Abstract
Purpose
Selenium (Se) and vitamin E (Vit‐E), as an integral part of antioxidant systems, play an important role in the motility and acrosome reaction (AR) of mammalian spermatozoa. The purpose of this study was to investigate the effect of Se and Vit‐E on motility, viability, AR and accumulation of ammonia in the culture medium during different incubation periods in porcine sperm.
Methods
Sperm samples were washed, swum‐up and incubated at 37°C for 1 and 3 h in Sp‐TALP medium supplemented with sodium selenite (SS), seleon l‐methionine (SeMet) and Vit‐E in the presence or absence of ammonia. Sperm motility was determined on the basis of movement quality examined by phase microscopy. Viability and AR of spermatozoa were assessed by Hoechst 33258 and chlortetracycline (CTC) staining technique, and accumulation of ammonia was measured by the indophenol method. The incorporation of 14C(U)‐glucose was assessed with a liquid scintillation counter.
Results
In experiment 1, the sperm motility, viability, AR and incorporation of 14C(U)‐glucose increased significantly (P < 0.05) in SS, SeMet and Vit‐E (5, 5 μg/l and 1.0 mM, respectively) compared with the control. In experiment 2, treatment of the sperm with SeMet and SeMet + Vit‐E in the presence of 300 μM ammonia also resulted in a significant increase (P < 0.05) in the rate of motility, viability, AR and incorporation of 14C(U)‐glucose. In contrast, the accumulation of ammonia was reduced by SeMet and SeMet + Vit‐E compared with the other treatments.
Conclusions
These findings indicate that SeMet and SeMet + Vit‐E may play an important role in reducing the accumulation of ammonia and subsequently in increasing the rate of AR and the utilization of glucose in porcine spermatozoa.
Keywords: Acrosome reaction, Ammonia, Porcine spermatozoa, Selenium, Vitamin E
Introduction
Ammonia produced from amino acids present in the cultured medium inhibits cell growth in vitro. The levels of ammonia increase significantly during the culture period because of the deamination and spontaneous breakdown of amino acids, especially glutamine, in in vitro fertilization of porcine oocytes [1]. The toxic effect of glutamine can be avoided by making use of the fact that dipeptides can be directly used by mammalian cells in vitro [2]. We reported previously that the accumulation of ammonia can be reduced in the medium by supplementation with the dipeptides l‐alanyl‐l‐glutamine and l‐glycyl‐l‐glutamine, which can play an important role in motility and acrosome reaction (AR) in porcine spermatozoa [3].
In recent years, the involvement of free radicals and oxidative damage has been proposed as an alternate mechanism for ammonia toxicity [4]. Selenium (Se), an essential trace element for mammals, is an integral part of antioxidant systems [5]. Se‐dependent glutathione peroxidase (GPx) has an important role in free radical protective mechanisms. Se may have a role in the motility of bovine spermatozoa by protecting them from oxidative damage [6]. Se, which commonly replaces sulfur in proteins, is normally incorporated through selenoamino acids (l‐selenomethionine, l‐selenocysteine) [7] and selenoenzymes, such as GPxs, which reduce glutathione in the antioxidant system and protect cells from oxidative damage [8]. Vitamin E (Vit‐E) is one of the most important antioxidative molecules, residing mainly in the cell membranes [9].
The AR involves the fusion of the outer acrosomal membrane with the plasma membrane and is of fundamental importance for the spermatozoa to fertilize an oocyte. Se positively regulates capacitation and the AR, both of which are required for spermatozoa to acquire fertilizing ability [10]. Although there are several studies that have shown the importance of Se levels in sperm function, the direct influence of Se and Vit‐E on reducing the accumulation of ammonia and AR in porcine spermatozoa in vitro have not yet been definitively demonstrated.
In this study, we have investigated the role of sodium selenite (SS), seleon l‐methionine (SeMet) and Vit‐E on the motility, viability, AR and accumulation of ammonia in the medium during different incubation periods in porcine spermatozoa, and assessed the utilization of glucose.
Materials and methods
Chemicals
Radioactive glucose was purchased from American Radiolabeled Chemicals Inc. (St Louis, MO). Ammonia, SS, SeMet, Vit‐E (α‐tocopheryl acetate, E) and all other chemicals were analytical grade and purchased from Nacalai Tesque (Kyoto, Japan). Vit‐E was dissolved in ethanol, and an emulsion was formed by vortex mixing before being added to the spermatozoa. The final concentration of ethanol was <2% by volume [11]. Control and the other treatments received an equivalent amount of ethanol.
Semen collection and preparation
Semen was collected from 2‐year‐old Duroc boars using the gloved‐hand technique at the Nagano Animal Industry Experiment Station, Nagano, Japan. The semen was diluted according to the method of Johnson et al. [12] with Modena extender to produce a sperm concentration of 1 × 108/ml at room temperature. The tube of semen was carried to the laboratory within 30 min after collection using a cork box to keep it at 21°C. The uppermost 10 ml of the semen sample was removed from the tube and incubated for 10 min in a water bath at 37°C for anabiosis. Then, 3 ml of the anabiosed sperm sample was washed twice with Sp‐TALP medium, a modified TALP medium used for the sperm cultures [13]. Sperm samples were then centrifuged at 900×g for 5 min. The sperm pellet was suspended in Sp‐TALP medium and pre‐incubated at 37°C in 95% humidified air and 5% CO2 for 1 h to swim‐up. A 50 μl sperm aliquot obtained from swim‐up separation was resuspended to the previously prepared drops with the specified medium to give a final concentration of spermatozoa 5 × 106/ml. The sperm was then incubated at 37°C in 95% humidified air and 5% CO2 for 3 h to evaluate the sperm motility [14, 15]. To evaluate the viability and acrosome status, swum‐up spermatozoa were resuspended separately at a concentration of 20 × 106/ml and incubated at 37°C for 3 h in the same condition.
