Abstract
Incidence of structural chromosome aberrations in mouse one‐cell embryos produced by intracytoplasmic sperm injection (ICSI) with mature epididymal spermatozoa were influenced by sperm incubation medium and time. When spermatozoa were incubated in bicarbonate‐buffered TYH for ≤0.5 h, the embryo aberration rates were significantly higher than in vitro fertilization (IVF) embryos. However, after the incubation of spermatozoa in the same medium for ≥2 h, the aberration rates were close to the IVF embryo level. When spermatozoa were incubated in bicarbonate‐buffered mCZB, hepes‐buffered H‐TYH and H‐mCZB, and phosphate‐buffered PB1, the increased incidences of aberrations were observed at any incubation time. In the case of sperm incubation in H‐TYH, H‐mCZB and PB1, the aberration rates increased in a time‐dependent manner. Chromosome aberrations generated by ICSI were transmissible to offspring. On the other hand, the aberration rate in embryos derived from testicular spermatozoa was independent of the medium type and incubation time. Thus, the incubation media appears to have no effect on sperm chromatin. TYH can effectively induce capacitation and acrosome reaction, while H‐TYH, H‐mCZB and PB1 never induce these spermatozoal events. It is probable that the cholesterol‐rich plasma membrane and intact acrosome injected into the ooplasm affect sperm chromatin remodeling, thus resulting in the generation of chromosome damage in ICSI embryos.
Keywords: Acrosome, ICSI, Mouse embryos, Sperm plasma membrane, Structural chromosome aberrations
Introduction
Since the mouse intracytoplasmic sperm injection (ICSI) technique was established in 1995 [1], it has been used for reproductive and cytogenetic research [2]. In addition, ICSI has superseded the interspecific in vitro fertilization (IVF) with zona‐free golden hamster oocytes in chromosome analysis of human spermatozoa [3, 4, 5, 6, 7, 8, 9]. However, little attention has been directed to chromosomal risks using ICSI. As far as previous chromosome studies of mouse one‐cell ICSI embryos are concerned, incidences (7.7 ± 3.4%) of structural chromosome aberrations are usually high even though live spermatozoa with normal morphology were used [10, 11, 12, 13, 14, 15, 16, 17]. The incidence was approximately six times as high as that (1.3 ± 0.6%) in one‐cell IVF embryos [18, 19, 20, 21, 22]. Thus, the detrimental effect of ICSI on chromosomes may be an issue.
There are several crucial differences between ICSI and conventional IVF. In ICSI, the oolemma is mechanically punctured by a fine glass needle, and then a small amount of medium and polyvinylpyrrolidone (PVP) are injected into the oocyte. Although a bicarbonate‐buffered medium with bovine serum albumin (BSA) is usually used under 5% CO2 to allow spermatozoa to capacitate in IVF, hepes‐buffered medium with polyvinyl alcohol (PVA) instead of BSA is used as a medium for sperm incubation under 100% air with ICSI because even uncapacitated spermatozoa can be fertilized after direct injection into the oocytes. However, our detailed chromosomal investigation of mouse ICSI embryos indicated that incidence of structural chromosome aberrations was relatively high when uncapacitated spermatozoa were used for ICSI [23]. As ICSI is essential to the treatment of human male infertility, the detection of causal factors of structural chromosome aberrations would be fundamental to further genetic security under this promising technique.
Experimental design for chromosome analysis of ICSI embryos
Mouse ICSI was basically performed according to Kimura and Yanagimachi [1], with the exception of sperm preparation. For sperm incubation, five types of media with a varying buffer system, i.e., bicarbonate‐buffered TYH and mCZB, hepes‐buffered H‐TYH and H‐mCZB, and phosphate‐buffered PB1, were prepared [23]. Both TYH and mCZB were used under 5% CO2, and others were used under 100% air. Spermatozoa were incubated in these media for 0 h (without incubation), 0.5, 2–2.5 or 6 h at 37°C. Regardless of the sperm incubation time, oocytes were collected approximately 16 h after hCG injection to avoid in vitro aging. To make a comparison of the chromosome aberration rate, IVF embryos were conventionally produced using spermatozoa incubated in TYH for 1.5–2.0 h. Spermatozoa incubated in other media were unavailable to produce IVF embryos due to insufficient induction of acrosome reaction (see Fig. 5). Oocytes that underwent ICSI and IVF were cultured in mCZB at 37°C. Six to eight hours later, fertilized eggs were transferred into mCZB containing vinblastine sulfate (0.02 μg/ml) to prevent syngamy and spindle formation, and cultured until they reached the first mitotic metaphase.
Figure 5.
