Abstract
Gonadotropin therapy is so central to infertility treatment that it is easy to overlook the considerable discovery and research that preceded production of the effective and safe products available today. The history underpinning this development spans over 300 years and provides a splendid example of how basic animal experimentation and technological advances have progressed to clinical application. Following the discovery of germ cells in 1677 and realizing, in 1870, that fertilization involved the merging of two cell nuclei, one from the egg and one from sperm, it took another 40 years to discover the interplay between hypothalamus, pituitary and gonads. The potential roles of gonadotropin regulation were discovered in 1927. Gonadotropin, such as pregnant mare serum gonadotropin (PMSG), was first introduced for ovarian stimulation in 1930. However, use of PMSG leads to antibody formation, and had to be withdrawn. Following withdrawal of PMSG, human pituitary gonadotropin (HPG) and urinary menopausal gonadotropin (hMG) appeared on the market, and 50 years ago the first child was delivered by our group in 1961 and opened the path to controlled ovarian stimulation. HPG produced good results, but its use came to an end in the late 1980s when it was linked to the development of Creutzfeldt–Jakob disease (CJD). HMG preparations containing a high percentage of unknown urinary proteins, making quality control almost impossible, were then the only gonadotropins remaining on the market. With the availability of hMG, clomiphene citrate, ergot derivatives, GnRH agonists and antagonists, as well as metformin, algorithms were developed for their optimal utilization and were used for the next four decades. Following the first human IVF baby in 1978 and ICSI in 1991, such procedures became standard practice. The main agents for controlled ovarian stimulation for IVF were gonadotropins and GnRH analogues, with batch to batch consistent gonadotropic preparations; methods could be developed to predict and select the correct dose and the optimal protocol for each patient. We are now seeing the appearance of gonadotropin with sustained action and orally active GnRH analogues as well as orally active molecules capable to stimulate follicle growth and inducing ovulation. These new developments may one day remove the need for the classical gonadotropin in clinical work.
Keywords: FSH, Gonadotropins, Human reproduction, Infertility, LH
Introduction
Use of gonadotropin therapy is so central to infertility treatment that it is easy to overlook the considerable discovery and research that preceded production of the effective and safe products available today. The history underpinning this development spans over 300 years and provides a splendid example of how basic animal experimentation and technological advances have progressed to clinical application.
An examination of the history in detail reveals that the road to efficient clinical use of gonadotropins developed a long and tedious pathway, which included many mistakes as well as important scientific encounters. This review will trace these events, from the past through to the present, and conclude with a glance towards the future.
Early understanding of the reproductive process
Our understanding of the human reproductive process began during the Renaissance, particularly in Italy, where Leonardo da Vinci's beautiful drawings illustrated detailed anatomy, and Vesale published his atlas Humani Corporis Fabrica in 1543, which included cross sections of the female pelvis [1]. The tubes, clitoris, vagina and placenta were described by Fallope [2], and the ovary and follicles by De Graaf in 1672 [3]. This was followed by many contributors to our knowledge of the reproductive process. Some of them included: Antoine Van Leeuwenhoek, a microscopist who, amongst his many other discoveries, was the first to conduct rigorous observations on human spermatozoa in 1677, and Karl Ernst von Baer, an embryologist, who, 150 years later, identified the mammalian oocyte during his studies on the ovary of dog. In exhaustive comparative studies, he catalogued the early stages of embryonic development. John Marion Sims (1876), often called the father of modern genecology, is credited for stating: “We must ascertain that sperm is present and enters the cervical canal with favorable secretions to vitality of spermatozoa” [4], and William Pancoast was the first doctor to perform insemination using a donor other than a husband in the late 1800s.
It was, however, Arnold Berthold who did the first study of hormones and their role in aggression [5] and it was E. Starling and W. Bayliss who introduced the term ‘hormone’ [6] (Fig. 1).
Figure 1.

The fathers of human reproduction
The twentieth century
Further diagnostic methods began to emerge at the beginning of the twentieth century; Huhner recommended the postcoital test in 1913 [7], and methods of tubal insufflation were devised to diagnose tubal obstruction [8]. Nevertheless, management of infertility remained empirical right up to the middle of the twentieth century. Knowledge about the physiological mechanisms governing reproduction was incomplete, and the therapeutic armamentarium was inadequate.
Early understanding of the hypothalamic–pituitary–ovarian axis
In the Western world, the first experimental evidence suggesting that the pituitary had a role in regulating the gonads stemmed from the studies of Crowe and his co‐workers. They showed that partial pituitary ablation resulted in atrophy of the genital organs in adult dogs, and a persistence of infantilism and sexual inadequacy in puppies [9]. Thus, by 1910 these early studies launched the idea that the reproductive organs were governed by the pituitary gland. Two years later, Aschner confirmed these findings and also postulated that pituitary function depends upon the function of higher centers in the brain. He observed that men and women with diseases, tumors or injuries of the hypophysis, pituitary stalk and centers including and above the medulla oblongata suffered hypopituitarism and, consequently, gonadal atrophy [10]. He further demonstrated that sectioning of the pituitary stalk affected the genital organs and, therefore, hypothesized that pituitary extracts may affect the gonads. He postulated that their use might have practical applications.
Another 15 years were to elapse before two different groups independently discovered the ‘gonadotrophic principle’. In 1926, Smith showed that daily implants of fresh anterior pituitary gland tissue from mice, rats, cats, rabbits and guinea pigs into immature male and female mice and rats rapidly induced precocious sexual maturity, marked enlargement of the ovaries and superovulation [11, 12]. In the same year, Zondek implanted anterior pituitary glands from adult cows, bulls and humans into immature animals, and this evoked a rapid development of sexual puberty [13]. These pioneering experiments revealed that ovarian function is regulated by the pituitary.
In 1927 Smith demonstrated that hypophysectomized immature male or female rats and mice failed to mature sexually, and that removal of the pituitary gland from adult animals without injury to the brain resulted in profound atrophy of genital organs, rapid regression of sexual characteristics and total loss of reproductive function in both sexes [14, 15]. Only 2 years later, Zondek proposed the idea that the pituitary secretes two hormones that stimulate the gonads. He named these biological substances ‘Prolan A’ and ‘Prolan B’. He postulated that Prolan A stimulated follicular growth, that Prolan A together with Prolan B stimulated the secretion of ‘foliculin’, and that Prolan B induced ovulation, the formation of the corpus luteum and the secretion of lutein and foliculin. These two hormones induced glandular transformation of the endometrium, with endometrial proliferation, and also caused changes in the vaginal epithelium. Zondek realized that the dynamics of Prolan A secretion by the anterior pituitary and the correct timing of Prolan B discharge are responsible for the rhythm of ovarian function: this in turn controlled the proliferation and function of the endometrium to create optimal conditions for nidation of the fertilized egg (Fig. 2). If we merely change the names of Prolan A and B to follicle stimulating hormone (FSH) and luteinising hormone (LH), and the names of foliculin and lutein to estrogen and progesterone, we can see that by 1930 Zondek had described the pituitary–gonadal relationship as we know it today [16].
Figure 2.
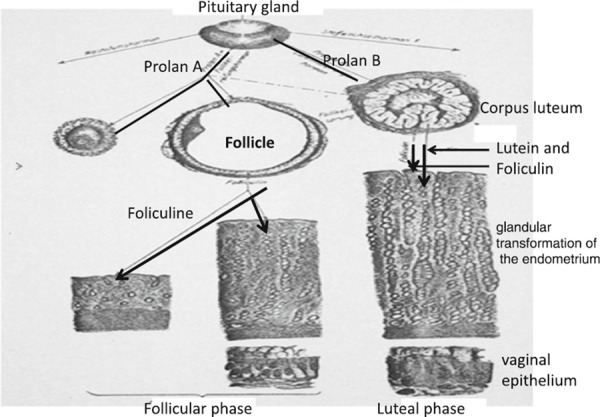
Schematic view of endocrine regulation of the ovarian cycle as seen by Bernard Zondek in 1929
The discovery of human chorionic gonadotropin
In 1927 Ascheim and Zondek [17] demonstrated that the blood and urine of pregnant women contained a gonad‐stimulating substance; injecting this substance subcutaneously into intact immature female mice produced follicular maturation, luteinization and hemorrhage into the ovarian stroma. This became known as the Ascheim‐Zondek Pregnancy test. Zondek believed that this gonadotropic substance was produced by the anterior pituitary. Subsequent work by Seegar Jones et al. [18] showed that this gonadotropin was produced in vitro in placental tissue culture, proving conclusively that the placenta and not the pituitary was responsible for the elaboration of the hormone. This gonad‐stimulating property was exhibited by the chorionic villi and was especially marked in the cytotrophoblastic Langerhans cells. Marius Tausk's book on the history of Organon [19] describes the gonadotropic hormone hCG (extracted from human placentas) as being very similar to the pituitary hormone Prolan B, or LH. Organon launched this extract on the market in 1931, and it continues to be commercially available even today.
