Abstract
The Energy Dispersive X-ray (EDX) microanalysis is a technique of elemental analysis associated to electron microscopy based on the generation of characteristic Xrays that reveals the presence of elements present in the specimens. The EDX microanalysis is used in different biomedical fields by many researchers and clinicians. Nevertheless, most of the scientific community is not fully aware of its possible applications. The spectrum of EDX microanalysis contains both semi-qualitative and semi-quantitative information. EDX technique is made useful in the study of drugs, such as in the study of drugs delivery in which the EDX is an important tool to detect nanoparticles (generally, used to improve the therapeutic performance of some chemotherapeutic agents). EDX is also used in the study of environmental pollution and in the characterization of mineral bioaccumulated in the tissues. In conclusion, the EDX can be considered as a useful tool in all works that require element determination, endogenous or exogenous, in the tissue, cell or any other sample.
Key words: Energy Dispersive X-ray (EDX) microanalysis, transmission electron microscopy, scanning electron microscopy, asbestos, calcifications, nanoparticles
Introduction
The Energy Dispersive X-ray (EDX) microanalysis is involved in different biomedical fields of study due to its high sensitivity in detecting the different elements in tissues. In fact, EDX technique is made particularly useful in the study of drugs delivery in which the EDX is an important tool in order to detect nanoparticles (generally, used to improve the therapeutic performance of some chemotherapeutic agents). EDX technique is also used in the study of environmental pollution. In particular, EDX microanalysis can carry a huge vantage in the detection of heavy metals pollutions as it has been demonstrated by Scimeca et al.1 In another study, authors investigated whether the impact of heavy metals accumulation in bone tissues could be related to the altered bone metabolism and architecture of osteoporotic patients.2 Otherwise, EDX was used by Barba et al. to study how infection associated to prosthesis could be linked to metallosis3 or in the case report of Khan et al. where they show a metallosis following a shoulder hemi - arthroplasty with a humeral component resurfacing shoulder replacement.4 Moreover, the EDX microanalysis on the pathological deposition of calcium has been useful to characterize the differences in the elemental composition of breast calcifications, in particular the composition of benign and malignant lesions5 or the tight association of asbestos nano-fibers and lung cancer cells.6 In fact, EDX microanalysis has greatly improved the characterization of the asbestos isotype and shed new light in the study of the possible bioaccumulation of polluting agents in different human organs and systems. In this review, we highlight the role of EDX microanalysis in medical and biological fields.
EDX microanalysis apparatus
Element detection is still a dark side of electron microscope (EM) studies. Indeed, few EM departments employ microanalysis in diagnostic or/and research purposes. There are three possible ways to detect the elements into the tissues using EM: i) wavelength dispersive spectroscopy (WDS); ii) electron energy-loss spectroscopy (EELS); and iii) energy dispersive spectroscopy (EDS), more commonly known as electron probe X-ray microanalysis (EDX).7 EDX microanalysis is a technique of elemental analysis that is based on the generation of characteristic Xrays in atoms of the specimen by the incident beam electrons.8 Afterwards the impact with the atoms, two fundamental physical events occur: elastic scattering and an inelastic scattering. The elastic scattering is a change in the direction of the electron with no noticeable energy loss, generally caused by interactions with the nucleus comprising the materials, whereas the inelastic scattering is the loss of energy with no noticeable change in direction, usually generated from interaction with both the bound electrons and the nucleus in the atoms.9 The elastic scattering events are the main determinants of the shape of the interaction volume while the inelastic scattering is the major determinant of the size of the interaction volume. Nevertheless, the atoms are ionized and when they return to their ground state, they will emit characteristic X-rays and the energy of the X-ray photon is the potential energy resulting from the difference between the two orbitals involved in the transition, which is thus characteristic of the element.7 The X-ray emission at different wavelengths may then be measured by a photon-energysensitive detector. These X-rays are characteristic for the element from which they originate and they contain information on the elements that are present in the specimen (Figure 1A). Indeed, the energy depends on the atomic number of the element in which the interaction is occurring.1 To collect X-rays of all energies simultaneously it has been used a semiconductor detector for X-ray microanalysis of biological specimens and the continuum intensity is not just related to the elemental composition of the specimen. This is due to heavy elements produce more continuum intensity than light elements.10
Figure 1.
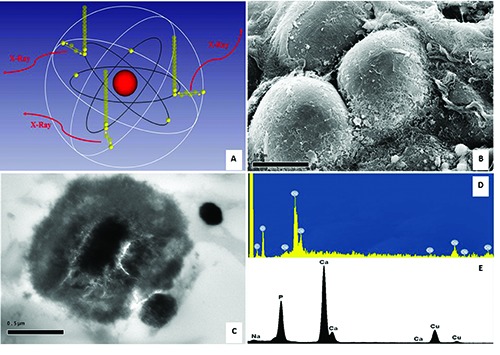
SEM and TEM EDX microanalysis. A) Characteristics X-rays was originated from the transitions of electrons from higher shells to lower shells by atoms of the specimen interacted with electrons beam. B) Scanning electron micrograph shows breast adipocyte cells (1000x). C) Transmission electron micrograph displays a psammomabody in a carotid plaque (30,000x). D) The Osmium used for lipid fixation is detected by SEM-EDX microanalysis. E) EDX spectrum demonstrate that psammoma-body is made of hydroxyapatite.
The EDX detector system performs a simultaneous display of all mid-energy (1-20 keV) X-rays collected during any individual analysis period9 and the energy of the X-rays is reproduced as a spectrum, which is a histogram plot of number of counts against X-ray energy. The spectrum contains both semi-qualitative and semiquantitative information. The position of a peak in the spectrum, its energy, identifies the element; the area under the peak is proportional to the number of atoms of the element in the irradiated area. X-rays are also produced when the electron beam is slowed by the electrostatic fields of the atomic nuclei of elements present in the specimen. These X-rays form a continuous radiation that appears below the peaks in that spectrum. Qualitative analysis, i.e., identification of elements in the spectrum, is usually achieved using manufacturer’s software. These systems are not foolproof, they are designed for materials applications rather than biology and misidentification of a peak by the software is a distinct possibility.9 Conventionally, EDX is a powerful technique allowing an elemental analysis of the surface of the samples (Figure 1 B,D).
This method implies some limitation in elemental analysis. Firstly, X-ray spectrometry detects elements and it is not capable of distinguishing between ionic and nonionic species. Moreover, in the EDX it is required that all samples are analysed under relatively vacuum and obviously this has serious implications for the preparation of the specimens because electron and X-rays are strongly absorbed by air molecules. Generally, X-ray detection is not influenced by the chemical state of elements but it is influenced by inter-elements interference, known in X-ray spectrometry as peak overlap, causing serious problems in the elemental analysis. Therefore, it is possible to detect those elements with atomic number larger than 10. The minimal detectable elemental concentration, which requires some signal averaging, is approximately 0.1 mmol per kg of dry specimen (i.e., 10 ppm), whereas spatial resolution ranges from about 10 nm to a few micrometers.1,10 Reducing the detection limit requires more counts, which can be obtained by increasing the counting time and /or the beam current. Values given here for detection limits refer to biological samples for which the mean atomic number (which determines continuum intensity) is generally quite low (e.g., C, H, N, Cl, P, K). Biological samples containing heavy elements give higher detection limits due to the higher background. Further, detection limits for heavy elements (using L or M lines) tend to be somewhat higher because the peak-to-background ratio is lower than for K lines.9
Biological sample preparation
Analytical results of EDX microanalysis are obtained from the specimen as it exists in the electron microscopy. Since that biological specimens undergo dehydration before electron microscopy analysis, the major aim in preparation for biological microanalysis is to maintain elements at their physiologically active site so that analysis gives true and meaningful results.7 For routine application of transmission electron microscopy, 1 mm3 of specimens are generally fixed in 4% paraformaldehyde and post-fixed in 2% osmium tetroxide1,11 and, after washing with 0.1 M phosphate buffer, the samples are dehydrated by a series of incubations in 30%, 50%, 70% ethanol. Dehydration continues by incubation steps in 95% ethanol, absolute ethanol and propylene oxide, after which samples are embedded in epoxidic resin.1,6
To prevent cell surface deformation, tissues that undergo scanning electron microscopy (SEM) analysis are dehydrated by using critical-point drying instruments with supercritical CO2.12 However, using these analytical methods diffusible atoms, elements and small molecules are easily lost during the dehydration and embedding steps.7 Thus, routine methods are only relevant if it is already known that the elements of interest are heavy and/or tightly bound to a biological structure (e.g., nuclear lamina). To prevent movement of elements, specimens are then maintained at low temperature throughout any further steps until they are either stabilized by freezedrying or analyzed in the frozen-hydrated state.7 In this context, cryofixation techniques developed for the study of antigens using immunocytochemistry provide a valid option for the study of in situ elements distribution by EDX microanalysis. Indeed, these techniques effectively prevent the lost or acquisition of elements during samples preparation. In addition, cryofixation techniques allow to quantify the detected elements by EDX microanalysis.7 Nevertheless EDX quantification techniques are very difficult to achieve and require the use of standard specimens. So, only few groups are using this approach. Indeed, in addition to the difficulty linked to the cryostatic techniques the preparation of standard specimens must be accurate. In particular, standard samples should be of similar overall composition to the specimen with the assumption that mass loss is similar in both specimen and standard. Standards are made by dissolving known concentrations of salts in a solution of either 15-20% protein such as gelatin or albumin, or 15-20% dextran, freezing droplets of the solution and taking them through exactly the same procedure used for specimen preparation.7 For each element, a number of standards of different concentration must be analyzed to obtain an accurate investigation. In addition, the accuracy of standardization must be checked either by the analysis of a specimen of known composition such as red blood cells or by analyzing a standard of known composition that was not used in the standardization procedure.
