Abstract
Background:
Urinary bladder carcinoma ranks ninth in worldwide cancer incidence. About 74,000 new cases were diagnosed in 2015 alone and 16,000 persons died of the disease. Since histopathology is considered gold standard for diagnosis, it is prudent to look for potential tumor proliferation and predictive markers in such a prevalent malignancy so as to alert surgical and medical oncologists for timely intervention and provide better patient-tailored therapy.
Aims:
This study is to analyze the role of potential biomarkers-proliferating cell nuclear antigen (PCNA) and angiogenesis using CD31 in urothelial neoplasms in relation to tumor grade and stage.
Methods:
Histopathology slides were prepared from transurethral resection of bladder tumor chips and assessed by three independent observers as per the WHO/International Society of Urologic Pathology criteria 2016. Representative sections were subjected to immunohistochemistry. PCNA labeling index (PCNA LI) and mean vessel density (MVD) were calculated.
Statistical Analysis:
Tests of analysis were applied as appropriate. A statistical P < 0.05 was considered significant.
Results:
Forty-nine patients were analyzed. PCNA LI increased with grade and stage. PCNA was significantly higher in noninvasive papillary urothelial carcinoma high grade (NIPUCHG) than in noninvasive papillary urothelial carcinoma low grade (NIPUCLG) and in infiltrating urothelial carcinoma as compared to NIPUCLG. MVD also increased with tumor grade and stage; however, a significant difference was observed only between infiltrating urothelial carcinoma and papillary urothelial neoplasm of low malignant potential. A cutoff value of 73% for PCNA and 49 vessels/high-power field for CD 31 showed 100% accuracy to differentiate between noninvasive papillary urothelial carcinoma high grade and NIPUCLG. No association was observed between tumor recurrence and PCNA or CD31 expression.
Conclusion:
PCNA and CD31 when used together are valuable markers to help classify urothelial neoplasms in limited tumor material. However, larger prospective studies are required for better prognostication.
Keywords: Angiogenesis, mean vessel density, proliferating cell nuclear antigen, urinary bladder, urothelial carcinoma
INTRODUCTION
Urothelial carcinoma comprises 90% of all bladder carcinomas.[1] Despite advances in surgical techniques and chemotherapy, the 10-year disease-free survival of this muscle invasive malignancy is only 50%–60%.[2] High recurrence rate is a cause of much concern and at present, there is no screening program for early detection of the disease. Therefore, histomorphological diagnosis plays a crucial role in tumor grading, assessing the extent of invasion, and providing additional information regarding tumor behavior. Pathological classification of urothelial lesions is subjective and marked inter-observer and intra-observer variability have been observed in microscopic grading.[3,4,5] In an effort to develop a classification that best reflects clinical behavior, the new WHO 2016 classification of tumors of the urinary tract has been introduced. However, this classification continues to recommend the use of the grading classification first put forth by the International Society of Urologic Pathology (ISUP) in 1997.[5]
Biomarkers of angiogenesis and cell proliferation may show differential expression in various grade and stage of malignancy and could help in early and timely diagnosis of urothelial carcinoma, when compared to clinical staging and grading as a reference standard. Proliferating cell nuclear antigen (PCNA) is a cell proliferation marker, whose rate of synthesis correlates directly with the rates of cellular proliferation and DNA synthesis.[6,7] PCNA is an independent marker of tumor progression in prostate carcinoma, more sensitive than ki-67.[8] Tumor angiogenesis provides oxygen and glucose supply to the developing tumor and facilitates metastasis.[9,10,11] Angiogenesis can be assessed by calculating mean vessel density (MVD). CD 31, a marker of endothelial differentiation among nonhematopoietic human neoplasms,[12] is used to assess MVD.[13]
In the present study, we aimed to assess the role of tumor proliferation and angiogenesis, as objective criteria for early and definitive diagnosis, in various grades of urothelial neoplasms.
