Abstract
Background:
Seasonal and circadian changes are two factors described to affect blood levels of some biological molecules. The Total Antioxidant Capacity (TAC) is one global measure of the antioxidant capacity of a system. There is no agreement about the existence of day/night changes in TAC levels as well as there is no information about seasonal changes in TAC levels.
Objective:
The aims of this research are studying if there are summer/winter changes in TAC con-centrations or if TAC concentrations have day/night changes.
Method:
Ninety-eight healthy subjects took part in the summer study of whom 64 participated in the winter one. Blood was sampled at 09:00, 12:00 and 00:00 h. TAC was measured by the ABTS radi-cal cation technique. Results are expressed in mmol/L of trolox equivalents.
Results:
The subjects had significantly higher TAC levels in summer than winter at the three-time point studied. Summer 09:00 TAC concentration was significantly higher than the 12:00 and 00:00 h concentrations (1.34±0.26 vs 0.83±0.19, 0.75±0.18). Summer TAC 12:00 h concentrations were significantly higher than the 00:00 h concentrations (0.83±0.19 vs. 0.75±0.18). Winter 09:00 TAC concentrations were significantly higher than the 12:00 and 00:00 h concentrations (1.24±0.16 vs. 0.73±0.10, 0.67±0.13). There were no significant differences between the 12:00 and 00:00 h TAC concentrations.
Conclusion:
Strong methodological biases may be made if the seasonal and circadian changes in se-rum TAC concentration are not taken into account when researching in this area.
Keywords: Antioxidants, total antioxidant capacity, biological variation, seasonal rhythms, circadian rhythms, methodology
1. Introduction
Measuring human biological variables is not an easy task because of the complications inherent to the analytical variability of the technique as well as the complications inherent to the variability of the biological sample. Among the first, the sensitivity and the specificity are some of the most studied ones. Among the second, apart from the specific biological sample (blood, urine, saliva, etc.), there are at least two other variables that have been shown to affect the results.
First, circadian changes, the variations of the concentrations along the day in the biological measure. For example, melatonin, malondialdehyde and cortisol [1-3], among others, present day/night changes. Second, seasonality, the variation between seasons in the level of the biological variable. Melatonin [4], S100B protein [5] and malondialdehyde [6] are some molecules that show seasonal changes in their levels.
Free radicals (FRs) are compounds with unpaired electrons or an open shell configuration that may have positive, negative, or zero charge. Depending on the atom placed at its core, the radical can be described as oxygen, carbon, nitrogen or metal centered radical [7]. The unpaired electrons produce chemical species highly reactive.
The research about FRs is complex. FRs are difficult to measure directly due to their short half-lives. In vivo human research on FRs has mainly been focused on two different areas. First, the measurement of the oxidant/antioxidant system as individual enzymes activity, such as the superoxide dismutase (SOD), glutathione peroxidase (GPX) or catalase (CAT) [8]. Second, the measurement of the FRs consequences on biological systems such as lipid peroxidation products, such as the malondialdehyde (MDA) and 4-hydroxyalkenals (HAE) [9].
FRs have been investigated in several medical specialties such as psychiatry [10], cardiology [11] and neurology [12] among others.
Data on individual antioxidant enzymes activity as well as global measures of the antioxidant defense system (AODS) in psychiatry are controversial. Increased, decreased or no change of their levels have been reported [13-16]. Furthermore, to complicate the panorama, circadian rhythms of global measures of the AODS have been reported as a possible influence of the variability of the results [17, 18].
One global measure of the AODS is the serum concentration of the total antioxidant capacity (TAC). The field will get even more complicated, thus, there is no agreement with respect to the terminology applied to the measurements of global antioxidant measures. TAC (Total Antioxidant Capacity), TAA (Total Antioxidant Activity), TAOP (Total Antioxidant Power), TAS (Total Antioxidant Status) and TAR (Total Antioxidant Response) have been published and all are considered synonymous [19].