In a recent work, we proposed a new TEM-EDX analytical protocol that can be a helpful trial to detect elements, asbestos nanofibers and more in general nanomaterials by using archival formalin fixed paraffin embedded (FFPE) tissues.6,13 Specifically, hematoxylin-and-eosin (H&E) stained sections were used in order to identify areas to be subjected to EDX analysis. As previously described, embedding beam capsules were placed over areas previously identified on H&E section and detached from the slide with thermal shock (liquid nitrogen) after resin polymerization.6 The application of this method opens the possibility to perform large-cohort EDX studies by analyzing FFPE tissues stored in the Anatomic Pathology departments’ archives. EDX spectra are acquired by analyzing 100 nmthick unstained ultrathin sections for TEM-EDX or unstained dried samples for SEM-EDX; staining whit heavy metal (e.g., U, Pb) or Au could compromise the analysis.
EDX microanalysis in heavy metals identification
The rapid growth of industries led to the increase of the environmental pollution specially with toxic substances.14 Heavy metals are among the most problematic pollutants because they are nonbiodegradable and they can accumulate in ecological systems.15 Most industries have been using substances containing heavy metals to manufacture of daily objects that we use every day. Small amounts of heavy metals are normally present in human tissues and some of them are also required for normal biological processes. Nevertheless, when the levels of heavy metals get too high, they became extremely toxic.16 In the light of this, the presence of high levels of heavy metals is a serious problem for human health.17 Unfortunately, there are still too many mining operations, industrial, domestic and agricultural production, that use metals and metal containing compounds and this has resulted in the release of toxic metals into the environment. Similarly, many industrial solid waste is mostly dangerous in nature because it can be highly inflammable, reactive or toxic, and consists of hazardous heavy metals.18,19 Numerous studies showed that some heavy metals are toxic and lethal in trace concentrations demonstrating their teratogenic, mutagenic, endocrine properties. Heavy metals can also cause behavioral and neurological disorders among infants and children.20-22 The highly relevant pollutants for their toxicity, non-degradability and their increasing presence in the environment as a consequence of their widespread industrial use are heavy metals such as cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), nickel (Ni), and zinc (Zn).23
Several studies demonstrated that the topsoil is a transfer interface for the accumulation of heavy metals in the air, plant, and water. The seasonal and spatial variations of heavy metals in the topsoil are attracting major concern across the world.24 There are differences between the heavy metal sources in urban regions and the pollution in agricultural areas. In fact, the heavy metal accumulation in agricultural land is associated with land use types and crop rotation types,25 while heavy metals in urban regions are mainly derived from industrial emissions, vehicle exhaust, and the weathering of building and pavement surfaces. In addition, an important role is also played by the atmospheric transport in the distribution of heavy metals in many regions. Identifying the sources of pollution has become a popular research topic in recent years and many different methods were introduced in previous studies. In this context, EDX microanalysis can be a useful tool to investigate the accumulation of heavy metals in tissues and in forensic science where for example it was used to identify the presence of different residue such us powders or metals that settles on the skin and clothing. In this field, some studies were designed to obtain comprehensive information on the prevalence of gunshot residue in various populations as a result of the primary and secondary transfers as well as to measure factors that influence these processes and states. They used a specific analytical method such as scanning electron microscopy coupled with energy dispersive X-ray spectrometry (SEM-EDX). SEMEDX being suitable for chemical and morphological examinations of the primer residue particles, which until now are thought to be the most characteristic features of a firearm discharge for the majority of ammunition in use.26
In a study of our group, EDX microanalysis was used to detect metal contaminants in non-small cell lung cancer. Our results show significant differences in the bioaccumulation of toxic elements, such as Co, Cr and Pb, between pulmonary cancer lesions and benign lesions. The ex vivo identification of toxic elements in the lung tissue could better clarify the environmental impact on lung carcinogenesis. To this aim we used the EDX microanalysis to associate morphological / ultrastructural data with the bio-accumulation of toxic elements related to air pollution.1
In another research, Scimeca et al., investigated whether heavy metals accumulation in bone tissues could be related to the altered bone metabolism and architecture of osteoporotic patients. Thanks to the SEM-EDX microanalysis, they demonstrated a specific accumulation of Pb, Cr and Cd in trabecular head biopsies. Toxic elements bio-accumulation appears a time dependent phenomenon, as demonstrated by the low presence of metals in under 50 patients. Indeed, authors demonstrated that trabecular tissues of osteoporotic patients harbor a higher number of heavy metals than patients with comparable age (osteoarthritis). It should be noticed that the heavy metals we detected coincide with those emitted in the atmosphere by natural and/or anthropic sources.2 Over the years, the EDX microanalysis has been increasingly used by the scientific community for the study of heavy metals pollution and its effects on the environment and the living organisms. For example, Bao et al. used the EDX technique to demonstrate that the underground subway stations in Shanghai were an important micro-environment which allowed the exposition to metal through aerosol.27 They highlighted that the aerosol composition in the metro station was quite different from the aboveground urban particles. In particular, thanks to SEM-EDX they demonstrated that the concentrations of Fe, Mn and Cr were higher than the averages of aboveground urban air particles.27 About pediatric dentistry research, Soteriou et al., evaluated the elemental alterations of Ag soldering alloys used in space maintainers after intraoral exposure.28 In this work, they compared the quality of two different Ag soldering alloys using Dentaurum Universal Silver Solder (US) and Leone Orthodontic Solder (OS). All devices were manufactured by the same technician while SEM-EDX assay demonstrated the oral elemental contamination before and after intra-oral placement in patients. In fact, they could prove a significant Cu and Zn reduction after intra-oral exposure that may raise biocompatibility concerns in both soldering alloys.28 Also, Ferraro et al. studied the presence of bioaccessible heavy metalssediment particles from Reconquista River (RR-PM), one of the most polluted watercourses in Argentina.29 The River receives effluent discharges from heavily industrialized and highly populated settlements. To this end, authors studied how this pollution induces lung inflammation in mice evaluating the biological impact of this particles on the respiratory system of BALB/c mice. Their results showed that animal exposure to RR-PM caused polymorphonuclear cell lung infiltration, increase of proinflammatory cytokines (tumor necrosis factor alpha TNFα, interleukin-6 IL-6) and apoptosis. Moreover, they obtained that the sensibilized and exposed to particles groups had more dramatic adverse response.29