METHODS
Study design and oversight
This cross-sectional study was done at the Department of Pathology. The Institutional Review Board and Institutional Ethical Committee approved the study. Informed consent was taken from individual participants allowing biological sample to be used for research purposes. The study was conducted on all consecutive 60 transurethral resection of bladder tumor (TURBT) specimens of histologically proven urothelial carcinomas of urinary bladder over a period of 1 year. Only sections prepared from TURBT specimens were analyzed, while benign bladder lesions and nontransitional cell malignancies were excluded from the study. To reduce the interobserver variability and increase sensitivity of diagnosis, three pathologists independently examined the H- and E-stained sections. Only cases where all three pathologists agreed in the grading and staging of urothelial carcinoma were included in this study.
Evaluation of immunohistochemistry staining
Immunohistochemistry (IHC) was done using avidin-biotin method. Anti-PCNA polyclonal antibody (Thermo Scientific, USA) at a dilution of 1:2000 and monoclonal anti-CD31 antibody (Biocare Medical, USA) at a dilution of 1:10 were used. For calculating PCNA labeling index (PCNA LI), tumor cells were counted manually at high power (×400), after identifying well-preserved areas of tumor (hot spots) at low power (×100) as per recommendation of Cohen et al.[14] One thousand cells per slide were counted in 5–10 microscopic fields from well-labeled areas to determine the average PCNA LI. PCNA LI was expressed as a percentage of PCNA labeled cells (nuclear positivity) to the total number of urothelial cells counted in each specimen. Semi-quantitative nuclear scoring was made as per the method used by Wolf and Dittrich[15] 0, no staining; 1, weak staining (positive nuclei visible only at higher magnification); 2, intermediate staining; and 3, strong staining (positive nuclei visible at scanning power).
Vessel count was done as per the method described by Weidner et al.[16] Criteria for assessing CD31 staining included strong and distinct predominantly membranous and/or cytoplasmic brown staining of normal vascular endothelial cells. Microvessel counting was performed within hot spots. Immunoreactive vessels in five fields were counted at ×200 magnification using the NIKON Eclipse 80i microscope. The average of five fields was then expressed as the number of blood vessels per high-power field (hpf), i.e., MVD.
Statistical analysis
Frequency tables were analyzed using Chi-square test or Fisher's exact test as appropriate, or likelihood ratio to assess the significance between categorical variables. Differences in the means of continuous variables between groups were analyzed using ANOVA (analysis of variance) or nonparametric tests (Mann–Whitney, Kruskal-Wallis) tests as appropriate. A statistical P < 0.05 was considered significant. A receiver operator curve analysis was done to determine any PNCA LI and MVD cutoff value in relation to tumor grading and staging. All data were analyzed using statistical package for social science program (SPSS) version 16.0. Chicago, SPSS Inc.
RESULTS
Out of 60 cases, initially included in the study, eight cases were excluded due to interobserver variability and three cases due to nonspecific immunohistochemical staining. Key patient data and tumor characteristics of 49 cases are enlisted in Table 1. Hematuria was the most common presenting complaint, followed by dysuria, pain lower abdomen, and acute urinary retention in descending order. Tumor location on cystoscopy was most commonly on the left lateral wall followed by posterior wall, dome, right lateral wall, and right VUJ.
Table 1.
