Abstract
Infertility affects ~7% of couples of reproductive age with little change in incidence in the last two decades. ART, as well as other interventions, have made major strides in correcting this condition. However, and in spite of advancements in the field, the age of the female partner remains a main factor for a successful outcome. A better understanding of the final stages of gamete maturation yielding an egg that can sustain embryo development and a pregnancy to term remains a major area for improvement in the field. This review will summarize the major cellular and molecular events unfolding at the oocyte-to-embryo transition. We will provide an update on the most important processes/pathways currently understood as the basis of developmental competence, including the molecular processes involved in mRNA storage, its recruitment to the translational machinery, and its degradation. We will discuss the hypothesis that the translational programme of maternal mRNAs plays a key role in establishing developmental competence. These regulations are essential to assemble the machinery that is used to establish a totipotent zygote. This hypothesis further supports the view that embryogenesis begins during oogenesis. A better understanding of the events required for developmental competence will guide the development of novel strategies to monitor and improve the success rate of IVF. Using this information, it will be possible to develop new biomarkers that may be used to better predict oocyte quality and in selection of the best egg for IVF.
Keywords: meiosis, oocyte, embryo, cytoplasm maturation, IVM of oocytes, mRNA, translation, gene expression
Introduction
According to the World Health Organization, infertility affects approximately one in six couples (WHO, 2007), and its incidence has not declined during the last two decades (Mascarenhas et al., 2012). Thus, infertility is a major global public health issue, which is growing in prominence worldwide. In spite of the remarkable progress made in ART over the last two to three decades, the treatment of infertility in general, and particularly that associated with an aging patient population, still is considered suboptimal. The increasing reliance on ART for family planning in western civilization is a further incentive for ART improvements. According to the most recent statistics (Calhaz-Jorge et al., 2016; Toftager et al., 2017), the live birth rate from IVF non-donor fresh embryo-transfers in patients <35 years old is ~29–33%, depending on the criteria applied. These rates are achieved under optimal conditions but success rate decreases substantially with the increasing age of the female partner (Gleicher et al., 2016). Using more stringent metrics, the pregnancy rate per retrieved oocyte is estimated at 4.5% (Stoop et al., 2012). Thus, there is general consensus that there still is room for improvement of the current ARTs at several levels. One area of further exploration is the development of technologies that more reliably predict oocyte quality and that may be able to quantify exactly the gamete potential to develop as an embryo and support a pregnancy to term. At the same time, a better understanding of the biological processes underlying gamete development into a fertilizable egg is an area of improvement that would further benefit ARTs.
Oocyte developmental competence is usually defined as the ability of a female gamete to mature into an egg with its potential to be fertilized and sustain embryo development to the blastocyst stage. In some cases, the definition is broadened to include the potential to sustain pregnancy up to a live birth. From the developmental biology standpoint, this gamete property encompasses some of the most critical and complex biological transitions. These include remodelling of the gamete to accept and integrate the male genome, nuclear reprogramming to totipotency in the zygote, and activation of the embryonic genome (EGA). In view of what is emerging from model organisms, such as worms and flies, some elements of gastrulation may be already programmed in the gamete (Farrell and O’Farrell, 2014). Given the complexity of the biological processes involved, it has been difficult to define the key events essential for the oocyte to acquire this potential. Nevertheless, it is generally agreed that generation of a healthy female gamete relies on the co-ordinated development of somatic and germ cells in the ovarian follicle. This co-ordination requires continuous exchange of information between the two cellular compartments. Many of the molecular entities mediating this dialog and the hormonal and paracrine controls are known, but the biological function of many still awaits clarification. Metabolic coupling of germ cells and surrounding granulosa or cumulus cells is an additional important facet of this interaction, which ultimately promotes developmental competence. Furthermore, nuclear and cytoplasmic maturation that take place during the last stages of oocyte maturation are important determinants of oocyte quality, but the molecular constituents involved, even if known, have yet to be arranged in a coherent blueprint of the machinery required for developmental competence.
Selection of the oocyte with the best developmental potential has been the focus of intense research in the last few decades, and a myriad of strategies have been proposed to accomplish this objective. Morphological criteria are the most widely used paradigms, but the realization that even the most normal appearing oocyte or embryo may conceal aneuploidy has shown the limits of this approach (Munne et al., 2007, 2009) and has prompted the search for more dynamic morphological criteria, such as the time required to progress through the most significant oocyte-to-embryo transitions. The most recent advancements in assessing gene expression through transcriptomics or genomics have also been applied to better understand oocyte quality (Jones et al., 2008; Labrecque and Sirard, 2014; Freour and Vassena, 2017), but some limitations including the invasive nature associated with this approach currently prevent its widespread use.
The aim of the present review is to summarize the major cellular and molecular events unfolding at the oocyte-to-embryo transition, as they represent key steps for the normal development of the future embryo. We will review the most current concepts and our present understanding of how the oocyte develops the competence to sustain embryo development. While covering most of the areas cited above, we will underscore available evidence linking developmental competence to the programme of maternal mRNA translation at the oocyte-to-embryo transition. An established characteristic of the fully grown female gamete is diminished reliance on transcription to control gene expression. Yet, very little is known about the properties of the translation programme in human oocytes. Also, we will build on the idea that understanding the temporal pattern of mRNA translation can ultimately provide a better understanding of the molecular details underlying gamete development, including the acquisition of developmental competence.
The final stages of follicle growth and oocyte maturation and preimplantation embryo development
Mammalian female germ cells enter meiosis during foetal life and arrest at the diplotene stage of prophase I for months or years, depending on the species. Meiosis will resume after puberty in response to a surge in LH. For most of post-natal life, oocytes are stored in a dormant pool, each enclosed by a layer of flat pre-granulosa cells, forming the primordial follicles. Upon activation, the oocyte initiates a growth phase while the surrounding cells become cuboidal and proliferate, giving rise to primary and secondary follicles (Rimon-Dahari et al., 2016). During this period of extensive growth, the chromatin in the oocyte nucleus (germinal vesicle: GV) is decondensed and transcriptionally active (Mattson and Albertini, 1990; Debey et al., 1993; Bouniol-Baly et al., 1999; De La Fuente and Eppig, 2001; Zuccotti et al., 1995), and the oocyte is unable to re-enter the meiotic cell cycle (Eppig and Schroeder, 1989).
The ability to resume meiosis I, also termed meiotic competence, is acquired during later stages of folliculogenesis, i.e. around the time of antrum formation in murine oocytes, later in other mammals (Eppig, 2001; Fair, 2003). This transition is accompanied by a progressive silencing of transcription and profound structural changes in the chromatin within the oocyte nucleus (GV), which gradually condenses in small clumps and then associates with the nucleolus (as reviewed in De La Fuente, 2006; Luciano and Lodde, 2013). Even though morphological and temporal differences have been described, this process is common to several mammalian species (Schramm et al., 1993; Combelles et al., 2003; Lodde et al., 2007; Franciosi et al., 2012; Dieci et al., 2013; Wang et al., 2009). In the mouse, for instance, the diffused chromatin configuration, also termed non-surrounded nucleolus (NSN), is typical (even though not exclusive) of preantral oocytes, while the condensed, surrounded nucleolus (SN) configuration becomes prevalent in antral oocytes, with the percentage of SN oocytes increasing with the increasing follicle diameter (Mattson and Albertini, 1990; Debey et al., 1993; Zuccotti et al., 1998). In the cow, four distinct patterns of chromatin condensation have been recognized, a property that has allowed a more detailed correlation with transcription and progressive acquisition of developmental competence (Lodde et al., 2007, 2008).
Meiotic competent oocytes assemble microtubule organizing centres (MTOCs) that will be critical for building a spindle (Wickramasinghe et al., 1991; Wickramasinghe and Albertini, 1992; Schuh and Ellenberg, 2007). However, and in spite of the presence of a functional meiotic machinery, progression through meiosis occurs only if the gametes are removed from the follicular environment (spontaneous maturation) because signals from the follicular cells maintain the oocyte arrested in Prophase I (Zhang et al., 2010; Conti et al., 2012) (see below). The follicle-imposed arrest ensures that the oocyte completes additional steps of the differentiation programme, leading to the acquisition of developmental competence. As stated above, this is defined as the oocyte ability to sustain embryo development. This final differentiation occurs during the late antral and periovulatory follicular stages, and the developmental potential of oocytes that fail this final differentiation process is severely compromised.
When compared to meiotic competence, the cellular events regulating the acquisition of full developmental competence are less well characterized. However, nuclear and cytoplasmic changes must take place in a co-ordinated fashion (Eppig, 1996) to ensure correct ploidy of the zygote, support fertilization, reprogramming of the parental genomes, DNA replication and EGA. These are all the properties associated with a ‘good egg’ fit to sustain a pregnancy to term.
Oocyte nuclear maturation
Cell cycle re-entry and GV breakdown
Upon hormonal stimulation, resumption of meiosis occurs with different timing that is species-specific. In mouse oocytes, GV breakdown (GVBD) occurs in a very short time, ~1–3 h after hCG/LH stimulation. In cows, ~6–8 h are required for GVBD (6.6–8.0 h) with chromosome condensation (also known as prometaphase I) occurring at 8.0–10.3 h, and metaphase I (MI) at 10.3–15.4 h. Porcine oocytes require ~18–24 h from GV to ProMetaphase I, and 36 h to reach metaphase II (MII) (Dieci et al., 2013). The exact timing of GVBD after the hormonal stimulus in humans is difficult to assess in vivo. Time-lapse microscopy of IVM of human GV oocytes derived from stimulated cycles showed considerable variability in the length of the GV stage, with a median of 6.5 h (range: 05–19.8 h) (Escrich et al., 2012). In the mouse, the time from GVBD to polar body extrusion (PBE) spans ~8–10 h. In humans, an additional 14 ± 0.3 h are needed from GVBD to PBE (Escrich et al., 2012); however, longer times up to 20.2 ± 2.6 h have been reported when using oocytes expressing microtubules and chromosome tags (Holubcova et al., 2015). The total duration of meiotic maturation in human, including the time to GVBD, during in vitro culture is estimated to be ~20–22 h (Escrich et al., 2012). These divergent time requirements reflect adaptive changes in the mechanisms controlling meiotic re-entry, the most notable being the different requirements for translation. In humans, the fact that the oocytes used for most of these studies are immature at retrieval should be borne in mind when interpreting the data.
In early antral and preovulatory follicles of humans and mice, the oocytes are competent to re-enter meiosis (Eppig, 1996; Gosden and Lee, 2010). However, they are maintained arrested in prophase unless they are removed from the follicle environment (Pincus and Enzmann, 1935; Edwards, 1965). The signalling pathways and second messengers maintaining meiotic arrest have been elucidated using the mouse as experimental model, but some of the most important components and functions have been detected also in human oocytes (Conti et al., 2012). Thus, it is expected that many of the mechanisms controlling meiotic arrest and meiotic resumption are shared among species with both short and long meiotic maturation.
