Abstract
A price increase of pyrimethamine tablets in the United States has made the life-saving drug difficult to acquire for hospitalized patients who need it most. We report the successful use of a pyrimethamine oral suspension compounded from an economical bulk powder in a patient with acute toxoplasmic encephalitis.
Keywords: bulk powder, encephalitis, oral suspension, pyrimethamine, Toxoplasma gondii
A staggering price increase of the pyrimethamine tablets in the United States has hindered accessibility of the life-saving drug for many medically vulnerable patients. Hospitals across the nation have removed the costly tablets from their inpatient drug formularies, limiting access to outpatient use. Pyrimethamine bulk powder, US Pharmacopeia (USP) grade, offers an affordable alternative accessible to hospitalized patients in need of a firstline regimen for toxoplasmic encephalitis (TE). However, in vivo efficacy of the powder prepared as an oral suspension for use in the management of TE has not been reported. We report a case of acute TE in a patient with acquired immunodeficiency syndrome (AIDS) and an observed dermatologic reaction to trimethoprim-sulfamethoxazole (TMP-SMX) who successfully responded to a preferred treatment regimen containing pyrimethamine suspension from an economical bulk powder.
CASE REPORT
In June 2017, a 55-year-old man, originally from El Salvador, with AIDS presented to an outside clinic after experiencing severe headaches and left-sided weakness with worsening cognitive dysfunction over the previous 3 months. He admitted to noncompliance with antiretroviral therapy (ART) since starting in 2013. His CD4+ lymphocyte count was 41 cells/mm3 (2.3%) with a viral load of 1.5 million copies/mL. A computed tomography (CT) scan of the head was recommended at clinic, but the patient refused due to lack of health insurance and returned home. Two weeks later, he was brought to an outside emergency department after being found unresponsive and actively seizing. Initial CT scan of the head revealed bilateral hypodensities and vasogenic edema. Intravenous (IV) dexamethasone and an anticonvulsant were initiated. Additionally, IV TMP-SMX was started due to a high suspicion for TE. Serologic testing for Toxoplasma gondii immunoglobulin G was later found to be positive. On day 5 of the outside hospital admission, his mental status worsened, and a repeat CT scan of the head showed stable brain edema with a midline shift. A new diffuse generalized blanching erythematous rash was also observed on the patient. As a result, he was transferred to our institution for escalation of care.
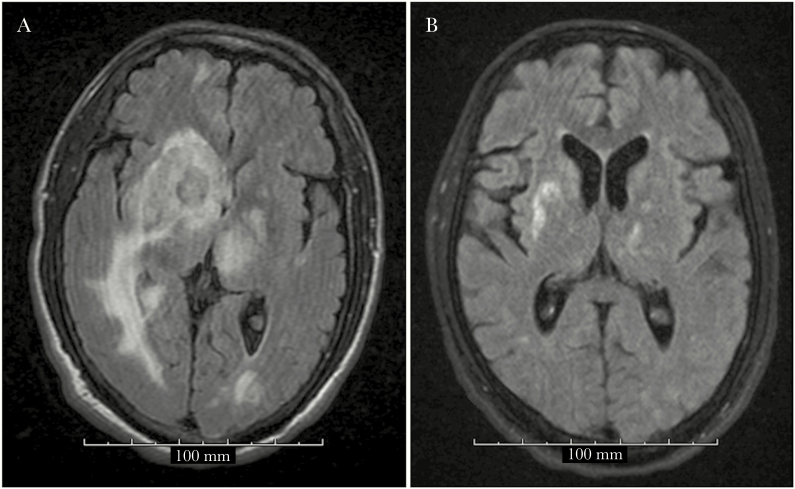
Upon transfer, the infectious diseases service was consulted and observed the patient to be awake but nonresponsive. A lumbar puncture could not be performed due to the midline shift, and his unresponsive state precluded consent for a brain biopsy. Upon rechallenge of TMP-SMX, the diffuse blanching erythematous rash worsened, involving the face, trunk, and extremities. Therefore, TMP-SMX was discontinued due to concern for a drug-related reaction. Attempts were made to obtain pyrimethamine tablets. However, an earlier change to nonformulary status due to high acquisition costs of the tablets limited the supply from the inpatient pharmacy. The patient was eligible to receive pyrimethamine tablets through the patient assistance program Daraprim Direct, but only upon discharge from the hospital. The patient remained unresponsive, requiring an enteric tube due to the inability to swallow from his altered mental status. As a result, on day 6, an alternative treatment for TE was initiated using high-dose atovaquone 1500-mg oral suspension twice daily. Magnetic resonance imaging (MRI) of the brain revealed numerous lesions in the supra- and infratentorial brain parenchyma, heterogeneous peripheral enhancement, and marked surrounding vasogenic edema with a 5-mm left midline shift (Figure 1A). In the interim, an oral suspension from pyrimethamine bulk powder was ascertained, offering the patient a procurable and cost-effective alternative to the tablets while hospitalized. On day 10, the patient remained awake but unresponsive. The pyrimethamine bulk powder (USP grade, Medisca Pharmaceuticals) was acquired through the inpatient pharmacy, and an oral suspension was prepared. The patient (62 kg) was given a 200-mg loading dose of pyrimethamine followed by daily doses of 75 mg, along with oral leucovorin 25 mg daily and IV clindamycin 600 mg every 6 hours (pyrimethamine, leucovorin, and clindamycin [PLC] regimen). The high-dose atovaquone was discontinued, and dapsone was started for the prevention of Pneumocystis pneumonia (PCP). A brain biopsy revealed necrotic brain tissue with innumerable Toxoplasma gondii bradyzoites and tachyzoites.
