Abstract
Objectives:
Primary urethral carcinoma (PUC) is rare, accounting for <1% of genitourinary malignancies. Current knowledge regarding is founded upon tertiary care centers reporting their experiences. We aim to identify factors predictive of outcomes using a nationwide registry database.
Materials and Methods:
The Surveillance, Epidemiology, and End Results-18 registries database was queried for cases of PUC ranging between 2004 and 2010. To identify PUC cases, ICD-O site code C68.0 was used as a filter, hence identifying PUC with histologic subtypes including urothelial carcinoma (UC), squamous cell carcinoma (SCC), and adenocarcinoma (AC). Tumor characteristics were compared using log-rank analysis, and survival outcomes were compared using Cox proportional hazards models.
Results:
A total of 419 PUC cases were identified, 250 (59.7%) male and 169 (40.3%) female patients. The most common histology in men was UC (134, 53.6%), followed by SCC (87, 34.8%) and AC (29, 11.6%). The most common histology in women was AC (79, 46.7%), followed by SCC (43, 25.4%) and UC (42, 24.9%). Log-rank analysis illustrated significant difference in cancer-specific survival (CSS) for T-stage, N-stage, M-stage, and stage of PUC with all histological variants combined (P < 0.001). Multivariate Cox proportional hazards model demonstrated that stage and age were significant for survival, with a risk ratio of 1.033 (95% confidence interval [CI], 1.020–1.046)/year of increased age (P < 0.001) and 3.71 (95% CI, 2.72–5.05) for patients with regional or distant spread.
Conclusions:
Knowledge of patient and tumor characteristics that influences survival is paramount in dictating management. The present study illustrates that age and stage are factors significantly associated with CSS in PUC.
Keywords: Gender influence, Surveillance, Epidemiology, and End Results, survival, urethral carcinoma
INTRODUCTION
Primary urethral carcinoma (PUC) is a rare disease. Hence, there are limited data and established reports defining the presenting characteristics and factors that influence survival for patients harboring urethral cancer. Urethral malignancy demonstrates well-recognized gender differences at the time of presentation due to the inherent anatomic differences between male and female urethras.
According to the 2016 cancer statistics,[1] malignancy of the urinary system other than bladder, kidney, and renal pelvis was projected to contribute 3,530 new cases and 910 deaths. The reported annual incidence for male and female PUC is at 4.3 and 1.5/million, respectively, according to a study done with the Surveillance, Epidemiology, and End Results (SEER) database.[2] Gender-specific histopathologic heterogeneity of PUC has also been previously described, as dictated by the variable histology and embryologic origin of different anatomical segments of the urethra.[3,4] As of the rare nature of this malignancy, literature regarding PUC is largely based on retrospective single-center case series. The body of current literature often reports the experience of single tertiary care centers regarding their cohort of PUC patients with descriptive characteristics and variable outcomes.[5,6,7,8,9,10,11,12,13]
Age, grade, TNM stage, histology, and extent of surgery have been suggested as predictors of cancer-specific survival (CSS) in prior work.[11,14] The National Cancer Institute (NCI) and the European Association of Urology have recently published guidelines on PUC diagnostic evaluation and management.[15,16] Both published guidelines make management recommendations based on stage of disease at the time of presentation, gender, tumor histology, and the presence of anatomical origin from the urethra versus invasion into the urethra from adjacent structures. We hypothesized that patient characteristics influence disease presentation, stage, and cancer-related survival of patients with PUC, which we aimed to address using a well-recognized, nationwide cancer registry maintained, and available through the NCI.
MATERIALS AND METHODS
SEER-18 registries database was queried for all PUC cases between 2004 and 2010. The year 2004 was chosen as a starting point due to more robust coding regarding tumor staging and histologic subtypes in the SEER database after that time point. The year 2010 was the last available update of the SEER before this dataset analysis. To identify PUC cases, ICD-O site code C68.0 was used which groups anterior and posterior urethral lesions together while excluding bladder and bladder neck tumors. The following three histologic subtypes were identified: squamous cell carcinoma (SCC) (8050–8052, 8070–8072, and 8074), urothelial carcinoma (UC) previously referred to as transitional cell carcinoma (8120, 8123–8124, and 8130), and adenocarcinoma (AC) (8140, 8200, 8240, 8260–8262, 8310, 8323, 8380, 8460, 8480–8481, 8490, 8500, 8542, 8560, and 8570).