Oocytes are arrested in meiotic prophase because high cAMP concentrations (micromolar levels) maintain a high protein kinase A (PKA) activity (Tsafriri et al., 1996; Conti et al., 1998; Mehlmann, 2005). In turn, PKA phosphorylates key cell cycle components that prevent M-phase promoting factor (MPF) activation, thus, enforcing the meiotic arrest. High cAMP levels in the mouse are dependent on the constitutive activity of the G protein coupled receptor GPR3 (Mehlmann et al., 2004; Hinckley et al., 2005), whereas GPR12 appears to be more abundant in rat oocytes (Hinckley et al., 2005). GPR3 expression has been reported also in human oocytes (DiLuigi et al., 2008). The scenario presently accepted is that GPR3 functions without a ligand produced by the surrounding somatic cells, by behaving as a constitutive receptor directly activating an adenylyl cyclase present in the oocytes (Freudzon et al., 2005). Thus, high cAMP levels in the oocyte are maintained autonomously by the GPR3 activity; conversely, cAMP degradation through phosphodiesterase 3A (PDE3A) inactivation is prevented by exogenous cGMP transferred to the oocytes, a property shared by mouse and humans (Nogueira et al., 2006; Norris et al., 2009; Vaccari et al., 2009). cGMP is synthesized in the somatic compartment, given that granulosa cells express the guanylyl cyclase receptor NPR2 and the cognate ligand C-type natriuretic peptide (CNP) (Zhang et al., 2010). Gap-junction permeability affords the translocation of cGMP from the somatic compartment to the oocyte (Sela-Abramovich et al., 2006; Norris et al., 2008). This arrangement provides a simple explanation of why mouse and human oocytes, when removed from the follicular compartment, undergo spontaneous maturation, thus, confirming the 5-decade-old hypothesis of an oocyte maturation inhibitor (Tsafriri and Pomerantz, 1986).
Oocyte re-entry into the meiotic cell cycle while still in the follicle is the result of complex endocrine and paracrine signals acting at different times and synergizing with each other. The LH surge produces rapid changes in mural granulosa cells through direct intrinsic intracellular pathways and extracellular paracrine loops. These include the dephosphorylation of NPR2 (Egbert et al., 2014; Shuhaibar et al., 2016), which shuts off cGMP production, as well as by the release of epidermal like growth factors. The growth factor amphiregulin (AREG) suppresses cGMP production in vitro through less well-defined pathways (Vaccari et al., 2009; Liu et al., 2014). Since this growth factor is produced in an LH-dependent fashion in vivo, it likely contributes to cGMP regulation in the intact follicle. A decrease in cGMP in the follicular compartment causes an efflux of cGMP from the oocyte (Shuhaibar et al., 2015), leading to activation of PDE3A and rapid decrease in cAMP. Other events contributing to a decrease in cGMP are the activation of PDE5 in granulosa cells (Egbert et al., 2016), suppression of the production of CNP (Kawamura et al., 2011; Liu et al., 2014), as well as closure of the gap-junction communication between granulosa cells (Gershon et al., 2008).
In mouse oocytes, the inactivation of PKA is triggered by the decrease in cAMP and leads to dephosphorylation of two key components that regulate the cyclin dependent kinase 1 (Cdk1)/cyclin complex, Cdc25 and Wee2 (Han et al., 2005; Pirino et al., 2009; Oh et al., 2010; Solc et al., 2008). In human oocytes, the extended time to GVBD is likely due to the fact that several components need to be synthesized de novo, most notably cyclins. Thus, a decrease in cAMP must somehow lead to unmasking and translation of a subset of maternal mRNAs in humans and other species with a long phase separating the LH surge from GVBD. Information on the nature of these transcripts and the mechanisms of translational activation in human is largely absent.
Spindle assembly and chromosome trafficking
Appropriate chromosome condensation, spindle assembly and chromosome trafficking are indispensable for generating an egg capable of developing into an embryo. Defects at several steps of nuclear maturation cause developmental arrest at best, or an oocyte that is fertilized but that cannot sustain normal embryo development (Hassold and Hunt, 2001; Nagaoka et al., 2012). A concept that is becoming prevalent and that explains the increased incidence in aneuploidy in oocyte aging is that chromosome cohesion is compromised with consequent errors in segregation (Herbert et al., 2015; Webster and Schuh, 2017). However, the exact sequence of events leading to homolog instability is not entirely clear. One facet that should be addressed is the possibility that disruption of the translation programme affects the concentrations of key molecular components necessary for spindle development and chromosome pairing, leading to genome instability and aneuploidy. Here, we will review the basic steps associated with spindle assembly and chromosome trafficking. For a detailed description of the mechanisms controlling spindle assembly and chromosome trafficking the reader is directed to recent reviews on the topic (Bennabi et al., 2016; Severson et al., 2016; Touati and Wassmann, 2016).
Over a period of ~10 h after GVBD, human oocytes condense the chromosomes, which may function as the site of microtubule nucleation followed by slow assembly of a spindle (Holubcova et al., 2015). The observation of a chromosome-dependent initiation of microtubule nucleation in human oocytes is in contrast with the most accepted hypothesis that spindle formation is driven by the organization of MTOCs, as demonstrated in mice (Schuh and Ellenberg, 2007). MTOCs are small cytoplasmic foci of pericentriolar material that compensate for the loss of centrioles occurring during early oogenesis (Szollosi et al., 1972; Luksza et al., 2013). Specifically, MTOCs are formed from an interphase-like microtubule network that extends throughout the cytoplasm of the GV oocyte. At GVBD, they migrate in proximity of the chromosomes, forming a sphere with the chromosome bivalents clustered at the surface, a configuration known as circular bivalents (Calarco et al., 1972; Schuh and Ellenberg, 2007). Even though chromosome-driven microtubule nucleation is also described in model organisms (Heald et al., 1996; Wilde and Zheng, 1999), further work is necessary to confirm the exact mechanism functioning in human oocytes. Although the only source possible, the human oocytes used by Holubcova et al. (2015) were oocytes retrieved after hCG stimulation that remained in GV and that did not respond to the gonadotrophin stimulus. Therefore, the possibility exists that these oocytes reflect a subpopulation not representative of the normal healthy oocyte population.
After microtubule nucleation, the organization of a stably bioriented meiotic spindle takes place with metaphase progression. The stage from GVBD and chromosome congression to the alignment of the chromosome on the first meiotic plate is defined as prometaphase I and lasts several hours owing to the multiple attempts that microtubules make to assemble into a spindle before reaching the final conformation at MI (Schuh and Ellenberg, 2007). When the MI spindle is formed, and its fibres stabilized, the spindle assembly checkpoint (SAC) is inactivated, and the cell cycle progresses to anaphase I. Owing to the high frequency of aneuploidy in mammalian oocytes, it was long believed that oocytes did not possess a functional SAC (LeMaire-Adkins et al., 1997). More recently, several studies showing delayed anaphase I onset in the presence of displaced chromosomes led to the hypothesis of a ‘weaker’ SAC or SAC-escape mechanisms in these cells (Gui and Homer, 2012; Kolano et al., 2012; Lane et al., 2012).
At anaphase I the bivalent homologue chromosomes, which were held together throughout meiotic arrest, are finally disjoined, and one set is eliminated with the first polar body (PBI) at telophase I. Faithful chromosomal segregation depends on a bipolar spindle attaching homologous kinetochores to microtubules emanating from opposite poles (Jang et al., 2007), while sister chromatids separation must be prevented. This is achieved by protecting the centromeric cohesion of sister kinetochores through the loading of the protein shugoshin, which in turn recruits a phosphatase that prevents cohesion phosphorylation (Lee et al., 2008). Recently, families with rare mutations in one of the tubulin genes (tubb8) that are associated with meiotic arrest and primary female infertility were identified (Feng et al., 2016), underscoring the importance of proper microtubule polymerization in spindle assembly and chromosome trafficking.
Finally, asymmetric cytodieresis is required to prevent loss of cytoplasm. One view is that this is achieved by migration and positioning of the meiotic spindle to the oocyte cortex, through a series of events that have been extensively studied in the last decade (Maro and Verlhac, 2002; Brunet and Verlhac, 2011; Fabritius et al., 2011; McNally et al., 2013). However, this idea that the meiotic spindle is assembled initially at the centre of the oocyte followed by cortex migration has been challenged. Instead, it has been proposed that the oocyte nucleus and spindle are always located close to the surface of the oocyte in vivo (Coticchio et al., 2015). The location to the centre of the oocyte is a consequence of the removal of cumulus cells and loss of oocyte/cumulus communication. This latter observation supports the hypothesis of oocyte polarity. Once the PBI is extruded, the oocyte reassembles the MII spindle with the chromosomes aligned on the equatorial plate, ready to segregate a set of sister chromatids in the second PB upon fertilization. Remarkably, all these transitions occur in the virtual absence of transcription (Liu and Aoki, 2002; De La Fuente, 2006), implying that timed translation of maternal mRNAs drives most of these events.
Oocyte cytoplasmic maturation
Concomitant with chromosome condensation and migration, maturation is accompanied by a remarkable reorganization of the oocyte cytoplasm. Extensive remodelling and repositioning of intracellular organelles takes place at GVBD, throughout the transitions to MI, PBE and MII, including movements of vesicles, mitochondria, Golgi and endoplasmic reticulum. This topic has been the subject of extensive recent reviews (Li and Albertini, 2013; Coticchio et al., 2015) and it will not be covered here. The exact functional significance of the extensive rearrangement of organelles during oocyte maturation is largely unknown. However, it has been argued that remodelling and repositioning of these structures is necessary for the acquisition of developmental competence. As an example, the position in the oocyte of the nucleus itself is highly regulated and thought to be dependent on the state of health of the oocyte cytoskeleton (Almonacid et al., 2015). Although the peripheral position in human oocytes could not be related to their quality in some cases, the position of the GV in mouse oocyte correlates with their health and age of the donor (Brunet and Maro, 2007; Bellone et al., 2009). Mitochondria morphology and repositioning around the spindle is another remodelling feature associated with oocyte competence, forming a boundary of organelles that encapsulates the nascent spindle and condensing chromosomes (Yu et al., 2010; Dalton and Carroll, 2013). This redistribution is thought to be necessary to establish high ATP concentrations in regions of high-energy demand, as reviewed in Van Blerkom (2011).