Pregnant mare serum gonadotropin
These early discoveries revealing the physiological action of gonadotropins in the normal ovarian cycle tempted many scientists to seek gonadotropic extracts with sufficient purity to allow their use in the treatment of infertile patients suffering from gonadotropin deficiency.
Pregnant mare serum gonadotropin (PMSG), secreted by structures known as endometrial cups in pregnant mares, was first described by Cole and Hart in 1930 [20]. PMSG was prepared by collecting blood from pregnant mares around the 65th day of pregnancy. In 1938 commercial preparations of PMSG appeared on the market. Clinical trials in women demonstrated an ovarian response to these gonadotropins [21], but attempts to induce ovulation produced inconsistent results and failed to result in progestational bleeding or progestational changes in the endometrium (as judged by endometrial biopsy studies) or in pregnancy [22, 23].
The concept of the ‘two‐step protocol’ was introduced in 1941: ovarian stimulation using gonadotropins (PMSG, or hog or sheep pituitary gonadotropins) to stimulate follicular growth and development, followed by the induction of ovulation using hCG [24]. In 1945 Hamblen et al. [25] defined the ideal treatment: ‘To permit effective therapy of hypofunctioning ovaries, a gonadotropin should evoke, in sequence, follicle stimulation, ovulation and corpus luteum development, and these phenomena should be in physiologic order compatible with fertility and conception.’ They demonstrated that administration of PMSG during the follicular phase, followed by the application of hCG 12–18 days later, resulted in secretory endometrium and pregnancies following correctly planned coitus.
In 1942 Ostergaard [26], Zondek and Sulman [27] demonstrated formation of ‘antihormones’ following the use of non‐primate gonadotropin extracts in women and in 1948 Leathem and Rakoff [28] suggested that non‐primate gonadotropins were of limited clinical value.
Human menopausal gonadotropin (hMG)
In 1950, we successfully extracted hMG from urine of post menopausal women using kaolin for adsorption and acetone for precipitation of proteins (gonadotropins). This method was accepted by many laboratories and used successfully for many years with minor variations. In 1953, we successfully used this extract of hMG for ovarian stimulation in female hypophisectomized infantile rats resulting in a linear increase in ovarian and uterine weights (Fig. 3). In male hypophisectomized infantile rats this same extract induced stimulation of leydig cells to produce testosterone, resulting in a linear increase of ventral prostate weight (Fig. 3). Stimulation with hMG also resulted in full spermatogenesis in male hypophysectomized infantile rats. Following these results, we predicted its clinical use [29]. However, in order to initiate clinical trials it was necessary to obtain large amounts of menopausal urine and to develop industrial extraction and purification methods.
Figure 3.
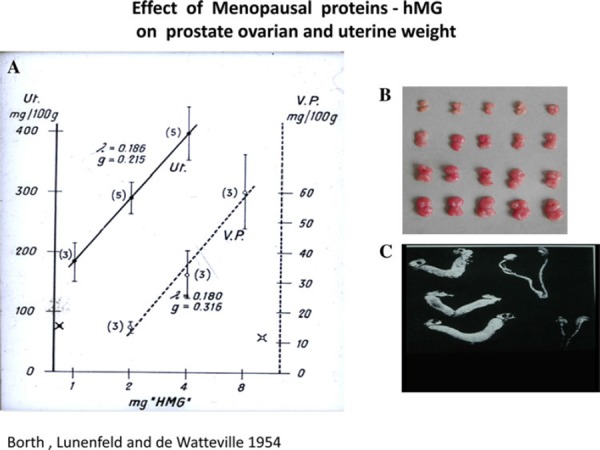
hMG administration to female hypophisectomized infantile rats results in linear increase on ovarian (a, b) and uterine weights (c) and in male hypophisectomized infantile rats this same extract results in a linear increase of ventral prostate weight (a)
In 1957, I contacted Pietro Donini, then a senior scientist at Serono Institute, who had already extracted hMG. He invited me to visit Istituto Farmacologico Serono and discuss with their board of directors the possibility of mass production of hMG and the initiation of clinical trials. I presented our findings to the board, but could not convince them to embark on this joint endeavor.
Since 1952, the Vatican has a major share of the Istituto Farmacologico Serono and, fortunately, one of the directors, Don Giulio Pacelli, a representative of the Vatican and nephew of the Pope, was interested in my proposal. Following long and interesting discussions with me and Dr. Donini, Pacelli returned to the board, declaring that old age homes of nuns would provide the required urine, and thus convinced Serono to undertake this project. Approximately 300 women contributed to these collection centers and this permitted Serono to produce enough hMG to start clinical trials. The purity of the available preparations was only 5%, containing both FSH and LH. However, in the absence of an alternative, the hMG preparation was accepted by both the regulatory agencies and the scientific community.
In 1960, we reported that the use of this preparation in humans led to the expected and desirable changes in endometrium, vaginal epithelium and steroid excretion [30]. In 1961, we noted that amenorrheic hypogonadotropic women needed 6.8–13.6 mg (75–150 IU/day) of this hMG product, known as Pergonal®, depending on individual sensitivity, in order to stimulate their ovaries to produce estrogens [31]. Thereafter, we reported the first successful induction of ovulation followed by pregnancies in hypogonadotrophic anovulatory women, using a sequential step‐up/step‐down regimen (Fig. 4). The starting dose in these women was 240 mg of the international reference preparation of human menopausal gonadotropins (IRP‐hMG). This corresponded to 150 IU daily, was then increased to 360 mg (225 IU), then to 480 mg IRP‐hMG (300 IU), and gradually reduced to 360 mg and finally to 240 mg IRP‐hMG daily. Ovulation was induced by administration of 10,000 IU of hCG followed by 10,000 and 5,000 IU of hCG on consecutive days (Fig. 4). This hCG dose was sufficient to maintain corpus luteum function until endogenous hCG appeared about 13 days later [32, 33, 34]. These results were confirmed by Palmer and Dorangeon [35] as well as by Salomon and Netter [36] in France, Rosenberg et al. [37] and Taymor et al. [38] in the USA, as well as by Tanaka in Japan [39].
Figure 4.
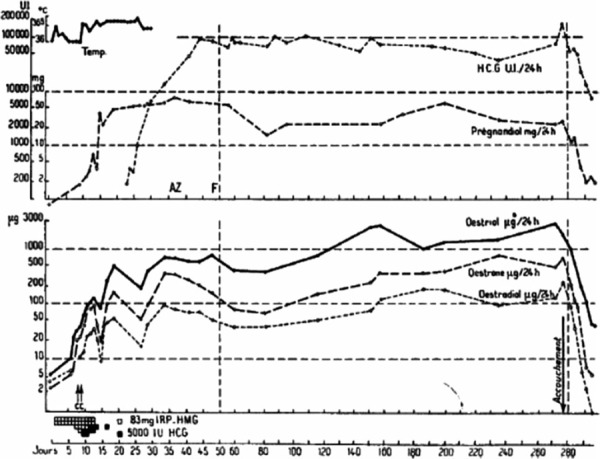
Hormonal pattern during follicular phase, ovulation, luteal phase and pregnancy following stimulation with hMG (step‐up, continuous and step‐down) of a primary amenoreic patient with hypopituitary hypogonadism
Following numerous reports of the successful induction of ovulation, Pergonal® 75 was registered in Israel in 1963 and in Italy in 1965. A similar preparation, Humegon®, was marketed by Organon in the Netherlands in 1963 and its first official registration was obtained in 1966. One ampoule of these hMG preparations contained approximately 75 IU of FSH and 75 IU of LH as measured by standard bioassays.
In 1972, the World Health Organization (WHO) Expert Committee of Biological Standardization, under my chairmanship, defined the international unit (IU) for FSH and the IU for interstitial cell stimulating hormone (ICSH) (LH) as the respective activities contained in 0.2295 mg of the IRP‐hMG [40].
In 1973, WHO convened a scientific group meeting in Geneva, chaired by me. During this meeting, guidelines for the diagnosis and management of infertile couples were developed [41]. The effective daily dose for hypogonadotrophic patients was reported to be in the range of 150–225 IU, and for anovulatory normogonadotrophic patients, 75–150 IU. It was also noted that the ratio of FSH to LH varies in different HMG preparations, but the available evidence indicated that preparations with ratios of 0.1–10 were acceptable therapeutic agents provided that a sufficient total dose of FSH was administered to the patient.