The information about circadian serum TAC concentrations is very scarce, higher nocturnal than diurnal levels [17] as well as higher diurnal than nocturnal levels [20] have been reported. As far as we know, there is no information about seasonal changes of TAC concentrations. The aims of this research consist in studying if serum TAC concentrations present summer/winter and day/night variations in healthy subjects.
2. Methods
The sample of subjects was recruited by an email announcement through the electronic mail of the University of La Laguna (ULL), the University Hospital of the Canary Islands and by word of mouth among the investigators´ acquaintances. Exclusion criteria were: 1) pregnancy, 2) being physically or mentally ill, 3) taking drugs of abuse, 4) being on a vegetarian diet and 5) taking vitamin supplements or melatonin.
Healthiness of the volunteers was checked by carrying out a general hematological, biochemical and urine analysis in July and December. Mental healthiness was assessed informally by asking the subjects if they had received psychiatric treatment in the past or were receiving treatment at present or if any first-degree relative had received psychiatric treatment in the past or at present. Psychological treatment was considered as if the subjects were on psychiatric treatment.
The study was carried out in July and December during two consecutive week-ends in order to minimize the interference of the study with the subjects working life. The volunteers arrived at the School of Medicine and laid in bed from 08:00 to 09:00 h when the first blood sample was drawn. This blood sample was drawn after fasting all night. After the first blood extraction, the subjects had breakfast and they were free to go around the School of Medicine until 11:00 h. Then, they laid in bed until 12:00 h when the second blood sample was extracted. Subjects were free to go home until 23:00 h when they had to be back at the School of Medicine. During the blood sampling at 09:00 and 12:00 h the subjects remained awake with their eyes open. The third blood sample was drawn at 00:00 h after having been in bed for one hour. Subjects were instructed to have a light dinner not later than 20:00 h. Nocturnal artificial light has been reported to affect TAC levels [17]. The nocturnal blood extraction was carried out in a room illuminated with a 4 lux light intensity. In order to avoid light contamination, the eyes of each subject were covered with a black mask. The rationale to lay in bed one hour before blood extraction was to allow the subjects to relax in order to minimize the psychological and physical stress they may have had [20]. The same routine was followed in December. All blood samples were extracted by venipuncture.
After each blood extraction, blood was placed in vacutainer tubes without anticoagulant. The blood clot at room temperature during 15 minutes and was then centrifuged at 3000 rpm during 10 minutes. Serum samples were aliquot in Eppendorf tubes and kept frozen at - 70º C until analysis. To minimize the assay variation, all serum samples were analyzed the same day with the same laboratory batch and by the same analyst. The analyst was blind with respect to the samples pertaining to summer/winter or the time of the day when samples were extracted.
Serum TAC was measured by the ABTS radical cation [21], with commercially available kits (Antioxidant Assay kit, SIGMA, Madrid, Spain). The principle of the antioxidant assay is based on the formation of a ferryl myoglobin radical from methamyoglobin and the hydrogen peroxide, which oxidizes the ABTS (2,2´-azino-bis [3-ethylbenzthiazoline-6-sulfonic acid]) to produce a radical cation, ABTS∙+, a soluble chromogen that is green color. This can be determined spectrophotometrically at 415 nm in a microplate spectrophotometer reader (Benchmark Plus, Bio-Rad, Hercules, CA, USA). Antioxidant compounds suppress the production of the radical cation in a concentration dependent manner and the color intensity decreases proportionally. Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid), a water soluble vitamin E analog, serves as a standard [21]. The intra- and inter-assay coefficients of variation (CV) were 6.96% and 9.13%, respectively. The results are expressed as mmol of Trolox/L.