It should be noted that oocytes of most species, including humans, contain unique cytoplasmic structures thought to be involved in storage of RNA, its metabolism and possibly the control of translation. In addition to the Balbiani body present early during development, rodent oocytes contain cytoplasmic structures called cytoplasmic lattices (Capco et al., 1993). This lattice is composed of fibrillary structures including intermediate filaments and other filamentous structures. It has been proposed that these structures function in the storage of ribosomes (Liu et al., 2017). Several genetic studies indicate that disruption of these structures leads to impaired developmental competence and defective embryo development. Given the phenotype of the knockout, the protein peptidyl arginine deiminase 6 (PADI6) is likely involved in the organization of these structures. Oocytes deficient in PADI6, lacking lattice organization, develop normally up to MII and fertilize (Esposito et al., 2007). However, early embryo development is compromised with a complete block at the two-cell stage (Esposito et al., 2007; Yurttas et al., 2008). This phenotype is associated with a decrease in protein synthesis, likely due to impaired zygote genome activation (Yurttas et al., 2008). Similarly, it has been proposed that maternal effect genes, such as Mater, are required for the organization of the cytoplasmic lattice (Kim et al., 2010). These observations in the mouse suggest that the cytoplasmic lattice and ribosomal biogenesis are somehow related to developmental competence. In humans, mutation in the PADI6 gene causes female infertility because of an arrest in early embryo development (Xu et al., 2016), confirming a critical role for this protein in oocyte developmental competence.
Genome-wide analysis of the oocyte transcriptome and developmental competence
With the development of powerful genomic techniques to assess transcription even in a single cell, it has become possible to address the question of whether oocyte transcriptomics may provide a signature for developmental competence. A large number of studies have been published on this topic for mouse, bovine and human oocytes (reviewed in Labrecque and Sirard, 2014; Robert, 2010; Svoboda et al., 2015). A comprehensive study of the mouse oocyte transcriptome at different stages of follicle development has been reported using microarray hybridization (Pan et al., 2005). This study identified major changes in gene expression comparing oocytes enclosed in primordial follicles and growing oocytes, with more than 5000 genes changing during this transition. When comparing oocytes from 17 to 24-day-old mice, a time when nuclear and cytoplasmic developmental competence is acquired, 180 genes changed at least 2-fold and only 3 changed 5-fold (Pan et al., 2005). The effect of hCG stimulation or in vitro culture was also small.
Additional transcriptomics studies in the mouse have investigated the effect of chromatin condensation during the NSN/SN transition (Ma et al., 2013) or aging (Hamatani et al., 2004; Pan et al., 2008). A recent review of the data in bovine oocytes has been published (Labrecque and Sirard, 2014). Numerous studies have probed the transcriptome of human oocytes in GV and/or MII stages under different conditions, testing the effect of maternal age (Steuerwald et al., 2007; Grondahl et al., 2010), the impact of IVM (Jones et al., 2008; Wells and Patrizio, 2008), and the aneuploidy state of the oocyte (Fragouli et al., 2010), all factors that are expected to negatively impact developmental competence. The maternal age affects the transcriptome of the oocyte with altered expression of genes involved in chromosome stability, the cell cycle, oxidative and stress responses as well as translation. A common theme emerging from the studies comparing in vitro and in vivo matured oocytes is that a substantial number of mRNAs is expressed more abundantly in in vitro matured oocytes. These aberrant levels may reflect the immaturity of the oocyte and/or the failure to execute correctly the programme of maternal mRNA degradation (see below). The latter hypothesis is in line with a similar observation in the mouse (Pan et al., 2008). Similarly, the transcriptome of oocytes that are aneuploid diverges from that of normal oocytes with the most affected pathways being those involved in spindle assembly, chromosome alignment and segregation (Fragouli et al., 2010). Some of the functions affected overlap with those detected comparing mouse oocytes from young and aged females (Hamatani et al., 2004; Pan et al., 2008).
A caveat in the interpretation of the above discussed data is warranted considering the fact that fewer changes have been detected in the mouse model compared to humans. Obviously, the mouse is a better controlled experimental model. Also, the variable quality of the human oocytes used and the inherent genetic variations found in the outbred human population should be considered when drawing conclusions. Moreover, an additional bias has been introduced in some of the studies because library preparations were made using oligo-dT priming. This strategy preferentially amplifies RNAs with long poly(A) tails. As will be discussed below, polyadenylation is a major mechanism of control of translation; therefore, differences detected using oligo-dT amplified libraries may be related to differences in polyadenylation rather than in mRNA levels.
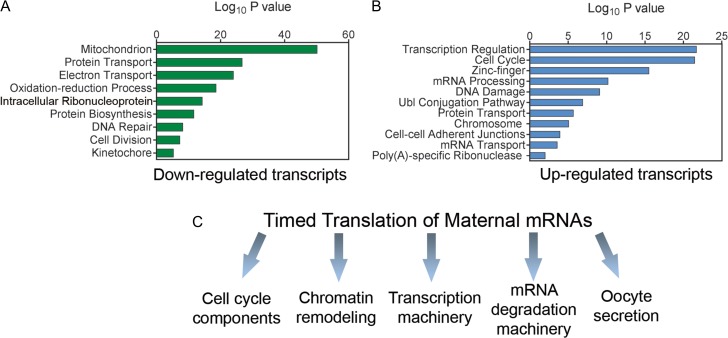
Given the fact that transcription has ceased in fully grown oocytes, the transcriptomic approach provides, in our opinion, only limited information on the state of differentiation of the gamete. Certainly, it is useful to monitor maternal mRNA accumulation during oocyte growth or mRNA degradation during the final stages of oocyte maturation. Indeed, a comparison of GV/MII mouse oocyte transcriptomes revealed a well concerted programme of maternal mRNA degradation, most notably degradation of mRNA related to ribosome and mitochondria biogenesis (Su et al., 2007). These are processes that undoubtedly have an impact on developmental competence (see below). However, the approach does not provide information as to whether the programme of maternal mRNA translation has been correctly executed during maturation. Indeed, transcriptomics comparing early antral and antral oocytes, the time when competence develops, show few changes (Pan et al., 2005; Bessa et al., 2013). Conversely, we have shown that if one investigates the pattern of maternal mRNA association with ribosomes, which probes the translational state of mRNAs, changes in translation of thousands mRNAs are observed during maturation. These mRNAs code for all the proteins involved in critical oocyte and embryo functions (Fig. 1). We also reported substantial differences in models of compromised developmental competence (Chen et al., 2011, 2013). Comparison of mRNA levels of maternal mRNAs with their translation pattern provides an example of how different conclusions can be drawn when comparing transcriptomics and translatomics (Fig. 2). Thus, we are convinced that a strategy of analysing patterns of maternal mRNA association with the polysomes would provide a more clear association with the oocyte quality and fitness to develop as an embryo. Although little experimental evidence was available at that time, this idea of translational regulation linked to developmental competence had been suggested 2 decades ago (Brevini-Gandolfi et al., 1999).
Figure 1.
Functional analysis of patterns of mRNA translation during oocyte maturation. A and B. Gene ontology (GO) analysis of mRNAs that are released from the polysomes (A) or recruited from the polysome fraction (B) during oocyte maturation. The analysis was performed comparing germinal vesicle and metaphase II polysome array data. N = 6. (C) Scheme of the function of timed translation of maternal mRNA. The components were derived from GO analysis and from manual curation of the data. The function of these regulated transcripts has been confirmed experimentally for component of the cell cycle, chromatin remodellers and secretory products of the oocyte (Chen et al., 2011, 2013; Cakmak et al., 2016).
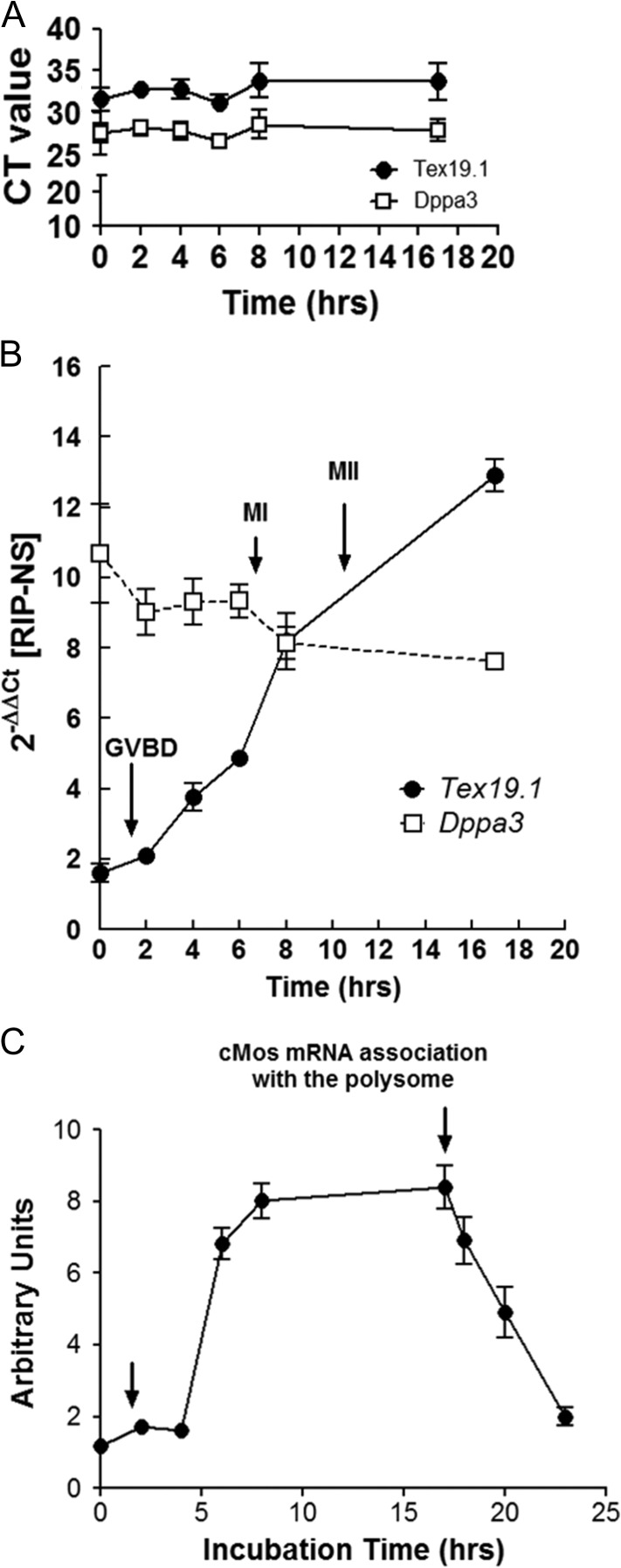
Figure 2.