Progressive motility
After completion of the 1‐ and 3‐h incubation periods, three subsamples (10 μl) of each sample were transferred to an examination chamber (Fujihira Industry Co., Ltd., Tokyo, Japan), placed on a warmer set (MPF‐10‐SZX, Kitazato Supply, Tokyo, Japan) and kept at 37°C, and the motility was examined under a light microscope (100×). Sperm motility was assessed by determining the percentage of spermatozoa in each of the following four categories of movement: (1) rapid progressive motility, (2) slow or sluggish progressive motility, (3) non‐progressive motility and (4) immotility. These were then expressed as a progressive motility percentage. The percentage of spermatozoa with progressive motility was determined subjectively by scoring 400 individual spermatozoa in each sample.
Evaluation of viability
Sperm viability was determined by Hoechst 33258 (Sigma, Chemical Co., St. Louis, Mo) staining as described by Wang et al. [14]. Briefly, 4 μl Hoechst (100 μg/ml in PBS) was mixed with 200 μl porcine semen and 196 μl PBS for 3‐min incubation at room temperature in the dark. After incubation, the slides were covered with 10 μl of sperm suspension and mounted on a glass slide under a coverslip. At least 200 sperm per slide were counted by fluorescent microscopy (FL‐microscopy, 420 nm). The live and dead sperm were separated by unstained and stained (blue‐colored) spermatozoa.
Evaluation of capacitation and acrosome reaction
The sperm capacitation and AR were evaluated by chlortetracycline (CTC) staining technique, slightly modified from that described by Ward and Storey [16], as shown in our previous study of boar sperm [17]. Briefly, the CTC stock solution containing 750 μM CTC–HCl (Sigma), 130 mM NaCl, 5 mM l‐cysteine and 20 mM Tris (pH 7.8) was prepared daily, wrapped in foil to protect against light, and stored at 4°C until required. Ten microliters of sperm suspension was mixed with 15 μl of CTC solution on a slide at room temperature. Then, 0.3 μl of 12.5% glutaraldehyde in 0.5 M Tris–HCl (pH 7.4) was added as a fixative. Samples were covered with coverslips and stored in the dark at 4°C. Sperm were observed within 24 h using a microscope (Nikon, Tokyo, Japan) with phase contrast and epifluorescence optics under blue‐violet illumination (excitation at 400–440 nm and emission at 470 nm). The percentage of F, B and AR pattern was determined at 1 and 3 h of incubation, and at least 200 spermatozoa were counted in each sample. Sperm were evaluated according to CTC staining patterns [18, 19]: fluorescence over the entire head (uncapacitated, pattern F), fluorescence‐free band in the postacrosomal region (capacitated, pattern B) and low fluorescence over the entire head except for a thin bright fluorescent band along the equatorial segment (acrosome‐reacted, pattern AR). For treatment of results, only the live cells were taken into consideration. Typical examples of these patterns are shown in Fig. 1.
Figure 1.
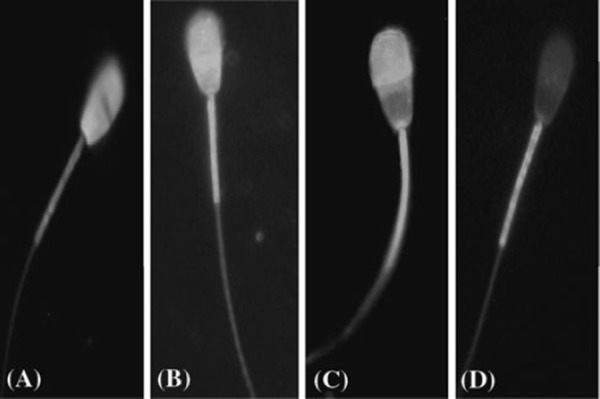
Hoechst 33258 and chlortetracycline (CTC) staining patterns. a Hoechst‐stained dead sperm, blue colored over the whole head. b F Pattern (non‐capacitated), uniform fluorescence on the head. c B pattern (capacitated), a fluorescence free band on the post acrosomal region. d AR pattern (acrosome reacted), a uniformly fluorescence‐free head
Evaluation of incorporation of 14C(U)‐glucose
The metabolic activity of spermatozoa was examined with or without ammonia by incorporating 14C(U)‐glucose in the semen samples, which were prepared in the same way as the samples for motility evaluation. A 100 μl aliquot of swim‐up spermatozoa (5 × 106/ml) from each treatment was incubated with 9.25 KBq/ml (specific activity 18.5 MBq/mM) of 14C(U)‐glucose and prepared for scintillation counting according to the method described in our previous study [15]. The incorporation of 14C(U)‐glucose by spermatozoa were determined using a liquid scintillation counter (LS‐6500, Beckman Instruments, Inc., Fullerton, CA). The incorporation values were expressed directly in counts per minute (cpm).
Ammonia assay
During the different incubation periods, the ammonia concentrations in the medium were assessed using the Bertholot‐indophenol method as described in our previous study [20]. To determine the ammonia concentration, 100 μl of the culture medium was removed every 1 and 3 h, and measurement was done immediately. The procedure was performed five times for each analysis. A calibration curve in the range of 0–0.40 mM ammonia was run with each experiment. The mean coefficient to determination for the calibration curve of five experiments was 0.994.
Experimental design
In the first experiment, the sperm motility, viability and AR were examined when sperm were treated with SS, SeMet and Vit‐E in various concentrations. The swim‐up separated spermatozoa were treated with SS, SeMet and Vit‐E (0, 2.5 and 5, 10 μg/l and 0.5, 1.0 and 2.0 mM, respectively) in Sp‐TALP medium. The sperm aliquots obtained from each treatment were then incubated at 37°C for 1 and 3 h.
In the second experiment, the effect of SS, SeMet, Vit‐E and their combinations on the motility, viability and AR was investigated in the presence of 300 μM ammonia in the medium. This concentration of 300 μM ammonia was selected from our previous study [3]. The optimum concentration of SS (5 μg/l), SeMet (5 μg/l), Vit‐E (1.0 mM) and their combination (2.5 + 2.5 μg/l + 1.0 mM, respectively) were selected from the first experiment. The spermatozoa were then incubated at 37°C for 3 h. After supplementation of all the chemicals, the pH (HM‐30s, Tokyo TOA Electronics, Tokyo, Japan) and osmolarity (Model 3300, Advanced Instruments, Norwood, MA) of these media were adjusted to 7.4 with 1 N HCl or 1 N NaOH and 270 mOsm with NaCl, respectively.