Patient demographic data and tumor characteristics (n=49)
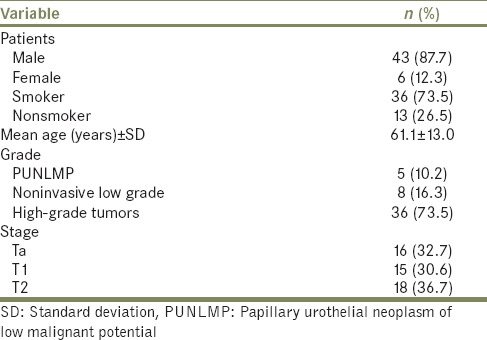
A total of 36 (73.5%) high-grade urothelial carcinomas were diagnosed, of which18 cases (36.7%) were muscle invasive, 15 cases (30.6%) were lamina propria invasive, and 3 (6.1%) were noninvasive. Due to limited number of cases in individual groups, the high-grade urothelial tumors have been clubbed together for analysis. A total of 8 cases (16.3%) were of noninvasive papillary urothelial carcinoma low-grade (NIPUCLG), and 5 cases (10.2%) were of papillary urothelial neoplasm of low malignant potential (PUNLMP) [Figure 1]. A significant correlation was observed between papillary architecture and tumor grade. PUNLMP had complex branching papillae throughout while non-invasive tumors showed occasionally fused papillae. Infiltrating tumors displayed complete loss or fused papillae configuration.
Figure 1.
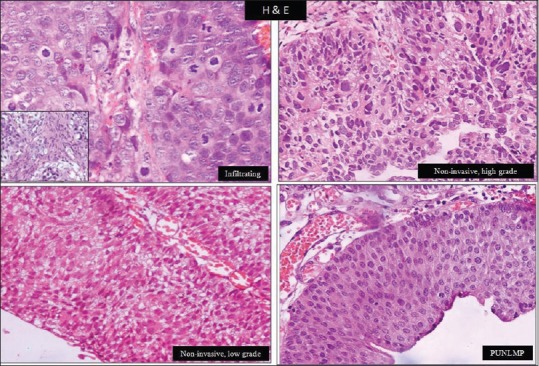
Grades of urothelial neoplasm. Inset: Muscle invasion in infiltrating urothelial neoplasm (H and E, ×400)
Overall mean PCNA LI was 79.5% ± 11.4%. We found an increasing trend of PCNA expression with various grades of urothelial neoplasms [Table 2 and Figure 2]. Mean PCNA LI was maximum in high-grade tumors (85.4% ± 6.3%) and minimum in PUNLMP (58% ± 5.4%, P < 0.001). Post hoc intergroup comparisons revealed a significant difference in PCNA expression between infiltrating urothelial carcinoma and other neoplasms.
Table 2.
Proliferating cell nuclear antigen labeling index expression with tumor grade and stage
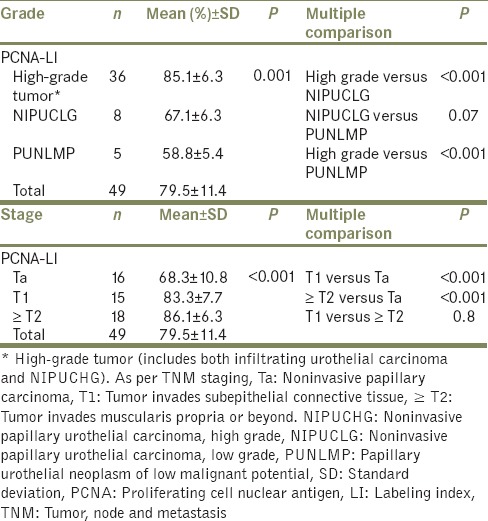
Figure 2.
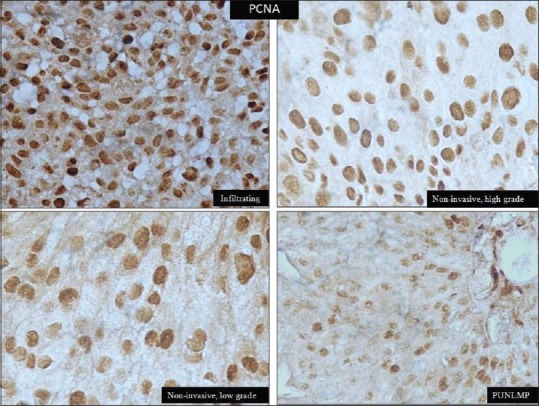
Nuclear positivity of proliferating cell nuclear antigen in urothelial carcinoma (immunohistochemistry, ×400)
Overall mean MVD was 55 ± 20.6/hpf. Correlation of MVD with various histological grades revealed increasing vessel count/hpf with increasing tumor grade [Figure 3]. However, a statistical significant difference was observed only between high-grade urothelial carcinoma and PUNLMP [Table 3].