Contrasting patterns of total mRNA levels versus mRNA bound to ribosomes during oocyte maturation. (A) Levels of the developmental pluripotency associated 3 (Dppa3) and testis expressed 19.1 (Text19.1) mRNA during oocyte maturation. The data were obtained by measuring total mRNA levels by quantitative PCR (qPCR). (B) Dppa3 and Tex19.1 mRNA bound to ribosomes measured by ribosome immunoprecipitation and quantification of the mRNA recovered in the pellet by qPCR. Details of the technique are reported in Sousa Martins et al. (2016). Note that no differences in transcript levels or transcript behavior are detected when using total mRNA (A). However, when mRNAs bound to ribosome are measured, clear differences are found: Dppa3 is constitutively translated during maturation whereas Tex19.1 mRNA translation increases up to 6-fold during oocyte maturation (B). (C) Pattern of ribosome loading on the mRNA coding for Mos, a kinase critical for meiotic maturation. Mos mRNA translation increases after germinal vesicle (GV) breakdown (GVBD), reaches a maximum at the end of metaphase I (MI), remains steady until metaphase II (MII), and its translation is shut off during egg activation. The data are composites of polysome arrays and ribosome immunoprecipitation data.
An additional approach to investigate how translation rates are associated with developmental competence is the measurement of protein accumulation by proteomic analysis. This approach is precluded for human oocytes, but improvement in the depth of this analysis is being been reported. Recently, 28 reprogramming factors have been identified in the oocyte by proteomic analysis (Pfeiffer et al., 2011), underscoring the oocyte cytoplasm potential in nuclear reprogramming. Moreover, an effect of maternal age on the oocyte proteome has been reported (Schwarzer et al., 2014).
The translational programme in oocytes: general properties
Given the widely accepted tenet that transcription is repressed in fully grown oocytes, the above-described restructuring that preludes gamete fertilization and zygote development relies on a programme of regulated translation. This programme controls mRNA synthesis and storage during oocyte growth, as well as selective translational activation, and/or degradation at different stages during oocyte maturation and embryo development. This programme is instrumental in generating the molecular machinery required for fertilization, reprogramming of the zygote to totipotency, and embryo development. Understanding all the facets of maternal mRNA translation should then provide a detailed molecular definition of developmental competence, an elusive property of the oocyte that until now could only be assessed retrospectively. In the same vein, any error in the execution of this programme is bound to affect the oocyte potential to sustain embryo development, providing powerful diagnostic tools.
A detailed description of the general mechanistic aspects of translational regulation is outside the scope of this review and the reader is directed to recent excellent surveys of this field (Hinnebusch et al., 2016; Reyes and Ross, 2016). Here, we will provide a brief account of the basic principles and the most important players involved in the regulation of maternal mRNA translation in the oocyte and provide information on how the overall programme may be structured. We will focus mostly on information available for mammalian gametes, which is still fragmented, and will fill the gaps with information derived from non-mammalian model organisms. It should be noted that a large body of work in worms and flies, which we cannot review here, has consolidated the concept that germ cell development relies more often on translational regulations rather than transcriptional regulations (Kimble and Crittenden, 2007; Slaidina and Lehmann, 2014).
During oocyte growth, transcription of the oocyte genome leads to the accumulation of large amounts of maternal mRNAs (Clarke, 2012). Many of these mRNAs are not translated immediately and remain dormant up to the GV oocyte stage. Indeed, turnover of mRNA in mouse oocytes is measured in days rather than minutes or hours (De Leon et al., 1983), consistent with a repressed or dormant state. Some understanding of the molecular components mediating this repression has emerged in the last decade (see below). Before ovulation and with the progression through the meiotic cell cycle, repression is relieved and a complex programme of maternal mRNA translation and degradation is executed during this transition.
Maternal mRNA translational activation
Activation of translation of dormant maternal mRNAs is thought to play a critical role in the oocyte-to-embryo transition. This translational activation and corresponding protein synthesis was detected early on, with the pioneer studies focusing on the tissue-specific plasminogen activator (Plat) mRNA (Huarte et al., 1987) as well as studies investigating total mRNA during maturation (de Vantery et al., 1997). This reawakening of dormant mRNA is not only important for assembling the machinery that drives the cell cycle. It is also necessary for nuclear reprogramming, transcription activation in the zygote, and paradoxically, to assemble the machinery involved in maternal mRNA destabilization and degradation (see below).
A well-established concept is that the extent of polyadenylation of mRNAs plays a predominant role in the activated translation in the oocytes (Richter, 2007; Ivshina et al., 2014). However, this is not the only mechanism involved in activation of maternal mRNA translation. The concept of cytoplasmic polyadenylation was developed early on, on the basis of observations in frog oocytes (Mendez and Richter, 2001) and confirmed in other species including the mouse (Tay et al., 2000; Reyes and Ross, 2016). After export from the nucleus, cytoplasmic maternal mRNAs retain short poly(A) tails of ~20–30 nucleotides. The length of the poly(A) tail in a subset of maternal mRNAs increases considerably at the time of activation of translation, which in frogs precedes GVBD. The increased length functions as a platform for recruitment of poly(A) binding proteins that establish a bridge with the 7 methyguanylate (7 mG) cap complex at the 5′ end to stabilize/promote the assembly of the translation initiation complexes. In the mouse, genetic data support the role of a gamete-specific poly(A) binding protein, termed embryonic polyadenylation binding protein (ePABP) (Seli et al., 2005; Guzeloglu-Kayisli et al., 2012). Mice deficient in ePABP are infertile because of aberrant oocyte development as well as compromised folliculogenesis, the latter phenotype likely an indirect consequence of oocyte dysfunction and aberrant secretion of oocyte factors (Lowther and Mehlmann, 2015), in some instances phenocopying the knockdown of cytoplasmic polyadenylation binding protein 1 (Cpeb1) (Racki and Richter, 2006).
The 3′ untranslated region (UTR), and to lesser extent the 5′UTR (see below), of maternal mRNAs encode the specificity for the control of polyadenylation through a combination of regulatory cis-acting elements present in this region. CPEBs are critical regulators of the poly (A) length in oocytes (Richter, 2007). The prototypic CPEB1 was identified as a protein that interacts with a specific sequence in the 3′UTR of mRNAs and assembles macromolecular complexes that prevent or promote polyadenylation and therefore translation. The nature of one complex assembled around the CPEB protein has been elucidated predominantly in frog oocytes. Although several macromolecular aggregates have been described, a prototypic complex includes a scaffold protein Symplekin (Barnard et al., 2004), the cleavage and polyadenylation factor (CPSF) protein which recognizes the polyadenylation signal, and a repressor of the cap complex, maskin (Cao and Richter, 2002). In addition, two enzymes, an adenylase (GLD2) and a deadenylase (PARN), are also part of the complex. The balance between the activities of these two enzymes determines the length of the poly(A) tail of the mRNA targeted by the complex. While this complex functions as a translational repressor during the quiescent GV state, it has been proposed that phosphorylation of CPEB signals rearrangements of the complex with expulsion of the deadenylase, allowing the adenylase to increase the poly(A) length (Hodgman et al., 2001). This model of translational regulation has been described for mRNAs involved in the regulation of the cell cycle, including cyclin B and Mos.
Genome-wide analysis of the mRNAs expressed in the oocyte indicates that a large number of transcripts are potential targets for CPEB regulation (Oh et al., 2000; Chen et al., 2011). It has also been confirmed at the genome-wide level that the length of the poly(A) tail controls mRNA stabilization and translation in the oocyte (Subtelny et al., 2014; Eichhorn et al., 2016). This relationship is not readily apparent for somatic cells (Subtelny et al., 2014). Globally, changes in polyadenylation have been detected in the cow (Tremblay et al., 2005) and indirectly in the mouse by assessing the oligo-dT priming. A recent report has added another dimension to polyadenylation in oocytes. Poly(A) tail length and 3′ terminal uridylation control the stability of mRNA in the oocyte and the enzymes involved (Tutases) have specific functions in shaping a functional maternal transcriptome (Morgan et al., 2017).
However, the waves of maternal mRNA translation are not dependent solely on CPEB1. Other RNA binding proteins (RBPs) contribute to the overall temporal programme of translation. Recently, several CPEB paralogs have been identified in mammals (Mendez and Richter, 2001; Huang et al., 2006; Ivshina et al., 2014) increasing the complexity of the regulatory circuits controlling translation. A zinc-finger domain is present in all CPEB proteins. It has been shown that zinc chelation derails oocyte maturation, opening the possibility that compromised functions of these translation regulators contributes to this phenotype (Bernhardt et al., 2012). Stem loop binding proteins are an additional class of RBPs required for histone mRNA translation (Arnold et al., 2008). This translational regulation is independent of polyadenylation.
Deleted in azoospermia like (DAZL) is a RBP initially identified as essential for primordial germ cell differentiation and entry into meiosis during foetal life, including human (Collier et al., 2005; Rosario et al., 2016). Female mice homozygous null for Dazl are sterile with gonads depleted of oocytes (Vogel et al., 2002; Kim et al., 2012). However, knockdown of DAZL in fully grown oocytes indicates that this RBP plays also a role in controlling translation during the final stages of oocyte maturation (Chen et al., 2011). Fully grown mouse oocytes depleted of DAZL fail to reach MII and display a large array of dysmorphic spindles (Chen et al., 2011). Even if able to extrude the PB and progress to MII, oocytes deficient in DAZL cannot be fertilized, strongly supporting the concept that translation is required for developmental competence. Indeed, Dazl mRNA translation and protein accumulation is impaired in a mouse model of oocyte decreased developmental competence (Chen et al., 2013). In many cases, DAZL functions in concert with CPEB1 to regulate maternal mRNAs (Sousa Martins et al., 2016). For instance, there is emerging evidence that CPEB and DAZL synergize in controlling the activation of a subset of maternal mRNAs. Using Tex19.1 as a prototypic mRNA, it has been reported that CPEB and DAZL are both required for activation of translation and function synergistically and likely in different waves of activation (Sousa Martins et al., 2016). In women, polymorphisms in the DAZL gene have been associated with decreased fertility and premature ovarian failure (Tung et al., 2006). Of note is the fact that DAZL-dependent activation of translation does not require an increase in polyadenylation of the mRNA (Collier et al., 2005), providing one example of alternative mechanisms of maternal mRNA activation.
Additional binding proteins shown to be essential for developmental competence are the zygote arrest 1 (ZAR1) and Zar-like proteins. Zar1 is an oocyte specific gene and its inactivation causes complete female infertility (Wu et al., 2003a, 2003b). Oocyte and ovary development are not affected by the loss of ZAR1 function in the mouse. However, most embryos from Zar1 null females arrest at the one-cell stage and few progress to the two-cell stage. These embryos show marked reduction in the synthesis of the transcription components at the zygotic genome activation (ZGA), indicating that Zar1 is a maternal effect gene. Although the exact function of this protein was not described in the original studies, it is now clear from studies in frog oocytes that Zar1 and 2 are RBPs involved in translational regulation (Charlesworth et al., 2012; Yamamoto et al., 2013).