Statistical analysis
Data were analyzed by one‐way ANOVA using the Turkey‐Kramer method as post hoc tests (Statcel2 software; OMS Publishing Inc., Japan). Motility, viability and CTC patterns were analyzed using the χ2 test. All percentage data were subjected to arc‐sine transformation before statistical analysis. Data are expressed as mean ± standard error and repeated five times. A value of P < 0.05 was considered to be statistically significant.
Results
The effect of SS, SeMet and Vit‐E on the percentages of progressive motility and viability at 1 h of incubation are shown in Fig. 2. The percentages of progressive motility and viability were significantly higher (P < 0.05) in media containing SS, SeMet and Vit‐E (5, 5 μg/l and 1.0 mM, respectively) compared with that in the control. The motility and viability were significantly decreased (P < 0.05) in spermatozoa at higher concentrations of SS, SeMet and Vit‐E (10, 10 μg/l and 2 mM, respectively) compared with the SS, SeMet and Vit‐E (5, 5 μg/l and 1.0 mM, respectively) treated spermatozoa. However, those showed no significant difference with the control.
Figure 2.
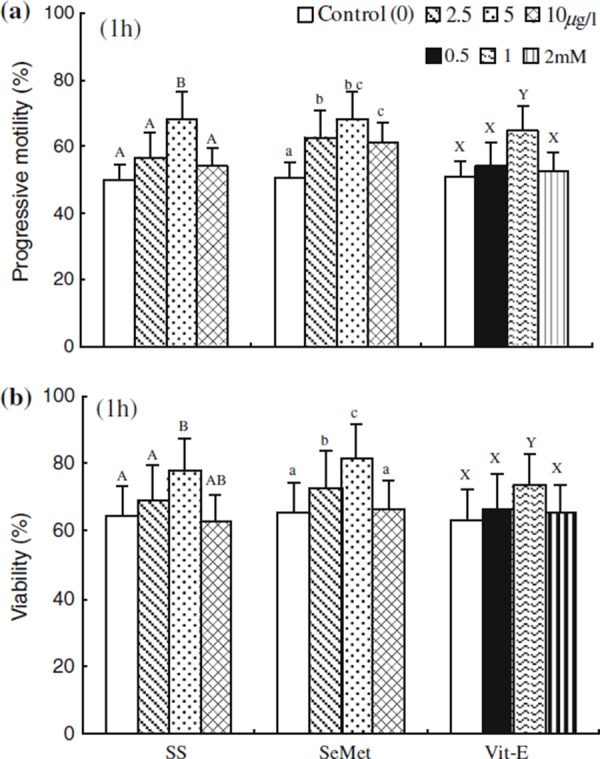
Effect of sodium selenite (SS), seleon l‐methionine (SeMet) and vitamin E (Vit‐E) on the percentage of a progressive motility under the phase microscopy and b viability obtained by Hoechst 33258 staining expressed as percentage of dead spermatozoa at 1 h incubation. Each column with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the different concentrations
The effect of SS, SeMet and Vit‐E on the percentages of CTC patterns F, B and AR at 1 and 3 h of incubation periods are shown in Table 1. Spermatozoa treated with SS, SeMet and Vit‐E (5, 5 μg/l and 1.0 mM, respectively) showed a significant increase in the capacitation and AR patterns (along with a decrease in non‐capacitated pattern, F) compared with control at 3 h incubation. However, the percentage of capacitated and acrosome reacted spermatozoa was significantly increased at 3 h from 1 h of incubation in all treated groups.
Table 1.
Effect of sodium selenite, seleon l‐methionine and vitamin E with various concentrations on the chlorteracyline (CTC) staining pattern of porcine spermatozoa
| Treatment | F pattern (%) | B pattern (%) | AR pattern (%) | |||
|---|---|---|---|---|---|---|
| 1 h | 3 h | 1 h | 3 h | 1 h | 3 h | |
| Sodium selenite (μg/l) | ||||||
| Control (0) | 60.1 ± 2.8a | 30.0 ± 1.1a | 24.1 ± 1.1 | 33.1 ± 1.7a | 16.1 ± 0.4 | 37.2 ± 0.8ab |
| 2.5 | 56.1 ± 1.7b | 17.2 ± 1.2b | 26.0 ± 1.7 | 42.1 ± 1.1bc | 18.1 ± 2.6 | 40.9 ± 0.8b |
| 5.0 | 53.1 ± 1.7b | 10.2 ± 1.1c | 27.0 ± 1.7 | 46.0 ± 1.1c | 20.7 ± 0.3 | 43.3 ± 0.6b |
| 10 | 61.0 ± 1.7a | 27.1 ± 1.1a | 23.0 ± 1.1 | 38.0 ± 1.1b | 16.3 ± 2.6 | 34.5 ± 2.6a |
| Seleone l‐methionine(μg/l) | ||||||
| Control (0) | 60.1 ± 2.8a | 30.0 ± 2.3a | 24.1 ± 1.1 | 33.1 ± 1.7a | 16.4 ± 2.6 | 37.2 ± 1.4a |
| 2.5 | 55.0 ± 1.1ab | 27.1 ± 1.1a | 27.0 ± 1.7 | 35.0 ± 1.1a | 17.9 ± 2.5 | 38.0 ± 2.6a |
| 5.0 | 50.0 ± 2.8b | 12.1 ± 1.2b | 28.0 ± 1.0 | 48.0 ± 1.7b | 22.0 ± 1.6 | 40.4 ± 0.9ab |
| 10 | 58.8 ± 2.0a | 11.1 ± 0.8b | 24.1 ± 1.2 | 45.1 ± 1.1ab | 17.1 ± 2.6 | 44.4 ± 0.4b |
| Vitamin E (mM) | ||||||
| Control (0) | 60.1 ± 2.8 | 30.0 ± 2.3a | 24.1 ± 1.1 | 34.1 ± 1.7a | 16.1 ± 0.4 | 36.2 ± 0.8ab |
| 0.5 | 58.1 ± 1.1 | 22.0 ± 1.1b | 25.3 ± 1.4 | 36.0 ± 1.0a | 17.3 ± 1.1 | 42.2 ± 1.1ab |
| 1.0 | 55.0 ± 1.2 | 12.1 ± 0.6c | 26.0 ± 1.1 | 43.0 ± 1.1b | 19.1 ± 1.1 | 45.1 ± 1.1b |
| 2.0 | 59.0 ± 0.4 | 27.4 ± 1.4ab | 23.0 ± 1.1 | 37.1 ± 1.1a | 18.0 ± 1.1 | 35.7 ± 0.9a |
Values with different letters (a–c) within the same column with different superscripts are significantly different (P < 0.05). All values percentages are expressed as mean ± standard error (n = 5)
The rate of incorporation of 14C(U)‐glucose supplemented with SS, SeMet and Vit‐E at 3 h of incubation periods are shown in Fig. 3. The incorporation rate of 14C(U)‐glucose was increased (P < 0.05) with SS, SeMet and Vit‐E (2.5, 2.5 μg/l and 0.5 mM, respectively) treated spermatozoa compared with the control. However, a higher (P < 0.05) rate of incorporation was observed with SS, SeMet and Vit‐E (5, 5 μg/l and 1.0 mM, respectively) compared with other treatments. The spermatozoa treated with SS, SeMet and Vit‐E showed a decreased rate of incorporation with the higher concentration of 10, 10 μg/l and 2 mM, respectively.