Data were analyzed using the 21st version of the SPSS statistical package (SPSS, Chicago, Illinois, USA). Day/night (09:00 h, 12:00 and 00:00 h) and summer/winter serum TAC differences were analyzed by means of an ANOVA. If the ANOVA resulted significant, the multiple-comparison of Bonferroni´s correction was performed. T-tests were applied to compare two quantitative variables. Pearson correlations were applied to study the relationships between quantitative variables while the chi-square statistics were applied to study the association between qualitative variables. All statistical tests were two-tailed. Statistical significance level was set at 0.05. Quantitative data are presented as mean ± SD.
3. Results
One hundred and one subjects, of whom three were excluded, comprised the initial summer sample. Thus, the final summer sample comprises 98 subjects. Of those 98 healthy subjects, 34 did not participate in winter. The final winter sample comprises 64 subjects. The seasonal demographic characteristics of the sample have been published elsewhere (Morera-Fumero et al., 2013). Summarizing, the sample comprised of 48 men and 50 women in summer, 30 men and 34 women in winter. The mean age was 39.8 (s.d.: 9.8) and the mean BMI was 24.8 (s.d.: 3.8). There were no significant differences in gender distribution, and BMI between the summer/winter samples.
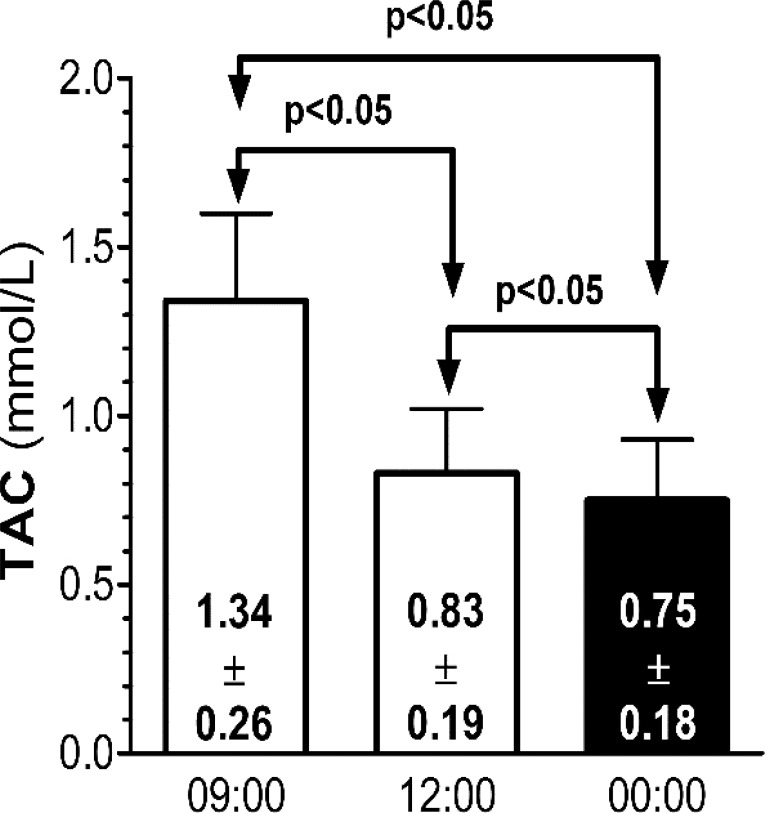
The summer ANCOVA model included TAC at 09:00, 12:00 and 00:00 h as intra-subject variables, gender as inter-subject factor and age, body mass index (BMI), number of salads, number of fish portions, meat portions and pieces of fruit per week acted as covariates. Table 1 shows the results of the ANCOVA. The only statistically significant variable was TAC by time of the day. None of the interactions was significant. The post-hoc Bonferroni’s comparison of serum TAC concentration at 09:00, 12:00 and 00:00 h is presented in Fig. (1). Morning TAC levels were significantly higher than midday and midnight levels. Midday TAC levels were significantly higher than midnight TAC levels.
Table 1.