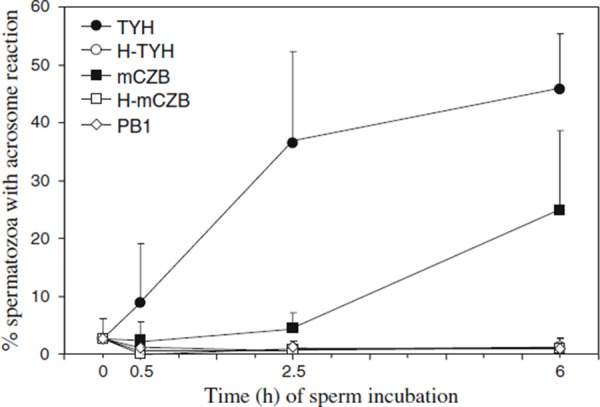
Change in percentage of spermatozoa with acrosome reaction after incubation in five different media
Chromosome slides of one‐cell embryos were made by the gradual fixation‐air drying method [24]. The chromosome slides were conventionally stained with 2% Giemsa solution to detect gap, acentric fragment, ring, and intra‐ and inter‐changes. Subsequently, C‐band staining was applied to detect dicentric chromosomes [12]. Structural chromosome aberrations reported here represent those in zygotes rather than in individual gametes because paternal chromosome complements were not always separated from maternal ones. However, in chromosome preparations in which paternal chromosome complements could be distinguished from maternal ones by less condensation of paternal chromosomes or presence of a Y chromosome, most structural chromosome aberrations were found to be of paternal origin. Regardless of the occurrence of structural aberrations in paternal chromosomes, incidences of structural chromosome aberrations of maternal origin in ICSI embryos remained fairly constant (0–2.8%) and they were similar to that (0.7%) in IVF embryos. Therefore, it is understandable that zygotic incidences of structural chromosome aberrations in one‐cell ICSI embryos are faithfully dependent on the occurrence of structural chromosome aberrations of paternal origin.
Changes in incidence of structural chromosome aberrations in one‐cell ICSI embryos with sperm medium, time of sperm incubation, and source of spermatozoa
Chromosome analysis of one‐cell ICSI embryos produced by motile spermatozoa from the cauda epididymis found that incidences of structural chromosome aberrations were greatly dependent on the kind of sperm medium and incubation time (Fig. 1). Major types of aberrations were breaks and dicentrics, regardless of sperm incubation conditions (Fig. 2). When spermatozoa were incubated in TYH for 0 and 0.5 h, the incidence of structural chromosome aberrations in the resultant ICSI embryos was 6.9 and 7.4%, respectively. These rates were significantly (P < 0.01; Chi‐square test) higher than that (2.3%: 8/355) in conventional IVF embryos. However, when spermatozoa were incubated for 2–2.5 and 6 h, the aberration rates (3.8 and 4.3%, respectively) were reduced to IVF embryo levels. Significantly (P < 0.05–0.001; Chi‐square test) high aberration rates compared to IVF embryos were found in ICSI embryos derived from spermatozoa incubated in other media in spite of incubation time. There was a clear time‐dependent increase in aberration rates when spermatozoa were incubated in hepes‐ and phosphate‐buffered media.
Figure 1.
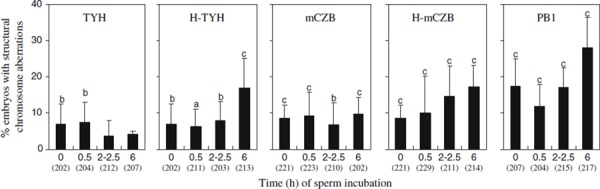
Occurrence of one‐cell ICSI embryos with structural chromosome aberrations when mature spermatozoa from the cauda epididymis were incubated in five different media for various times before ICSI. Number of embryos analyzed is indicated in parentheses. Statistical significance compared to IVF embryos (2.3%) is shown by a (P < 0.05), b (P < 0.01) and c (P < 0.001) (Chi‐square test)
Figure 2.