Similarly, El-Mufleh et al. used a combination of density fractionation procedure and in particular microanalysis techniques to evaluate the distribution of polycyclic aromatic hydrocarbons (PAHs) and trace metals (i.e. Cd, Cr, Cu, Ni, Pb, and Zn) for three urban storms water basin sediments.30 The sensibility of microanalysis allows the authors to identify pollutantbearing phases in their samples. Their results confirm that PAHs are found in the lightest fractions (d < 1.9, 1.9 < d < 2.3 g cm−3) whereas trace metals are equally distributed within the light, intermediary, and highest fractions (d < 1.9, 1.9 < d < 2.3, 2.3 < d < 2.6, and d > 2.8 g cm−3) and are mostly in the 2.3 < d < 2.6 g cm−3 fraction.30
EDX microanalysis in asbestos isotype characterization
In the last years, the scientific community has developed a wide interest in the study of asbestos and the effects in exposure to it. Asbestos is one such mineral which has been shown to be a potent hazard for human health, being listed as the third most abundant pollutant on a global scale.31,32 The internalization of micro- to nano-sized asbestos fibers suspended in air can lead to their gradual accumulation particularly in lungs.33 In addition, it is known their later manifestation as lung carcinoma, pleural mesothelioma or asbestosis.34 As best method for physicochemical characterization of asbestos, the literature survey on the analytical techniques best suited indicated electron microscopy techniques among which the most suitable one resulted to be the EDX in comparison to more convenient techniques of light microscopy or phase contrast optical microscopy.35-45 It is possible to classify asbestos, a generic term, into six distinct types of fibrous silicate minerals, which are easily separated in long, thin, flexible fibers when crushed or elemental composition.46,47 There are two groups or classes, the serpentine and amphibole asbestos. Just one type was found in the serpentine group and it is chrysotile (commonly known as white asbestos) that possesses relatively long and flexible fibers with the capacity of being woven and five amphibole asbestoses: crocidolite (blue asbestos), amosite (brown asbestos), actinolite, anthophyllite, and tremolite.48 TEM-EDX and/or SEM-EDX allow to detect six crystalline structures of asbestos and in particular, asbestoscontaining materials, as reported in many studies.35-45 Our group performed EDX microanalysis studies to associate asbestos nanofibers and lung cancer cells. In particular, we applied TEM-EDX microanalysis to show the presence of asbestos nanofibers in histological specimens (paraffin blocks) of patients with possible occupational exposure to asbestos. This method allowed us to re-evaluated paraffin-embedded lung biopsies of lung cancer patients with an history of possible exposure to asbestos. The EDX microanalysis showed that the identified nanofibers were mainly made of silicon and iron in patients with possible occupational exposure to asbestos. In particular, fibers detected were compatible with an asbestiform variety of amosite, the gedrite. Due to its relevance in different fields, such as environment, medical, legal, the research on asbestos is carried worldwide out and the EDX microanalysis was a fundamental step to improve the information about it.6
In Iran, Marioryad et al. sought to characterize asbestos subtypes, based on evaluations of raw materials and airborne asbestos samples. Authors used SEM-EDX analysis to analyze chemical composition of the fibers. They demonstrated through the fiber morphology and EDX spectrum that all samples examined contained only chrysotile asbestos and in particular tremolite and actinolite asbestos are present in or around certain chrysotile mines.49 Hippeli et al. investigated relationships between redox properties and bio-durability of crocidolite asbestos fibers and three different man-made vitreous fibers. In particular, they examined changes of fiber morphology and chemical composition using SEM-EDX technology. 50 In the last decade, the use of the EDX analysis as a support tool in the study of asbestos fibers is increasing. In fact, some author studied the applicability of a UV micro-Raman setup for a rapid identification of fibrous asbestos minerals. To this aim, the authors used a SEM-EDX spectrometer to analyze the elemental composition of asbestos reference samples. Their results and the use of EDX analysis shed new light on the structural and vibrational consequences of cation distribution in asbestos minerals.51 Moreover, in 1989 a paper reported the use of EDX method to measure the environmental air concentrations of asbestos and total mineral fibers during the period June-July 1985 in several locations near a large asbestos-cement factory located in the proximity of a northern Italian town. Specifically, authors did the measurements of the number and type of fibers by SEM-EDX analysis. According to Recommended Technical Method No. 2 (RTM2) of the Asbestos International Association, EDX analysis allowed them to show that about 65% of fibers were sulfate fibers, 20% were aluminum silicates or other silicates, and only 15% were asbestos fibers (mainly chrysotile and tremolite-group amphiboles). In conclusion, in this study authors demonstrated large differences in day-to-day concentrations, suggesting that they were affected by the rate of production in the plant and by weather conditions.52 Finally, Barbieri et al. used EDX microanalysis to provide information on the intensity of environmental asbestos exposure through the evaluation of the lung fibers burden in mesothelioma patients who live around asbestos-cement factories.53
Identification of elemental composition of tissue calcification
Calcium is a very important mineral in the human body. Altered levels of this mineral could have different effects on the tissues. Several major diseases, such as cancer and cardiovascular abnormalities, may be linked to pathological deposition of minerals or organic compounds in various tissues (Figure 1 C,E).54,55 Thus, the detection of such minerals or compounds and understanding the physicochemical processes associated with their formation are essential. In this field, the EDX is a useful technique to detect possible pathological deposition of materials in tissues. Pathologic calcifications are defined as the abnormal tissue deposition of calcium salts, together with smaller amounts of iron, magnesium, and other mineral salts. These elementary lesions may occur in various parts of the body, namely joints,56 brain,57-59 breast,60-64 cartilage,65 cardiac valves,66,67 middle ear,68 gallbladder,69 gastric system,70,71 heart,72,73 intestine,74 kidney,75,76 larynx,77 liver,78 lungs,79,80 pancreas,81,82 prostate,83,84 tendons,85 testicles,85,86 thyroid,87,88 and artery or vessels affecting adults, as well as fetuses and newborns.89,90 Calcifications of placenta have also been observed.78 Calcifications of medical devices made of polyurethane, silicones, and hydrogels have been widely reported.
Different aspects of such calcifications must be taken into account to establish a significant relationship with the disease. These include chemical diversity, morphologic features at the microscopic and macroscopic scale, location in the organ, presence of trace elements (which could be catalytic agents), presence of molecular groups, such as carbonate groups in apatites and, finally, proteins.