Figure 3.
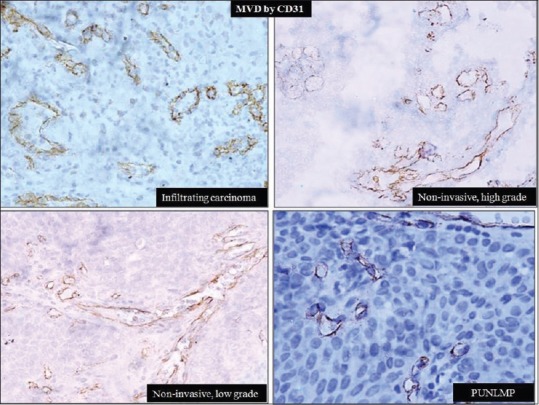
Vessels with cytoplasmic and/or membranous staining of CD31 (immunohistochemistry, ×400)
Table 3.
Mean vessel density expression with tumor grade and stage
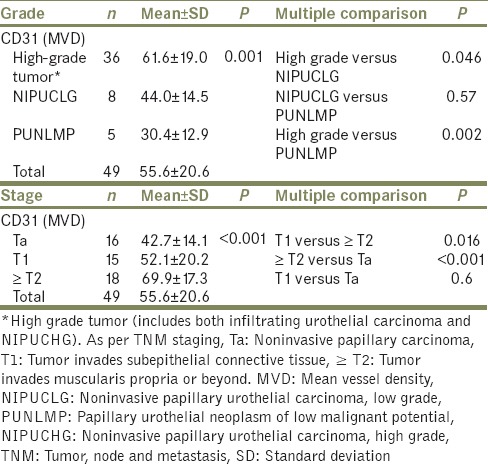
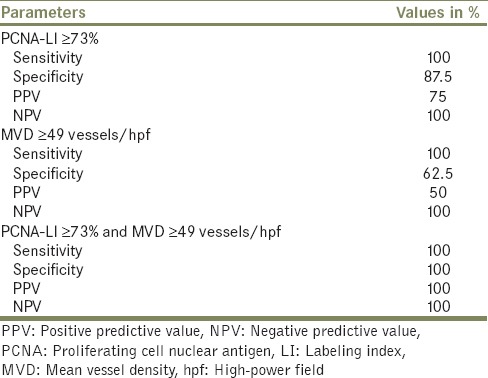
The two immunostains were also assessed with tumor stage. PCNA LI was significantly higher in T1 as compared to Ta and in ≥T2 as compared to Ta [Table 2]. An attempt to score the intensity of PCNA staining with tumor grade and stage proved unfruitful, as heterogeneous staining was observed within most tumor sections and no significant difference was discerned. MVD was higher in ≥T2 stage than in T1 and higher in T1 than in Ta [Table 3]. PCNA LI ≥73% and MVD ≥49 vessels/hpf were the best cutoff values for differentiating high-grade tumors from NIPUCLG [Table 4]. When both PCNA LI ≥73% and MVD ≥49 vessels/hpf were present, the accuracy to differentiate was 100%. Seven (14.2%) patients had recurrent tumor. Smoking had no association with PCNA LI and MVD.
Table 4.