All statistical analyses were performed using Minitab version 17 (Minitab, Inc., State College, PA). Patient and tumor characteristics were compared using Chi-square analyses. CSS and overall survival were compared using log-rank analyses. A predetermined P < 0.05 was considered statistically significant. Cox proportional hazards models were used for multivariate analysis.
RESULTS
A total of 419 PUC cases were identified, including 250 (59.7%) males and 169 (40.3%) females with PUC over the time period assessed. There appears to be a dichotomy in the histologic variant of PUC based on patient sex with more than half of men with PUC harboring UC variant and nearly half of women with PUC harboring AC. In male patients, the most common histology was UC (134, 53.6%), followed by SCC (87, 34.8%) and AC patients (29, 11.6%). Alternatively, in female patients, the most common histology was AC (79, 46.7%), followed by SCC (43, 25.4%) and UC (42, 24.9%) [Table 1].
Table 1.
Gender influence on primary urethral carcinoma histology and stage at presentation
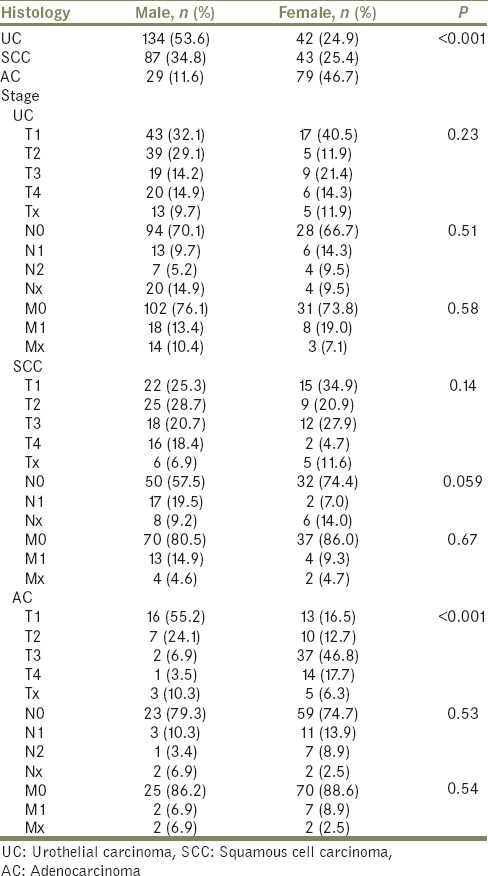
The TNM stage for males and females as stratified by PUC histologic subtype is also depicted in Table 1. Locally advanced carcinoma (T3 and T4) for male patients presented in 39.1% of SCC, 29.1% of UC, and 10.3% of AC. However, locally advanced carcinoma for female patients presented in 64.5% of AC, 32.6% of SCC, and 35.7% of UC [Table 1].
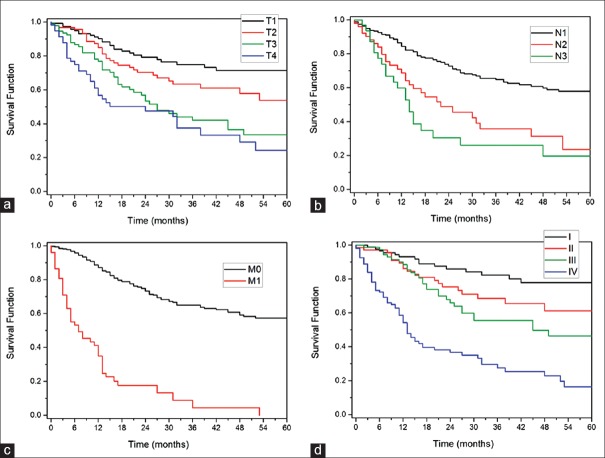
On univariate analysis, gender, age, race, and subtype of urethral cancer did not have a statistically significant impact on cancer-specific or overall survival. However, log-rank analysis illustrated a significant difference in CSS based on T-stage, N-stage, M-stage, and overall pathologic stage of PUC for the entire cohort, when all histological subtypes were included (P < 0.001) [Figure 1a–d].