The morphologic features of the calcification must be considered,87,88 especially for concretions. From a physicochemical point of view, the terms nanocrystals and crystallites is used to define the structural hierarchy of these mineral concrements. Crystallites (measuring typically some tens of micrometres) are made of a collection of nanocrystals (measuring typically some hundreds of nanometres).For as much as the biological role of calcification is concerned, special attention must be paid to the presence of trace elements and molecular groups on the surface or inside the mineral.91,92 These trace elements have been the object of numerous studies in chemistry or medicine to elucidate their role regarding the crystal formation kinetics or the morphologic features of the mineral.93,94 A recent study highlighted that Zn may have an inhibitory effect on calcium oxalate (CaOx) stone formation,95 whereas Fe and Cu could promote CaOx stone formation.96-99
The EDX microanalysis made possible to do an elemental analysis on different isotype of calcification and gave additional information about linkage between calcification and disease. In addition, EDX also allowed significant progress on the knowledge of molecular process and the effects that calcification involves. For example, Sonou et al. for the first time did a report on the accurate determination of the chemical composition of aortic medial calcification using the SEM-EDX. Before this study the composition of medial calcification has not been accurately determined.100 Few studies have been done about the compounds in atheromatous plaques.101-106 Schmid et al.101 demonstrated that apatite (71%), carbonates (9%), and a relatively high percentage of protein (15%) were found in the plaque calcifications. Marra et al.103 results showed the same chemical composition of surface and interior of the mineral deposit by SEM-EDX. However, SEM revealed that the deposits were heterogeneous, consisting of five different structures.103 Information of Marra’s study about the composition and properties of calcified deposits helped to determine the risk associated with their presence. Starting from these evidence, Lara et al. studied the composition and genesis of calcium deposits in atheroma plaques. In particular, they used the SEM-EDX to determine the organic and mineral components of atheroma as a first step toward establishing the genesis of these deposits.104
Over time the EDX techniques has been increasingly used in this field of research, Chang et al. used this method to study the characteristics of topographical microstructure and elemental mapping of human cardiac calcified deposition. Their data showed that calcium hydroxyapatite (HA) and cholesterol were the main components of the cardiac calculus. The authors demonstrated the presence of an area with a rich calcium HA deposition that exhibited higher amounts of C, O, P, and Ca elements as well as trace amounts of N, Na, Mg, and Al, whereas the major concentration of C, minor concentrations of N and O, and trace amounts of P and Ca. Furthermore, this area was generally observed in a cholesterol-rich area. In this study, the use of electron microscopy associated with EDX was performed to provide useful information on the microstructural characteristics and spatial distribution of elements on the surface of human cardiac calculi.105
In another studie, the EDX has been used to investigate the bone matrix calcification during embryonic and postembryonic development.107 In the same field, Okata et al. investigated the process of calcification during bone healing in a standardised rat calvarial bone defect model. Thanks to EDX, authors could examine the calvaria of rats in weeks 1, 2, 4 and 8 demonstrating that initially the bone healing was not completely calcified and gradually proceded.108 Khan et al. studied a case of an otherwise-healthy 4.5-year-old girl with primary congenital glaucoma who had multiple nummular calcium deposits on the dorsal plate of a previously-implanted Ahmed glaucoma valve. In this case, they found multiple white nummular lesions on the dorsal plate surface and the EDX microanalysis confirmed that the lesions were made of calcium.109
Moreover, Henmi et al. used this technique to investigate the process of calcification during rat calvarial bone development. In particular, the mineral crystal matures and the Ca/P ratio increases in developing bone but is still not known how an increase in the Ca/P ratio is involved in the maturation of the crystal and are also unclear the relationships among organic components and mineral changes. The authors analyzed the atomic distribution and concentration of Ca, P, and C of calcification by using the SEM-EDX microanalysis. In fact, their results showed that crystallization of hydroxyapatite during the bone matrix calcification process started around embryonic day 20 where the Ca/P ratio had increased and the C/Ca and C/P ratios had decreased significantly. Finally, thanks to with X-ray diffraction, Fourier transform infrared spectroscopy and histological analysis but also thanks to EDX analysis, authors demonstrated that the Ca/P molar ratio increases and the proportion of organic components such as proteins of the bone matrix decreases during the early stage of calcification, whereas crystal maturation continues throughout embryonic and postembryonic bone development.107 Also EDX microanalysis can be used to identify micro-HA crystal produced by osteoblast cultures.110,111
Recently, we characterized the elemental composition of microcalcifications by TEM-EDX microanalysis describing for the first time the presence of magnesium-substituted HA (Mg-HA) in breast microcalcifications.5,112,113 It is important to underline that the complex forms of calcification (HA and Mg-HA) are strictly related to malignant lesions whereas CO is mainly reported in benign lesions. Mg-HA microcalcifications were mainly detected in breast cancers. These data on microcalcifications composition let to speculate about an active deposition of calcium minerals in breast carcinogenesis, since such complex form of minerals cannot be due to a mere degenerative process but rather resemble a process of mineralization similar to that of bone. All these works allow to understand the importance of EDX microanalysis and the help which brought in the study of many pathologies related to the process of calcification.
EDX microanalysis for the development of the nanotechnologies
The general alarm on the possible hazard associated to the exposure to nanomaterials is often related to the change of toxicity occurring when nano-dimension is achieved. For instance, substances generally considered safe for humans in the micrometric range (e.g., TiO2, FexOy, amorphous SiO2 and carbon) might induce detrimental effects on human health when at the nanometric scale.114-116 In recent year, nanoparticles (NPs) became increasingly important in different application fields such as biomedical imaging, diagnostic tests, and drug delivery. Recently, the U.S. Food and Drug Administration approval an increasing number of nanotechnology products. Thus, understanding biological responses to nanoparticles is an important area in which to address the safety of nanoscale materials.117 The literature defined as nanomaterial a small-scale substance that are less than 100 nm in at least one dimension.118 These technologies are rapidly developing and a lot of products, such as NPs, is expected to further increase in the near future.118,119 Particularly silver nanoparticles (AgNPs) are becoming more and more interesting for scientific community since they are used in a wide range of applications (e.g., textiles and wound dressings), mostly because of their antimicrobial properties. AgNPs are applied also in food industry for processing and packaging of food (kitchenware coated with AgNPs or foils and containers with incorporated AgNPs) but also in food itself, for example, as health supplements.120-123 In addition, a special attraction for biological applications is given to gold nanoparticles (AuNPs) due to their simple synthesis methods, fast preparation and easy bioconjugation.124 An important application of AuNPs is represented by biological diagnostics,125 cell tracers,126 drug delivery,127 and cell imaging.128 Recently, some scientist used AuNPs conjugated to anti-epidermal growth factor receptor (EGFR) antibodies to selectively target cancer cells that overexpress EGFR on their surface and to highlight whole cancer cells.129 All together, these data allow us to speculate that the use of nanotechnology in cancer biology has provided hope within scientific communities of developing novel cancer therapeutic strategies.130 Recently, vaccine and drug delivery were perfectioned by the use of nanotechnologies because they have proprieties to give protection of payloads from both metabolism and offtarget effects and the ability to facilitate specific delivery of cargo to immune cells.131 To this aim, the knowledge of NPs potential toxicity is essential for biomedical purposes and applications. Motskin et al. using the EDX studied the sequestration of HS NPs by human monocyte-macrophages in a compartment that allows free diffusion with the extracellular environment. They studied this nanoparticle because calcium phosphate and hydroxyapatite nanoparticles are commonly used for medical applications, including bone implant materials, DNA and SiRNA delivery vectors and slow release vaccines. To this aim, authors used SEM and EDX to elucidate the mechanisms by which cells internalize this NP. The authors demonstrated that hydrophilic HA NPs are sequestered within a specialized compartment called surface-connected compartment (SCC), an elaborate labyrinthlike structure directly connected to the extracellular space. This data show that this specialized compartment could remove large amounts of foreign material from the extracellular space, followed by slow degradation, and could avoid excessive damage to surrounding cells or tissues.132
To develop these new technologies, the EDX was fundamental to study the different materials. For example, Adachi et al. investigated how TiO2 NPs influenced on rat skin exposed. They used the EDX to prove the presence of the particles in the viable skin. They demonstrated that TiO2 particles neither penetrate into viable cell layers nor biologically cause any cellular changes 133. In the same field, Walczak et al. did a study to test the behaviour of well-characterised 60 nm AgNPs and Ag+ ions derived from AgNO3 in an in vitro model of human gastrointestinal digestion where they used the SEM-EDX analysis to characterize morphology and elemental composition of particles.134
Especially in the last decade the aim of the use of nanoparticles was, cell and tissue distribution and biological effects of drugs or toxic substances and interesting results were obtained thanks to the EDX microanalysis. Indeed, Jimeno-Romero et al. used the ultrastructural observations and EDX microanalysis to understand the bioaccumulation, cell and tissue distribution and biological effects of disodium laureth sulfosuccinate (DSLS)-stabilised TiO2 NPs in marine mussels (Mytilus galloprovincialis). The authors showed significant Ti accumulation that was observed in mussels exposed to TiO2 NPs, which were localised in endosomes, lysosomes and residual bodies of digestive cells, and in the lumen of digestive tubules. The data of this study highlighted that the effects on the cellular structures generally depended on the nanoparticle size and on the biological effects of the NPs elemental composition.135
Finally, Van den Brule et al. worked on the possible impact of dietary Ag nanoparticles on the gut microbiota and using the EDX microanalysis demonstrated that oral exposure to human relevant doses of AgNPs can induce microbial alterations in the gut.136
Conclusions and future prospective
The use of EDX microanalysis in biomedical research and diagnosis can contribute at the realization of the “dream” of a personalized medicine that take into account the uniqueness of every human being.
Indeed, EDX microanalysis could represent a powerful tool to:
investigate the accumulation of heavy metals in tissues and in forensic science;
identify specific asbestos isotype for linking the pulmonary asbestos related-disease to previous workplace exposure;
characterize different isotype of calcification and gave additional information about linkage between calcification and disease;
study the toxic affect and potential drug delivery of NPs.