Optimal cut-offs of proliferating cell nuclear antigen labeling index and mean vessel density expression to differentiate high-grade tumors versus low-grade noninvasive papillary urothelial carcinoma
DISCUSSION
The present study was undertaken to assess two of the commonly used immunohistochemical markers in supporting the diagnostic categories of urothelial neoplasms of urinary bladder. PCNA has now been recognized as a potential prognostic and therapeutic biomarker in several malignancies.[17,18] CD31 is a known marker of neovascularization resulting in increased tumor metastases.[19,20] Histologic grade and stage are considered to be basic parameters that determine the prognosis of bladder urothelial carcinomas and are directly related to biological behavior and tendency for recurrence.[21] However, a diagnosis based on the WHO and ISUP classification (2004) is often considered subjective.[22] To overcome this, Sangwan et al.[4] proposed a multivariate analysis model using mitotic activity index (MAI), Ki-67 IHC, and morphometry for calculating mean nuclear area of 10 nuclei. However, among the three parameters, MAI alone was the single most important markers for tumor grading. This MAI can be difficult to assess in limited biopsy material. In the present study, a more practical manual reporting on all H- and E-stained sections was done by 3 individual pathologists using the new WHO/ISUP criteria proposed in 2016.
Other researchers have also tried to study the utility of biomarkers for prognosticating urothelial carcinomas. Yıldırım et al.[21] carried out a subjective quantitative assessment on bladder urothelial carcinomas using C-erB2 and CD44 in addition to PCNA. The results were similar to ours and PCNA immunostaining significantly increased with grade and stage of urothelial carcinoma. The increasing trend of PCNA LI with tumor grade and stage observed in our study is similar to the study done by Hattori et al.,[23] Chen et al.,[6] and Lipponen and Eskelinen[7] These previously published studies were done using the 1973 WHO classification that divided urothelial carcinomas into Grade 1, 2, and 3, which has now been replaced by the ISUP classification. We observed much higher mean PCNA LI values using the latest classification. All nuclei whether staining strongly or weakly by PCNA were counted as positive in the current study and could have resulted in comparative but higher values. Our methodology of cell counting has been supported by Kemp et al.,[24] who in an extensive review demonstrated that the assessment of an overall PCNA nuclear staining provides a better representation of histological grades as compared to counting limited to intensely staining cells.
We observed a wide variation in vessel counts, suggesting a spectrum of angiogenic activity within different grades and stages. However, a significant higher MVD was only evident in infiltrating urothelial carcinoma as compared with PUNLMP and not between low-grade and high-grade papillary urothelial neoplasms. We identified a cutoff MVD value of 49/hpf to differentiate between high-grade tumors and NIPUCLG, with a negative predictive value of 100%. This correlation with tumor grading is in coherence with previous studies by El Gehani et al.[9] and Philp et al.[25] MVD was also significantly associated with tumor stages in our study similar to others.[9,26] This is easily understandable since, when regarded as a composite variable, tumor stage is dependent on both tumor size and degree of spread (i.e., presence of metastasis), both of which rely on angiogenesis. In a recent study on superficial bladder cancers, Goddard et al. found a strong association between intensity of angiogenesis and clinical stage.[26] Papadopoulos et al. concluded that vascular survival ability and tumor angiogenic activity are associated with an aggressive tumor behavior in transitional cell carcinoma of the urinary bladder.[10] In contrast, Bochner et al. found no association between tumor angiogenesis and tumor grade, depth of tumor invasion or lymph node status.[27] However, they found that MVD was significantly associated with an increased risk of disease recurrence and decreased overall survival. This variability in observations of microvessel counts may be attributed to certain crucial differences: sample selection for IHC staining, antibody characteristics, objectivity of the method used in counting vessels, and inherent biological differences.
As per the NCCN 2017 guidelines, TURBT has primary role in both tumor staging and management.[28] Other available therapies include intravesical chemotherapy, BCG or cystectomy, depending on the grade and stage. The upcoming role of biomarkers can be helpful in revolutizing the treatment of urothelial bladder tumors.
The need to further study predictive biomarkers of bladder cancer was highlighted by the recent systematic review.[29] Interestingly, we observed that combining two markers, PCNA LI and CD31, with specific cutoff values helped in differentiating low-grade and high-grade noninvasive tumors. The results of our single-center study are encouraging. However, larger prospective studies are required to validate these findings, which can lead to better diagnosis and prognostication of urothelial carcinomas.