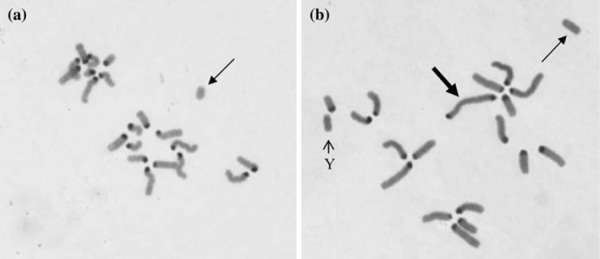
Major types of structural chromosome aberrations found in ICSI embryos. Centromeric regions of chromosomes except Y chromosome are positively stained with the C‐band method. a A chromosome break with acentric fragment (arrow). b A dicentric chromosome (thick arrow) and a derivative fragment (fine arrow)
To confirm whether such medium type‐ and incubation time‐dependent changes are found in chromosome aberration rates in ICSI embryos derived from testicular spermatozoa, the spermatozoa were incubated in TYH, H‐mCZB, and PB1 for 0.5 and 6 h before ICSI [25]. Unlike the results on embryos derived from mature epididymal spermatozoa, the aberration rates (7.4–11.7%) in embryos derived from testicular spermatozoa were considerably independent of the medium type, and there was no time‐dependent change in incidence of structural chromosome aberrations even though sperm incubation was extended to 6 h (Fig. 3).
Figure 3.
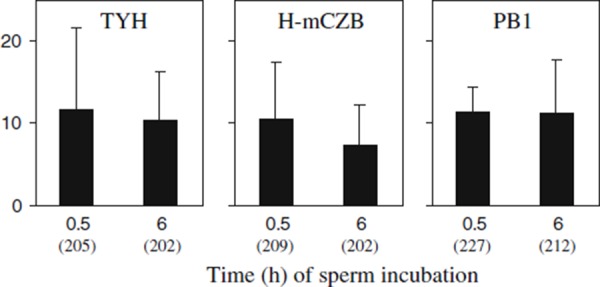
Comparison of occurrence of structural chromosome aberrations in one‐cell ICSI embryos derived from testicular spermatozoa after incubation in three different media for 0.5 and 6 h. Number of embryos analyzed is indicated in parentheses
Transmission of structural chromosome aberrations to offspring
Embryos with imbalanced chromosome aberrations such as breaks and asymmetric exchanges usually die in the pre‐implantation and early post‐implantation stages, while most embryos with balanced chromosome aberrations, such as reciprocal translocation and inversion, can survive [26]. These aberrations, however, often disturb future gametogenesis. To evaluate heritable risk, chromosomal analysis of ICSI fetuses was carried out [23, 25].
On the basis of chromosome analysis in one‐cell embryos (Fig. 1), mature epididymal spermatozoa were incubated in TYH for 2–2.5 h (low aberration rate: 3.8%), H‐mCZB for 2–2.5 h (intermediate aberration rate: 14.7%), and PB1 for 6 h (high aberration rate: 28.1%) to produce embryos. When fertilized ova became two‐ or four‐cell embryos, they were transferred into the oviducts of pseudopregnant females. The surrogate females were killed on day 16 of pregnancy to examine fetus morphology and chromosomes.
There were neither morphological anomalies nor chromosome aberrations in fetuses developed from IVF embryos (Table 1). However, external anomalies (umbilical hernia and polydactyly) were found in fetuses developed from ICSI embryos. Because both of the malformed fetuses had a normal karyotype, the cause of teratogenicity remains unclear. In a chromosomal survey, one (1.8%) fetus in TYH group had a mosaic consisting of normal cells and hyperploid cells [23], and four (6.7%) fetuses in H‐mCZB group displayed structural chromosome aberrations including a deletion, an inversion, and two different types of reciprocal translocations [25]. Thus, structural chromosome aberrations generated by ICSI are transmissible to offspring. Although there was no fetus with chromosome aberrations in PB1 group, more than 40% of embryos transferred were lost due to pre‐ and post‐implantation death. The result of chromosome analysis of one‐cell embryos indicates that the developmental defect is mostly due to structural chromosome aberrations.
Table 1.
Morphological and chromosomal analysis of fetuses developed from 2‐ to 4‐cell embryos using IVF and ICSI techniques [23, 24]
| Methods | Sperm medium (incubation time) | No. of embryos transferred | No. of developing fetuses | Morphological analysis | Chromosome analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| Live fetuses | Dead fetuses | No. of fetuses karyotyped | Normal | Aberration | |||||
| Normal | Malformation | ||||||||
| IVF | TYH (1.5–2.0 h) | 75 | 60 | 60 | 0 | 0 | 59 | 59 | 0 |
| ICSI | TYH (2–2.5 h) | 79 | 57 | 56 | 0 | 1 (1.7%) | 56 | 55 | 1 (1.8%) |
| H‐mCZB (2–2.5) | 87 | 62 | 59 | 1 a (1.6%) | 2 (3.2%) | 60 | 56 | 4 (6.7%) | |
| PB1 (6 h) | 62 | 35 | 34 | 1 b (2.9%) | 0 | 35 | 35 | 0 | |
aUmbilical hernia
bPolydactyly
The prenatal diagnosis of human ICSI fetuses revealed that incidence (0.44%) of de novo structural chromosome aberrations was approximately twice as high as the general population (0.16–0.22%) [27], and a higher rate (0.9%) of de novo structural chromosome aberrations was recently reported in ICSI fetuses, compared with that (0.2%) in IVF fetuses [28]. Although there is no available data on the incidence of de novo structural chromosome aberrations of paternal origin in human ICSI embryos, the results of the prenatal diagnosis suggest that embryos with structural chromosome aberrations may be significantly produced by ICSI.