Figure 1.
(a-d) Kaplan–Meier curves for impact of overall stage, T-stage, N-stage, and M-stage of primary urethral carcinoma on cancer-specific survival as a function of months. (a) Survival based on T-stage. (b) Survival based on N-stage (c) Survival based on M-stage. (d) Survival based on overall stage
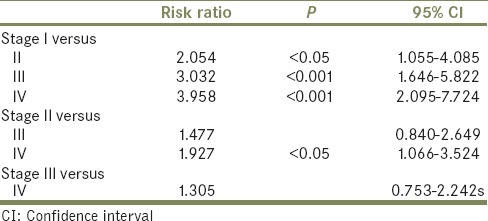
Multivariate Cox proportional hazards models predicted that stage and age have a significant influence on cancer-specific survival. Per each year of age, the risk ratio is 1.033 (95% confidence interval [CI], 1.020–1.046; P < 0.001) and for disease that is not localized is 2.71 (95% CI, 2.72–5.05; P < 0.0001) [Table 2].
Table 2.
Stage influence on survival
DISCUSSION
PUC is a rare malignancy accounting for <1% of all genitourinary cancers.[17] Current literature largely consists of case series with only a few multi-institutional experiences and analyses of large cancer databases. Furthermore, there is a paucity of literature regarding patient characteristics at the time of clinical presentation and limited data on the factors that influence survival.
We found patient gender had a significant influence on the histologic variant of PUC found at the time of presentation and diagnosis. Previous analyses illustrate the most common PUC histology as UC (54%–65%), followed by SCC (16%–22%) and AC (10%–16%), according to studies using both the SEER and the RARECARE databases, irrespective of gender.[2,15,18] Interestingly, a SEER study of 2065 male only PUC illustrated different rates of histopathology with 78% UC, 12% SCC, and 5% AC.[11] Furthermore, smaller a study of 91 females, only cases using the Cancer Registry of the Netherlands illustrated rates of 45% UC, 19% SCC, and 29% AC. These results differ from those published by single-center studies, which cite SCC as the predominant histological type found. In addition, traditional teaching cites PUC as a malignancy more commonly diagnosed in women. However, our SEER analysis, which is consistent with that of Swartz et al. and Visser et al., indicates that the majority of PUC cases are in men.[2,18]
Our data further illustrate that AC cases of PUC have the highest proportion of locally advanced (T3 and T4) disease for males and females, at 41% and 65%, respectively. In male PUC, nodal and metastatic spread is most common in SCC, at 37% and 15%, respectively. However, in female PUC, nodal and metastatic spread is most common in UC cases, at 26% and 19%, respectively.
According to previous SEER and RARECARE analyses, the range of the mean at 1-year and 5-year overall survival was 46%–71% and 29%–54%, respectively.[11,18] A more recent SEER analysis further demonstrated that CSS at 5 and 10 years was 68.0% and 60.1%, respectively.[11] However, these analyses were on data exclusively derived from cases of men with PUC, emphasizing the need for simultaneous analysis of PUC in both genders.