References
- 1.Scimeca M, Orlandi A, Terrenato I, Bischetti S, Bonanno E. Assessment of metal contaminants in non-small cell lung cancer by EDX microanalysis. Eur J Histochem 2014;58:2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scimeca M, Feola M, Romano L, Rao C, Gasbarra E, Bonanno E, et al. Heavy metals accumulation affects bone microarchitecture in osteoporotic patients. Environ Toxicol 2017; 32:1333-42. [DOI] [PubMed] [Google Scholar]
- 3.Barba T, Wach J, Lustig S, Laurent F, Devouassoux-Shisheboran M, Valour F, et al. Metallosis-associated prosthetic joint infection. Med Mal Infect 2015;45:484-7. [DOI] [PubMed] [Google Scholar]
- 4.Khan H, Hurworth M, Kop A. Metallosis following a dual coat porous hydroxyapatite shoulder hemiarthroplasty. J Orthop 2015;12:266-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scimeca M, Giannini E, Antonacci C, Pistolese CA, Spagnoli LG, Bonanno E. Microcalcifications in breast cancer: an active phenomenon mediated by epithelial cells with mesenchymal characteristics. BMC Cancer 2014; 14:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scimeca M, Pietroiusti A, Milano F, Anemona L, Orlandi A, Marsella LT, et al. Elemental analysisof histologicalspecimens: a method to unmask nanoasbestos fibers. Eur J Histochem 2016;60:2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Segura E, Warley A. Electron probe X-ray microanalysis for the study of cell physiology. Methods Cell Biol 2008;88:19-43. [DOI] [PubMed] [Google Scholar]
- 8.Samuelson DA. Energy dispersive Xray microanalysis. Methods Mol Biol 1998;108:413-24. [DOI] [PubMed] [Google Scholar]
- 9.Morgan AJ. X-ray microanalysis in electron microscopy for biologists. Oxford University Press; 1985. [Google Scholar]
- 10.Pivovarova NB, Andrews SB. Measurement of total calcium in neurons by electron probe X-ray microanalysis. J Vis Exp 2013;e50807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Pietro G, Capuani S, Manenti G, Vinicola V, Fusco A, Baldi J, et al. Bone marrow lipid profiles from peripheral skeleton as potential biomarkers for osteoporosis: A 1HMR spectroscopy study. Acad Radiol 2016;23:273-83. [DOI] [PubMed] [Google Scholar]
- 12.Carpentieri A, Cozzoli E, Scimeca M, Bonanno E, Sardanelli AM, Gambacurta A. Differentiation of human neuroblastoma cells toward the osteogenic lineage by mTOR inhibitor. Cell Death Dis 2015;6: e1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scimeca M, Antonacci C, Colombo D, Bonfiglio R, Buonomo OC, Bonanno E. Emerging prognostic markers related to mesenchymal characteristics of poorly differentiated breast cancers. Tumour Biol 2016;37:5427-35. [DOI] [PubMed] [Google Scholar]
- 14.Wang P, Zhang Q, Li Y, Matsiko J, Zhang Y, Jiang G. Airborne persistent toxic substances (PTSs) in China: occurrence and its implication associated with air pollution. Environ Sci Process Impacts 2017;19:983-99. [DOI] [PubMed] [Google Scholar]
- 15.Rosner D, Markowitz G. "Ain't necessarily so!": The Brake Industry's Impact on Asbestos Regulation in the 1970s. Am J Public Health 2017;107: 1395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon KR, Velders GJ, Wilson SR, Madronich S, Longstreth J, Aucamp PJ, et al. Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto Protocols. J Toxicol Environ Health B Crit Rev 2016;19:289-304. [DOI] [PubMed] [Google Scholar]
- 17.Akpomie KG, Dawodu FA. Treatment of an automobile effluent from heavy metals contamination by an ecofriendly montmorillonite. J Adv Res 2015;6:1003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ioannidis TA, Zouboulis AI. Selective removal of lead and bromide from a hazardous industrial solid waste using limited acid demand and separation factor at ambient conditions. J Hazard Mater 2006;131:46-58. [DOI] [PubMed] [Google Scholar]
- 19.Mahar A, Wang P, Ali A, Awasthi MK, Lahori AH, Wang Q, et al. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol Environ Saf 2015;126:111-21. [DOI] [PubMed] [Google Scholar]
- 20.Savabieasfahani M, Ali SS, Bacho R, Savabi O, Alsabbak M. Prenatal metal exposure in the Middle East: imprint of war in deciduous teeth of children. Environ Monit Assess 2016;188:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roeva NN, Rovinskii FY, Kononov EY. Special features of the behavior of heavy metals in various natural environments. J Anal Chem 1996;51:352-64. [Google Scholar]
- 22.Pathak AK, Yadav S, Kumar P, Kumar R. Source apportionment and spatialtemporal variations in the metal content of surface dust collected from an industrial area adjoining Delhi, India. Sci Total Environ 2013;443:662-72. [DOI] [PubMed] [Google Scholar]
- 23.Sun R, Chen L. Assessment of heavy metal pollution in topsoil around Beijing metropolis. PLoS One 2016; 11:e0155350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guagliardi H, Cicchella D, Rosa RD. A geostatistical approach to assess concentration and spatial distribution of heavy metals in urban soils. Water Air Soil Poll 2012;223:5983-8. [Google Scholar]
- 25.Melaku S, Morris V, Raghavan D, Hosten C. Seasonal variation of heavy metals in ambient air and precipitation at a single site in Washington, DC. Environ Pollut 2008;155:88-98. [DOI] [PubMed] [Google Scholar]
- 26.Brożek-Mucha Z. On the prevalence of gunshot residue in selected populations - an empirical study performed with SEM-EDX analysis. Forensic Sci Int 2014;237:46-52. [DOI] [PubMed] [Google Scholar]
- 27.Bao LM, Lei QT, Tan MG, Li XL, Zhang GL, Liu W, et al. Study on transition metals in airborne particulate matter in Shanghai city's subway. Huan Jing Ke Xue 2014;35:2052-9. [PubMed] [Google Scholar]
- 28.Soteriou D, Ntasi A, Papagiannoulis L, Eliades T, Zinelis S. Decomposition of Ag-based soldering alloys used in space maintainers after intra-oral exposure. A retrieval analysis study. Acta Odontol Scand 2014;72:130-8. [DOI] [PubMed] [Google Scholar]
- 29.Ferraro SA, Curutchet G, Tasat DR. Bioaccessible heavy metals-sediment particles from Reconquista River induce lung inflammation in mice. Environ Toxicol Chem 2012;31:2059-68. [DOI] [PubMed] [Google Scholar]
- 30.El-Mufleh A, Béchet B, Basile- Doelsch I, Geffroy-Rodier C, Gaudin A, Ruban V. Distribution of PAHs and trace metals in urban stormwater sediments: combination of density fractionation, mineralogy and microanalysis. Environ Sci Pollut Res Int 2014;21:9764-76. [DOI] [PubMed] [Google Scholar]
- 31.Greenberg M. Experimental asbestos studies in the UK: 1912-1950. Am J Ind Med 2017;60:956-62. [DOI] [PubMed] [Google Scholar]
- 32.Yates DH. Asbestos: insights from women. Lancet Respir Med 2017;pii:S2213-2600(17)30333-8. [DOI] [PubMed] [Google Scholar]
- 33.Iliopoulou M, Bostantzoglou C, Nenna R, Skouras VS. Asbestos and the lung: highlights of a detrimental relationship. Breathe (Sheff) 2017;13:235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marioryad H, Kakooei H, Shahtaheri SJ, Yunesian M, Azam K. Assessment of airborne asbestos exposure at an asbestos cement sheet and pipe factory in Iran. Regul Toxicol Pharmacol 2011;200-5. [DOI] [PubMed] [Google Scholar]