Figure 3.
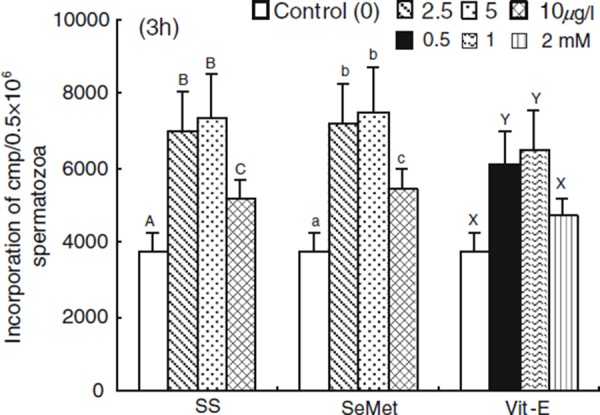
Effect of sodium selenite (SS), seleon l‐methionine (SeMet) and vitamin E (Vit‐E) on the rates of incorporation of 14C(U)‐glucose in porcine spermatozoa at 3 h of incubation. Each column with an error bar represents the mean ± standard error (n = 5). Different letters indicate significant differences (P < 0.05) among the treatments in the different concentrations
In the presence of 300 μM ammonia in the medium, the effects of SS, SeMet, Vit‐E and their combinations on the percentages of progressive motility, viability and ammonia concentration at 1 and 3 h of incubation periods are shown in Table 2. The percentages of progressive motility and viability significantly increased when sperm was treated with SeMet and SeMet + Vit‐E, and less percentage was observed with Vit‐E at 1 h of incubation compared with the control. The SS, SeMet, Vit‐E and their combinations used in the present study showed individually higher (P < 0.05) percentages of progressive motility and viability compared with the ammonia treated sperm.
Table 2.
Effect of sodium selenite, seleon l‐methionine and/or vitamin E on progressive motility, viability in porcine spermatozoa and ammonia concentrations in the culture medium
| Treatment | Progressive motility (%) | Viability (%) | Ammonia concentration (mM) | |||
|---|---|---|---|---|---|---|
| 1 h | 3 h | 1 h | 3 h | 1 h | 3 h | |
| Control (without NH3) | 50.5 ± 1.4ab | 33.2 ± 1.4a | 63.6 ± 0.5abc | 53.6 ± 5.2a | 0.15 ± 0.005a | 0.17 ± 0.005a |
| NH3 | 36.5 ± 1.0c | 26.0 ± 1.4a | 51.8 ± 0.5c | 39.0 ± 5.2b | 0.36 ± 0.008b | 0.39 ± 0.01d |
| NH3 + SS | 45.5 ± 1.4bc | 27.8 ± 1.5a | 63.6 ± 0.5abc | 45.4 ± 5.2ab | 0.31 ± 0.005c | 0.34 ± 0.01c |
| NH3 + SeMet | 63.5 ± 1.4a | 32.4 ± 0.6a | 67.2 ± 0.5ab | 53.3 ± 4.9a | 0.24 ± 0.001d | 0.24 ± 0.01b |
| NH3 + Vit‐E | 40.7 ± 1.4bc | 34.7 ± 2.2a | 55.4 ± 5.2bc | 41.5 ± 5.5ab | 0.28 ± 0.01cd | 0.30 ± 0.02bc |
| NH3 + SS + Vit‐E | 51.0 ± 2.8ab | 39.7 ± 1.4ab | 65.4 ± 5.2abc | 43.6 ± 5.2ab | 0.29 ± 0.01cd | 0.29 ± 0.01bc |
| NH3 + SeMet + Vit‐E | 61.7 ± 5.0a | 47.2 ± 0.2b | 71.8 ± 0.5a | 49.0 ± 5.2ab | 0.26 ± 0.01cd | 0.24 ± 0.01b |
| NH3 + SS + SeMet + Vit‐E | 42.5 ± 1.4bc | 32.3 ± 2.0a | 56.3 ± 5.3abc | 43.6 ± 5.2ab | 0.35 ± 0.01b | 0.38 ± 0.01cd |
Values with different letters (a–e) within the same column with different superscripts are significantly different (P < 0.05). Spermatozoa were incubated 1 and 3 h in the presence of 300 μM ammonia (NH3) in medium (Sp‐TALP) by supplementing with sodium selenite (SS; 5μg/l), seleon l‐methionine (SeMet; 5 μg/l), vitamin E (Vit‐E; 1.0 mM) and SS + SeMet + Vit‐E (0.25 + 0.25 μg/l + 1.0 mM). All values percentages are expressed as mean ± standard error (n = 5)
This study examined whether SeMet and SeMet + Vit‐E reduced the amount of ammonia produced over incubation periods of 1 and 3 h of spermatozoa with 300 μM ammonia in the medium (shown in Table 2). The accumulation of ammonia in the medium over different incubation periods was significantly reduced (P < 0.05) after the addition of the SS, SeMet, Vit‐E and their combinations, with the exception of SS + SeMet + Vit‐E compared with the ammonia‐treated medium. Among the treatments, SeMet and SeMet + Vit‐E play important roles in reducing the accumulation of ammonia.