CONCLUSION
PCNA expression and MVD show a parallel trend with increasing grades and stage of urothelial carcinoma. Combining both markers, rather than stand-alone, helps in differentiating NIPUCHG and NIPUCLG with excellent accuracy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to Dr. Neeraj Gupta, Consultant, Institute of Child Health, Sir Ganga Ram Hospital, New Delhi, for his constant motivation for completion of this manuscript.
REFERENCES
- 1.Khochikar MV. Rationale for an early detection program for bladder cancer. Indian J Urol. 2011;27:218–25. doi: 10.4103/0970-1591.82841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thalmann GN, Stein JP. Outcomes of radical cystectomy. BJU Int. 2008;102:1279–88. doi: 10.1111/j.1464-410X.2008.07971.x. [DOI] [PubMed] [Google Scholar]
- 3.Bol MG, Baak JP, van Diermen B, Buhr-Wildhagen S, Janssen EA, Kjellevold KH, et al. Proliferation markers and DNA content analysis in urinary bladder TaT1 urothelial cell carcinomas: Identification of subgroups with low and high stage progression risks. J Clin Pathol. 2003;56:447–52. doi: 10.1136/jcp.56.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangwan M, Singh S, Kumar S, Chabbra S, Sen R, Rana P, et al. Role of morphometry and proliferative parameters in grading of urothelial neoplasms. Cent European J Urol. 2015;68:37–44. doi: 10.5173/ceju.2015.01.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part B: Prostate and bladder tumours. Eur Urol. 2016;70:106–19. doi: 10.1016/j.eururo.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Lin MS, Li RC. Expression and prognostic value of proliferating cell nuclear antigen in transitional cell carcinoma of the urinary bladder. Urol Res. 1997;25:25–30. doi: 10.1007/BF00941902. [DOI] [PubMed] [Google Scholar]
- 7.Lipponen PK, Eskelinen MJ. Cell proliferation of transitional cell bladder tumours determined by PCNA/cyclin immunostaining and its prognostic value. Br J Cancer. 1992;66:171–6. doi: 10.1038/bjc.1992.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taftachi R, Ayhan A, Ekici S, Ergen A, Ozen H. Proliferating-cell nuclear antigen (PCNA) as an independent prognostic marker in patients after prostatectomy: A comparison of PCNA and Ki-67. BJU Int. 2005;95:650–4. doi: 10.1111/j.1464-410X.2005.05356.x. [DOI] [PubMed] [Google Scholar]
- 9.El Gehani K, Al-Kikhia L, Mansuri N, Syrjänen K, Al-Fituri O, Elzagheid A, et al. Angiogenesis in urinary bladder carcinoma as defined by microvessel density (MVD) after immunohistochemical staining for Factor VIII and CD31. Libyan J Med. 2011;6:6016. doi: 10.3402/ljm.v6i0.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papadopoulos I, Giatromanolaki A, Koukourakis MI, Sivridis E. Tumour angiogenic activity and vascular survival ability in bladder carcinoma. J Clin Pathol. 2004;57:250–5. doi: 10.1136/jcp.2003.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar V, Abbas AK, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. India: Elsevier; 2015. [Google Scholar]
- 12.DeYoung BR, Swanson PE, Argenyi ZB, Ritter JH, Fitzgibbon JF, Stahl DJ, et al. CD31 immunoreactivity in mesenchymal neoplasms of the skin and subcutis: Report of 145 cases and review of putative immunohistologic markers of endothelial differentiation. J Cutan Pathol. 1995;22:215–22. doi: 10.1111/j.1600-0560.1995.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 13.DeLisser HM, Christofidou-Solomidou M, Strieter RM, Burdick MD, Robinson CS, Wexler RS, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–7. [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen MB, Waldman FM, Carroll PR, Kerschmann R, Chew K, Mayall BH, et al. Comparison of five histopathologic methods to assess cellular proliferation in transitional cell carcinoma of the urinary bladder. Hum Pathol. 1993;24:772–8. doi: 10.1016/0046-8177(93)90015-9. [DOI] [PubMed] [Google Scholar]