The search for pure preparations
In the early 1970s, clinicians began to voice the opinion that different patient populations and individuals may benefit from tailored treatment regimens, with variations in protocols and in dosages of FSH and LH. Such individually adjusted treatment regimens would require therapeutic gonadotropin preparations that contained pure, or almost pure, FSH and LH.
In the 1960s [42], by using an immuno‐column with polyclonal anti‐hCG antibodies (cross reacting with LH), an FSH preparation could be obtained from hMG. The final product (Metrodin®, Serono Laboratories, Aubonne, Switzerland) contained 150 IU of FSH and approximately 1 IU of LH per mg of protein. Further technological advances made it possible to replace polyvalent antibodies with highly specific monoclonal antibodies. As a result of the improved processing, this FSH preparation (Metrodin® HP, Serono Laboratories, Aubonne, Switzerland) contained less than 0.1 IU of LH activity and less than 5% of unidentified urinary proteins [43]. The specific activity of the FSH was increased from approximately 100–150 IU/mg of protein in purified urinary FSH preparations (Metrodin®) to about 9000 IU/mg protein in the highly purified product (Metrodin® HP). The purity was also increased from 1–2% to 95%. This enhanced purity means that the total amount of injected protein is very small, making the highly purified urinary FSH preparation suitable for subcutaneous administration.
In the past, human‐derived pituitary gonadotropin (hPG) extracted human pituitary glands [44] and menopausal urine were the sole sources for the production of human‐derived gonadotropin preparations; hPG preparations were abandoned when cases of iatrogenic Creutzfeldt–Jakob disease (CJD) were recognized [45, 46], and until 1995, menopausal urine represented the only primary source. With the availability of hMG, urinary FSH, clomiphene citrate, ergot derivatives such as bromocriptine, gonadotropin releasing hormone (GnRH) agonists and antagonists, as well as metformin, diagnostic algorithms were developed and have been used successfully to select treartment protocols (Fig. 5).
Figure 5.
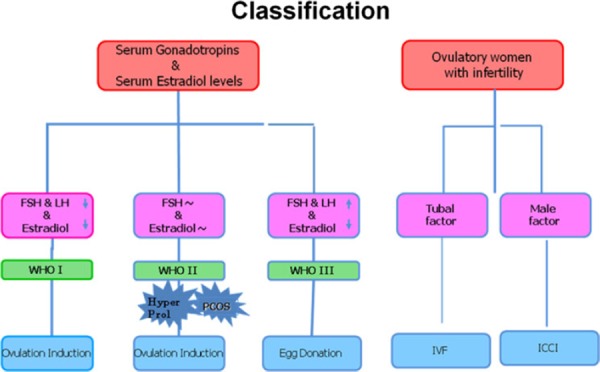
Classification of infertile patients
In vitro fertilization used in treatment for infertility
Following Heape's first rabbit embryo transfers in 1880, Pincus's attempts at IVF in rabbits in 1934 [47], Menkinˈs and John Rockˈs in vitro fertilization of human eggs in 1944 [48], and IVF in rabbits [49], the first birth following IVF in a rabbit [50] was described. Following Shettleˈs publication on a human blastocyst grown in vitro [51], the first pregnancy following in vitro fertilization (IVF) (although resulting in a miscarriage) was published by the Monash research team in Australia [52]. The first baby was born following IVF in a natural cycle in 1978 [53]. However, the first IVF pregnancies in a gonadotropin stimulated cycle were performed only in 1981 [54, 55] (Fig. 6). Protocols were then adopted for controlled ovarian stimulation (COS) using human menopausal gonadotropin (hMG). Exogenous human chorionic gonadotropin (hCG) was used to substitute for the midcycle luteinizing hormone (LH) surge. Laparoscopy for follicular aspiration was scheduled 36–38 h after hCG administration.
Figure 6.
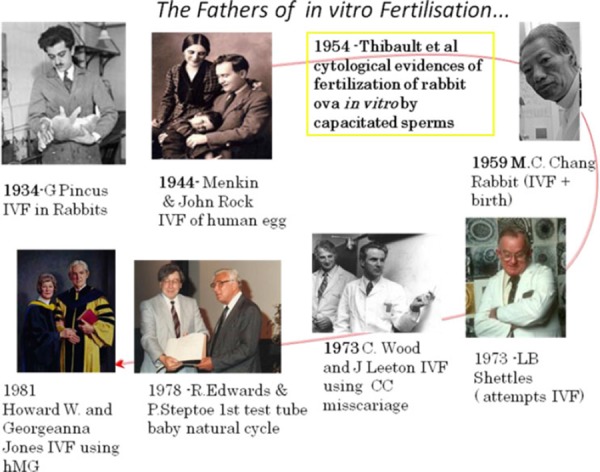
The fathers of in vitro fertilization
One of the main drawbacks with these ‘super‐ovulation’ protocols was the precipitation of a premature LH surge and premature follicular luteinization, which frequently resulted in treatment cancellation. GnRH analogues suppress LH and, to a lesser extent, FSH, referred to as pituitary desensitization and down‐regulation following an initial flareup effect. The use of GnRH‐agonists combined with human menopausal gonadotropins (hMG) treatment was then widely implemented for induction of follicular growth in infertile women for both in vivo and in vitro fertilization. The co‐utilization of GnRH analogues [56] and hMG offered improved clinical control over the process of ovulation induction and the phenomenon of premature luteinization could be essentially eliminated. Since 1988, the main agents for COS associated with IVF have been gonadotropins and GnRH analogues.
The introduction of GnRH agonist and gonadotropin protocols in IVF for patients with tubal factor infertility increased the demand for menopausal urine. The demand for gonadotropins became even greater with the introduction of intracytoplasmic sperm injection (ICSI) for male factor infertility [57]. Increasing the pool for potential patients, it soon became evident that there would never be a sufficient urine supply to cover the increasing world demand for gonadotropins and that there were serious shortcomings in the use of menopausal urine as a source. When the urinary extraction process was started, there were four urine collecting centers: in The Netherlands, Spain, Israel and one in Italy. Altogether, 600 women participated in these collection centers, and each woman was well known by the collectors. If any of these women fell ill or was treated with drugs such as antibiotics, their urine samples were rejected. Over a period of 1 year, these women produced 120,000 L of urine sufficient for the production of 40,000 ampoules with 75 IU FSH, each. This amount was enough for treating hypopituitary–hypogonadotrophic amenorrheic women [WHO Group I (Fig. 5)] worldwide at that time. As indications for the use of gonadotropins increased to patients of WHO Group II who failed to ovulate or become pregnant with clomiphene citrate 10 million liters of urine needed to be collected. The amount necessary increased to 30 million liters following the use of gonadotropins in patients with tubal factor infertility requiring IVF and then further to 70 million liters when ICSI was introduced for male factor infertility.
At the beginning of this millennium, 120 million liters of urine were necessary to satisfy the worldwide need—an increase of 100‐fold, which required 600,000 donors (Fig. 7). In Buenos Aires alone 200,000 women collected 70 million liters of urine resulting in the production of 25 million ampoules of hMG [58]. Urine donors are also recruited from countries in Europe, as well as Korea, China, and India. Since this process is no longer based upon individual collections from well‐known donors, an increasing number of safety measures had to be included in the production process.
Figure 7.
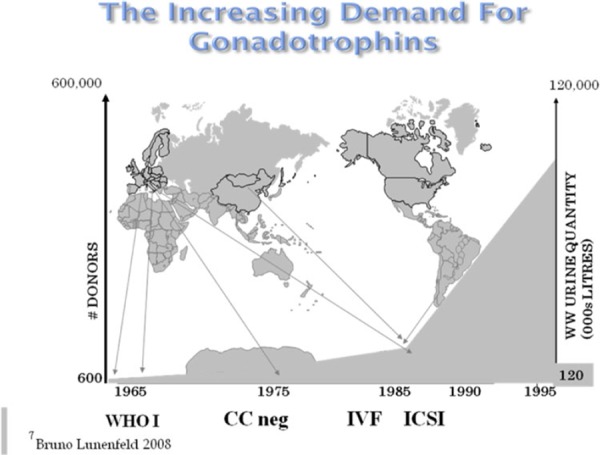
Growing demands for gonadotropins requires expanding urine collections by increased recruitment of donors
Nonetheless, besides the limited source, some of the potential shortcomings of urine extracts are lack of regulatory control; unlike for blood derived products, it is impossible to trace donor source, and cross contamination between urine samples cannot be avoided. Furthermore some decontamination procedures may denature proteins.