In the presence of 300 μM ammonia in the medium, the effects of SS, SeMet, Vit‐E and their combinations on the percentages of CTC patterns F, B and AR at 0, 1 and 3 h of incubation periods are shown in Table 3. The percentage of F pattern (corresponding to non‐capacitated sperm) to about 76% before incubation and the number of spermatozoa with this pattern gradually reduced in the presence of both SeMet and SeMet + Vit‐E after 3 h of incubation. The B pattern spermatozoa accounted for 15% at 0 h and gradually increased approximately 40% with SeMet and SeMet + Vit‐E treated group after 3 h of incubation. The percentages of B and AR pattern were significantly increased when sperm were treated with SeMet and SeMet + Vit‐E compared with that of the ammonia‐treated control at both 1 and 3 h of incubation. When SeMet and SeMet + Vit‐E treated spermatozoa were incubated for 3 h in medium, approximately 35% of the sperm showed an AR pattern.
Table 3.
Effect of sodium selenite, seleon l‐methionine and/or vitamin E on F, B and AR pattern in the chlorteracyline (CTC) staining of porcine spermatozoa
| Treatment | F pattern (%) | B pattern (%) | AR pattern (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 3 h | 0 h | 1 h | 3 h | 0 h | 1 h | 3 h | |
| Control (without NH3) | 78.0 ± 4.6a | 60.3 ± 1.3a | 30.0 ± 2.8ab | 14.0 ± 1.7 | 24.1 ± 1.2a | 34.1 ± 1.7a | 8.1 ± 2.6 | 15.4 ± 2.6a | 36.2 ± 1.4a |
| NH3 | 83.0 ± 1.1b | 76.0 ± 1.1b | 46.0 ± 1.1c | 10.0 ± 1.1 | 15.0 ± 1.1b | 25.0 ± 1.1b | 7.2 ± 2.6 | 9.9 ± 0.2b | 29.9 ± 0.8b |
| NH3 + SS | 81.0 ± 3.3ab | 62.3 ± 1.8a | 30.9 ± 1.5ab | 11.0 ± 1.7 | 22.0 ± 0.6a | 36.1 ± 1.2a | 8.9 ± 2.6 | 16.3 ± 0.2a | 32.7 ± 0.3a |
| NH3 + SeMet | 79.6 ± 2.1ab | 57.7 ± 1.5ac | 24.0 ± 1.1bd | 10.0 ± 1.1 | 24.0 ± 1.3a | 39.0 ± 1.7a | 10.8 ± 2.6 | 18.7 ± 0.8a | 36.9 ± 0.1a |
| NH3 + Vit‐E | 81.3 ± 1.9ab | 62.0 ± 2.1a | 36.1 ± 2.3a | 11.0 ± 1.2 | 23.5 ± 0.8a | 33.0 ± 1.7ab | 8.1 ± 2.6 | 15.4 ± 0.8a | 30.6 ± 1.1ab |
| NH3 + SS + Vit‐E | 80.6 ± 1.5ab | 60.3 ± 3.9a | 34.0 ± 1.7a | 11.0 ± 1.7 | 24.0 ± 1.7a | 34.6 ± 1.4a | 8.6 ± 2.3 | 16.0 ± 0.7a | 31.8 ± 0.8a |
| NH3 + SeMet + Vit‐E | 74.0 ± 1.2a | 51.3 ± 1.7c | 23.1 ± 1.2d | 13.0 ± 1.2 | 28.0 ± 1.1a | 42.0 ± 1.2a | 12.9 ± 0.8 | 20.9 ± 0.3a | 35.5 ± 0.3a |
| NH3 + SS + SeMet + Vit‐E | 81.3 ± 1.3ab | 63.3 ± 1.3a | 32.0 ± 1.7a | 10.2 ± 0.5 | 22.0 ± 1.2a | 36.0 ± 1.1a | 9.3 ± 2.6 | 15.5 ± 2.6a | 31.8 ± 2.6a |
Values with different letters (a–d) within same the column with different superscripts are significantly different (P < 0.05). Spermatozoa were incubated 0, 1 and 3 h in the presence of 300 μM ammonia (NH3) in medium (Sp‐TALP) by supplementing with sodium selenite (SS; 5 μg/l), seleon l‐methionine (SeMet; 5 μg/l), vitamin E (Vit‐E; 1.0 mM) and SS + SeMet + Vit‐E (0.25 + 0.25 μg/l + 1.0 mM). All values percentages are expressed as mean ± standard error (n = 5)
The rates of incorporation of 14C(U)‐glucose and the accumulation of ammonia treated with SS, SeMet, Vit‐E and their combinations in medium containing 300 μM ammonia at 1 and 3 h of incubation periods are shown in Table 4. The incorporation rate of 14C(U)‐glucose was higher (P < 0.05) in SeMet and SeMet + Vit‐E treated spermatozoa compared with the ammonia supplemented control at 1‐ and 3‐h incubation periods. When compared with the ammonia‐supplemented media, all treatments showed higher rates with the exception of Vit‐E and SS + SeMet + Vit‐E treatments.
Table 4.