In the current analysis, the only significant predictors of CSS were age and the stage of PUC, with a 3.3% increased risk of cancer-specific death/year of increasing age at the time of diagnosis. Interestingly, even though older patients have a shorter life expectancy, this does not always correlate to lower CSS in younger patients who may present with a more aggressive variant of a given malignancy, as is the case in liver malignancy.[19] However, the most urologic malignancy follows the pattern of the data reported in this study, where age is a significant predictor in cancer-specific survival.[20]
Finally, having nonlocalized disease, defined as any T3 or T4 staging at the time of presentation, yielded a risk ratio of 3.7 when compared to those patients who had localized disease on diagnosis. The need for multimodal therapy including chemotherapy, radiotherapy, and surgery in contemporary management of PUC, especially for advanced disease, has been well described, although the ideal combination is more contested.[21,22,23] According to the international collaboration on PUC, in analyzing 124 patients at ten centers undergoing a number of treatment combinations, neoadjuvant chemotherapy may provide a benefit to advanced disease.[24] Limitations in our study include possible coding discrepancies, including UC extending from the bladder neck that may be coded as PUC, although efforts were made to exclude codes for bladder and bladder neck pathologies. Studies have demonstrated up to 8% of UC involvement in postcystectomy females.[25] The SEER database does not provide the ability to differentiate between anterior and posterior PUC. In addition, the database does not provide any information on quality of life outcomes, a significant consideration for this and studies involving other malignancies. Further study consisting of prospective multicenter trials is necessary to evaluate determinants of survival and effects of various management strategies.
CONCLUSIONS
Knowledge of patient and tumor characteristics that influence survival is paramount in counseling patients and dictating management. The present study illustrates that age and stage are driving factors in survival. Furthermore, we highlight gender-based differences in PUC presentation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Swartz MA, Porter MP, Lin DW, Weiss NS. Incidence of primary urethral carcinoma in the United States. Urology. 2006;68:1164–8. doi: 10.1016/j.urology.2006.08.1057. [DOI] [PubMed] [Google Scholar]
- 3.Ray B, Canto AR, Whitmore WF., Jr Experience with primary carcinoma of the male urethra. J Urol. 1977;117:591–4. doi: 10.1016/s0022-5347(17)58546-8. [DOI] [PubMed] [Google Scholar]
- 4.Bolduan JP, Farah RN. Primary urethral neoplasms: Review of 30 cases. J Urol. 1981;125:198–200. doi: 10.1016/s0022-5347(17)54965-4. [DOI] [PubMed] [Google Scholar]
- 5.Champ CE, Hegarty SE, Shen X, Mishra MV, Dicker AP, Trabulsi EJ, et al. Prognostic factors and outcomes after definitive treatment of female urethral cancer: A population-based analysis. Urology. 2012;80:374–81. doi: 10.1016/j.urology.2012.02.058. [DOI] [PubMed] [Google Scholar]
- 6.Dalbagni G, Zhang ZF, Lacombe L, Herr HW. Male urethral carcinoma: Analysis of treatment outcome. Urology. 1999;53:1126–32. doi: 10.1016/s0090-4295(98)00659-1. [DOI] [PubMed] [Google Scholar]
- 7.Dimarco DS, Dimarco CS, Zincke H, Webb MJ, Bass SE, Slezak JM, et al. Surgical treatment for local control of female urethral carcinoma. Urol Oncol. 2004;22:404–9. doi: 10.1016/S1078-1439(03)00174-1. [DOI] [PubMed] [Google Scholar]
- 8.DiMarco DS, DiMarco CS, Zincke H, Webb MJ, Keeney GL, Bass S, et al. Outcome of surgical treatment for primary malignant melanoma of the female urethra. J Urol. 2004;171:765–7. doi: 10.1097/01.ju.0000104671.20863.47. [DOI] [PubMed] [Google Scholar]