In addition to these drawbacks, a further cause for concern was raised when a protease resistant form of prion protein was found in the urine of scrapie‐infected hamsters, bovine spongiform encephalopathy (BSE)–infected cattle and humans suffering from CJD [59]. Although CJD has never been reported in a recipient of urine‐derived fertility hormones, a recent study [60] found protease‐sensitive prion protein in urine‐derived injectable fertility products containing hCG, hMG, and hMG‐HP. This suggests that prions may co‐purify in these products. Intramuscular injection is a relatively efficient route of transmission of human prion disease, and young women exposed to prions can be expected to survive an incubation period associated with a minimal inoculum. The current urinary gonadotropin preparations, however, are most often injected by the subcutaneous route. We are unaware of any risk of prion transmission following a subcutaneous injection.
Van Dorsselaer et al. [60] in their publication claim that the risks of urine‐derived fertility products could now outweigh their benefits, particularly considering the availability of recombinant products.
Concerns about disease transmission led a number of countries including Switzerland and France to apply the precautionary principle: in 1996 the Australian Drug Evaluation Committee published its resolution on replacement of urinary with recombinant gonadotropins in view of their higher standard of purity and safety.
Recombinant DNA technology now allows the production of pharmacologically active pure FSH, LH and hCG preparations in unlimited quantities, minimizing any potential risk of disease transmission via biological contamination.
Recombinant gonadotropins
The general principle behind obtaining recombinant human gonadotropin relies, first of all, upon identification and separation of the appropriate protein molecules. The amino acid sequences of the FSH α‐ and β‐subunits were described in the late 1970s by Rathnam and Saxena [61] and Saxena and Rathnam [62], respectively. In 1985, the gene encoding the FSH beta‐subunit was cloned. Under appropriate conditions, this gene is incorporated into the nuclear DNA of a host cell via a viral vector, using spliced DNA strings containing the FSH gene and segments of bacterial DNA. Chinese hamster ovarian cells (CHO) were selected as recipient cells, since they are easily transfected with foreign DNA, and are capable of post‐translational modification of the molecule by protein folding and glycosylation, and made functional glycoproteins protein production possible. Furthermore, they can be grown in cell cultures on a large scale.
In the manufacture of Gonal F®, Serono used two separate vectors to construct an FSH‐producing cell line, one vector for each subunit [63] Puregon® was manufactured by NV Organon, using a single vector containing the coding sequences of both subunit genes [64].
Following transfection, a genetically stable tranformed cell line producing biologically active FSH was isolated. The CHO line used for the production of Puregon had 150–450 gene copies present. For the purpose of bioproduction, stable cell lines were selected that expressed FSH dimer in relatively abundant amounts. A master cell bank (MCB) was established, which contains identical cell preparations of the clone that was selected on the basis of high FSH productivity. The resulting recombinant FSH (rFSH) was more homogenous than the most highly purified pituitary FSH preparations, providing a basis for clinical use.
Specific cell clones have also been selected for large scale production of recombinant FSH, LH and hCG. The resultant preparations have high purity and high biological potency (FSH 10,000, LH 9,000 and hCG 20,000 IU/mg protein, respectively). These cell preparations are stored in individual vials and cryopreserved until needed. A working cell bank is established by growing cells from a single vial of the MCB, and aliquots of this culture are then cryopreserved in vials. Cells from one or more vials are cultured for each production cycle. Figure 8 summarizes the steps involved in bulk production of rhFSH.
Figure 8.
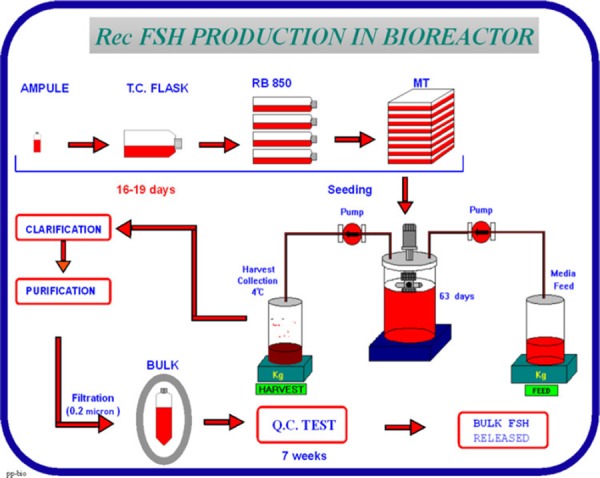
Steps involved in bulk production of rhFSH
The FSH producing CHO cells are grown on microcarriers in a bioreactor, perfused with growth promoting medium for a period of up to 3 months. The cell culture supernatant medium is collected from the bioreactor for isolation of recombinant FSH. The downstream purification process differs for the two commercially available recombinant FSH preparations. The Puregon process uses a series of anion and cation exchange chromatographic steps, hydrophobic chromatography and size exclusion chromatography. The Gonal F process uses a similar series of five chromatographic steps and also includes an immuno‐affinity step with a specific monoclonal antibody. Each purification step is rigorously controlled in order to ensure batch to batch consistency of the purified product.
The world's first recombinant human FSH (rhFSH; follitropin alfa) preparation for clinical use was produced by Serono Laboratories in 1988, and was licensed for marketing in the European Union as Gonal‐F® in 1995. A similar rhFSH (follitropin beta, (Puregon®) product was licensed by Organon Laboratories in 1996 [65].
Recent advances in the manufacturing process for the rFSH (follitropin alfa) result in high batch‐to‐batch consistency in both isoform profile and glycan species distribution. The most significant advantage of this preparation over urinary‐derived FSH is that it permits FSH to be quantified reliably by protein content (mass in mg) rather than by biological activity; this indication of purity offers optimal risk reduction as well as superior quality assurance and batch‐to‐batch consistency. The coefficient of variation for an in vivo bioassay is typically 20%, compared with 2% for physico‐chemical analytical techniques such as size exclusion high‐performance liquid chromatography (SE‐HPLC; [66]). As a result, Serono International now quantify their rFSH (Gonal‐Fw) protein by SE‐HPLC, a precise and robust assay that results in a significant improvement in batch‐to‐batch consistency over batches quantified by the Steelman–Pohley bioassay [67].
During the early 1990s, several IVF programmes reported pregnancies following the use of recombinant FSH to induce ovulation for IVF [68, 69, 70]. In 1997, Agrawal et al. [71] reported the first birth following stimulation of follicular growth in a hypopituitary–hypogonadotrophic woman (WHO Group I) with recombinant FSH and recombinant LH; recombinant hCG was used to induce ovulation. In 2002, Donderwinkel et al. [72] reported a pregnancy following ovulation induction with rFSH in a patient with polycystic ovaries.
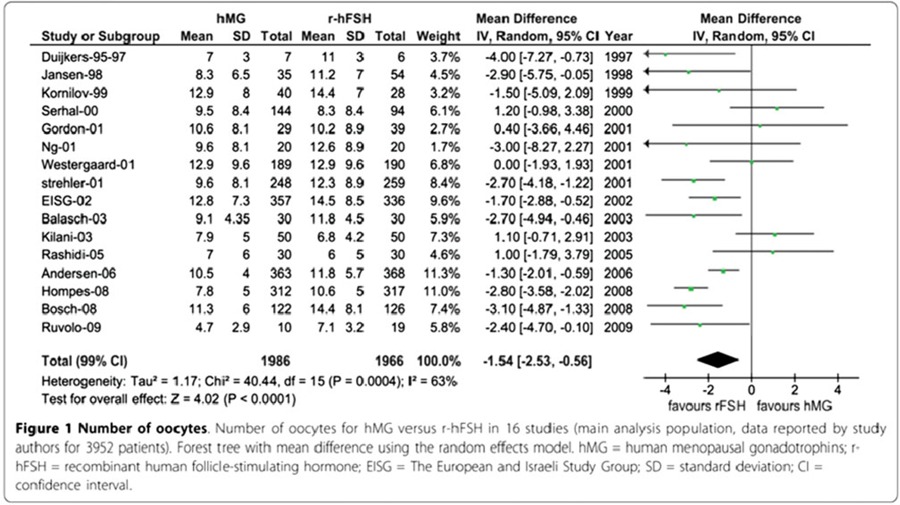
At the present, human menopausal gonadotrophins and recombinant human follicle stimulating hormone are the two main gonadotrophin products utilized for COS in assisted reproductive technologies. To compare the results of both products, the most relevant endpoint directly resulting from ovarian stimulation, and therefore where the drug effect may be estimated with the best sensitivity are the number of oocytes A meta‐analysis involving 4040 patients, and designating the number of oocytes as the endpoint, showed that the treatment with human menopausal gonadotropins is characterized by the utilization of more IU of gonadotropins to produce fewer total oocytes (Tables 1, 2). When considering only fresh transfers, pregnancy rates were similar with both products [73] (Table 3). When comparing urinary and recombinant FSH in every day practice using for example the German IVF directory similar on about 3 million cycles pregnancy rates were better with rFSH in both women below and above 35 years (Fig. 9).