- 35.Green FH, Attfield M. Pathology standards for asbestosis. Scand J Work Environ Health 1983;9:162-8. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg SD. Cytopathology of asbestos-associated pulmonary disease. Diagn Cytopathol 1985;1:177-82. [DOI] [PubMed] [Google Scholar]
- 37.Craighead JE. 1987 H. P. Smith award lecture. Eyes for the epidemiologist: the pathologist's role in shaping our understanding of the asbestos-associated diseases. Am J Clin Pathol 1988; 89:281-7. [DOI] [PubMed] [Google Scholar]
- 38.Baur X, Woitowitz HJ, Budnik LT, Egilman D, Oliver C, Frank A, et al. Asbestos, asbestosis, and cancer: The Helsinki criteria for diagnosis and attribution. Critical need for revision of the 2014 update. Am J Ind Med 2017;60:411-21. [DOI] [PubMed] [Google Scholar]
- 39.Manners D, Wong P, Murray C, Teh J, Kwok YJ, de Klerk N, et al. Correlation of ultra-low dose chest CT findings with physiologic measures of asbestosis. Eur Radiol 2017;27:3485-90. [DOI] [PubMed] [Google Scholar]
- 40.Roggli V, Gibbs AR, Attanoos R, Churg A, Popper H, Corrin B, et al. Pathology of Asbestosis: An Update of the Diagnostic Criteria Response to a Critique. Arch Pathol Lab Med. 2016;140:950-2. [DOI] [PubMed] [Google Scholar]
- 41.Roggli VL, Sporn TA. Carcinoma of the lung in the absence of asbestosis: The value of lung fiber burden analysis. Ultrastruct Pathol 2016;40:151-4. [DOI] [PubMed] [Google Scholar]
- 42.Capella S, Bellis D, Belluso E. Diagnosis of asbestos-related diseases: The mineralogist and pathologist's role in medicolegal field. Am J Forensic Med Pathol 2016;37:24-8. [DOI] [PubMed] [Google Scholar]
- 43.Roggli VL. Fiber analysis vignettes: Electron microscopy to the rescue! Ultrastruct Pathol 2016;40:126-33. [DOI] [PubMed] [Google Scholar]
- 44.Asahi T, Kobayashi S, Nakayama K, Konya T, Fujinawa G, et al. Preparation and evaluation of a chrysotile asbestos-containing standard material for validating x-ray diffractometric quantitation. Anal Sci 2011;27:1217-21. [DOI] [PubMed] [Google Scholar]
- 45.Sheehy JW, Cooper TC, D’Brien DM. Control of asbestos exposure during brake drum services. National Institute for Occupational Safety and Health; Cincinnati; 1989. [Google Scholar]
- 46.Paustenbach DJ, Finley BL, Lu ET, Brorby GP, Sheehan PJ. Environmental and occupational health hazards associated with the presence of asbestos in brake lining and pads (1900 to present): a state-ofthe- art. J Toxicol Environ Health 2004;7:25-80. [DOI] [PubMed] [Google Scholar]
- 47.Kakooei H1, Sameti M, Kakooei AA. Asbestos exposure during routine brake lining manufacture. Ind Health 2007;45:787-92. [DOI] [PubMed] [Google Scholar]
- 48.Kakooei H, Yunesian M, Marioryad H, Azam K. Assessment of airborne asbestos fiber concentrations in Urban area of Tehran, Iran. Air. Air Qual Atmos Health 2009;2:39. [Google Scholar]
- 49.Marioryad H, Kakooei H, Shahtaheri SJ, Yunesian M, Azam K. Assessment of airborne asbestos exposure at an asbestos cement sheet and pipe factory in Iran. Regul Toxicol Pharmacol 2011;60:200-5. [DOI] [PubMed] [Google Scholar]
- 50.Hippeli S, Dornisch K, Wiethege T, Gillissen A, Müller KM, Elstner EF. Biological durability and oxidative potential of man-made vitreous fibres as compared to crocidolite asbestos fibres. Z Naturforsch C 2001;56:633-48. [DOI] [PubMed] [Google Scholar]
- 51.Petry R, Mastalerz R, Zahn S, Mayerhöfer TG, Völksch G, Viereck- Götte L, et al. Asbestos mineral analysis by UV Raman and energy-dispersive X-ray spectroscopy. Chem - physchem 2006;7:414-20. [DOI] [PubMed] [Google Scholar]
- 52.Marconi A, Cecchetti G, Barbieri M. Airborne mineral fibre concentrations in an urban area near an asbestoscement plant. IARC Sci Publ 1989;90:336-46. [PubMed] [Google Scholar]
- 53.Barbieri PG, Mirabelli D, Somigliana A, Cavone D, Merler E. Asbestos fibre burden in the lungs of patients with mesothelioma who lived near asbestos-cement factories. Ann Occup Hyg 2012;56:660-70. [DOI] [PubMed] [Google Scholar]
- 54.Lee SM, Ha DH, Kang H, Rho JY. Intraarticular calcifying aponeurotic fibroma of the wrist: mimicking gout or calcium pyrophosphate dihydrate deposition disease. Skeletal Radiol 2017. [DOI] [PubMed] [Google Scholar]
- 55.Azpiazu D, Gonzalo S, González- Parra E, Egido J, Villa-Bellosta R. Role of pyrophosphate in vascular calcification in chronic kidney disease. Nefrologia 2017;pii:S0211-6995(17)30189-3. doi: 10.1016/j.nefro.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Ando M, Bando H, Endo T, Ichihara S, Hashimoto E, Hyodo K, et al. Refraction-based 2D, 2.5D and 3D medical imaging: stepping forward to a clinical trial. Eur J Radiol 2008;68:S32-6. [DOI] [PubMed] [Google Scholar]
- 57.Ramonet D, de Yebra L, Fredriksson K, Bernal F, Ribalta T, Mahy N. Similar calcification process in acute and chronic human brain pathologies. J Neurosci Res 2006;83:147-56. [DOI] [PubMed] [Google Scholar]
- 58.Betsholtz C, Keller A. PDGF, pericytes and the pathogenesis of idiopathic basal ganglia calcification (IBGC). Brain Pathol 2014;24:387-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCartney E, Squier W. Patterns and pathways of calcification in the developing brain. Dev Med Child Neurol 2014;56:1009-15. [DOI] [PubMed] [Google Scholar]
- 60.Tot T. Correlating the ground truth of mammographic histology with the success or failure of imaging. Technol Cancer Res Treat 2005;4:23-8. [DOI] [PubMed] [Google Scholar]
- 61.Holmberg L, Wong YN, Tabár L, Ringberg A, Karlsson P, Arnesson LG, et al. Mammography casting-type calcification and risk of local recurrence in DCIS: analyses from a randomised study. Br J Cancer 2013;108:812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henrot P, Leroux A, Barlier C, Génin P. Breast microcalcifications: the lesions in anatomical pathology. Diagn Interv Imaging 2014;95:141-52. [DOI] [PubMed] [Google Scholar]
- 63.Boisserie-Lacroix M, Bullier B, Hurtevent-Labrot G, Ferron S, Lippa N, Mac Grogan G. Correlation between imaging and prognostic factors: molecular classification of breast cancers. Diagn Interv Imaging 2014; 95:227-33. [DOI] [PubMed] [Google Scholar]
- 64.Tamaki K, Ishida T, Miyashita M, Amari M, Ohuchi N, Tamaki N, et al. Correlation between mammographic findings and corresponding histopathology: potential predictors for biological characteristics of breast diseases. Cancer Sci 2011;102:2179-85. [DOI] [PubMed] [Google Scholar]
- 65.Gutierrez M, Di Geso L, Salaffi F, Carotti M, Girolimetti R, De Angelis R, et al. Ultrasound detection of cartilage calcification at knee level in calcium pyrophosphate deposition disease. Arthritis Care Res (Hoboken) 2014;66:69-73. [DOI] [PubMed] [Google Scholar]
- 66.Trenson S, de Ceuninck M. A calcified left ventricular mass. Acta Cardiol 2014;69:316-8. [DOI] [PubMed] [Google Scholar]
- 67.Okada Y. Surgical management of mitral annular calcification. Gen Thorac Cardiovasc Surg 2013;61:619-25. [DOI] [PubMed] [Google Scholar]
- 68.Gossner J. Prevalence of the petrified ear: a computed tomographic study. Eur Arch Otorhinolaryngol 2014; 271:195-7. [DOI] [PubMed] [Google Scholar]