ANCOVA’S results of the summer TAC levels.
| Origin | F | P |
|---|---|---|
| TAC by time | 14.214 | 0.001 |
| TAC by age | 0.345 | 0.559 |
| TAC by meat | 0.484 | 0.618 |
| TAC by salads | 0.011 | 0.915 |
| TAC by fruits | 0.023 | 0.880 |
| TAC by fish | 0.973 | 0.327 |
| TAC by BMI | 0.187 | 0.667 |
| TAC by gender | 1.823 | 0.181 |
Fig. (1).
Summer post-hoc comparison of total antioxidant capacity concentrations by time of the day.
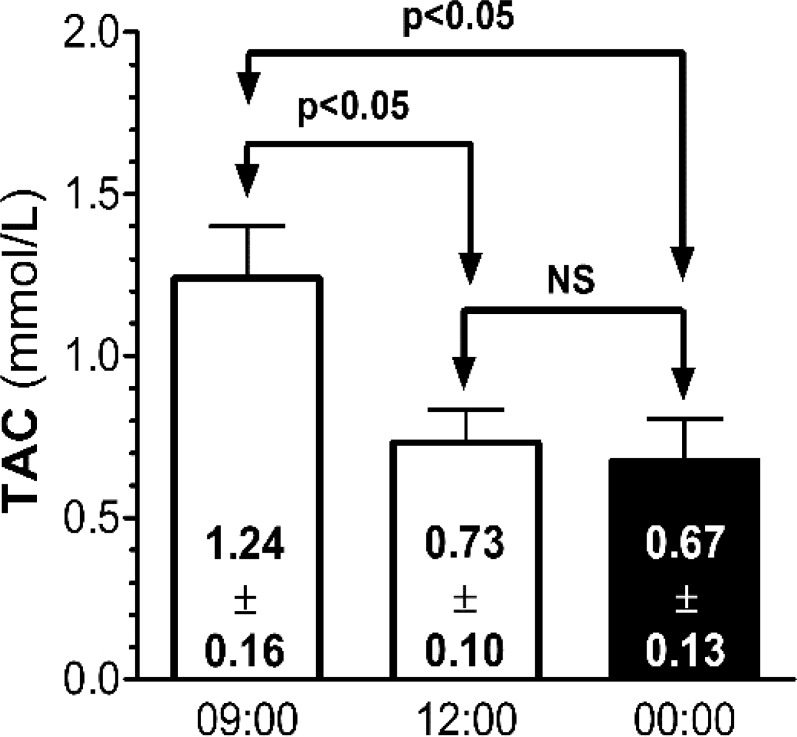
Table 2 shows the result of the ANCOVA for the TAC winter levels. The model included the same variables as the summer model. TAC by time of the day was the only significant variable. None of the interactions was significant. Fig. (2) represents the post-hoc Bonferroni’s comparison of serum TAC concentration at 09:00, 12:00 and 00:00 h. The morning TAC levels were significantly higher than midday and midnight TAC concentrations. There were no significant differences in serum TAC concentrations between midday and midnight.
Table 2.
ANCOVA’S results of the winter TAC levels.
| Origin | F | P |
|---|---|---|
| TAC by time | 4.858 | 0.036 |
| TAC by age | 3.635 | 0.070 |
| TAC by meat | 0.184 | 0.671 |
| TAC by salads | 0.220 | 0.643 |
| TAC by fruits | 3.017 | 0.093 |
| TAC by fish | 0.118 | 0.734 |
| TAC by BMI | 0.044 | 0.834 |
| TAC by gender | 0.648 | 0.427 |
Fig. (2).
Winter post-hoc comparison of total antioxidant capacity concentrations by time of the day.