Effect of sodium selenite, seleon l‐methionine and/or vitamin E on incorporation of 14C(U)‐glucose in porcine spermatozoa
| Treatment | Incorporation of cmp/0.5 × 106 spermatozoa | |
|---|---|---|
| 1 h | 3 h | |
| Control (without NH3) | 3021.2 ± 287.85ab | 3735.3 ± 288.0a |
| NH3 | 2508.6 ± 33.6a | 3368.4 ± 150.1ae |
| NH3 + SS | 2983.8 ± 60.4ab | 3806.7 ± 193.5ab |
| NH3 + SeMet | 3325.8 ± 214.7ab | 5056.7 ± 46.1c |
| NH3 + Vit‐E | 2861.5 ± 136.2ab | 3748.7 ± 85.9a |
| NH3 + SS + Vit‐E | 3102.0 ± 130.9ab | 4492.7 ± 35.0b |
| NH3 + SeMet + Vit‐E | 3626.7 ± 307.7b | 6859.0 ± 215.6d |
| NH3 + SS + SeMet + Vit‐E | 2735.2 ± 99.1a | 2742.5 ± 133.1e |
Values with different letters (a–f) within the same column with different superscripts are significantly different (P < 0.05). Spermatozoa were incubated 1 and 3 h in the presence of 300 μM ammonia (NH3) in medium (Sp‐TALP); by supplementing with sodium selenite (SS; 5 μg/l), seleon l‐methionine (SeMet; 5 μg/l), vitamin E (Vit‐E; 1.0 mM) and SS + SeMet + Vit‐E (0.25 + 0.25 μg/l + 1.0 mM). All value percentages are expressed as mean ± standard error (n = 5)
Discussion
The results obtained in this study show that supplementation of SS, SeMet and Vit‐E in Sp‐TALP medium can improve motility, viability and AR of porcine spermatozoa. SS and SeMet (5 μg/l) showed higher percentages of motility, viability and AR in ammonia‐free medium. The optimum concentration of Se and Vit‐E used in the present study is similar to that of the concentration used by porcine embryos and human sperm [11, 21]. These results were in agreement with the study of bovine [22] and ram [23] sperm, the results of which showed that SS and SeMet significantly stimulated motility and AR without affecting viability. Aitken et al. [10] observed that Se positively regulates sperm capacitation and AR for the human spermatozoa. In our present study, spermatozoa were evaluated for pattern B and AR staining at 0, 1 and 3 h of incubation. The AR pattern after CTC staining was used as a probe for sperm's ability to undergo the AR [16]. Using modified Hoechst 33258 staining, the number of dead spermatozoa was also established [24]. During incubation of human [25], pig [26] and Japanese black cattle [27] sperm suspensions under conditions known to support capacitation and AR in vitro, there are changes in the distribution of the three CTC fluorescence patterns, with a sequential alteration from the F to the pattern and then to the AR pattern. In this study, treatment of the sperm with Vit‐E (1.0 mM) improved the motility, viability and AR compared with ammonia‐free medium. Geva et al. [28] showed that supplementation of Vit‐E in the culture medium can enhance oxidative stability and functional properties such as motility and AR of human spermatozoa. Although there are several studies that show the importance of Se and Vit‐E levels in sperm function, this is the first report of the direct influence of Se and Vit‐E on motility, viability and AR of porcine spermatozoa.
In general, Se plays an important role in cellular antioxidant defenses, since it forms the catalytic site of antioxidant enzymes (mainly as selenocysteine) such as GPx. GPx has been assumed to play a role in protecting cells from the harmful effects of toxic metabolites and free radicals by preventing lipid peroxidation of membranes in mammalian spermatozoa [29]. In the cell culture, Se in the form of selenite helps the cells to detoxify the medium to protect them from oxidative damages [30]. Se and Vit‐E have been shown to modulate adenylate cyclase and cyclic adenosine 3′, 5′‐monophosphate (cAMP)‐related signaling events, as well as protein kinases [31]. The inclusion of Se and Vit‐E in the medium may act in the stimulation of sperm motility, viability and AR. Adenosine triphospate (ATP), cAMP, adenylate cyclase and phosphodiesterase are known to be intricately involved in the motility, AR and survival of spermatozoa [32]. In this study, supplementation of SS, SeMet and Vit‐E (5 μg/l and 1.0 mM) increased incorporation rates compared with ammonia‐free medium. We find that the higher rates of incorporation of 14C(U)‐glucose agree with the results of higher rates of motility, viability and AR. It is well known that either glucose or a combination of glucose and other monosaccharides is the most important exogenous energy source to promote AR in vitro [33]. Hichs et al. [34] also stated that cAMP plays an important role in the glycolytic pathway of spermatozoa and that it can influence the generation required for sperm motility through its effect on glycolysis.
This study showed that SS, SeMet and SeMet + Vit‐E protect and accelerate the motility and viability and induced AR when the medium was supplemented with 300 μM ammonia. The media supplemented with SeMet and SeMet + Vit‐E were higher in motility, viability and AR in 300 μM ammonia containing medium. These results were in agreement with the study of Keskes‐Ammar et al. [35], who reported that the combination of Se and Vit‐E, in comparison to single supplementation, improves motility in the human sperm in vitro. Kosenko et al. [4] suggested that ammonia toxicity has been proposed as an alternate mechanism for the involvement of free radicals and oxidative damage. GPx, GSH and Vit‐E (enzymatic and non‐enzymatic antioxidant) are involved in reducing ammonia toxicity and the production of free radicals [36, 37]. We suggest that SeMet and SeMet + Vit‐E might protect oxidative damage of ammonia. Thus, the present results support the findings of El‐Demerdash [38], who reported that the amino acids SeMet and seleoncysteine can spare Vit‐E and Se through their antioxidation role. Various agents (such as Se, Vit‐E and amino acids) principally act by directly neutralizing free radical activity in the culture medium; they also provide protection against oxidative damage [39]. In our previous study, we also reported that the accumulation of ammonia can be reduced in the medium through supplementation with dipeptides, which can play an important role in the AR of porcine spermatozoa [3]. In this study, we also observed that the results are almost the same when sperm was treated with Se and Vit‐E.
In this study, the supplementation of SS, SeMet and SeMet + Vit‐E stimulated the rate of incorporation of 14C(U)‐glucose at 1 and 3 h of incubation in 300 μM ammonia‐containing medium. That the rates of incorporation and oxidation of glucose (as an energy source) became the same as the motility, viability and AR is supported by the findings of our previous study [3]. We found that the higher rates of incorporation of 14C(U)‐glucose agreed with the results of higher rates of motility, viability and AR. As an exogenous energy source, glucose [40] has various effects on spermatozoa and is required for the stimulation of the motility, viability and AR in some species. Shamberger [41] demonstrated that sperm motility was enhanced by Se supplementation as SeMet and seleoncysteine, which were assessed by ATP‐utilizing and ATP‐regenerating pathways in the human sperm.