Possible causal factors of structural chromosome aberrations and the production mechanism
There was a clear time‐dependent increase in chromosome aberration rates when mature epididymal spermatozoa were incubated in hepes‐ and phosphate‐buffered media (Fig. 1). Initially these media were suspected as one of causal factors of structural chromosome aberrations. However, the result of chromosome analysis of ICSI embryos derived from testicular spermatozoa following incubation in H‐mCZB and PB1 (Fig. 3) decreased the suspicion. Sperm chromatin is compacted in a small head; nevertheless, chromatin of testicular spermatozoa is less stable than that of mature epididymal spermatozoa because the former spermatozoa have poor disulfide (S–S) bonds in their nucleoprotein [29]. Some studies suggest that testicular sperm chromatin is more vulnerable to chemical and physical treatments than mature epididymal sperm chromatin [30, 31, 32]. If sperm culture media directly exerted some detrimental effects on sperm DNA, more structural chromosome aberrations would be generated in embryos derived from testicular spermatozoa. However, even when testicular spermatozoa were incubated in H‐mCZB and PB1 for 6 h, aberration rates in the resultant ICSI embryos were significantly lower than those in ICSI embryos derived from mature epididymal spermatozoa (Figs. 2, 3). It is unlikely that hepes‐ and phosphate‐buffered media are possible causal factors of structural chromosome aberrations. In addition, the great fluctuation in chromosome aberration rate depending on sperm incubation conditions does not support the original hypothesis that mechanical puncture of the oolemma, and injection of a small amount of medium with PVP, cause structural chromosome aberrations in mouse ICSI embryos.
Mouse mature epididymal spermatozoa have an intact acrosome that covers nearly two‐thirds of the sperm head (Fig. 4), and the cholesterol distinctively distributes in the plasma membrane overlying the acrosomal region [33, 34]. Because uncapacitated spermatozoa are usually used in ICSI, these components are injected into oocytes. Precocious induction of the acrosome reaction by calcium ionophore reportedly improves fertilization and development in ICSI of mice [35]. A similar result was observed by mechanically disrupting the acrosome in human spermatozoa [36]. Furthermore, the injection of intact acrosome into the ooplasm altered nuclear decondensation in human [37], mouse [38], pig [39], and rhesus monkey spermatozoa [40, 41, 42]. The acrosome enzymes could potentially induce deformation and degeneration of mouse oocytes [43]. However, the simultaneous removal of the sperm plasma membrane and acrosome before ICSI improved oocyte activation and embryonic development [44].
Figure 4.
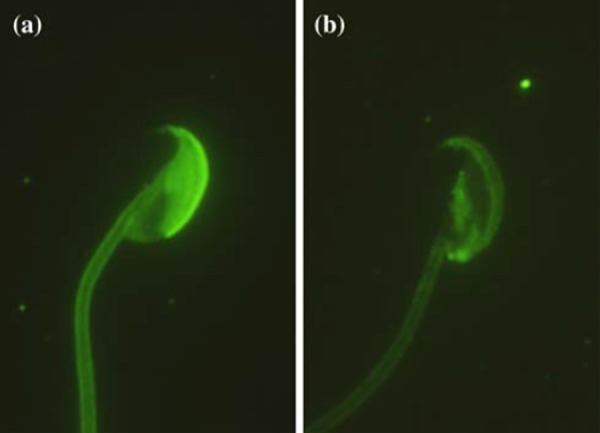
Fluorescent patterns of mouse sperm acrosomes stained with FITC‐conjugated peanut agglutinin. a Spermatozoon with the intact acrosome. b Spermatozoon showing acrosome reaction
Here, we observed a reduction in the incidence of structural chromosome aberrations in ICSI embryos derived from mature epididymal spermatozoa when spermatozoa were incubated in TYH for at least 2 h before ICSI (Fig. 1). TYH is widely used in the mouse IVF program because it can effectively induce sperm capacitation by promotion of cholesterol efflux from the plasma membrane and trigger acrosome reaction [45, 46]. Immunocytological staining of acrosome [47] also revealed that acrosome reaction was effectively induced when TYH was used for sperm incubation (Figs. 4, 5). Although mCZB is a bicarbonate‐buffered medium for mouse embryo culture, sperm incubation in this medium delayed progression of the acrosome reaction, most likely because mCZB contains the Ca2+‐chelating agent, EDTA. In the sperm incubation in H‐TYH, H‐mCZB and PB1, the acrosome reaction was never induced even after a lapse of 6 h. These results strongly suggest that the removal of cholesterol from the plasma membrane and/or the induction of acrosome reaction before ICSI may prevent the generation of structural chromosome aberrations in mouse ICSI embryos.