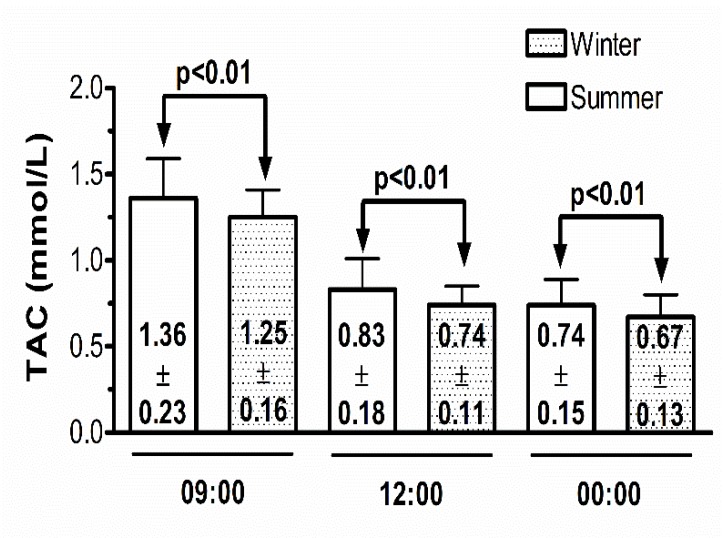
The comparisons of summer/winter changes in serum TAC levels by time of the day are represented in Fig. (3). Summer serum TAC concentrations were significantly higher than winter concentrations at 09:00, 12:00 and 00:00 h.
Fig. (3).
Comparison of total antioxidant capacity concentrations in summer and winter by time of the day.
4. Discussion
As far as we know, this is the first time that a summer/winter variation of TAC concentrations has been reported. The fact that healthy subjects present higher TAC levels in summer than winter at 09:00, 12:00 and 00:00 h has not been reported previously. In a preliminary report, we compared the summer and winter serum TAC levels at 12:00 and 00:00 hours [22]. There were no significant differences between summer and winter but the subjects had higher TAC levels in summer than winter. There is no other research on this topic to compare our results, though some speculative thoughts may be made. One of the main differences between summer and winter is the number of hours that a person may be exposed to sunlight. It is well known that humans have lower levels of melatonin (MLT) in summer (light is the main key inhibitor of MLT secretion) than winter [4]. Among the many functions of MLT, the antioxidant properties have been reported [23]. We could think that subjects in summer have lower MLT levels and a way of counteracting the effect of MLT could be by increasing TAC concentrations. On the other hand, in winter, because of the higher level of MLT, the antioxidant capacity of the subjects is more preserved and the levels of TAC are more reduced compared to summer.
With respect to the day/night difference in TAC concentrations, we have found that both in summer and winter, the subjects had significantly higher levels of TAC at 09:00 h than at midday and midnight, but only in summer the midday and midnight difference reached statistical significance. The available information about circadian TAC levels is very scarce. The paper of Benot et al. [17] reported significant higher serum TAC levels at 01:00 than those at 13:00, 19:00 and 07:00 h. Those results are opposite to our results. The authors suggested that the TAS rhythm was related to the MLT rhythm, so when MLT levels were high TAC levels were also high. A second paper published by our own research group [22] reported no significant differences in serum TAC levels between blood samples taken at 12:00 and 00:00 h. Although no significant differences were found in this research, a higher serum TAC levels in the morning sample than in the midnight sample were reported. A recent paper that studied day/night TAS changes in schizophrenia patients and in a control group of healthy subjects found that healthy subjects had significantly higher levels of TAC at 12:00 than 00:00 h [18].
Methodological differences may explain those discrepancies. First, our sample is bigger (N 09:00, 12:00 and 00:00 h. = 98 in summer and N 09:00, 12:00 and 00:00 h. = 64 in winter) than the sample of Benot et al. [17] (N 13:00, 19:00, 01:00 and 07:00 = 12). Second, our sample comprises the same subjects at each time-point of the day (paired design) while in the study of Benot et al. [17] the subjects at each time of the day were different ones (independent or unpaired design). Third, we kept subjects relaxed one hour before blood extraction. In the case of Benot et al. [17] this information has not been considered or reported in their paper. With respect to the second paper [22], the study was carried out on five male subjects, a very small sample of subjects. Despite that there were no significant differences between midday/midnight and summer/winter TAC concentrations, the samples presented a pattern without statistical significance. The control group of the third paper [18] comprised thirty healthy subjects that were studied at 12:00 and 00:00 h. The pattern of TAC levels (at 12:00 and 00:00 h) was the same as the pattern that we have found in this research.