In conclusion, our results provide evidence that Se and Vit‐E are involved in modulating porcine sperm motility and AR. Furthermore, the combination of SeMet and SeMet + Vit‐E may be involved in reducing the accumulation of ammonia, and subsequently in increasing the rate of AR and the utilization of glucose in porcine spermatozoa.
References
- 1. Tareq KMA, Miah AG, Salma U, Yoshida M, Tsujii H. Effect of amino acids and dipeptides on accumulation of ammonia in the medium during in vitro maturation and fertilization of porcine oocytes. Rep Med Biol, 2007, 6, 165–170 10.1111/j.1447‐0578.2007.00180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eagle H. Utilization of dipeptides by mammalian cells in tissue culture. Proc Soc Exp Biol Med, 1955, 89, 96–99 [DOI] [PubMed] [Google Scholar]
- 3. Tareq KMA, Hossain MS, Akter QS, Sawada T, Afrose S, Hamano K et al. Effect of amino acids and dipeptides on acrosome reaction and accumulation of ammonia in porcine spermatozoa. Rep Med Biol, 2008, 7, 123–133 10.1111/j.1447‐0578.2008.00209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kosenko E, Kaminsky Y, Lopata O, Muravyov N, Kaminsky A, Hermenegildo C et al. Nitroarginine, an inhibitor of nitric oxide synthase, prevents changes in superoxide radical and antioxidant enzymes induced by ammonia intoxication. Metab Brain Dis, 1998, 13, 29–41 10.1023/A:1020626928259 [DOI] [PubMed] [Google Scholar]
- 5. Sánchez‐Gutiérrez M, García‐Montalvo EA, Izquierdo‐Vega JA, Del Razo LM. Effect of dietary selenium deficiency on the in vitro fertilizing ability of mice spermatozoa. Cell Biol Toxicol, 2008, 24, 321–329 10.1007/s10565‐007‐9044‐8 [DOI] [PubMed] [Google Scholar]
- 6. Siegel RB, Murray FA, Julien WE, Moxon AL, Conrad HR. Effect of in vitro selenium supplementation on bovine sperm motility. Theriogenology, 1980, 13, 357–367 10.1016/0093‐691X(80)90048‐5 [DOI] [PubMed] [Google Scholar]
- 7. Olson GE, Winfrey VP, Hill KE, Burk RF. Sequential development of flagellar defects in spermatids and epididymal spermatozoa of selenium‐deficient rats. Reproduction, 2004, 127, 335–342 10.1530/rep.1.00103 [DOI] [PubMed] [Google Scholar]
- 8. Rivera RE, Christensen VL, Edens FW, Wineland MJ. Influence of selenium on heat shock protein 70 expression in heat stressed turkey embryos (Meleagris gallopavo). Comp Biochem Physiol A Mol Integr Physiol, 2005, 142, 427–432 10.1016/j.cbpa.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 9. Palamanda JR, Kehrer JP. Involvement of vitamin E and protein thiols in the inhibition of microsomal lipid peroxidation by glutathione. Lipids, 1993, 28, 427–431 10.1007/BF02535941 [DOI] [PubMed] [Google Scholar]
- 10. Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine DS. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox‐regulated, cAMP‐mediated induction of tyrosine phosphorylation. J Cell Sci, 1998, 111, 645–656 [DOI] [PubMed] [Google Scholar]
- 11. Verma A, Kanwar KC. Effect of vitamin E on human sperm motility and lipid peroxidation in vitro. Asian J Androl, 1999, 1, 151–154 [PubMed] [Google Scholar]
- 12. Johnson LA, Aalbers JG, Grooten HJG. Artificial insemination of swine: fecundity of boar semen stored in Beltsville TS (BTS), modified Modena (MM), or MR‐A and inseminated on 1, 3 and 4 days after collection. Zuchthygiene, 1988, 23, 49–55 [Google Scholar]
- 13. Parrish JJ, Susko‐Parrish J, Winer MA, First NL. Capacitation of bovine sperm by heparin. Biol Reprod, 1988, 38, 1171–1180 10.1095/biolreprod38.5.1171 [DOI] [PubMed] [Google Scholar]
- 14. Wang WH, Abeydeera LR, Fraser LR, Niwa K. Functional analysis using chlortetracycline fluorescence and in vitro fertilization of frozen‐thawed ejaculated boar spermatozoa incubated in a protein‐free chemically defined medium. J Reprod Fertil, 1995, 104, 305–313 [DOI] [PubMed] [Google Scholar]
- 15. Miah AG, Tareq KMA, Hamano K, Kohsaka T, Tsujii H. Effect of relaxin on acrosome reaction and utilization of glucose in boar spermatozoa. J Reprod Dev, 2006, 52, 773–779 10.1262/jrd.18037 [DOI] [PubMed] [Google Scholar]
- 16. Ward CR, Storey BT. Determination of the time course of capacitation in mouse sperm using chlortetracycline fluorescence assay. Dev Biol, 1984, 104, 287–296 10.1016/0012‐1606(84)90084‐8 [DOI] [PubMed] [Google Scholar]
- 17. Miah AG, Salma U, Takagi Y, Kohsaka T, Hamano K, Tsujii H. Effect of relaxin and IGF‐I on capacitation, acrosome reaction, cholesterol efflux and utilization of labeled and unlabeled glucose in porcine spermatozoa. Repord Med Biol, 2008, 7, 29–36 10.1111/j.1447‐0578.2007.00198.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adachi J, Tate S, Miyake M, Harayama H. Effects of protein phosphatase inhibitor calyculin a on the postacrosomal protein serine/threonine phosphorylation state and acrosome reaction in boar spermatozoa incubated with a cAMP analog. J Reprod Dev, 2008, 54, 171–176 10.1262/jrd.19172 [DOI] [PubMed] [Google Scholar]