Musashi is a RBP shown to be involved in the translation of mos in Xenopus oocytes (Charlesworth et al., 2006; MacNicol et al., 2008, 2011). Although present, little is known about its function in rodent and human oocytes. The cis-acting element recognized by Musashi is enriched in those maternal mRNAs whose translation is activated during oocyte maturation (Chen et al., 2011). Musashi2 has been recently implicated in cancer, and inhibitors of its function are being developed. If these efforts are successful, a question that will need to be addressed is how these inhibitors would affect oogenesis, oocyte maturation and developmental competence.
Pumilios are additional RBPs expressed in the oocyte, and a recent report implicates this family of RBP in developmental competence (Mak et al., 2016). These proteins are considered repressors of translation, are critical for germ cell development in model organisms (Kimble and Crittenden, 2007), and may be involved in maternal mRNA destabilization in the oocyte. Pum2 mRNA translation increases during oocyte maturation whereas Pum1 mRNA is barely detectable in the oocyte (Chen et al., 2011). Additional RBPs likely involved in mRNA destabilization include proteins that bind to U-rich elements (AREs) within their 3′UTR. One of these proteins is the CCCH tandem zinc-finger protein ZFP36l2, which in the mouse is essential for embryo development and fertility (see below).
In sum, the emerging picture is that activation of translation at different steps of oocyte maturation depends on the coordinated action of different RBPs. Mining of available transcriptomic data indicates that more than 200 RBPs are expressed in the oocyte (Conti, unpublished), suggesting that the oocyte-to-embryo transition is controlled by a complex network of translational regulators.
Degradation of maternal mRNAs
Early studies investigating RNA levels in oocytes showed a remarkable decrease in RNA content (by ~30–50%) during oocyte maturation, suggesting a programme of regulated mRNA degradation (Paynton et al., 1988). In agreement with this initial observation, a genome-wide analysis comparing GV and MII transcript levels in the oocyte has confirmed the degradation of a large number of transcripts (Su et al., 2007). Interestingly, mRNAs coding for mitochondria and ribosome components were enriched among the destabilized transcripts. Additional waves of degradation have been observed at the oocyte-to-embryo transition up to the two-cell stage of embryo development. These waves of degradation, particularly those that occur in the zygote and in the two-cell embryo, are thought to be essential to erase the products of the maternal genome and allow expression of the zygotic genome in the mouse (Svoboda et al., 2015) and model organisms (Yartseva and Giraldez, 2015). These waves of degradation are likely transcript-specific. However, the code defining the timing of degradation is, with few exceptions, still poorly defined. Of note, there is now ample evidence from mouse models that disrupting these waves of degradation precludes correct oocyte development and compromises embryo development (see below).
Destabilization and degradation of mRNA follow modification at the 5′ or the 3′ end of the mRNA. Destruction of the cap complex at 5′ end through decapping proteins induces 5′–3′ exoribonuclease 1 (XRN1)-mediated exonucleases and exosome-complex mediated hydrolysis. At the 3′ end deadenylation is induced by recruitment of PARN or CCR4-NOT proteins causing mRNA destabilization. Conversely, mRNA stabilization is achieved by preventing the above modifications.
An example of how important the timed translation and degradation are for progression through the oocyte-to-embryo transition is the pattern of regulation of c-Mos (Fig. 2), a key component of the meiotic cell cycle. Mos mRNA translation begins after GVBD in mouse oocytes and continues to increase up to MII. At fertilization the association of Mos mRNA with the polysomes decreases dramatically within 6 h after egg activation. MOS protein is absent in the zygote.
Several proteins functioning as repressors of translation and mRNA stability have been identified. In quiescent oocytes, a role in mRNA stabilization/repression has been proposed for ELAVL2, an oocyte specific AU-binding protein (Chalupnikova et al., 2014). Its ablation is associated with decreased developmental competence. Y-box-binding protein 2 (MSY2) is a protein that recognizes both DNA and RNA and likely serves to stabilize mRNA and functions as global repressor of translation. Its ablation in rodent causes infertility and disruption of oocyte growth and maturation (Yang et al., 2005; Medvedev et al., 2011). It has been proposed that MSY2 functions to protect maternal mRNAs from degradation and that its phosphorylation by CDK1 leads to loss of this protective function (Medvedev et al., 2011), leading to maternal mRNA destabilization.
As a first step toward degradation, decapping of some mRNAs is necessary. In this regards, the decapping proteins DCP1/2 are expressed in a pattern consistent with a role in maternal mRNA destabilization late during oocyte maturation. The Dcp1/2 mRNAs are recruited to the polysomes, and the corresponding proteins accumulate from GV to MII (Chen et al., 2011; Ma et al., 2013). These proteins are also phosphorylated in M phase. Oocyte depletion of DCP1/2 prevents mRNA degradation and ultimately affects zygote genome activation (Ma et al., 2013). Other components of the degradation machinery include CCR4-NOT transcription complex subunit 7 (CNOT7), and CCR4-NOT transcription complex subunit 6 like (CNOT6l). Their mRNA is again recruited to the polysomes and, when their translation is prevented, deadenylation of the mRNAs is reduced (Ma et al., 2015b).
Recently, two reports have pointed to an important function of B-cell translocation gene-4 (BTG4) in maternal mRNA destabilization and degradation in mouse oocytes (Pasternak et al., 2016; Yu et al., 2016). BTG4 serves as a scaffold to bring the CNOT7 catalytic subunit of the CCR4-NOT deadenylase to the translation initiation factor eIF4E or PABPN1. Thus, BTG4 recruits a deadenylation complex that eventually leads to decay of maternal mRNA. Oocytes depleted of BTG4 show mRNA stabilization and untimely polyadenylation, probably because the wave of deadenylation that occurs between MI and MII is disrupted. According to one report, BTG4 depleted oocytes progress to anaphase II spontaneously before fertilization (Pasternak et al., 2016). These authors proposed that this failure to maintain MII arrest is linked to the inability to accumulate the anaphase-promoting complex (APC) inhibitor Emi2. More recent reports have added further details on the mechanisms mediating oocyte mRNA decay in the mouse. In one case, it has been shown that mouse oocytes contain shorter polyA tails than somatic cells and that these mRNA are 3′ terminal uridylated. Uridylation of oocyte mRNA requires the enzymes TUT4 and TUT7 (Morgan et al., 2017). Terminal uridylation of short poly(A) tails in mRNA is required for culling maternal mRNAs (Morgan et al., 2017), and knockout of TUT4/TUT7 yields an oocyte unable to fertilize or progress to a two-cell embryo. Furthermore, an additional post-transcriptional modification of RNA has been implicated in mRNA stability. N6-methyladenosine (m6A) is a common modification of the mRNA, and at least five different readers of this modification have been found in mammals (Wang and He, 2014). Ivanova et al. (2017) have shown that the cytoplasmic YTHDF2 reader binds and destabilizes N6-methyladenosine (m6A)-modified mRNA, and conditional inactivation of this gene in the oocyte leads to an aberrant programme of mRNA degradation and infertility. All these findings are consistent in supporting the concept that the machinery required to destabilize maternal mRNAs is assembled during oocyte maturation through concerted activation of translation of a subset of mRNA and that this is one of the components of developmental competence. No data are available for human oocytes; given the infertility phenotypes associated with defective maternal mRNA destabilization, it is likely that a subgroup of infertile patients carries mutations in any one of the above genes. Transcriptomic analysis of human oocytes with compromised developmental competence indicates upregulation of a subset of maternal mRNAs. One could speculate that the above-described mechanisms of maternal mRNA degradation are defective in these oocytes, thus, yielding an egg unable to sustain embryo development, as documented in model organisms.
The zinc-finger protein ZFP36l2 binds to and decreases the stability of mRNAs containing an ARE. Little is known about the biochemistry of this protein in oocytes. However, mice with deletions in this gene are completely infertile. They ovulate normally and their oocytes can be fertilized. However, embryos do not develop beyond the two-cell stage (Ramos et al., 2004). Understanding how this ARE binding protein affects the programme of maternal mRNA translation would provide important insight into the role of mRNA degradation in developmental competence and embryo development.
Given the well-established concept that microRNAs function to inhibit translation and to promote mRNA destabilization, it would be conceivable that this is a mechanism involved in maternal mRNA culling (Yartseva and Giraldez, 2015). However, substantial evidence suggests that this mechanism is inactive in mouse oocytes (Ma et al., 2010; Suh et al., 2010). Conversely siRNA-mediated mRNA degradation is functional, as supported by the Dicer knockout phenotype (Murchison et al., 2007).
Regulated translation of components of the cell cycle
The development of oocyte meiotic competence begins during the growth phase in preantral and early antral follicles, likely in concert with transcription of cell cycle related genes. However, the ability to progress through the two meiotic divisions requires a well-orchestrated pattern of mRNA translation. Genome-wide analysis of maternal mRNA transcript association with the polysomes predicts regulation of a large number of the cell cycle components (105 transcripts from David gene ontology analysis of dataset reported in Fig. 1). Undoubtedly, and as mentioned above, changes in polyadenylation driven by CPEBs play a critical role in translational activation in mammalian oocytes. Again, much of what is known about the mechanisms of translational regulation of meiotic cell cycle components has been derived from studies in Xenopus oocytes. By investigating the maternal mRNA translation of cyclins as prototypic core cell cycle components in this model organism, different temporal patterns of translation have been observed (Pique et al., 2008). The mRNA coding for the Cdk1 atypical activator Ringo/Speedy is a very early-translated mRNA (Padmanabhan and Richter, 2006) followed by translation of cyclin B2 and B5. Ringo/speedy homologs have been described for mouse and humans (Cheng et al., 2005). Other transcripts are translated later, around GVBD, when Cdk1 is already activated, including cyclin CcnB1/B4. These time-dependent translations have led to the proposal of a combinatorial code of cytoplasmic polyadenylation elements (CPE) present in the 3′UTR of these mRNAs. The presence and position of these elements defines repression or activation, as well as the timing of activation. A shift in CPEB1-to-CPEB4 driven polyadenylation has been described in frog oocytes as an additional mechanism to account for late translations (Igea and Mendez, 2010). Additional elements and related binding proteins, such as Musashi and ZAR1/2, contribute to these transcript-specific patterns of translation in frogs (see below). Specifically, Musashi is likely involved in early activation of translation of Mos and cyclin B5 prior to GVBD (Charlesworth et al., 2006).