Table 1.
Pregnancy rate for hMG versus r‐hFSH in 16 studies (4040 patients)
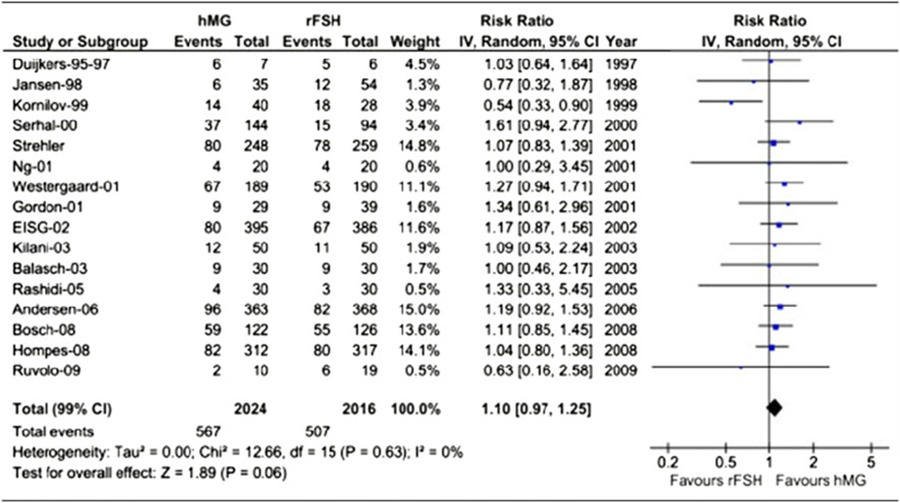
A meta‐analysis of sixteen studies involving 4040 patients. Treatment with human menopausal gonadotrophins resulted in fewer oocytes (−1.54; 95% Cl −2.53 to −0.56; P < 0.0001) compared to recombinant human follicle‐stimulating hormone. When adjusting for baseline conditions, the mean difference estimate was −2.10 (95% Cl −2.83 to −1.36; P < 0.001)
Table 2.
Number of oocytes for hMG versus r‐ hFSH
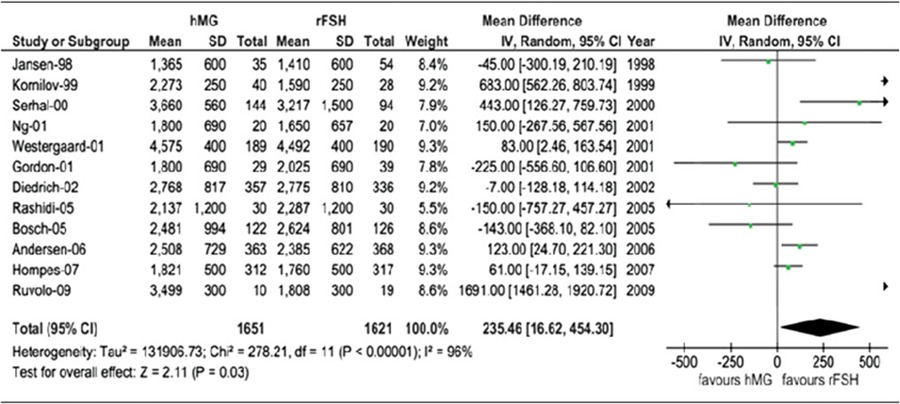
A meta‐analysis of 14 studies (3272 patients) A higher total dose of human menopausal gonadotrophin was necessary (mean difference, 235.46 IU [95% Cl 16.62–454.30; P = 0.03]; standardized mean difference, 0.33 [95% Cl 0.08–0.58; P = 0.01])
Table 3.
Total gonadotrophins dose for hMG versus r‐hFSH
A meta‐analysis of sixteen studies involving 4040 patients. The pregnancy absolute risk difference (RD [hMG‐r‐hFSH]) for fresh transfers was 3% (P = 0.051), and the relative risk 1.10 (P = 0.06). When adjusted for baseline conditions, the relative risk was 1.04 (P = 0.49) and absolute difference was 0.01 (P = 0.34), respectively
Figure 9.
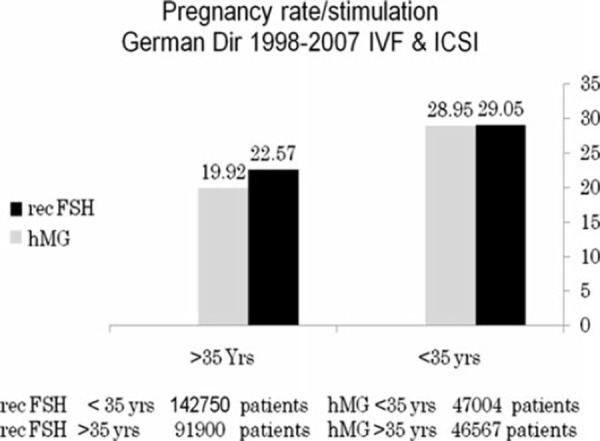
Comparison of pregnancy rate/cycle in patients stimulated with either hMG or rFSH. Calculated for patients below and above the age of 35 years from data published in the German IVF directories, 1998–2007
In 2007, a fixed 2:1 combination of r‐hFSH (150 IU) and r‐hLH (75 IU) Pergoveris was registered in Europe. First experiences in daily use of this combination for the stimulation of follicular development in women with severe FSH and LH deficiency (defined in clinical trials by an endogenous serum LH level of 1.2 IU/l) [74, 75] shows promising results [76]. By avoiding the need for two separate injections or mixing of gonadotropins prior to injection, the 2:1 formulation of follitropin α and lutropin α offers potential benefits for such patients.
Recombinant DNA technology permits the design of potent therapeutically active gonadotropin agonists and antagonists by altering key proteins and carbohydrate regions in the α‐ and β‐subunits of FSH and LH [77]. FSH has a relatively short half‐life, and hCG has a relatively long half‐life. The long half‐life of hCG is in part due to the presence of four serine O‐linked oligosaccharides attached to an extended hydrophilic carboxy‐terminus. Site‐directed mutagenesis and gene transfer techniques made it possible to fuse the carboxy‐terminal extension of hCGb (CTP) to the 30 end of the FSH coding sequence. This newly developed product, corifollitropin α, is a new biological entity developed for patients undergoing fertility treatment and has the same pharmacological activity as pure FSH preparations. It is a recombinant gonadotropin molecule in which the FSH chain is fused with the carboxy‐terminal peptide of the hCG β‐unit, and consequently has a longer elimination half life. One injection is able to keep the circulating FSH activity above the threshold necessary to support multi‐follicular growth for an entire week, thereby sustaining multiple follicular development during the first week of IVF treatment [78]. In fact, one single injection of corifollitropin alfa replaces the first seven daily injections of rFSH. The first live birth following IVF after ovarian stimulation using this chimeric long‐acting human rFSH agonist (rFSH‐CTP) was reported by Beckers [79].
The clinical trial program for corifollitropin alfa, that includes Engage, Ensure and others, is presently the largest in ART and includes more than 2500 patients in 78 IVF centers in 23 countries. These trials demonstrated that the treatment with a single injection of corifollitropin alfa was safe and well tolerated. The number of cases of ovarian hyperstimulation syndrome (OHSS) was low and comparable with that reported for current rFSH regimens. Corifollitropin alfa was also shown to be non‐immunogenic, with no antibody formation or hypersensitivity reaction being observed. In their trial, the conclusion, was that a single dose of corifollitropin alfa can sustain multifollicular growth for an entire week. The dose of 150 μg of corifollitropin alfa is the most appropriate dose for achieving an optimal outcome in terms of the number of oocytes for patients with a body weight >60 kg. In women weighing ≤60 kg a lower dose of 100 mg showed similar results [80, 81, 82, 83, 84]. Corifollitropin alfa is used for ART proceduderes only and is not meant to be used for ovulation induction in un‐ovulatory or PCOD patients.
