on internode growth in light-grown mustard: occurrence, kinetics and locus of perception. Planta 175: 214–220. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Smith H. 1988b The loci of perception for phytochrome control of internode growth in light-grown mustard: promotion by low phytochrome photoequilibria in the internode is enhanced by blue light perceived by the leaves. Planta 176: 277–282. [DOI] [PubMed] [Google Scholar]
- Dudley SA, Schmitt J. 1996. Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. American Naturalist 147: 445–465. [Google Scholar]
- Dybzinski R, Farrior CE, Ollinger S, Pacala SW. 2013. Interspecific vs intraspecific patterns in leaf nitrogen of forest trees across nitrogen availability gradients. New Phytologist 200: 112–121. [DOI] [PubMed] [Google Scholar]
- Dybzinski R, Farrior C, Wolf A, Reich PB, Pacala SW. 2011. Evolutionarily stable strategy carbon allocation to foliage, wood, and fine roots in trees competing for light and nitrogen: an analytically tractable, individual-based model and quantitative comparisons to data. American Naturalist 177: 153–166. [DOI] [PubMed] [Google Scholar]
- Evers JB. 2016. Simulating crop growth and development using functional–structural plant modeling. In: Hikosaka K, Niinemets Ü, Anten N, eds. Canopy photosynthesis: from basics to applications. Dordrecht: Springer, 219–236. [Google Scholar]
- Evers JB, Bastiaans L. 2016. Quantifying the effect of crop spatial arrangement on weed suppression using functional–structural plant modelling. Journal of Plant Research 129: 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers JB, Vos J. 2013. Modeling branching in cereals. Frontiers in Plant Science 4: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falster DS, Westoby M. 2003. Plant height and evolutionary games. Trends in Ecology and Evolution 18: 337–343. [Google Scholar]
- Farrior CE, Dybzinski R, Levin SA, Pacala SW. 2013. Competition for water and light in closed-canopy forests: a tractable model of carbon allocation with implications for carbon sinks. American Naturalist 181: 314–30. [DOI] [PubMed] [Google Scholar]
- Gautier H, Mech R, Prusinkiewicz P, Varlet-Grancher C. 2000. 3D architectural modelling of aerial photomorphogenesis in white clover (Trifolium repens L.) using L-systems. Annals of Botany 85: 359–370. [Google Scholar]
- Huber H, de Brouwer J, von Wettberg EJ, During HJ, Anten NPR. 2014. More cells, bigger cells or simply reorganization? Alternative mechanisms leading to changed internode architecture under contrasting stress regimes. New Phytologist 201: 193–204. [DOI] [PubMed] [Google Scholar]
- Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R. 2010. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proceedings of the National Academy of Sciences, USA 107: 22740–22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaer LP, Weisbach AN, Weiner J. 2013. Root and shoot competition: a meta-analysis. Journal of Ecology 101: 1298–1312. [Google Scholar]
- Kozuka T, Kobayashi J, Horiguchi G et al. . 2010. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiology 153: 1608–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstler G, Falster D, Coomes DA et al. . 2016. Plant functional traits have globally consistent effects on competition. Nature 529: 1–15. [DOI] [PubMed] [Google Scholar]
- Maddonni GA, Otegui ME, Andrieu B, Chelle M, Casal JJ. 2002. Maize leaves turn away from neighbors. Plant Physiology 130: 1181–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNickle GG, Dybzinski R. 2013. Game theory and plant ecology. Ecology Letters 16: 545–555. [DOI] [PubMed] [Google Scholar]
- Olsen J, Kristensen L, Weiner J, Griepentrog HW. 2005. Increased density and spatial uniformity increase weed suppression by spring wheat. Weed Research 45: 316–321. [Google Scholar]
- Pierik R, de Wit M. 2014. Shade avoidance: phytochrome signalling and other aboveground neighbour detection cues. Journal of Experimental Botany 65: 2815–2824. [DOI] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, De Kroon H, Voesenek LACJ. 2003. Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant, Cell and Environment 26: 1229–1234. [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193: 30–50. [DOI] [PubMed] [Google Scholar]
- Sasidharan R, Chinnappa CC, Staal M et al. . 2010. Light quality-mediated petiole elongation in Arabidopsis during shade avoidance involves cell wall modification by xyloglucan endotransglucosylase/hydrolases. Plant Physiology 154: 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt J, Mccormac AC, Smith H. 1995. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. American Naturalist 146: 937–953. [Google Scholar]
- Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5: 537–542. [DOI] [PubMed] [Google Scholar]
- Vermeulen PJ. 2015. On selection for flowering time plasticity in response to density. New Phytologist 205: 429–439. [DOI] [PubMed] [Google Scholar]
- Vos J, Evers JB, Buck-Sorlin GH, Andrieu B, Chelle M, de Visser PHB. 2010. Functional–structural plant modelling: a new versatile tool in crop science. Journal of Experimental Botany 61: 2101–2115. [DOI] [PubMed] [Google Scholar]
- Weijschede J, Berentsen R, De Kroon H, Huber H. 2008. Variation in petiole and internode length affects plant performance in Trifolium repens under opposing selection regimes. Evolutionary Ecology 22: 383–397. [Google Scholar]
- de Wit M, Kegge W, Evers JB et al. . 2012. Plant neighbor detection through touching leaf tips precedes phytochrome signals. Proceedings of the National Academy of Sciences, USA 109: 14705–14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit M, Ljung K, Fankhauser C. 2015. Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin levels. New Phytologist 3: 198–209. [DOI] [PubMed] [Google Scholar]
- de Wit M, Keuskamp DH, Bongers FJ et al. . 2016. Integration of phytochrome and cryptochrome signals determines plant growth during competition for light. Current Biology 26: 1–7. [DOI] [PubMed] [Google Scholar]
- Yu Y, Stomph TJ, Makowski D, van der Werf W. 2015. Temporal niche differentiation increases the land equivalent ratio of annual intercrops: a meta-analysis. Field Crops Research 184: 133–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.