The main limitations of our study are that we did not analyze biological markers of seasonality and circadian rhythms. Future research should include such markers, e.g., cortisol, testosterone, MLT, etc. On the other hand, our results also have some strengths. First, the fact that the same group of subject was studied at three time-points the same day and in summer and winter, give consistency to our results. Second, our morning results (09:00) coincide quantitatively with the value for adults reported in the analytical technique developed to measure TAC by other authors [21].
conclusion
In conclusion, our research reports two new important findings, the existence of a summer/winter difference in serum TAC concentrations and the day/night differences in serum TAC concentrations.
Methodological pitfalls, such as, quantitative changes in the variable along the day or along the year, may be committed when those differences are not taken into account when researching on this topic. Therefore, the inclusion of those differences in the research protocol is strongly advisable in designing a study. Future research on this field is guaranteed and the addition of biological markers of seasonality and circadian rhythms is strongly recommended.
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are base of this research.
Consent for Publication
Not applicable.
Acknowledgements
We would like to thank to Dr. Aram Morera-Mesa (freelance translator) for his assistance in the translation of this article.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Klerman E.B., Gershengorn H.B., Duffy J.F., Kronauer R.E. Comparisons of the variability of three markers of the human circadian pacemaker. J. Biol. Rhythms. 2002;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- 2.Morera A.L., Abreu P. Daytime/night-time and summer/winter melatonin and malondialdehyde rhythms: An inverse relationship. J. Pineal Res. 2007;43:313–314. doi: 10.1111/j.1600-079X.2007.00467.x. [DOI] [PubMed] [Google Scholar]
- 3.Waller K.L., Mortensen E.L., Avlund K., Fagerlund B., Lauritzen M., Gammeltoft S., Jennum P. Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nat. Sci. Sleep. 2016;8:47–53. doi: 10.2147/NSS.S75946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morera A.L., Abreu P. Seasonality of psychopathology and circannual melatonin rhythm. J. Pineal Res. 2006;41:279–283. doi: 10.1111/j.1600-079X.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 5.Morera-Fumero A.L., Abreu-Gonzalez P., Henry-Benitez M., Yelmo-Cruz S., Diaz-Mesa E. Summer/winter changes in serum s100b protein concentration as a source of research variance. J. Psychiatr. Res. 2013;47:791–795. doi: 10.1016/j.jpsychires.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Morera A.L., Intxausti A., Abreu-Gonzalez P. Winter/summer seasonal changes in malondialdehyde formation as a source of variance in oxidative stress schizophrenia research. World J. Biol. Psychiatry. 2009;10:576–580. doi: 10.1080/15622970801901802. [DOI] [PubMed] [Google Scholar]
- 7.International Union of Pure and Applied Chemistry Radicals (Free Radicals) Compendium of Chemical Terminology. Oxford: Gold Book; 1997. [Google Scholar]
- 8.Raffa M., Atig F., Mhalla A., Kerkeni A., Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry. 2011;11:124. doi: 10.1186/1471-244X-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berzosa C., Gomez-Trullen E.M., Piedrafita E., Cebrian I., Martinez-Ballarin E., Miana-Mena F.J., Fuentes-Broto L., Garcia J.J. Erythrocyte membrane fluidity and indices of plasmatic oxidative damage after acute physical exercise in humans. Eur. J. Appl. Physiol. 2011;111:1127–1133. doi: 10.1007/s00421-010-1738-6. [DOI] [PubMed] [Google Scholar]