Less is known about the activation of translation of these cell cycle components in mammalian oocytes. As inferred by mRNA recruitment to the polysomes, translation of a relatively small group of maternal mRNAs is activated early in mouse oocytes at the GVBD-prometaphase transition (Chen et al., 2011) whereas translation of up to 1500 mRNAs is activated at MII. At least 100 of these MII-activated mRNAs code for components of the cell cycle, including cyclins and Mos.
Three M-phase cyclins are expressed in mouse oocytes: Ccnb1, Ccnb2 and Ccnb3. While Ccnb3 mRNA association with ribosomes decreases during maturation, Ccnb1 and Ccnb2 mRNA translation follows a pattern not too dissimilar to that described in frog oocytes with some notable differences (Han, 2017). Ccnb1 mRNA translation becomes markedly activated ~2–4 h after GVBD, and this activation is associated with increased polyadenylation (Tay et al., 2000; Sousa Martins et al., 2016; Han, 2017). Ccnb1 mRNA is recovered by CPEB1 IP, while CPEB1 knockdown leads to a decreased Ccnb1 association with ribosomes (Sousa Martins et al., 2016) and decreases Ccnb1 3′UTR reporter translation (Han et al., 2017; Han, 2017). Recent data from our laboratory suggest an additional level of complexity related to Ccnb1 and Ccnb2 3′UTR heterogeneity (Yang et al., 2017). Heterogeneity of the 3′UTR of CCNB1 has been reported also for bovine (Tremblay et al., 2005) and porcine (Zhang et al., 2010) oocytes, even though how these different 3′UTRs direct translation during oocyte maturation has not been defined in these species. We show that Ccnb1 mRNAs with 3′UTRs of different lengths contribute to the pattern of translation of this critical cell cycle protein during maturation (Yang et al., 2017). Little information is available on the translation of cyclin mRNAs in human oocytes; this would be important information to gather because defects in the regulation of these cell cycle components might be a cause of GV or MI arrest in oocytes as well as defects in chromosome segregation.
c-MOS is a MAPK-kinase–kinase expressed exclusively in the oocyte and is necessary for the activation of MAP/ERK kinases during oocyte maturation. In agreement with its different functions in frog and mammals, Mos mRNA translation follows different temporal patterns in mouse and frog. In mouse oocytes, Mos mRNA translation is activated at advanced prometaphase, whereas in frog oocytes it is activated early in prophase. CPE elements have been identified in the 3′UTR of this mRNA in mice and frogs. Studies conducted by the Richter group showed that the CPE functions in concert with the polyadenylation element to promote polyadenylation and translation of the Mos mRNAs (Gebauer et al., 1994). Mutation of these elements prevents translation. Ablation of the endogenous c-Mos mRNA with antisense oligonucleotides also prevents oocyte progression to the MII stage, a phenotype that can be rescued by injection of a wild-type c-Mos mRNA, but not of a similar RNA lacking functional CPEs. In a classical experiment, the phenotype of a mutant Mos mRNA could be partially rescued if the polyadenylation elements were provided in trans by co-injection of an RNA that hybridizes to the 3′ end of c-Mos mRNA (Barkoff et al., 1998). Further phenotypic analysis of the Mos knockout mouse shows additional defects in the MI spindle, and leaky MII arrest (Araki et al., 1996). The knockout of MAPK in oocytes is consistent with some of these phenotypes. The role of Mos/MAPK regulation in developmental competence remains to be determined in any species.
A report on the properties of human MOS mRNA has further contributed to understanding the translation control of this mRNA (Prasad et al., 2008). The human MOS 3′UTR contains a functional CPE, and the endogenous MOS mRNA undergoes maturation-dependent cytoplasmic polyadenylation in human oocytes, as in Xenopus. When a reporter with the 3′UTR of human MOS mRNA is expressed in frog oocytes, it directs translation with a delayed time course when compared to the Xenopus mos 3′UTR. This observation is consistent with the idea that the temporal pattern of translation is encoded in the 3′UTR of these cell cycle components. Given the limited amount of tissue available, any mechanistic studies in oocytes are precluded. However, translation of reporters, including the 3′UTR of these mRNAs, should be possible even in human oocytes.
The mRNAs coding for components of the spindle, such as TPX2, chromosomes and SAC, such as BUB1B and CDC20, follow a well-defined pattern of translation during mouse oocyte maturation (Chen et al., 2011, 2013). The consequent accumulation of the corresponding proteins is required for an orderly progression through the cell cycle. In human, little is known about the consequences of disruptions that target these translations.
Synthesis and degradation of a myriad of components of the cell cycle must be co-ordinated during meiosis. Although the general consensus is that protein synthesis is not required for mouse oocytes to re-enter the cell cycle, steady-state protein levels are necessary to create a poised state that allows oocytes to rapidly reinitiate meiosis upon receiving signals from the soma. These steady states depend on the co-ordinated synthesis and degradation of key proteins. For example, there is evidence that the prophase arrest is, in part, dependent on an active APC and continuous degradation of CCNB1 to prevent build-up of CDK1 activity (Reis et al., 2006). If this is the case, the rate of translation of the Ccnb1 mRNA must balance the rate of degradation by the APC. Any imbalance between synthesis and degradation ultimately yields an oocyte unable to re-enter meiosis or an oocyte prone to untimely resumption of meiosis. Similarly, the equilibrium that allows high levels of CCNB1 in MII arrest is characterized by a high turnover of synthesis and degradation as described in frogs but likely true also for mammals (Yamamoto et al., 2005). Protein synthesis is required for re-entry into the cell cycle of human, cow and pig oocytes (Ferrell, 1999a,b), but little is known of whether and how synthesis and degradation, are co-ordinated.
Other examples suggesting tightly coupled mRNA translation and protein synthesis and degradation are available. The APC activator CDH1 is present only in the first part of the meiotic cell cycle, and Cdh1 mRNA translation decreases dramatically during oocyte maturation (Chen et al., 2011). In parallel, CDH1 is ubiquitinated and targeted to the proteasome as it is degraded in metaphase. Conversely, the mRNA for the other APC activator Cdc20 is recruited to the polysomes late during MI and MII.
In summary, some of the concepts developed in frogs on cell cycle component translation have been applied to mammalian oocytes. However, the picture that is emerging is that translational control of these components in mammals is enriched by additional layers of regulation. Understanding these differences will be important to understand and better manage IVF patients with immature (GV) oocytes at retrieval and, perhaps to learn how to promote cell cycle component synthesis and meiotic resumption during IVM procedures.
Role of translation in nuclear reprogramming and zygote genome activation
Evidence that the machinery for nuclear reprogramming is assembled earlier during oocyte maturation
Gene ontology analysis of the transcripts recruited to the polysomes during oocyte maturation in the mouse shows enrichment in mRNAs coding for components of the transcription machinery and for chromatin remodelling (Chen et al., 2011) (Fig. 1). This finding points to a critical role of the oocyte translation programme in preparation for embryo development, including the events necessary to reprogramme the oocyte and sperm nucleus to that of a totipotent cell. Here, we will review some of the most informative examples of early expression for later use in the embryo, even though this should not be considered an exhaustive survey of the accumulation of chromatin remodelling components occurring at this transition (Svoboda et al., 2015).
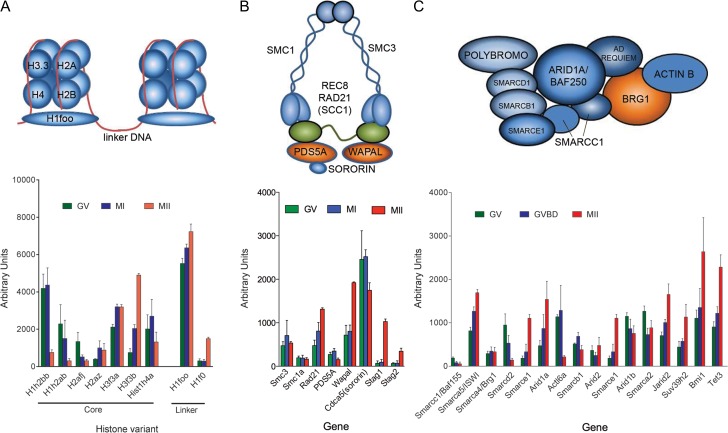
As a first example, a survey of the polysome array data shows different patterns of translation for the transcripts coding for histones (Fig. 3). Although H1h2bb, H1h2ab, H2afj, H1h4a and H1foo mRNAs are constitutively associated with or released from the polysomes, the histone H3.3 mRNA polysome association increases 6-fold during maturation, predicting a large increase in H3.3 protein synthesis. Histone H3.3 plays a critical role in chromatin decondensation and transcription in the zygote (Wen et al., 2014). If H3.3 mRNA translation is blocked with morpholino oligonucleotides, no apparent effect of H3.3 depletion is seen during oocyte maturation. Conversely, early embryo development is disrupted (Lin et al., 2013), with most zygotes unable to proceed beyond the two-cell stage. At the same time, decondensation of the male, and to lesser extent of the female, pronucleus is prevented and DNA replication disrupted. Reprogramming of a somatic nucleus also requires maternal H3.3 (Wen et al., 2014). A phenocopy of the H3.3 knockdown is observed with the genetic deletion of Hira, the chaperone required for H3.3 loading onto the chromatin (Lin et al., 2014), confirming the critical role of H3.3 synthesized during meiotic maturation for preimplantation embryo development.
Figure 3.
Dynamic changes in components of the chromatin during oocyte maturation due to contrasting patterns of components translation. Schematic representation of the components of the nucleosome, cohesins, and the chromatin switch/sucrose non-fermentable (SWI/SNF) complex are reported on top. The lower panel reports the level of mRNAs bound to polysomes for the above components as well as additional chromatin remodelers thought to function at the maternal to zygote transition. The data are derived from deposited data (GEO dataset Accession: GSE35106 ID:200 035 106). SMC1: Structural Maintenance Of Chromosomes 1; SMC3: Structural Maintenance Of Chromosomes 3; REC8: REC8 Meiotic Recombination Protein; RAD21: Rad21 (S.Pompe) Homolog (Scc1); SCC1: alternative name for RAD21 (used interchangeably) ; PDS5A: Cohesin Associated Factor A; WAPAL: Wings Apart-Like Homolog; SORORIN: Cell Division Cycle-Associated Protein 5 (CDCA5); STAG1: Stroma Antigen 1; STAG2: Stromal Antigen 2; POLYBROMO1: BRG1-Associated Factor 180 (Baf180); SMARCD1: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily D, Member 1; SMARCB1: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily B, Member 1; SMARCE1: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily E, Member 1; SMARCC1: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin Subfamily C Member 1; ARID1A: AT-Rich Interaction Domain 1A (BAF250); AD REQUIEM: Double PHD Fingers 2; BRG1: Brahma-related gene-1 (known as SMARCA4) ; SMARCA4: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily A, Member 4 (BRG1); SMARCA5: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator of Chromatin, Subfamily A, Member 5; SMARCD2: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily D, Member 2; ACTl6A: Actin Like 6A; ARID2: AT-Rich Interaction Domain 2; ARID1B: AT-Rich Interaction Domain 1B; SMARCA2: SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily A, Member 2; JARID2: Jumonji And AT-Rich Interaction Domain Containing 2; SUV39H2: Suppressor Of Variegation 3–9 Homolog 2; BMI1: Polycomb Ring Finger Proto-Oncogene; TET3: Tet Methylcytosine Dioxygenase 3.