There is a possible reason why injection of the cholesterol‐rich plasma membrane and/or intact acrosome can affect chromosomes of paternal origin. During normal fertilization, sperm chromatin begins to decondense immediately after incorporation into the ooplasm. After passing through recondensation, the sperm chromatin drastically expands to form an enlarged male pronucleus. It has been found that decondensing mouse sperm chromatin during fertilization was highly vulnerable to topoisomerase II (topo‐II) inhibitor teniposide [48]. When mouse sperm chromatin during fertilization was exposed to topo‐II inhibitors etoposide and merbarone, nearly 100% of embryos displayed structural chromosome aberrations [49]. Thus, sperm DNA is cut and rejoined in the course of chromatin remodeling during normal fertilization and the process is mediated by ooplasmic topo‐II. If the topological rearrangement of DNA during sperm chromatin remodeling was physically or chemically interfered with by the plasma membrane cholesterol and/or acrosome enzymes, structural chromosome aberrations would be produced in ICSI embryos. Whether removal of cholesterol from the plasma membrane and the induction of acrosome reaction before ICSI can reduce the occurrence of structural chromosome aberrations still remains to be investigated.
It has been shown that in vitro aging of mouse spermatozoa caused structural chromosome aberrations of paternal origin. The aberration rate increased from 1 to 6% after 6 h of in vitro aging and 12% after 12 h of in vitro aging when spermatozoa were stored in unsupplemented Tyrode medium [50, 51]. Estop et al. [51] also found that in vitro incubation of mouse mature spermatozoa altered the chromatin structure. The authors proposed that the change of chromatin structure makes DNA susceptible to denaturation, thus leading to structural chromosome aberrations. Chromatin alteration by in vitro aging of spermatozoa may contribute to an increased incidence of structural chromosome aberrations when mature epididymal spermatozoa were incubated for 6 h in hepes‐ and phosphate‐buffered media.
Conclusions
In mouse ICSI, injection of uncapacitated spermatozoa with intact acrosome was linked with the generation of structural chromosome aberrations in resultant embryos. In contrast, precocious induction of capacitation and acrosome reaction by incubating spermatozoa in bicarbonate‐buffered TYH before ICSI was effective in repressing the occurrence of structural chromosome aberrations. Long incubation periods of spermatozoa in hepes‐ and phosphate‐buffered media, which are unable to induce capacitation and acrosome reactions, increase chromosomal damage in ICSI embryos. To further improve genetic security using ICSI, sperm preparation and incubation conditions should be refined.
Although this article exclusively concentrated on the generation of structural chromosome aberrations in mouse ICSI embryos, concurrent chromosomal analysis of mouse ICSI embryos concludes that ICSI has no relation to the production of aneuploid embryos [23, 25].