For companies committed to developing a wider range of innovative medicines for infertility, a generation of orally bioavailable gonadotropin mimetics has been the ‘holy grail’ of drug development research for several years. As knowledge about the activating sites of gonadotropin and GnRH analogues has increased, it has become possible to create small, non‐peptide molecules that induce signal transduction without binding to the extracellular domains of membrane proteins. Such molecules will ultimately be converted into highly potent orally active therapeutic preparations, and will either replace the dimeric glycoprotein hormones or act as antagonists. The first report of a bioactive low molecular weight (LMW) gonadotropin described an FSH‐R antagonist [85] as a potential compound for female contraception. Others reported small molecule modulators of the FSH receptor [86] and LMW compounds (thiazolidinones) with high affinity for the FSH receptor [87, 88].
The first report of a LMW (Org 43553) compound with a nanomolar potency on the LH‐R, showing oral bioavailability and ovulation induction in various animals was reported by van de Lagemaat et al. [89]. Org 43553 is a single, pure synthetic molecule lacking the variability between batches as observed for proteins of urinary origin. It is completely protein free, thus totally excluding the minimal current risk for diseases like Creutzfeldt–Jakob's, originating from recombinant gonadotropin production in the presence of bovine serum in the culture media. This compound has been developed as a safe oral alternative to the current injectable LH/hCG preparations for clinical use to induce ovulation or oocyte maturation for both in vivo and IVF therapy. TVUS measurements and progesterone data showed that this compound induced ovulation within 2 days following 300 and 900 mg doses (Fig. 10). In addition, the compound could also be developed for male indications such as hypogonadism.
Figure 10.
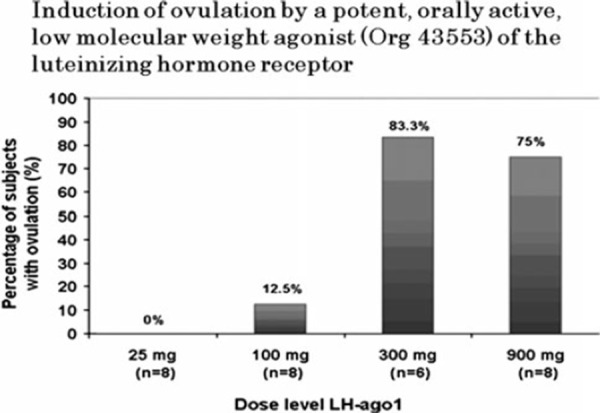
Induction of ovulation by a potent, orally active, low molecular weight agonist (Org 43553) of the luteinizing hormone receptor—% of patients ovulating with different doses of Org 43553
We have come a long way from when Frank Stabler wrote in 1954, “I think I can say that I know of no hormone available to me that will make a woman ovulate naturally.” Today we have a consensus that hormones helped to develop protocols which enabled more the 90% of infertile couples to have their genetic offspring. We now need to encourage our colleagues not only to develop and improve methods to permit even more infertile couples to have healthy offspring, but to encourage them to prevent infertility. Prevention is cheaper than the cure, even when using the mildest stimulation protocols. It may be less profitable to the service providers and pharmaceutical companies, but this must not be our concern. Prevention of STD by the use of condoms, delaying adolescence pregnancies and unwanted pregnancies by contraception and sex education in schools, may all reduce mechanical causes of infertility for which IVF was created. We create better and safer methods for IVF, a major part of budgets toward women's health are spent for these important issues, and a large part of government programs are filled with these issues. Should we not spend some time and money to prevent the conditions which make IVF necessary? ICSI was a blessing, but again we were so impressed with a method that could help nearly all men to have genetic heirs, that we forgot to invest in andrology. Let us promote health education, fight the global epidemic of obesity, promote a healthy life style with proper nutrition and physical exercise and help to reduce stress—this might be a very good investment and may decrease hypothalamic–pituitary and metabolic conditions related to male and female sub fertility or infertility. It is my sincere hope that this short historical overview spanning over 300 years will stimulate us all to continue our efforts to help every couple to enjoy the fruits of parenthood.
Conflict of interest
None to declare.
References
- 1.Vesalius, A. De humani corporis fabrica libri septem . Basileae [Basel]: Ex officina Joannis Oporini; 1543.
- 2.Fallopius, G. Observationes anatomicae, Venise; 1561.
- 3.De Graaf, R. De Mullerium Organis. Paris; 1672.
- 4. Sims HM Sterility and the value of microscope in its diagnosis and treatment, vol 13, 1888. Boston: Trans Am Gynecol Soc; [Google Scholar]
- 5. Soma K. Testosterone and aggression: berthold, birds and beyond. J Neuroendocrinol, 2006, 19, 543–551 10.1111/j.1365‐2826.2006.01440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gregory R. The gastrointestinal hormones: a review of recent advances. J Physiol., 1974, 241, 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huhner M The diagnosis and treatment of sexual disorders in the male and female, 1937. Philadelphia: Davis; [Google Scholar]
- 8. Rubin IC. Non‐operative determination of the patency of Fallopian tubes in sterility. JAMA, 1920, 74, 1017 10.1001/jama.1920.02620150025009 [Google Scholar]
- 9. Crowe SJ, Cushing H, Homans J. Experimental hypophysectomy. Bull Johns Hopkins Hosp, 1910, 21, 127–167 [Google Scholar]
- 10. Aschner B. Ueber die Beziehung zwischen Hypophysis und Genitale. Arch Gynäkol, 1912, 97, 200–227 10.1007/BF01726121 [Google Scholar]
- 11. Smith PE. Hastening of development of female genital system by daily hemoplastic pituitary transplants. Proc Soc Exp Biol Med, 1926, 24, 1311–1333 [Google Scholar]
- 12. Smith PE, Engle ET. Experimental evidence of the role of anterior pituitary in development and regulation of gonads. Am J Anat, 1927, 40, 159 10.1002/aja.1000400202 [Google Scholar]
- 13. Zondek B. Ueber die Funktion des Ovariums. Zeitschr Geburtsh Gynäkol, 1926, 90, 327 [Google Scholar]
- 14. Smith PE. The disabilities caused by hypophysectomy and their repair. JAMA, 1927, 88, 158–161 10.1001/jama.1927.02680290020005 [Google Scholar]
- 15. Smith PE. Hypophysectomy and replacement therapy in the rat. Am J Anat, 1930, 45, 205–274 10.1002/aja.1000450203 [Google Scholar]
- 16. Zondek B. Weitere Untersuchungen zur Darstellung, Biologie und Klinik des Hypophysenvorderlappenhormones (Prolan). Zentralblatt für Gynäkologie, 1929, 14, 834–848 [Google Scholar]
- 17. Ascheim S, Zondek B. Hypophysenvorderlappenhormone und Ovarialhormone im Harn von Schwangeren. Klin Wochenschr, 1927, 6, 13–21 10.1007/BF01736848 [Google Scholar]
- 18. Seegar‐Jones GE, Gey GO, Ghisletta M. Hormone production by placental cells maintained in continuous culture. Bull Johns Hopkins Hosp, 1943, 72, 26–38 [Google Scholar]
- 19.Tausk M. Organon. De geschiedenis van een bijzondere Nederlandse onderneming. In: Nijmegen: Dekker, Van de Vegt; 1978.
- 20. Cole HH, Hart GH. The potency of blood serum of mares in progressive stages of pregnancy in affecting the sexual maturity of the immature rat. Am J Physiol, 1930, 93, 57 [Google Scholar]
- 21. Fevold HL. The gonadotropic hormones. Cold Spring Harbor Symp (Quantitative Biology), 1937, 5, 93 [Google Scholar]
- 22. Hamblen EC. Results of preoperative administration of extract of pregnancy urine: study of ovaries and endometria in hyperplasia of endometrium following such administration. Endocrinology, 1935, 19, 169–178 10.1210/endo‐19‐2‐169 [Google Scholar]
- 23. Hamblen EC. Clinical evaluation of ovarian responses to gonadotropic therapy. Endocrinology, 1939, 24, 848–857 10.1210/endo‐24‐6‐848 [Google Scholar]
- 24. Mazer C, Ravetz E. The effect of combined administration of chorionic gonadotropin and the pituitary synergist on the human ovary. Am J Obstet Gynaecol, 1941, 41, 474–588 [Google Scholar]
- 25. Hamblen EC, Davis CD, Durham NC. Treatment of hypo‐ovarianism by the sequential and cyclic administration of equine and chorionic gonadotropins—socalled one–two cylic gonadotropic therapy. Summary of 5 years’ results. Am J Obstet Gynecol, 1945, 50, 137–146 [Google Scholar]
- 26. Ostergaard E Antigonadotrophic substances, 1942. Copenhagen: Ejnar Munksgaard; [Google Scholar]
- 27.Zondek B, Sulman F. The antigonadotropic factor. Baltimore: Williams & Wilkins; 1942. p. 1–185.