- 69.Chen MY, Bechtold RE, Bohrer SP, Zagoria RJ, Dyer RB. Abnormal calcification on plain radiographs of the abdomen. Crit Rev Diagn Imaging 1999;40:63-202. [PubMed] [Google Scholar]
- 70.van Rossum PS, Haverkamp L, Verkooijen HM, van Leeuwen MS, van Hillegersberg R, Ruurda JP. Calcification of arteries supplying the gastric tube: a new risk factor for anastomotic leakage after esophageal surgery. Radiology 2015;274:124-32. [DOI] [PubMed] [Google Scholar]
- 71.Zamir O, Mogle P, Udassin R. Multiple gastrointestinal atresias with intraluminal calcifications. Am J Perinatol 1988;5:293-4. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen T, Phillips C, Movahed A. Incidental findings of pericardial calcification. World J Clin Cases 2014; 2:455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenbush SW, Parker JM. Height and heart disease. Rev Cardiovasc Med 2014;15:102-8. [DOI] [PubMed] [Google Scholar]
- 74.Tu HY, Hsia CC, Chen T, Chen RC. Extensive peritoneal calcification and small intestinal perforation in a peritoneal dialysis patient: a case report. Kaohsiung J Med Sci 2011;27:199-202. [DOI] [PubMed] [Google Scholar]
- 75.Lee FC, Dunmire B, Harper JD, Cunitz BW, Paun M, Bailey M, Sorensen MD. Ultrasound acoustic shadow width is an accurate predictor of kidney stone size. J Acoust Soc Am 2014;135:2267. [Google Scholar]
- 76.Yang B, Dissayabutra T, Ungjaroenwathana W, Tosukhowong P, Srisa-Art M, Supaprom T, er al. Calcium oxalate crystallization index (COCI): an alternative method for distinguishing nephrolithiasis patients from healthy individuals. Ann Clin Lab Sci 2014;44:262-71. [PubMed] [Google Scholar]
- 77.Di Nunno N, Lombardo S, Costantinides F, Di Nunno C. Anomalies and alterations of the hyoid-larynx complex in forensic radiographic studies. Am J Forensic Med Pathol 2004;25:14-9. [DOI] [PubMed] [Google Scholar]
- 78.Kidron D, Sharony R. Fetal liver calcifications: an autopsy study. Virchows Arch 2012;460:399-406. [DOI] [PubMed] [Google Scholar]
- 79.Prokop M. Lung cancer screening: the radiologist's perspective. Semin Respir Crit Care Med 2014;35:91-8. [DOI] [PubMed] [Google Scholar]
- 80.Murai T, Hara M, Ozawa Y, Shibamoto Y, Shimizu S, Yano M. Mucinous colloid adenocarcinoma of the lung with lymph node metastasis showing numerous punctate calcifications. Clin Imaging 2011;3:151-5. [DOI] [PubMed] [Google Scholar]
- 81.Gupta RA, Udwadia FE, Agrawal P, Doctor N. Pancreatic glucagonoma with pancreatic calcification. Pancreatology 2013;13:327-9. [DOI] [PubMed] [Google Scholar]
- 82.Domínguez-Muñoz JE, Alvarez- Castro A, Lariño-Noia J, Nieto L, Iglesias-García J. Endoscopic ultrasonography of the pancreas as an indirect method to predict pancreatic exocrine insufficiency in patients with chronic pancreatitis. Pancreas 2012; 41:724-8. [DOI] [PubMed] [Google Scholar]
- 83.Hama Y. Detection of prostate calcification with megavoltage helical CT. Acad Radiol 2014;21:565-8. [DOI] [PubMed] [Google Scholar]
- 84.Bai Y, Wang MY, Han YH, Dou SW, Lin Q, Guo Y, et al. Susceptibility weighted imaging: a new tool in the diagnosis of prostate cancer and detection of prostatic calcification. PLoS One 2013;8:e53237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silengo M, Defilippi C, Belligni E, Biamino E, Flex E, Brusco A, et al. Progressive extreme heterotopic calcification. Am J Med Genet A 2013; 161A:1706-13. [DOI] [PubMed] [Google Scholar]
- 86.Nistal M, Paniagua R, Gonzalez- Peramato P, Reyes-Múgica M. Perspectives in pediatric pathology, Chapter 13. Calcifications in the testis and paratesticular structures. Pediatr Dev Pathol 2016;19:173-82. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z, Zhang H, Zhang P, He L, Dong W. Diagnostic value of ultrasound- detected calcification in thyroid nodules. Ann Acad Med Singapore 2014;43:102-6. [PubMed] [Google Scholar]
- 88.Lee J, Lee SY, Cha SH, Cho BS, Kang MH, Lee OJ. Fine-needle aspiration of thyroid nodules with macrocalcification. Thyroid 2013;23:1106-12. [DOI] [PubMed] [Google Scholar]
- 89.Virmani R, Joner M, Sakakura K. Recent highlights of ATVB: calcification. Arterioscler Thromb Vasc Biol 2014;34:1329-32. [DOI] [PubMed] [Google Scholar]
- 90.McCarty MF, Di Nicolantonio JJ. The molecular biology and pathophysiology of vascular calcification. Postgrad Med 2014;126:54-64. [DOI] [PubMed] [Google Scholar]
- 91.Giachelli CM. Ectopic calcification: gathering hard facts about soft tissue mineralization. Am J Pathol 1999;154:671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daudon M, Bader CA, Jungers P. Urinary calculi: review of classification methods and correlations with etiology. Scanning Microsc 1993;7:1081-104; discussion 1104-6. [PubMed] [Google Scholar]
- 93.Bazin D, Chevallier P, Matzen G, Jungers P, Daudon M. Heavy elements in urinary stones. Urol Res 2007;35:179-84. [DOI] [PubMed] [Google Scholar]
- 94.Słojewski M, Czerny B, Safranow K, Jakubowska K, Olszewska M, Pawlik A, at al. Microelements in stones, urine, and hair of stone formers: a new key to the puzzle of lithogenesis? Biol Trace Elem Res 2010;137:301-16. [DOI] [PubMed] [Google Scholar]
- 95.Ozgurtas T, Yakut G, Gulec M, Serdar M, Kutluay T. Role of urinary zinc and copper on calcium oxalate stone formation. Urol Int 2004;72:233-6. [DOI] [PubMed] [Google Scholar]
- 96.Touryan LA, Lochhead MJ, Marquardt BJ, Vogel V. Sequential switch of biomineral crystal morphology using trivalent ions. Nat Mater 2004;3:239-43. [DOI] [PubMed] [Google Scholar]
- 97.Durak I, Kilic Z, Perk H, Sahin A, Yurtarslani Z, Yaar A, ar al. Iron, copper, cadmium, zinc and magnesium contents of urinary tract stones and hair from men with stone disease. Eur Urol 1990;17:243-7. [DOI] [PubMed] [Google Scholar]
- 98.Meyer JL, Thomas WC JR. Trace metal-citric acid complexes as inhibitors of calcification and crystal growth. II. Effects of Fe (III), Cr (III) and Al (III) complexes on calcium oxalate crystal growth. J Urol 1982; 128:1376-8. [DOI] [PubMed] [Google Scholar]
- 99.Meneghini C, Dalconi MC, Nuzzo S, Mobilio S, Wenk RH. Rietveld refinement on x-ray diffraction patterns of bioapatite in human fetal bones. Biophys J 2003;84:2021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sonou T, Ohya M, Yashiro M, Masumoto A, Nakashima Y, Ito T, at all. Mineral composition of phosphate- induced calcification in a rat aortic tissue culture model. J Atheroscler Thromb 2015;22:1197-206. [DOI] [PubMed] [Google Scholar]
- 101.Schmid K, McSharry WO, Pameijer CH, Binette JP. Chemical and physiochemical studies on the mineral deposits of the human atherosclerotic aorta. Atherosclerosis 1980;37:199-210. [DOI] [PubMed] [Google Scholar]
- 102.Tomazic BB. Characterization of mineral phases in cardio vascular calcification. Brown PW, Constanz B, editors. Hydroxy apatite and related materials. Boca Raton; CRC Press; 1994. p. 93-115. [Google Scholar]
- 103.Marra SP, Daghlian CP, Fillinger MF, Kennedy FE. Elemental composition, morphology and mechanical properties of calcified deposits obtained from abdominal aortic aneurysms. Acta Biomater 2006;2:515-20. [DOI] [PubMed] [Google Scholar]