Acknowledgments
This study was supported by a Grant‐in‐Aid for Scientific Research (C): No. 19591886 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod, 1995, 52, 709–720 10.1095/biolreprod52.4.709 [DOI] [PubMed] [Google Scholar]
- 2. Yanagimachi R. Intracytoplasmic injection of spermatozoa and spermatogenic cells: its biology and applications in humans and animals. Reprod Biomed Online, 2005, 10, 247–286 [DOI] [PubMed] [Google Scholar]
- 3. Lee JD, Kamiguchi Y, Yanagimachi R. Analysis of chromosome constitution of human spermatozoa with normal and aberrant morphologies after injection into mouse oocytes. Hum Reprod, 1996, 11, 1942–1946 [DOI] [PubMed] [Google Scholar]
- 4. Ogawa S, Araki S, Araki Y, Ohno M, Sato I. Chromosome analysis of human spermatozoa from an oligoasthenozoospermic carrier for a 13,14 Robertsonian translocation by their injection into mouse oocytes. Hum Reprod, 2000, 15, 1136–1139 10.1093/humrep/15.5.1136 [DOI] [PubMed] [Google Scholar]
- 5. Tsuchiya K, Kamiguchi Y, Sengoku K, Ishikawa M. A cytogenetic study of in vitro matured murine oocytes after ICSI by human sperm. Hum Reprod, 2002, 17, 420–425 10.1093/humrep/17.2.420 [DOI] [PubMed] [Google Scholar]
- 6. Watanabe S. A detailed cytogenetic analysis of large numbers of fresh and frozen‐thawed human sperm after ICSI into mouse oocytes. Hum Reprod, 2003, 18, 1150–1157 10.1093/humrep/deg224 [DOI] [PubMed] [Google Scholar]
- 7. Watanabe S. Frequent structural chromosome aberrations in immotile human sperm exposed to culture media. Hum Reprod, 2004, 19, 940–947 10.1093/humrep/deh148 [DOI] [PubMed] [Google Scholar]
- 8. Fedorova ID, Kuznetsova TV, Baranov VS, Rybouchkin AV, Elst J, Dhont M. Cytogenetic analysis of human spermatozoa using intracytoplasmic sperm injection into mouse oocytes. Genetika, 2005, 41, 396–404 [PubMed] [Google Scholar]
- 9. Araki Y, Yoshizawa M, Araki Y. A novel method for chromosome analysis of human sperm using enucleated mouse oocytes. Hum Reprod, 2005, 20, 1244–1247 10.1093/humrep/deh757 [DOI] [PubMed] [Google Scholar]
- 10. Kishikawa H, Tateno H, Yanagimachi R. Chromosome analysis of BALB/c mouse spermatozoa with normal and abnormal head morphology. Biol Reprod, 1999, 61, 809–812 10.1095/biolreprod61.3.809 [DOI] [PubMed] [Google Scholar]
- 11. Kishikawa H, Tateno H, Yanagimachi R. Fertility of mouse spermatozoa retrieved from cadavers maintained at 4°C. J Reprod Fertil, 1999, 116, 217–222 [DOI] [PubMed] [Google Scholar]
- 12. Tateno H, Kimura Y, Yanagimachi R. Sonication per se is not as deleterious to sperm chromosomes as previously inferred. Biol Reprod, 2000, 63, 341–346 10.1095/biolreprod63.1.341 [DOI] [PubMed] [Google Scholar]
- 13. Kusakabe H, Szczygiel MA, Whittingham DG, Yanagimachi R. Maintenance of genetic integrity in frozen and freeze‐dried mouse spermatozoa. Proc Natl Acad Sci USA, 2001, 98, 13501–13506 10.1073/pnas.241517598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szczygiel MA, Ward WS. Combination of dithiothreitol and detergent treatment of spermatozoa causes paternal chromosomal damage. Biol Reprod, 2002, 67, 1532–1537 10.1095/biolreprod.101.002667 [DOI] [PubMed] [Google Scholar]
- 15. Kusakabe H, Kamiguchi Y. Chromosome analysis of mouse zygotes after injecting oocytes with spermatozoa treated in vitro with green tea catechin (−)‐epigallocatechin gallate (EGCG). Mutat Res, 2004, 564, 195–200 [DOI] [PubMed] [Google Scholar]
- 16. Suganuma R, Walden CM, Butters TD, Platt FM, Dwek RA, Yanagimachi R, Spoel AC. Alkylated imino sugars, reversible male infertility‐inducing agents, do not affect the genetic integrity of male mouse germ cells during short‐term treatment despite induction of sperm deformities. Biol Reprod, 2005, 72, 805–813 10.1095/biolreprod.104.036053 [DOI] [PubMed] [Google Scholar]
- 17. Tateno H, Kamiguchi Y. How long do parthenogenetically activated mouse oocytes maintain the ability to accept sperm nuclei as a genetic partner?. J Assist Reprod Genet, 2005, 22, 89–93 10.1007/s10815‐005‐1498‐0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin‐DeLeon PA, Boice ML. Sperm aging in the male after sexual rest: contribution to chromosome anomalies. Gamete Res, 1985, 12, 151–163 10.1002/mrd.1120120206 [Google Scholar]