In a similar fashion, the mRNA coding for one member of the ten-eleven translocation (TET) family of enzymes (Tet3) is regulated during oocyte maturation with a marked increase in the association of the mRNA with the polysomes (Fig. 3). TET proteins belong to the family of 2-oxoglutarate (2OG) and iron (Fe2+)-dependent dioxygenases, and TET3 is responsible for the generation of 5hmC in the paternal genome (Gu et al., 2011). Whether this is the first step required for demethylation of the paternal genome is a matter of debate (Inoue et al., 2015). However, the TET3- mediated oxidation is likely biologically important for the embryo, given the fact that female mice depleted of Tet3 in the germ line display reduced fecundity (Gu et al., 2011). Also, the development of heterozygous mutant embryos is impaired. TET3 is also involved in zygotic reprogramming of the paternal genome (Ladstatter and Tachibana-Konwalski, 2016). With a pattern opposite to Tet3, mRNAs coding for the DNA methyl transferases Dnmt1, Dnmt3a and Dnmt3l are released from the polysomes, indicating decreased synthesis during oocyte maturation. It has been reported that a DNMT3A2-HDAC2 complex is essential for genome integrity in mouse oocytes as well as for imprinting (Ma et al., 2015a). Hamatani et al. (2004) have reported a significant decrease in the expression DNMTs in oocytes derived from old female mice (9 months) and propose that this aberrant expression may be one cause of the defective embryo development associated with aging.
Components of the SWItch/sucrose non-fermentable (SWI/SNF) chromatin remodelling complex have been detected in the oocyte of different species, including mouse, pig and monkey (Zheng et al., 2004; Lisboa et al., 2012). BRG1/Smarca4 (brahma-related gene 1) is the prototypic maternal SWI/SNF component investigated at the oocyte-to-embryo transition in the mouse. Its depletion in the oocyte causes a block in embryo development and failure to activate the embryonic genome (Bultman et al., 2006), underscoring the importance of maternal effect genes involved in chromatin remodelling. BAFL55 (SMARCC1) (Guidi et al., 2001) and BAF47 (SMARCB1) (Kim et al., 2001) are also essential for embryo development. On the basis of the polysome array data generated in mouse oocytes, the mRNAs coding for several SWI/SNF proteins are differentially translated during oocyte maturation (Fig. 3). The Brg1/Smarca4 and Smarcb1 mRNAs are constitutively translated during oocyte maturation, whereas Smarca5/Swi and Smarce1 mRNAs are progressively recruited to the polysomes; Smarcc1, Smarced2 and Smarca2 translation is downregulated. The mRNA coding for Arid1a (Baf250a) is markedly recruited to the polysomes. Although the physiological significance of the divergent pattern of translation is not known, it is conceivable that a remodelling of the SWI/SNF complex is taking place during oocyte maturation. Indeed, different SWI/SNF complexes are assembled during differentiation (Ho and Crabtree, 2010). A changing pattern of expression of the different SWI/SNF mRNAs has been reported also for the oocyte-to-embryo transition in monkey oocytes (Zheng et al., 2004). Of interest is the disrupted translation of some of these components in models of compromised developmental competence. Oocytes from mice deficient in the growth factor AREG (Chen et al., 2013) show altered patterns of translation (see below) for Smarcad1a, a SWI/SNF component required for genome stability, and for Arid5b (Lahoud et al., 2001). In an additional model of compromised oocyte developmental competence induced by postovulatory aging, decreased accumulation of BRG1 has been reported (Trapphoff et al., 2016). N-ethyl-N-nitrosourea (ENU) induced mutations in Brwd1, which interacts with the SWI/SNF complex, have also been associated with oocyte-to-embryo transition failure (Philipps et al., 2008). Given the relevance of this complex in remodelling of chromatin in the early embryo, correct accumulation of these components is likely a major factor in determining the histone synthesis in cow GV oocytes (Labrecque et al., 2015).
SIN3A is a scaffold protein that interacts with HDAC1/2, functioning as co-repressor necessary to stabilize a chromatin repressive conformation. It is encoded by an mRNA recruited to the polysomes during oocyte maturation, leading to accumulation of the protein in the oocytes (Jimenez et al., 2015). The suppression of translation during oocyte maturation using siRNA affects embryo development with a block at the two to four-cell transition. Sin3a knockout die by embryonic day (ED) 6.5 (Dannenberg et al., 2005). Thus, this protein should be considered a maternal factor involved in chromatin reconfiguration during embryo development.
Assembly of the transcription machinery and ZGA
Activation of the embryo genome is a critical step for the development of a new organism. This transition has been extensively studied in model organisms including the mouse, but information is also emerging for human embryos. Genes whose transcription is activated at this stage are known for both mouse and human. Very recent reports have proposed a role for DUX4 in human and DUX transcription factor in the transcriptional activation of a subset of genes, the prototype being Zscan4 and the transposon mERVL (Hendrickson et al., 2017). Expression of Dux is sufficient to activate the ‘2 cell genes’ programme in embryonic stem cells. This activation is embryo cell-autonomous, as none of the downstream genes are expressed in oocytes at significant levels. Regulations functioning upstream of Dux are at present unknown, but it is conceivable that translational regulation during oocyte maturation may be part of the programme necessary to activate this cascade in the preimplantation embryo. Certainly, the oocyte contributes to ZGA by providing the core machinery of transcription. TfIIa1, components of TafIId including Taf1, Taf2, Taf4, Taf6, Taf7 and Taf 15, and TfIIh, and TfIIh3 are all mRNAs whose translation is activated during oocyte maturation. Additional co-activators or repressors of translation are also regulated in a co-ordinated fashion at the level of translation during oocyte maturation, including members of the Krupple-like factor, Gata, forkhead and Glis families of proteins.
In some species such as the zebrafish, pluripotency factors are involved in EGA, and some of these core pluripotency factors may be accumulating during oocyte maturation (Lee et al., 2013). Pouf5f1 (Oct4) is constitutively translated during mouse oocyte maturation (Conti, unpublished observation). A maternal mRNA role for Pouf5f1 in early embryo development has been proposed (Foygel et al., 2008; Zuccotti et al., 2011), but this possibility is inconsistent with genetic data (Wu and Scholer, 2014). Klf4 and Sox2 mRNA translation are activated at the GV/MII transition (Conti, unpublished). Whether the increase in protein levels of these two pluripotency network components predicted by analysis of the polysome array plays any role in embryo development remains to be determined. Using proteomic analysis of mouse oocytes, it has been proposed that protein arginine methyltransferase 7 (PRMT7) may be an important reprogramming factor present in the oocyte (Wang et al., 2016). In the same vein, the enrichment of GLIS1 in unfertilized oocytes has also prompted its use to direct somatic cell reprogramming (Maekawa et al., 2011). Glis1 mRNA is constitutively translated in maturing oocytes (Conti, unpublished observation). These data again support the view that transcription factors necessary for embryo development are being already synthesized in the oocyte.
Lin28 mRNA becomes progressively translated during oocyte maturation, suggesting that increased amounts of protein are delivered to the zygote (Conti, unpublished). Depletion of Lin28 triggers an increase in let7 expression during ZGA (Flemr et al., 2014). However, this depletion does not affect embryo development and fertility. In spite of the lack of an overt phenotype, these findings further support the idea that mRNAs translated during oocyte maturation play a role later during embryo development.
Role of somatic cells in promoting maternal mRNA translation and oocyte developmental competence
Oocyte growth until ovulation occurs in the protected environment of the follicle, and there is ample evidence that cumulus–oocyte communication is critical for oocyte acquisition of competence to fertilize and to support embryo development. In addition to being essential for termination of transcription at the end of the growth phase (De La Fuente and Eppig, 2001; Lodde et al., 2008), oocyte–somatic cell communication is critical for oocyte developmental potential. For instance, maintenance of cumulus oocyte communication is indispensable for an efficient IVM (Gilchrist, 2011); indeed, it is commonly accepted that oocyte denudation leads to impaired fertilization and few embryos are produced. Gap-junction mediated metabolic coupling between somatic cells and the oocyte obviously is an important facet of this interaction but other exchanges also likely mediate this dialogue (Gilchrist, 2011). A possibility that has been put forward is that cyclic nucleotides exchanged with the oocyte are not only essential to maintain meiotic arrest but also they are also indispensable for developmental competence (Ali and Sirard, 2005; Shu et al., 2008). Patent gap junctions are necessary to maintain this cyclic communication, and cyclic AMP seems to be necessary to maintain permeable gaps in bovine and porcine cumulus oocyte complexes (COC). FSH, which increases cAMP in cumulus cells, is one way to promote cAMP accumulation in the oocyte and promote developmental competence; however, additional mechanisms are also functional (see below). Of note, little is known about the effect of cyclic nucleotide signalling on maternal mRNAs, their stability, and translation, an area that should be investigated particularly in those species where translational activation takes place prior to GVBD and CDK1 activation.
It has long been recognized that epidermal growth factor (EGF) signalling improves oocyte fitness to sustain embryo development. Early on, De la Fuente and colleagues (De La Fuente et al., 1999) described how exposure of mouse COC to EGF improved the rate of fertilization and embryo development. Similarly, several other studies using EGF-like growth factors (Richani et al., 2013) are consistent with this concept. It should be noted that EGF and related growth factors do not signal directly to the oocyte. Hence, the EGF effects on oocyte quality are dependent on stimulation of cumulus or granulosa cells and that these cells in turn convey signals that promote oocyte functions.
Recently, it has been proposed that the cumulus cell crosstalk with the oocyte required for oocyte competence is through regulation of translation in the oocyte (Fig. 4).
Figure 4.
Integration of cumulus–oocyte functions via local feedbacks targeting maternal mRNA translation in the oocyte. Regulation of maternal mRNA translation in the oocyte plays a critical role in mediating protein secretion and these feedback regulations. In the follicle microenvironment, there is bidirectional exchange of signals between the oocyte and the surrounding somatic cells. The signals from the somatic cells stimulate the fully grown oocytes to increase translation of selected mRNAs. In turn, oocyte secreted factors may control cumulus cell function. AREG, amphiregulin; OSF, oocyte secreted factor; EGFR, epidermal growth factor receptor.