- 104.Lara MJ, Ros E, Sierra M, Dorronsoro C, Aguilar J. Composition and genesis of calcium deposits in atheroma plaques. Ultrastruct Pathol 2014;38:167-77. [DOI] [PubMed] [Google Scholar]
- 105.Chang HH, Cheng CL, Huang PJ, Lin SY. Application of scanning electron microscopy and X-ray microanalysis: FE-SEM, ESEM-EDS, and EDS mapping for studying the characteristics of topographical microstructure and elemental mapping of human cardiac calcified deposition. Anal Bioanal Chem 2014;406:359-66. [DOI] [PubMed] [Google Scholar]
- 106.Bischetti S, Scimeca M, Bonanno E, Federici M, Anemona L, Menghini R, et al. Carotid plaque instability is not related to quantity but to elemental composition of calcification. Nutr Metab Cardiovasc Dis 2017;27:768-74. [DOI] [PubMed] [Google Scholar]
- 107.Henmi A, Okata H, Anada T, Yoshinari M, Mikami Y, Suzuki O, et al. Bone matrix calcification during embryonic and postembryonic rat calvarial development assessed by SEMEDX spectroscopy, XRD, and FTIR spectroscopy. J Bone Miner Metab 2016;34:41-50. [DOI] [PubMed] [Google Scholar]
- 108.Okata H, Nakamura M, Henmi A, Yamaguchi S, Mikami Y, Shimauchi H, et al. Calcification during bone healing in a standardised rat calvarial defect assessed by micro-CT and SEM-EDX. Oral Dis 2015;21:74-82. [DOI] [PubMed] [Google Scholar]
- 109.Khan AO, Al-Katan H, Edward DP. Nummular dystrophic calcification of an Ahmed glaucoma valve in a child. J AAPOS 2012;16:401-2. [DOI] [PubMed] [Google Scholar]
- 110.Scimeca M, Salustri A, Bonanno E, Nardozi D, Rao C, Piccirilli E, et al. Impairment of PTX3 expression in osteoblasts: a key element for osteoporosis. Cell Death Dis 2017;8:e3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Arianna C, Eliana C, Flavio A, Marco R, Giacomo D, Manuel S, et al. Rapid Rapamycin-only induced osteogenic differentiation of blood-derived stem cells and their adhesion to natural and artificial scaffolds. Stem Cells Int 2017;2017:2976541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scimeca M, Antonacci C, Toschi N, Giannini E, Bonfiglio R, Buonomo CO, et al. Breast osteoblast-like cells: A reliable early marker for bone metastases from breast cancer. Clin Breast Cancer 2017;pii: S1526-8209(17)30209-4. [DOI] [PubMed] [Google Scholar]
- 113.Scimeca M, Bonfiglio R, Montanaro M, Bonanno E. Osteoblast-like cells in human cancers: new cell type and reliable markers for bone metastasis. Future Oncol 2018;14:9-11. [DOI] [PubMed] [Google Scholar]
- 114.Voinov MA1, Sosa Pagán JO, Morrison E, Smirnova TI, Smirnov AI. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J Am Chem Soc 2011;133:35-41. [DOI] [PubMed] [Google Scholar]
- 115.Jiang J, Oberdörster G, Elder A, Gelein R, Mercer P, Biswas P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008;2:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Turci F, Colonna M, Tomatis M, Mantegna S, Cravotto G, Gulino G, et al. Surface reactivity and cell responses to chrysotile asbestos nanofibers. Chem Res Toxicol 2012;25:884-94. [DOI] [PubMed] [Google Scholar]
- 117.De Jong WH, Borm PJA. Drug delivery and nanoparticles: Applications and hazards. Int J Nanomed 2008;3: 133-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boverhof DR, Bramante CM, Butala JH, Clancy SF, Lafranconi M, West J, et al. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul Toxicol Pharmacol 2015;73:137-50. [DOI] [PubMed] [Google Scholar]
- 119.Campagnolo L, Massimiani M, Vecchione L, Piccirilli D, Toschi N, Magrini A, et al. Silver nanoparticles inhaled during pregnancy reach and affect the placenta and the foetus. Nanotoxicology 2017;11:687-98. [DOI] [PubMed] [Google Scholar]
- 120.Chaudhry Q, Scotter M, Blackburn J, Ross B, Boxall A, Castle L, et al. Applications and implications of nanotechnologies for the food sector. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2008;25: 241-58. [DOI] [PubMed] [Google Scholar]
- 121.Tien DC, Tseng KH, Liao CY, Tsung TT. Colloidal silver fabrication using the spark discharge system and its antimicrobial effect on Staphylococcus aureus. Med Eng Phys 2008;30:948-952. [DOI] [PubMed] [Google Scholar]
- 122.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 2009;27:76-83. [DOI] [PubMed] [Google Scholar]
- 123.Bouwmeester H1, Dekkers S, Noordam MY, Hagens WI, Bulder AS, de Heer C, et al. Review of health safety aspects of nanotechnologies in food production. Regul Toxicol Pharmacol 2009;53:52-62. [DOI] [PubMed] [Google Scholar]
- 124.Daniel MC, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 2004;104:293-346. [DOI] [PubMed] [Google Scholar]
- 125.Storhoff JJ, Mirkin CA. Programmed materials synthesis with DNA. Chem Rev 1999;99:1849-62. [DOI] [PubMed] [Google Scholar]
- 126.Yi H, Leunissen JLM, Shi GM, Gutekunst CA, Hersch SM. A novel procedure for pre-embedding double immunogold-silver labeling at the ultrastructural level. J Histochem Cytochem 2001;49:279-83. [DOI] [PubMed] [Google Scholar]
- 127.Voskerician G, Shive MS, Shawgo RS, von Recum H, Anderson JM, Cima MJ, et al. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials 2003;24:1959-67. [DOI] [PubMed] [Google Scholar]
- 128.Sharma P, Brown SC, Bengtsson N, Zhang Q, Walter GA, Grobmyer SR, et al. Gold-speckled multimodal nanoparticles for noninvasive bioimaging. Chem Mater 2008;20: 6087-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: Applications in oral cancer. Nano Lett 2005;5:829-34. [DOI] [PubMed] [Google Scholar]
- 130.Jabir NR, Tabrez S, Ashraf GM, Shakil S, Damanhouri GA, Kamal MA. Nanotechnology-based approaches in anticancer research. Int J Nanomed 2012;7:4391-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Glass JJ, Yuen D, Rae J, Johnston AP, Parton RG, Kent SJ, et al. Human immune cell targeting of protein nanoparticles - caveospheres. Nanoscale 2016;8:8255-65. [DOI] [PubMed] [Google Scholar]
- 132.Motskin M, Müller KH, Genoud C, Monteith AG, Skepper JN. The sequestration of hydroxyapatite nanoparticles by human monocytemacrophages in a compartment that allows free diffusion with the extracellular environment. Biomaterials 2011;32:9470-82. [DOI] [PubMed] [Google Scholar]
- 133.Adachi K, Yamada N, Yamamoto K, Yoshida Y, Yamamoto O. In vivo effect of industrial titanium dioxide nanoparticles experimentally exposed to hairless rat skin. Nanotoxicology 2010;4:296-306. [DOI] [PubMed] [Google Scholar]
- 134.Walczak AP, Fokkink R, Peters R, Tromp P, Herrera Rivera ZE, Rietjens IM, et al. Behaviour of silver nanoparticles and silver ions in an in vitro human gastrointestinal digestion model. Nanotoxicology 2013;7:1198-210. [DOI] [PubMed] [Google Scholar]
- 135.Jimeno-Romero A, Oron M, Cajaraville MP, Soto M, Marigómez. Nanoparticle size and combined toxicity of TiO2 and DSLS (surfactant) contribute to lysosomal responses in digestive cells of mussels exposed to TiO2 nanoparticles. Nanotoxicology 2016;10:1168-76. [DOI] [PubMed] [Google Scholar]
- 136.Van den Brule S, Ambroise J, Lecloux H, Levard C, Soulas R, De Temmerman PJ, et al. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part Fibre Toxicol 2016;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]