- 9.Dinney CP, Johnson DE, Swanson DA, Babaian RJ, von Eschenbach AC. Therapy and prognosis for male anterior urethral carcinoma: An update. Urology. 1994;43:506–14. doi: 10.1016/0090-4295(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 10.Ouzaid I, Hermieu JF, Dominique S, Fernandez P, Choudat L, Ravery V, et al. Management of adenocarcinoma of the female urethra: Case report and brief review. Can J Urol. 2010;17:5404–7. [PubMed] [Google Scholar]
- 11.Rabbani F. Prognostic factors in male urethral cancer. Cancer. 2011;117:2426–34. doi: 10.1002/cncr.25787. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KA, McAninch JW. Primary squamous cell carcinoma of anterior male urethra. Urology. 1984;23:134–40. doi: 10.1016/0090-4295(84)90006-2. [DOI] [PubMed] [Google Scholar]
- 13.Gheiler EL, Tefilli MV, Tiguert R, de Oliveira JG, Pontes JE, Wood DP, Jr, et al. Management of primary urethral cancer. Urology. 1998;52:487–93. doi: 10.1016/s0090-4295(98)00199-x. [DOI] [PubMed] [Google Scholar]
- 14.Eng TY, Naguib M, Galang T, Fuller CD. Retrospective study of the treatment of urethral cancer. Am J Clin Oncol. 2003;26:558–62. doi: 10.1097/01.coc.0000037764.72722.07. [DOI] [PubMed] [Google Scholar]
- 15.Gakis G, Witjes JA, Compérat E, Cowan NC, De Santis M, Lebret T, et al. EAU guidelines on primary urethral carcinoma. Eur Urol. 2013;64:823–30. doi: 10.1016/j.eururo.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 16.PDQ ® Urethral Cancer Treatment. Bethesda, MD: National Cancer Institute; [Last accessed on 2013 May 08; Last modified on 2013 May 24]. National Cancer Institute. Available from: http://www.cancer.gov/cancertopics/pdq/treatment/urethral/HealthProfessional . [Google Scholar]
- 17.Gatta G, van der Zwan JM, Casali PG, Siesling S, Dei Tos AP, Kunkler I, et al. Rare cancers are not so rare: The rare cancer burden in europe. Eur J Cancer. 2011;47:2493–511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Visser O, Adolfsson J, Rossi S, Verne J, Gatta G, Maffezzini M, et al. Incidence and survival of rare urogenital cancers in europe. Eur J Cancer. 2012;48:456–64. doi: 10.1016/j.ejca.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Chen CH, Chang TT, Cheng KS, Su WW, Yang SS, Lin HH, et al. Do young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patients. Liver Int. 2006;26:766–73. doi: 10.1111/j.1478-3231.2006.01309.x. [DOI] [PubMed] [Google Scholar]
- 20.Feng H, Zhang W, Li J, Lu X. Different patterns in the prognostic value of age for bladder cancer-specific survival depending on tumor stages. Am J Cancer Res. 2015;5:2090–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Dayyani F, Hoffman K, Eifel P, Guo C, Vikram R, Pagliaro LC, et al. Management of advanced primary urethral carcinomas. BJU Int. 2014;114:25–31. doi: 10.1111/bju.12630. [DOI] [PubMed] [Google Scholar]
- 22.Dayyani F, Pettaway CA, Kamat AM, Munsell MF, Sircar K, Pagliaro LC, et al. Retrospective analysis of survival outcomes and the role of cisplatin-based chemotherapy in patients with urethral carcinomas referred to medical oncologists. Urol Oncol. 2013;31:1171–7. doi: 10.1016/j.urolonc.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deberne M, Timsit MO, Verkarre V, Eiss D, Kreps S, Dupont S, et al. Multimodal management of primary adenocarcinoma of the female urethra: About four cases. Cancer Radiother. 2016;20:169–75. doi: 10.1016/j.canrad.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Gakis G, Morgan TM, Daneshmand S, Keegan KA, Todenhöfer T, Mischinger J, et al. Impact of perioperative chemotherapy on survival in patients with advanced primary urethral cancer: Results of the international collaboration on primary urethral carcinoma. Ann Oncol. 2015;26:1754–9. doi: 10.1093/annonc/mdv230. [DOI] [PubMed] [Google Scholar]
- 25.Chen ME, Pisters LL, Malpica A, Pettaway CA, Dinney CP. Risk of urethral, vaginal and cervical involvement in patients undergoing radical cystectomy for bladder cancer: Results of a contemporary cystectomy series from M. D. Anderson cancer center. J Urol. 1997;157:2120–3. [PubMed] [Google Scholar]