- 19. Matsuda Y, Seki N, Utsugi‐Takeuchi T, Tobari I. Changes in X‐ray sensitivity of mouse eggs from fertilization to the early pronuclear stage, and their repair capacity. Int J Radiat Biol, 1989, 55, 233–256 10.1080/09553008914550281 [DOI] [PubMed] [Google Scholar]
- 20. Matsuda Y, Tobari I. Repair capacity of fertilized mouse eggs for X‐ray damage induced in sperm and mature oocytes. Mutat Res, 1989, 210, 35–47 [DOI] [PubMed] [Google Scholar]
- 21. Santaló J, Badenas J, Calafell JM, Catalá V, Munné S, Egozcue J, Estop AM. The genetic risks of in vitro fertilization techniques: the use of an animal model. J Assist Reprod Genet, 1992, 9, 462–474 10.1007/BF01204053 [DOI] [PubMed] [Google Scholar]
- 22. Yoshizawa M, Takada M, Nakamoto S, Muramatsu T, Okamoto A. Analyses of developmental ability of (BALB/c × C57BL/6)F1 and ICR mouse embryos fertilized in vitro and their chromosome at the first cleavage division. J Reprod Dev, 1993, 39, 115–122 10.1262/jrd.39.115 [Google Scholar]
- 23. Tateno H, Kamiguchi Y. Evaluation of chromosomal risk following intracytoplasmic sperm injection in the mouse. Biol Reprod, 2007, 77, 336–342 10.1095/biolreprod.106.057778 [DOI] [PubMed] [Google Scholar]
- 24. Mikamo K, Kamiguchi Y Ishihara T. Sasaki MS. A new assessment system for chromosomal mutagenicity using oocytes and early zygotes of the Chinese hamster. Radiation‐induced chromosome damage in man, 1983. New York: Alan R Liss; 411–432 [Google Scholar]
- 25. Tateno H. Chromosome aberrations in mouse embryos and fetuses produced by assisted reproductive technology. Mutat Res, 2008, 657, 26–31 [DOI] [PubMed] [Google Scholar]
- 26. Marchetti F, Bishop JB, Cosentino L, Moor D II, Wyrobek AJ. Paternally transmitted chromosomal aberrations in mouse zygotes determine their embryo fate. Biol Reprod, 2004, 70, 616–624 10.1095/biolreprod.103.023044 [DOI] [PubMed] [Google Scholar]
- 27. Bonduelle M, Assche E, Joris H, Keymolen K, Devroey P, Steirteghem A, Liebaers I. Prenatal testing in ICSI pregnancies: incidence of chromosomal anomalies in 1586 karyotypes and relation to sperm parameters. Hum Reprod, 2002, 17, 2600–2614 10.1093/humrep/17.10.2600 [DOI] [PubMed] [Google Scholar]
- 28. Gjerris AC, Loft A, Pinborg A, Christiansen M, Tabor A. Prenatal testing among women pregnant after assisted reproductive techniques in Denmark 1995–2000: a national cohort study. Hum Reprod, 2008, 23, 1545–1552 10.1093/humrep/den103 [DOI] [PubMed] [Google Scholar]
- 29. Bedford JM, Calvin HI. The occurrence and possible functional significance of –S–S– crosslinks in sperm heads, with particular reference to eutherian mammals. J Exp Zool, 1974, 188, 137–155 10.1002/jez.1401880203 [DOI] [PubMed] [Google Scholar]
- 30. Yanagida K, Yanagimachi R, Perreault SD, Kleinfeeld RG. Thermostability of sperm nuclei assessed by microinjection into hamster oocytes. Biol Reprod, 1991, 44, 440–447 10.1095/biolreprod44.3.440 [DOI] [PubMed] [Google Scholar]
- 31. Kosower NS, Katayose H, Yanagimachi R. Thiol‐disulfide status and acrigine orange fluorescence of mammalian sperm nuclei. J Androl, 1992, 13, 342–348 [PubMed] [Google Scholar]
- 32. Kaneko T, Whittingham DG, Overstreet JW, Yanagimachi R. Tolerance of the mouse sperm nuclei to freeze‐drying depends on their disulfide status. Biol Reprod, 2003, 69, 1859–1862 10.1095/biolreprod.103.019729 [DOI] [PubMed] [Google Scholar]
- 33. Toshimori K, Higashi R, Ōura C. Distribution of intramembranous particles and filipin–sterol complex in mouse sperm membranes: polyene antibiotic filipin treatment. Am J Anat, 1985, 174, 455–470 10.1002/aja.1001740408 [DOI] [PubMed] [Google Scholar]
- 34. Lin Y, Kan FWK. Regionalization and redistribution of membrane phospholipids and cholesterol in mouse spermatozoa during in vitro capacitation. Biol Reprod, 1996, 55, 1133–1146 10.1095/biolreprod55.5.1133 [DOI] [PubMed] [Google Scholar]
- 35. Lacham‐Kaplan O, Trounson A. Intracytoplasmic sperm injection in mice: increased fertilization and development to term after induction of the acrosome reaction. Hum Reprod, 1995, 10, 2642–2649 [DOI] [PubMed] [Google Scholar]