- 19. Fraser LR, Abeydeera LR, Niwa K. Ca2+ regulating mechanisms that modulate bull sperm capacitation and acrosomal exocytosis as determined by chlortetracycline analysis. Mol Reprod Dev, 1995, 40, 233–241 10.1002/mrd.1080400213 [DOI] [PubMed] [Google Scholar]
- 20. Tareq KMA, Obata R, Miah AG, Hamano K, Tsujii H. Ammonia concentration in porcine ovarian developing follicles. J Mamm Ova Res, 2005, 22, 185–189 10.1274/jmor.22.185 [Google Scholar]
- 21. Jeong YW, Hossein MS, Bhandari DP, Kim YW, Kim JH, Park SW et al. Effects of insulin‐transferrin‐selenium in defined and porcine follicular fluid supplemented IVM media on porcine IVF and SCNT embryo production. Anim Reprod Sci, 2008, 106, 13–24 10.1016/j.anireprosci.2007.03.021 [DOI] [PubMed] [Google Scholar]
- 22. Pratt WD, Murray FA, Conrad HR, Moxon AL, Kinder JE. Effects of selenium supplementation on bull sperm metabolism in vitro. Theriogenology, 1980, 13, 369–379 10.1016/0093‐691X(80)90049‐7 [DOI] [PubMed] [Google Scholar]
- 23. Alabi NS, Whanger PD, Wu AS. Interactive effects of organic and inorganic selenium with cadmium and mercury on spermatozoal oxygen consumption and motility in vitro. Biol Reprod, 1985, 33, 911–919 10.1095/biolreprod33.4.911 [DOI] [PubMed] [Google Scholar]
- 24. Song X, Li F, Cao G, Zhang J, Han Y. Distribution of alpha‐d‐mannose residues on zona pellucida and their role(s) in fertilization in pigs. Sci China C Life Sci, 2007, 50, 170–177 10.1007/s11427‐007‐0029‐x [DOI] [PubMed] [Google Scholar]
- 25. DasGupta S, Mills CL, Fraser LR. Ca2+‐related changes in the capacitation state of human spermatozoa assessed by a chlortetracycline fluorescence assay. J Reprod Fertil, 1993, 99, 135–143 10.1530/jrf.0.0990135 [DOI] [PubMed] [Google Scholar]
- 26. Mattioli M, Barboni B, Lucidi P, Seren E. Identification of capacitation in boar spermatozoa by chlortetracycline staining. Theriogenology, 1996, 45, 373–381 10.1016/0093‐691X(96)81099‐5 [DOI] [PubMed] [Google Scholar]
- 27. Kuroda K, Fukushima M, Harayama H. Premature capacitation of frozen‐thawed spermatozoa from subfertile Japanese black cattle. J Reprod Dev, 2007, 53, 1079–1086 10.1262/jrd.19031 [DOI] [PubMed] [Google Scholar]
- 28. Geva E, Lessing JB, Bartoov B, Lenergeva I, Zabludovsky N, Amit A. The effect of antioxidant treatment on human spermatozoa and fertilization rate in an in vitro fertilization program. Fertil Steril, 1996, 66, 430–434 [DOI] [PubMed] [Google Scholar]
- 29. Alvarez JG, Storey BT. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res, 1989, 23, 77–90 10.1002/mrd.1120230108 [DOI] [PubMed] [Google Scholar]
- 30. Zhang J, Robinson D, Salmon P. A novel function for selenium in biological system: selenite as a highly effective iron carrier for Chinese hamster ovary cell growth and monoclonal antibody production. Biotechnol Bioeng, 2006, 95, 1188–1197 10.1002/bit.21081 [DOI] [PubMed] [Google Scholar]
- 31. Kempná P, Reiter E, Arock M, Azzi A, Zingg JM. Inhibition of HMC‐1 mast cell proliferation by vitamin E: involvement of the protein kinase B pathway. J Biol Chem, 2004, 279, 50700–50709 10.1074/jbc.M410800200 [DOI] [PubMed] [Google Scholar]
- 32. Garbers DL, Kopf GS. The regulation of spermatozoa by calcium cyclic nucleotides. Adv Cyclic Nucleotide Res, 1980, 13, 251–306 [PubMed] [Google Scholar]
- 33. Bracket BG Hawk HW. In vitro fertilization and its assessment with embryo culture. Beltsville symposia in agricultural research 3: animal reproduction, 1979. Montclair: Allen Held, Osman Co; 171–193 [Google Scholar]
- 34. Hichs JJ, Pedron N, Rosado A. Modification of human spermatozoa glycolysis by cyclic adenosine monophosphate (cAMP), estrogens, and follicular fluid. Fertil Steril, 1972, 23, 886–893 [PubMed] [Google Scholar]
- 35. Keskes‐Ammar L, Feki‐Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, Hammami S et al. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch Androl, 2003, 49, 83–94 10.1080/01485010390129269 [DOI] [PubMed] [Google Scholar]
- 36. Kosenko E, Kaminsky A, Valencia M, Lee L, Heraenegildo C, Felipo V. Superoxide production and antioxidant enzymes in ammonia intoxication in rats. Free Radic Res, 1997, 27, 637–644 10.3109/10715769709097867 [DOI] [PubMed] [Google Scholar]
- 37. Velvizhi S, Dakshayani KB, Subramanian P. Effects of alpha ketoglutarate on antioxidants and lipid peroxidation in rats treated with ammonium acetate. Nutrition, 2002, 18, 747–750 10.1016/S0899‐9007(02)00825‐0 [DOI] [PubMed] [Google Scholar]
- 38. El‐Demerdash FM. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminium. J Trace Elem Med Biol, 2004, 18, 113–121 10.1016/j.jtemb.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 39. Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull, 1993, 49, 481–493 [DOI] [PubMed] [Google Scholar]
- 40. Vandevoort CA, Overstreet JW. Effects of glucose and other energy substrates on the hyperactivated motility of macaque sperm and the zona pellucida‐induced acrosome reaction. J Androl, 1995, 16, 327–333 [PubMed] [Google Scholar]
- 41. Shamberger RJ Feieden E. Biological interactions of selenium with other substances. Biochemistry of selenium, 1983. New York: Plenum Press; 125–166 [Google Scholar]