- 28. Leathem JH, Rakoff A. Gonadotrophic hormone therapy in man complicated by antihormone formation. Am J Obstet Gynecol, 1948, 56 (3) 521–526 [DOI] [PubMed] [Google Scholar]
- 29. Borth R, Lunenfeld B, Watteville H. Activité gonadotrope d'un extrait d'urines de femmes en menopause. Experientia, 1954, 10, 266–270 10.1007/BF02157401 [DOI] [PubMed] [Google Scholar]
- 30.Lunenfeld B, Menzi A, Volet B. Clinical effects of human post‐menopausal gonadotropin. In: Fuchs F, editor. Advance abstracts of short communications, 1st International Congress of Endocrinology (Copenhagen 1960).
- 31. Lunenfeld B, Rabau E, Rumney G, Winkelsberg G. The responsiveness of the human ovary to gonadotropin (Hypophysis III). Proc Third World Cong Gynecol Obstet (Vienna), 1961, 1, 22 [Google Scholar]
- 32.Lunenfeld B, Sulimovici S, Rabau E. Les effets des gonadotrophins urinaires des femmes menopausees sur l'ovaire humain. CR Soc Franc Gynecol. 1962;32/5:291.
- 33.Lunenfeld B, Sulimovici S, Rabau E, Eshkol A. L'induction de l'ovulation dans les amenorrheas hypophysaires par un traitement combiné de gonadotropins urinaires menopausiques et de gonadotropins chorioniques. CR Soc Franc Gynecol. 1962;32/5:346.
- 34. Lunenfeld B. Treatment of anovulation by human gonadotropins. J Int Fed Gynecol Obstet, 1963, 1, 15 [Google Scholar]
- 35. Palmer R, Dorangeon P. Les gonadotropines dans les traitements de la stérilité féminine. CR Soc Franc Gynecol, 1962, 32, 407–415 [Google Scholar]
- 36.Salomon Y, Netter A. Traitement de l'anovulation par les gonadotrophines humaines. In: Gazette Medicale de France; 1965, t.72. p. 3.615–28. [PubMed]
- 37. Rosenberg E, Coleman J, Damani M, Garcia CR. Clinical effect of post menopausal gonadotropin. Clin Endocrinol Metab, 1962, 23, 181–189 [Google Scholar]
- 38. Taymor ML, Sturgis SH, Lieberman BL, Goldstein DP. Induction of ovulation with human postmenopausal gonadotropin. I. Case selection and results of therapy. Fertil Steril., 1966, 17, 731–735 [DOI] [PubMed] [Google Scholar]
- 39. Tanaka Y. Combined effect of HMG and HCG on ovulaton. Sanfujinka No Jissai., 1969, 18, 314–319 [PubMed] [Google Scholar]
- 40.WHO. Expert Committee on biological Standardization (Chair B. Lunenfeld), vol 565. Technical Report Series. Geneva: World Health Organization, 1972; p. 56–7.
- 41.WHO Expert Committee. Agents stimulating gonadal function in human (Chair B. Lunenfeld). Technical Report Series. Geneva: World Health Organization; 1973. [PubMed]
- 42. Eshkol A, Lunenfeld B. Purification and separation of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from human menopausal gonadotropin (HMG). Part III. Acta Endocrinol, 1967, 54, 919 [DOI] [PubMed] [Google Scholar]
- 43. Lunenfeld B, Eshkol A. Immunology of follicle stimulating hormone and luteinizing hormone. Vitam Horm, 1970, 27, 131–159 10.1016/S0083‐6729(08)61126‐7 [DOI] [PubMed] [Google Scholar]
- 44. Gemzell CA, Diczfalusy E, Tillinger G. Clinical effect of human pituitary follicle stimulating hormone (FSH). J Clin Endocrinol Metab, 1958, 18, 1333 10.1210/jcem‐18‐12‐1333 [DOI] [PubMed] [Google Scholar]
- 45. Dumble LD, Klein RD. Creutzfeld–Jakob disease legacy for Australian women treated with human pituitary gonadotropins. Lancet, 1992, 330, 848 [DOI] [PubMed] [Google Scholar]
- 46. Cochius JI, Mack K, Burns RJ. Creutzfeld–Jakob disease in a recipient human pituitary derived gonadotropin. Aust NZ J Med, 1990, 20, 592 10.1111/j.1445‐5994.1990.tb01322.x [DOI] [PubMed] [Google Scholar]
- 47. Pincus G, Enzmann EV. Can mammalian eggs undergo normal development in vitro?. Proc Natl Acad Sci USA, 1934, 20 (2) 121–122 10.1073/pnas.20.2.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rock J, Menkin MF. In vitro fertilization and cleavage of human ovarian eggs. Science, 1944, 100 (2588) 105–107 10.1126/science.100.2588.105 [DOI] [PubMed] [Google Scholar]
- 49. Dauzier L, Thibault C, Wintenberger S. Fecundation in vitro of rabbit egg. C R Hebd Seances Acad Sci, 1954, 238 (7) 844–845 [PubMed] [Google Scholar]
- 50.Chang Mc. Fertilization of rabbit ova in vitro. Nature. 1959; 184(Suppl 7):466–7. [DOI] [PubMed]
- 51. Shettles LB. Human blastocyst grown in vitro in ovulation cervical mucus. Nature, 1971, 229 (5283) 343 10.1038/229343a0 [DOI] [PubMed] [Google Scholar]
- 52. Kretzer D, Dennis P, Hudson B, Leeton J, Lopata A, Outch K, Talbot J, Wood C. Transfer of a human zygote. Lancet, 1973, 2 (7831) 728–729 10.1016/S0140‐6736(73)92553‐1 [DOI] [PubMed] [Google Scholar]
- 53. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet, 1978, 2 (8085) 366 10.1016/S0140‐6736(78)92957‐4 [DOI] [PubMed] [Google Scholar]
- 54. Jones GS, Andrews MC, Acosta A et al. The program for in vitro fertilization at Norfolk. Fertil Steril, 1982, 38 (1) 14–21 [PubMed] [Google Scholar]
- 55. Trounson AO, Leeton JF, Wood C, Webb J, Wood J. Pregnancies in humans by fertilization in vitro and embryo transfer in the controlled ovulatory cycle. Science, 1981, 212 (4495) 681–682 10.1126/science.7221557 [DOI] [PubMed] [Google Scholar]
- 56. Fleming R, Jamieson ME, Hamilton MP, Black WP, Macnaughton MC, Coutts JR. The use of GnRH analogues in combination with exogenous gonadotropins in infertile women. Acta Endocrinol Suppl (Copenh), 1988, 288, 77–84 [PubMed] [Google Scholar]
- 57. Palermo G, Joris H, Devroey P, Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet, 1992, 340 (8810) 17–18 10.1016/0140‐6736(92)92425‐F [DOI] [PubMed] [Google Scholar]
- 58.Massone R Urine collection and processing, a 40 year experience 2011 10th International symposium on GnRH. http://www.kenes.com/gnrh.