- 36. Takeuchi T, Colombero TC, Neri QV, Rosenwaks Z, Palermo GD. Does ICSI require acrosomal disruption? An ultrastructural study. Hum Reprod, 2004, 19, 114–117 10.1093/humrep/deg511 [DOI] [PubMed] [Google Scholar]
- 37. Terada Y, Luetjens CM, Sutovsky P, Schatten G. Atypical decondensation of the sperm nucleus, delayed replication of the male genome, and sex chromosome positioning following intracytoplasmic human sperm injection (ICSI) into golden hamster eggs: does ICSI itself introduce chromosomal anomalies?. Fertil Steril, 2000, 74, 454–460 10.1016/S0015‐0282(00)00671‐3 [DOI] [PubMed] [Google Scholar]
- 38. Ajduk A, Yamauchi Y, Ward MA. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol Reprod, 2006, 75, 442–451 10.1095/biolreprod.106.053223 [DOI] [PubMed] [Google Scholar]
- 39. Katayama M, Koshida M, Miyake M. Fate of the acrosome in ooplasm in pigs after IVF and ICSI. Hum Reprod, 2002, 17, 2657–2664 10.1093/humrep/17.10.2657 [DOI] [PubMed] [Google Scholar]
- 40. Sutovsky P, Hewitson L, Simerly CR, Tengowski MW, Navara CS, Haavisto A, Schatten G. Intracytoplasmic sperm injection for rhesus monkey fertilization results in unusual chromatin, cytoskeletal, and membrane events, but eventually leads to pronuclear development and sperm aster assembly. Hum Reprod, 1996, 11, 1703–1712 [DOI] [PubMed] [Google Scholar]
- 41. Hewitson L, Dominko T, Takahashi D, Martinovich C, Ramalho‐Santos J, Sutovsky P, Fanton J, Jacob D, Monteith D, Neuringer M, Battaglia D, Simerly C, Schatten G. Unique checkpoints during the first cell cycle of fertilization after intracytoplasmic sperm injection in rhesus monkeys. Nat Med, 1999, 5, 431–433 10.1038/7430 [DOI] [PubMed] [Google Scholar]
- 42. Ramalho‐Santos J, Sutovsky P, Simerly C, Oko R, Wessel GM, Hewitson L, Schatten G. ICSI choreography: fate of sperm structures after monospermic rhesus ICSI and first cell cycle implications. Hum Reprod, 2000, 15, 2610–2620 10.1093/humrep/15.12.2610 [DOI] [PubMed] [Google Scholar]
- 43. Morozumi K, Yanagimachi R. Incorporation of the acrosome into the oocyte during intracytoplasmic sperm injection could be potentially hazardous to embryo development. Proc Natl Acad Sci USA, 2005, 102, 14209–14214 10.1073/pnas.0507005102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morozumi K, Shikano T, Miyazaki S, Yanagimachi R. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc Natl Acad Sci USA, 2006, 103, 17661–17666 10.1073/pnas.0608183103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Toyoda Y, Yokoyama M, Hosi T. Studies on the fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperm (in Japanese). Jpn J Anim Reprod, 1971, 16, 147–151 [Google Scholar]
- 46. Ward CR, Storey BT. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev Biol, 1984, 104, 287–296 10.1016/0012‐1606(84)90084‐8 [DOI] [PubMed] [Google Scholar]
- 47. Mendoza C, Carreras A, Moos J, Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one‐step staining method using Pisum sativum agglutinin. J Reprod Fertil, 1992, 95, 755–763 10.1530/jrf.0.0950755 [DOI] [PubMed] [Google Scholar]
- 48. Bizzaro D, Manicardi G, Bianchi PG, Sakkas D. Sperm decondensation during fertilisation in the mouse: presence of DNase I hypersensitive sites in situ and a putative role for topoisomerase II. Zygote, 2000, 8, 197–202 10.1017/S0967199400000988 [DOI] [PubMed] [Google Scholar]
- 49. Tateno H, Kamiguchi Y. Chromosome analysis of mouse one‐cell androgenones derived from a sperm nucleus exposed to topoisomerase II inhibitors at pre‐ and post‐fertilization stages. Mutat Res, 2004, 556, 117–126 [DOI] [PubMed] [Google Scholar]
- 50. Munné S, Estop A. The effect of in vitro aging on mouse sperm chromosomes. Hum Reprod, 1991, 6, 703–708 [DOI] [PubMed] [Google Scholar]
- 51. Estop AM, Munné S, Jost LK, Evenson PD. Studies on sperm chromatin structure alterations and cytogenetic damage of mouse sperm following in vitro incubation, studies on in vitro‐incubated mouse sperm. J Androl, 1993, 14, 282–288 [PubMed] [Google Scholar]