Figure 1.
Pre–pyrimethamine, leucovorin, and clindamycin (PLC) regimen: Magnetic resonance imaging (MRI) showing multiple bilateral T2 isodense round lesions with surrounding extensive edema and midline shift to the left (A). Post-PLC regimen: MRI showing interval decrease in lesion sizes and interval near complete resolution of the previously seen vasogenic edema (B).
On day 14 (day 4 of the PLC regimen), the patient appeared more alert, answering questions and following basic commands for the first time during his hospitalization. Due to progressing leukopenia and thrombocytopenia thought to be due to pyrimethamine, leucovorin was increased to 75 mg daily, and dapsone prophylaxis was changed to atovaquone because of concerns of contributing agranulocytosis. A repeat MRI without contrast on day 23 (day 14 of the PLC regimen) showed an interval decrease in the size of the multiple necrotic lesions, a marked decrease in the surrounding vasogenic edema, and resolution of mass effect and midline shift (Figure 1B). Interval re-expansion of the lateral and third ventricles was also noted. Given his clinical and radiological improvements, ART using dolutegravir, tenofovir disoproxil fumarate, and emtricitabine was initiated on day 24 (day 15 of the PLC regimen). The patient was then discharged to a long-term care facility and supplied with pyrimethamine tablets via Daraprim Direct to finish a tentative 6-week treatment course for TE. The patient successfully completed induction therapy with continued radiographic and clinical improvement and was transitioned to pyrimethamine maintenance therapy during immune reconstitution.
DISCUSSION
In August 2015, Turing Pharmaceuticals (now Vyera Pharmaceuticals) acquired exclusive rights to Daraprim (pyrimethamine 25-mg tablets) in the United States and increased the price of the drug more than 5000%, from $13.50 to $750 per tablet. With this initial price increase, a 6-week treatment induction phase for TE would cost up to $94 000 for pyrimethamine tablets alone. As a result, many hospitals across the nation have removed the tablets from inpatient formularies, limiting its availability to outpatient specialty 340B pharmacies. After appreciable protest and criticism from various channels, Turing Pharmaceuticals reduced the price of the tablets by 50% and currently offers an outpatient access program known as Daraprim Direct. However, hospitalized patients without insurance are ineligible, limiting the supply of the tablets to those discharged from inpatient stay.
Pyrimethamine is a highly selective agent for the tachyzoite form of Toxoplasma gondii with efficient brain parenchyma penetration [1]. The synergistic activity of pyrimethamine when used in combination with sulfadiazine or clindamycin is highly effective for TE in AIDS patients [2, 3] and is strongly recommended for initial therapy [4]. Mortality due to TE in immunodeficient patients reaches close to 100% if treatment is delayed [5]. Therefore, timely initiation of the most optimal regimen for patients hospitalized with acute TE is critical. Limited access to preferred, firstline agents for acute TE may compel clinicians to prescribe alternative, less effective therapy. TMP-SMX is the recommended alternative treatment option when pyrimethamine is unobtainable [4], with studies indicating similar efficacy and improved tolerability compared with pyrimethamine-based regimens [6–8]. However, use of TMP-SMX may also be complicated by intolerance or allergic reactions. High-dose atovaquone is moderately recommended as an alternative treatment option, mostly used as salvage therapy for acute TE, supported by a limited quality of evidence [4].