- 10.Flatow J., Buckley P., Miller B.J. Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh R., Ray U., Jana P., Bhattacharya R., Banerjee D., Sinha A. Reduction of death rate due to acute myocardial infarction in subjects with cancers through systemic restoration of impaired nitric oxide. PLoS One. 2014;9:e88639. doi: 10.1371/journal.pone.0088639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanabrocki E.L., Ryan M.D., Hermida R.C., Ayala D.E., Scott G.S., Murray D., Bremner W.F., Third J.L., Johnson M.C., Foley S., Van C.J., Shah F., Shirazi P., Nemchausky B.A., Hooper D.C. Altered circadian relationship between serum nitric oxide, carbon dioxide, and uric acid in multiple sclerosis. Chronobiol. Int. 2004;21:739–758. doi: 10.1081/cbi-200025981. [DOI] [PubMed] [Google Scholar]
- 13.Akyol O., Herken H., Uz E., Fadillioglu E., Unal S., Sogut S., Ozyurt H., Savas H.A. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:995–1005. doi: 10.1016/s0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 14.Al-Chalabi B.M., Thanoon I.A., Ahmed F.A. Potential effect of olanzapine on total antioxidant status and lipid peroxidation in schizophrenic patients. Neuropsychobiology. 2009;59:8–11. doi: 10.1159/000202823. [DOI] [PubMed] [Google Scholar]
- 15.Pazvantoglu O., Selek S., Okay I.T., Sengul C., Karabekiroglu K., Dilbaz N., Erel O. Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin. Neurosci. 2009;63:693–700. doi: 10.1111/j.1440-1819.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 16.Ustundag B., Atmaca M., Kirtas O., Selek S., Metin K., Tezcan E. Total antioxidant response in patients with schizophrenia. Psychiatry Clin. Neurosci. 2006;60:458–464. doi: 10.1111/j.1440-1819.2006.01532.x. [DOI] [PubMed] [Google Scholar]
- 17.Benot S., Goberna R., Reiter R.J., Garcia-Maurino S., Osuna C., Guerrero J.M. Physiological levels of melatonin contribute to the antioxidant capacity of human serum. J. Pineal Res. 1999;27:59–64. doi: 10.1111/j.1600-079x.1999.tb00597.x. [DOI] [PubMed] [Google Scholar]
- 18.Morera-Fumero A.L., Diaz-Mesa E., Abreu-Gonzalez P., Fernandez-Lopez L., Cejas-Mendez M.R. Low levels of serum total antioxidant capacity and presence at admission and absence at discharge of a day/night change as a marker of acute paranoid schizophrenia relapse. Psychiatry Res. 2017;249:200–205. doi: 10.1016/j.psychres.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable abts radical cation. Clin. Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Buyukhatipoglu H., Kirhan I., Vural M., Taskin A., Sezen Y., Dag O.F., Turan M.N., Aksoy N. Oxidative stress increased in healthcare workers working 24-hour on-call shifts. Am. J. Med. Sci. 2010;340:462–467. doi: 10.1097/MAJ.0b013e3181ef3c09. [DOI] [PubMed] [Google Scholar]
- 21.Miller N.J., Rice-Evans C., Davies M.J., Gopinathan V., Milner A. A Novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci., (Lond) . 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 22.Morera A., Abreu P., Henry M., Garcia-Hernandez A., Guillen-Pino F., Orozco A., Intxausti A., Diaz-Mesa E., De la Varga M., Gracia R. Absence of circadian and seasonal rhythms of total antioxidant status in healthy subjects. Eur. Neuropsychopharmacol. 2007;17(Suppl. 4):S587–S588. [Google Scholar]
- 23.Adamczyk-Sowa M., Pierzchala K., Sowa P., Mucha S., Sadowska-Bartosz I., Adamczyk J., Hartel M. Melatonin acts as antioxidant and improves sleep in MS patients. Neurochem. Res. 2014;39:1585–1593. doi: 10.1007/s11064-014-1347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]