- 59. Shaked GM, Shaked Y, Jaruv‐Inbal A et al. A proteaseresistant prion protein isoform is present in urine of animals and humans affected with prion diseases. J of Biol Chem, 2001, 276, 1479–1482 10.1074/jbc.C100278200 [DOI] [PubMed] [Google Scholar]
- 60. Dorsselaer A, Carapito C, Delalande F, Schaeffer‐Reiss C, Thierse D, Diemer H, McNair DS, Krewski D, Cashman NR. Detection of prion protein in urine‐derived injectable fertility products by a targeted proteomic approach. PLoS One, 2011, 6 (3) 17815 10.1371/journal.pone.0017815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rathnam P, Saxena B. Primary amino acid sequence of folliclestimulating hormone from human pituitary glands I alpha subunit. J Biol Chem, 1975, 250, 6735–6746 [PubMed] [Google Scholar]
- 62. Saxena BB, Rathnam P. Amino acid sequence of the beta subunit of follicle‐stimulating hormone from human pituitary glands. J Biol Chem, 1976, 251, 993–1005 [PubMed] [Google Scholar]
- 63. Howles CM. Genetic engineering of human FSH (Gonal‐F). Hum Reprod Update, 1996, 2 (2) 172–191 10.1093/humupd/2.2.172 [DOI] [PubMed] [Google Scholar]
- 64. Olijve W, Boer W, Mulders JW, Wezenbeek PM. Molecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon). Mol Hum Reprod, 1996, 2 (5) 371–382 10.1093/molehr/2.5.371 [DOI] [PubMed] [Google Scholar]
- 65. Olijve W, Boer W, Mulders JW, Wezenbeek PM. Molecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon). Mol Hum Reprod, 1996, 2 (5) 371–382 10.1093/molehr/2.5.371 [DOI] [PubMed] [Google Scholar]
- 66. Driebergen R, Baer G. Quantification of follicle stimulating hormone (follitropin alfa): is in vivo bioassay still relevant in the recombinant age?. Curr Med Res Opin, 2003, 19 (1) 41–46 10.1185/030079902125001344 [DOI] [PubMed] [Google Scholar]
- 67. Hugues J‐N, Barlow DH, Rosenwaks Z et al. Improvement in consistency of response to ovarian stimulation with recombinant human follicle stimulating hormone resulting from a new method for calibrating the therapeutic preparation. Reprod Biomed Online, 2003, 6, 185–190 10.1016/S1472‐6483(10)61709‐9 [DOI] [PubMed] [Google Scholar]
- 68. Devroey P, Steirteghem A, Mannaerts A, Coelingh Bennink H. Successful in vitro fertilization and embryo transfer after treatment with recombinant human FSH. Lancet, 1992, 339, 1170–1171 10.1016/0140‐6736(92)90770‐4 [PubMed] [Google Scholar]
- 69. Germond M, Dessole S, Senn A et al. Successful in vitro fertilization and embryo transfer after treatment with recombinant human FSH. Lancet, 1992, 339, 1170 10.1016/0140‐6736(92)90770‐4 [PubMed] [Google Scholar]
- 70. Out HJ, Mannaerts BMJL, Driessen SGAJ et al. A prospective, randomized, assessor‐blind, multicentre study comparing recombinant and urinary follicle‐stimulating hormone (Puregon versus Metrodin) in in vitro fertilization. Hum Reprod, 1995, 10, 2534–2540 [DOI] [PubMed] [Google Scholar]
- 71. Agrawal R, West C, Conway GS, Page ML, Jacobs HS. Pregnancy after treatment with three recombinant gonadotropins. Lancet, 1997, 349, 29–30 10.1016/S0140‐6736(05)62162‐9 [DOI] [PubMed] [Google Scholar]
- 72. Donderwinkel PFJ, Schoot DC, Coelingh I, Bennink HJT, Fauser CJM. Pregnancy after induction of ovulation with recombinant human FSH in polycystic ovary syndrome. Lancet, 2002, 340, 983 10.1016/0140‐6736(92)92879‐K [DOI] [PubMed] [Google Scholar]
- 73. Lehert P, Schertz JC, Ezcurra D. Recombinant human follicle‐stimulating hormone produces more oocytes with a lower total dose per cycle in assisted reproductive technologies compared with highly purified human menopausal gonadotrophin: a meta‐analysis. Reprod Biol Endocrinol, 2010, 16 (8) 112 10.1186/1477‐7827‐8‐112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kaufmann R, Dunn R, Vaughn T, Hughes G, O'Brien F, Hemsey G, Thomson B, O'Dea LS. Recombinant human luteinizing hormone, lutropin alfa, for the induction of follicular development and pregnancy in profoundly gonadotrophin‐deficient women. Clin Endocrinol (Oxf), 2007, 67, 563–569 [DOI] [PubMed] [Google Scholar]
- 75. O'Dea L, O'Brien F, Currie K, Hemsey G. Follicular development induced by recombinant luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) in anovulatory women with LH and FSH deficiency: evidence of a threshold effect. Curr Med Res Opin, 2008, 24, 2785–2793 10.1185/03007990802374815 [DOI] [PubMed] [Google Scholar]
- 76.Bühler K, Naether O. A 2:1 formulation of follitropin alfa and lutropin alfa in routine clinical practice: a large, multicentre, observational study. Gynecol Endocrinol. 2010 (Epub ahead of print). PMID: 20849209. [DOI] [PMC free article] [PubMed]
- 77. Boime I, Keene J, Galway AB, Fares FM. Expression of recombinant human FSH LH and CG in mammalian cells, a structure‐function model for therapeutic drug design. Semin Reprod Endocrinol, 1990, 10, 45–50 10.1055/s‐2007‐1018859 [Google Scholar]
- 78.Devroey P, Koper N, Mannaerts B. A randomized, dose‐finding trial to establish the ovarian response to a single injection of Org 36286 for sustained follicular stimulation. Abstracts of the 22nd Annual Meeting of the ESHRE, Prague, Czech Republic, 18–21 June 2006. Free Communication. Session 45—ART—Ovarian stimulation. O‐172; 2006.
- 79. Beckers NGM, Macklon NS, Devroey P, Platteau P, Boerrigter PJ, Fauser BCJM. First live birth after ovarian stimulation using a chimeric long‐acting human recombinant follicle‐stimulating hormone (FSH) agonist (recFSH‐CTP) for in vitro fertilization. Fertil Steril, 2003, 79, 621–623 10.1016/S0015‐0282(02)04804‐5 [DOI] [PubMed] [Google Scholar]
- 80.The Corifollitropin Alfa Dose‐finding Study Group. A randomized dose–response 482 trial of a single injection of corifollitropin alfa to sustain multifollicular growth 483 during controlled ovarian stimulation. Hum Reprod. 2008;23:2484–92. [DOI] [PubMed]
- 81. Greef R, Zandvliet AS, Haan AF, Ijzerman‐Boon PC, Marintcheva‐Petrova M, Mannaerts BM. Dose selection of corifollitropin alfa by modeling and simulation in controlled ovarian stimulation. Clin Pharmacol Ther, 2010, 88 (1) 79–87 10.1038/clpt.2010.54 [DOI] [PubMed] [Google Scholar]
- 82. Devroey P, Boostanfar R, Koper N, Mannaerts B, IJzerman‐Boon P, Fauser B. ENGAGE investigators. A double‐blind, non‐inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Hum Reprod, 2009, 24 (12) 3063–3072 10.1093/humrep/dep291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mannaerts B. The corifollitropin alpha ENSURE study group. Corifollitropin alfa for ovarian stimulation in IVF: a randomized trial in lower‐body‐weight women. Reproductive Biomed Online, 2010, 21 (1) 66–76 10.1016/j.rbmo.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 84. Fatemi HM, Oberyé J, Popovic‐Todorovic B, Witjes H, Mannaerts B, Devroey P. Corifollitropin alfa in a long GnRH agonist protocol: proof of concept trial. Fertil Steril, 2010, 94 (5) 1922–1924 10.1016/j.fertnstert.2009.12.070 [DOI] [PubMed] [Google Scholar]
- 85. Arey BJ, Deecher DC, Shen ES, Stevis PE, Meade EH, Wrobel J, Frail DE, Lopez FJ. Identification and characterization of a selective, nonpeptide follicle‐stimulating hormone receptor antagonist. Endocrinology, 2002, 143, 3822–3829 10.1210/en.2002‐220372 [DOI] [PubMed] [Google Scholar]
- 86. Guo T, Adang AE, Dolle RE, Dong G, Fitzpatrick D, Geng P, Ho KK, Kultgen SG, Liu R, McDonald E et al. Small molecule biaryl FSH receptor agonists. Part 2. Lead optimization via parallel synthesis. Bioorg Med Chem Lett, 2004, 14, 1717–1720 10.1016/j.bmcl.2004.01.043 [DOI] [PubMed] [Google Scholar]
- 87. Maclean D, Holden F, Davis AM, Scheuerman RA, Yanofsky S, Holmes CP, Fitch WL, Tsutsui K, Barrett RW, Gallop MA. Agonists of the follicle stimulating hormone receptor from an encoded thiazolidinone library. J Comb Chem, 2004, 6, 196–206 10.1021/cc0300154 [DOI] [PubMed] [Google Scholar]
- 88. Yanofsky SD, Shen ES, Holden F, Whitehorn E, Aguilar B, Tate E, Holmes CP, Scheuerman R, MacLean D, Wu MM et al. Allosteric activation of the follicle‐stimulating hormone (FSH) receptor by selective, nonpeptide agonists. J Biol Chem, 2006, 281, 13226–13233 10.1074/jbc.M600601200 [DOI] [PubMed] [Google Scholar]
- 89. Lagemaat R, Timmers CM, Kelder J, Koppen C, Mosselman S, Hanssen RGJM. Induction of ovulation by a potent, orally active, low molecular weight agonist (Org 43553) of the luteinizing hormone receptor. Hum Reprod., 2009, 24, 640–648 10.1093/humrep/den412 [DOI] [PubMed] [Google Scholar]