The stability of pyrimethamine compounded into a liquid dosage formulation has been demonstrated out to 91 days [10]; however, this method used crushed pyrimethamine tablets. Lewis et al. were the first to investigate the stability of pyrimethamine oral suspension compounded from a USP grade bulk powder (Medisca Pharmaceuticals) [11]. A stability-indicating high-performance liquid chromatographic (HPLC) method with ultraviolet (UV) detection was performed to determine the beyond-use date of the oral suspension compounded from this bulk pyrimethamine powder. Six batches of 2-mg/mL concentrated oral suspension compounded in amber bottles were equally divided and stored at room (20°C–21.4°C) and refrigerated temperatures (4.3°C–5.2°C) for at least 30 days. The concentration of pyrimethamine in all samples of the compounded oral suspension was tested and found to be stable within the 90% to 110% labeled potency claim for up to 90 days when refrigerated and 48 days at room temperature. Additionally, the HPLC-UV method met stability-indicating criteria set in USP General Chapter <1225>, demonstrating that pyrimethamine bulk powder is >99% pure [12]. Therefore, 75 mg of powder is equal to 75 mg of pyrimethamine. Using this data, a 5-g bottle of pyrimethamine USP bulk powder (Medisca Pharmaceuticals; Lot no. 138577H; expiration date: 4/2021) was acquired through the inpatient pharmacy, and a 2-mg/mL liquid suspension was prepared using equal portions of Ora-Plus and Ora-Sweet (480 mg/240 mL). The 5-g bulk powder cost the inpatient pharmacy $13.50 and would supply more than 6 weeks of TE therapy based on the ordered daily dose of pyrimethamine for this patient.
Pyrimethamine is a folic acid antagonist. Increased doses of leucovorin (folinic acid) were administered in this case to abate hematologic toxicities known to be associated with pyrimethamine, including leukopenia and thrombocytopenia [9]. Plasma pyrimethamine concentrations were not drawn in this patient. However, future studies comparing pyrimethamine plasma concentrations obtained from the tablets vs compounded suspension would offer valuable insight into the relationship between drug levels and clinical outcomes.
To our knowledge, this is the first reported case of clinical and radiological improvement in an AIDS patient with TE while using a pyrimethamine oral suspension compounded from bulk powder. A preferred pyrimethamine-based regimen was made available early when clinical outcomes of this hospitalized AIDS patient with TE were the most vulnerable. This accessible and stable formulation offers a significant cost benefit for hospitalized patients requiring timely initiation of pyrimethamine for a life-threatening infection.
Acknowledgments
Financial support. No external funding was received.
Potential conflicts of interest. All authors: no relevant conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Leport C, Meulemans A, Robine D et al. Levels of pyrimethamine in serum and penetration into brain tissue in humans. AIDS 1992; 6:1040–1. [DOI] [PubMed] [Google Scholar]
- 2. Dannemann B, McCutchan JA, Israelski D et al. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med 1992; 116:33–43. [DOI] [PubMed] [Google Scholar]
- 3. Katlama C, De Wit S, O’Doherty E et al. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis 1996; 22:268–75. [DOI] [PubMed] [Google Scholar]
- 4. Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed 31 October 2017. [Google Scholar]
- 5. Montoya JG, Boothroyd JC, Kovacs JA. Toxoplasma gondii. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principle and Practice of Infectious Diseases. Vol. 2, 8th ed Philadelphia, PA: Elsevier Saunders, Inc; 2015:3122–53. [Google Scholar]
- 6. Torres D, Casari S, Speranza F et al. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Italian Collaborative Study Group. Antimicrob Agents Chemother 1998; 42:1346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beraud G, Pierre-Francois S, Foltzer A et al. Cotrimoxazole for treatment of cerebral toxoplasmosis: an observational cohort study during 1994–2006. Am J Trop Med Hyg 2009; 80:583–87. [PubMed] [Google Scholar]
- 8. Kongsaengdao S, Samintarapanya K, Oranratnachai K et al. Randomized controlled trial of pyrimethamine plus sulfadiazine versus trimethoprim plus sulfamethoxazole for treatment of toxoplasmic encephalitis in AIDS patients. J Int Assoc Physicians AIDS Care (Chic) 2008; 7:11–6. [DOI] [PubMed] [Google Scholar]
- 9. Van Delden C, Hirschel B. Folinic acid supplements to pyrimethamine-sulfadiazine for Toxoplasma encephalitis are associated with better outcome. J Infect Dis 1996; 173:1294–5. [DOI] [PubMed] [Google Scholar]
- 10. Nahata MC, Morosco RS, Hipple TF. Stability of pyrimethamine in a liquid dosage formulation stored for three months. Am J Health Syst Pharm 1997; 54:2714–6. [DOI] [PubMed] [Google Scholar]
- 11. Lewis PO, Cluck DB, Huffman JD et al. Stability of a pyrimethamine suspension compounded from bulk powder. Am J Health Syst Pharm 2017; 74:2060–4. [DOI] [PubMed] [Google Scholar]
- 12. US Pharmacopeia. United States Pharmacopeia and National Formulary (USP 37 – NF 32). Rockville, MD: US Pharmacopeia; 2014. [Google Scholar]