- 15.Wolf HK, Dittrich KL. Detection of proliferating cell nuclear antigen in diagnostic histopathology. J Histochem Cytochem. 1992;40:1269–73. doi: 10.1177/40.9.1354677. [DOI] [PubMed] [Google Scholar]
- 16.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, et al. Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–87. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 17.Müller R, Misund K, Holien T, Bachke S, Gilljam KM, Våtsveen TK, et al. Targeting proliferating cell nuclear antigen and its protein interactions induces apoptosis in multiple myeloma cells. PLoS One. 2013;8:e70430. doi: 10.1371/journal.pone.0070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malkas LH, Herbert BS, Abdel-Aziz W, Dobrolecki LE, Liu Y, Agarwal B, et al. A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc Natl Acad Sci U S A. 2006;103:19472–7. doi: 10.1073/pnas.0604614103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao Y, Kubota T, Takeuchi H, Sato K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: Comparison with an anti-CD31 monoclonal antibody. Neuropathology. 2005;25:201–6. doi: 10.1111/j.1440-1789.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 20.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Yıldırım A, Kösem M, Sayar I, Gelincik I, Yavuz A, Bozkurt A, et al. Relationship of PCNA, C-erbB2 and CD44s expression with tumor grade and stage in urothelial carcinomas of the bladder. Int J Clin Exp Med. 2014;7:1516–23. [PMC free article] [PubMed] [Google Scholar]
- 22.Kapur U, Antic T, Venkataraman G, Durazo-Arvizu R, Quek MM, Flanigan RC, et al. Validation of World Health Organization/International Society of Urologic Pathology 2004 classification schema for bladder urothelial carcinomas using quantitative nuclear morphometry: Identification of predictive features using bootstrap method. Urology. 2007;70:1028–33. doi: 10.1016/j.urology.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Hattori K, Uchida K, Akaza H, Koiso K, Nemoto R, Harada M, et al. Proliferating cell nuclear antigen cyclin in human transitional cell carcinoma. Br J Urol. 1995;75:162–6. doi: 10.1111/j.1464-410x.1995.tb07304.x. [DOI] [PubMed] [Google Scholar]
- 24.Kemp C, Alberti VN, de Lima GR, de Carvalho FM. How should PCNA be assessed? Total of stained cells or only the most intensely stained ones. Sao Paulo Med J. 1998;116:1667–74. doi: 10.1590/s1516-31801998000200005. [DOI] [PubMed] [Google Scholar]
- 25.Philp EA, Stephenson TJ, Reed MW. Prognostic significance of angiogenesis in transitional cell carcinoma of the human urinary bladder. Br J Urol. 1996;77:352–7. doi: 10.1046/j.1464-410x.1996.08475.x. [DOI] [PubMed] [Google Scholar]
- 26.Goddard JC, Sutton CD, Furness PN, O'Byrne KJ, Kockelbergh RC. Microvessel density at presentation predicts subsequent muscle invasion in superficial bladder cancer. Clin Cancer Res. 2003;9:2583–6. [PubMed] [Google Scholar]
- 27.Bochner BH, Cote RJ, Weidner N, Groshen S, Chen SC, Skinner DG, et al. Angiogenesis in bladder cancer: Relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87:1603–12. doi: 10.1093/jnci/87.21.1603. [DOI] [PubMed] [Google Scholar]
- 28.Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, et al. Bladder cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1240–67. doi: 10.6004/jnccn.2017.0156. [DOI] [PubMed] [Google Scholar]
- 29.Miyata Y, Sakai H. Predictive markers for the recurrence of nonmuscle invasive bladder cancer treated with intravesical therapy. Dis Markers 2015. 2015:857416. doi: 10.1155/2015/857416. [DOI] [PMC free article] [PubMed] [Google Scholar]