Exploration of this pathway was prompted by the finding that the oocyte levels of a critical component of the spindle, TPX2, are dependent on the environment in which the oocyte matures (Chen et al., 2013). TPX2 is an anchoring protein deposited on spindle microtubules and functions as the anchor for Aurora A kinase, another component critical for spindle function. TPX2 levels are highest in oocytes matured in vivo and the least when the denuded oocyte is cultured in vitro. Subsequent studies have demonstrated that cumulus cell stimulation promotes accumulation of several proteins in the oocyte, and reporter assays strongly suggest that an increase in translation is at the basis of this increased accumulation. The phosphatidyl-inositol-3-kinase-AKT-mechanistic target of rapamycin (PI3K-AKT-mTOR) pathway plays a role in the cumulus-dependent regulation of translation in the oocyte (Chen et al., 2013).
mTOR signalling is involved in sensing nutrients and other growth signals and orchestrating the complex set of adaptive changes during growth and survival (as reviewed in Gonzalez and Hall, 2017). mTOR also functions downstream of the stress response pathways. Thus, it is no surprise that manipulation of this pathway affects oocyte function and developmental competence. Among the adaptive changes induced by mTOR signalling is the regulation of translation. mTOR is a kinase component of two complexes, Torc1 and Torc2, which function by regulating phosphorylation of the 4E-BP protein, an inhibitor of the cap complex. Once phosphorylated, 4E-BP dissociates from eIF4E, allowing assembly of the cap complex and recruitment of the small ribosomal subunit. The complex also functions by regulating the activity of S6-kinase. Some of the effects on translation are global, but some specificity of the response is engendered in the presence of TOP sequences in the 5′UTR of regulated transcripts. Cumulus cells send signals to the oocytes to activate PI3K, AKT and mTOR and enhance translation. This increased translation synergizes with the cell cycle-dependent translational activation measured as endogenous protein levels and luciferase reporter accumulation driven by the 3′UTR of selected mRNAs such as Tpx2, Dazl or Il7. The mechanism is unclear and may be in part due to facilitation of the interaction of the 3′UTR complex with the 5′ Cap. Other oocyte transcripts appear to be unaffected, suggesting regulation of a subset of mRNAs. Constitutive activation of AKT, such as that found in the phosphatase and tensin homolog (Pten) knockout oocytes, also causes constitutive translation of some transcripts, including those mentioned above. Interestingly, Pten-null oocytes show improved developmental competence independent of exposure to growth factors (Franciosi et al., 2016). A role for mTOR in translation in the oocyte has been confirmed by other reports in which it was proposed that the effects are mediated via the classic mTOR dependent enhanced translation of mRNAs containing the TOP sequence (Susor et al., 2016, 2015). However the mechanism of activation of mTOR has not been addressed. It should be noted that Cdk1, which is activated during oocyte maturation, also phosphorylates 4E-BP (Velasquez et al., 2016). Thus, it is possible that activation of Cdk1 at the G2/M transition in the oocytes is also coupled to increased mRNA translation through mTor regulation.
Similar to that observed with EGF-like growth factors, FSH also enhances translation of maternal mRNAs in the oocytes (Fig. 4) and improves embryo development. This activity requires the presence of functional EGF on cumulus cells (Franciosi et al., 2016) and underscores the link between developmental competence and translational regulation during oocyte maturation. Moreover, these findings might explain the positive effect that FSH has on embryo development when administered in vivo during the ovulatory period in humans and in cows (Sirard et al., 1999; Sugimura et al., 2012), (Lamb et al., 2011) or during IVM (Schroeder et al., 1988; Merriman et al., 1998; Modina et al., 2007; Schoevers et al., 2003).
Oocyte signals affecting cumulus cells and, indirectly, developmental competence: regulation of translation of oocyte secreted factors
It has long been debated whether the oocyte predominantly receives messages coming from the somatic cells, or if the gamete acts to regulate the surrounding environment. Even though the nature of the molecules involved was only discovered years later, studies conducted in the early 1990s clearly demonstrated that the oocyte controls the phenotype of the surrounding somatic cells (Buccione et al., 1990; Vanderhyden et al., 1990). Even more striking, this regulation is exerted from the assembly of the primordial follicle until ovulation, with the oocytes ultimately participating in feedback regulatory loops necessary for developmental competence (Eppig, 2001; Matzuk et al., 2002; McNatty et al., 2004). The oocyte-to-somatic cell signals are mediated by oocyte secreted factors (OSFs), the prototypic ones being growth and differentiation factor 9 (GDF9) (Dong et al., 1996) and bone morphogenetic protein 15 (BMP15) (Dube et al., 1998). Both factors signal through the BMP receptors, activating intracellular cascades that lead to the phosphorylation of SMA and MAD related intracellular proteins (SMADs) (Mazerbourg et al., 2004; Mazerbourg and Hsueh, 2006). The function, mechanisms of action and effects of GDF9 and BMP15 during folliculogenesis and oogenesis have been thoroughly investigated and the reader is directed to extensive reviews on the topic (Shimasaki et al., 2004; Juengel and McNatty, 2005; Gilchrist et al., 2008). Notably genetic mutations in GDF9 and BMP15 loci have been associated with female fertility disorders (Galloway et al., 2002; Di Pasquale et al., 2004, 2006; Kovanci et al., 2007; Qin et al., 2015). However, GDF9 and BMP15 are not the only signalling molecules secreted by the female gamete. Members of the fibroblast growth factor (FGF) family (Valve et al., 1997; Sugiura et al., 2007), oocyte secreted protein 1 (Yan et al., 2001) and extracellular proteolytic components, such as tissue plasminogen activator (PLAT) (Huarte et al., 1985), were identified. Recent insights into the pattern of OSF secretion came from mining of transcripts bound to the polysomes during mouse oocyte maturation. Putative OSFs were identified based on the presence of signal peptides, through bioinformatic analysis. This genome-wide approach indicated a shift in the secretion during the periovulatory period (Cakmak et al., 2016) with 45 OSFs being constitutively present in the polysomal fraction, among which were Gdf9, Bmp15 and some FGF family members. Eighty-seven transcripts coding for secreted proteins showed a decreased association with the polysomes, as in the case of zona pellucida proteins and of the cytokine IK. The translation of another 38 transcripts, including cytokines such as interleukin 7 (Il7), and Plat (in agreement with previous reports) was instead greatly induced by meiotic progression. Notably, this study demonstrates that different sets of signals are exchanged with the surrounding cellular environment while differentiating into a developmentally competent egg, and reveals possible candidates to be used in assisted reproduction to non-invasively assess the fitness of the retrieved eggs (discussed below).
Another relevant finding is that the activation of translation positively responds, at least for the prototypic target Il7, to signals coming from the somatic compartment, such as AREG or FSH (Cakmak et al., 2016; Franciosi et al., 2016). This stimulation induces a maximal accumulation of endogenous protein as well as of the synthetic reporter, confirming a regulatory loop between the somatic and germinal compartment that is likely necessary for the final acquisition of developmental competence during the periovulatory stage (Fig. 4).
Brief description of currently available strategies to assess gamete developmental potential of the oocyte
Conventional embryo selection methods are based on morphological evaluations (Gardner et al., 2015). More recently, quantitative approaches have been developed, including time-lapse imaging of the kinetics of embryonic cellular division (Montag et al., 2011; Kirkegaard et al., 2012) and genetic screening of blastomeres (Twisk et al., 2006; Wells et al., 2008). To allow sufficient time to score morphokinetic parameters or for cell biopsy and DNA analysis, embryo-based selection prolongs the exposure to in vitro culture conditions, for to up to 5–6 days after fertilization (blastocyst stage). However, this lengthened culture is associated with an increased rate of large-for-gestational age newborns (Makinen et al., 2013) and a higher birthweight (Zhu et al., 2014), raising concerns of possible disturbances in the epigenetic status of the embryo induced by in vitro culture (Feuer et al., 2016).
We have identified IL7, a maturation-specific OSF, as a potential candidate for the development of a screening test of egg quality (Cakmak et al., 2016). IL7 protein content measured by ELISA is increased in the follicular fluid of follicles yielding a mature egg and is higher in the follicular fluid of normally fertilized eggs than abnormally fertilized ones (Cakmak et al., 2016). Preliminary experiments indeed show that IL7 is detectable in spent media of human cumulus enclosed oocytes cultured for 3 h before ICSI, opening the possibility that IL7 may be used as a non-invasive biomarker that is predictive of egg quality. Studies in the mouse model show that the accumulation of IL7 protein depends on the polysomal recruitment and translation of Il7 transcript during oocyte maturation. Whether a similar mechanism is in place in human oocytes needs to be verified but, if confirmed, measuring IL7 protein accumulation in the spent medium would be a potential read-out of the ability of an oocyte to regulate translation during meiotic maturation. Mining of the mouse translation data yielded a sufficient number of secreted products from the oocyte predicted to be translated during maturation that could be used, together with IL7, to assess translation in oocytes in a non-invasive manner. Further studies are required to assess the predictive value of secretion/translation for human oocyte quality.
Conclusion
The survey of the major cellular and molecular events associated with oocyte developmental competence documents the major advancements made in this field during the last few years. Genetic studies in mouse models of the oocyte-to-zygote transition are building a catalogue of genes whose products accumulate in the oocyte but that play a function necessary later on for embryo development. At the same time, genomic and proteomic probing of the properties of the female gamete are revealing molecular details that drive oocyte differentiation into a fertilizable egg. In this context, we believe that an important facet of oocyte development is the programme of maternal mRNA translation, and eventually degradation, which takes place at the maternal-to-zygote transition. With this review, we have provided an account of the state of the field, at least in mammals. Additional explorations of this programme should provide new and critical information about this developmental transition. Efforts should be devoted to assemble this vast body of information generated in model organisms into a molecular blueprint of the processes necessary for making a ‘good egg’. Although most manipulations are not feasible for human oocytes for ethical and logistic reasons, one should be able to overlay the limited human data available to the high density maps of gene networks and regulations that have been built for the mouse, to eventually identify all the processes that occur in human oocytes. There is no doubt in our mind that this wealth of information will provide improvements in ART in two ways. On one hand, the discovery of new biological features will facilitate the development of biomarkers to precisely predict the quality of an egg or an embryo. On the other, improvements will be possible in the culture conditions for IVM of the oocyte, for fertilization, and for embryo development. Together, these advancements should yield the tools to promote and monitor the fitness of an egg to develop into an embryo and to sustain a pregnancy to term.
Acknowledgements
The authors are indebted to Drs Paolo Rinaudo, Alex Tsafriri and Linda Giudice for advice and critical reading of the manuscript.
Authors’ roles
M.C. and F.F. conceived the review and worked together on the different sections.
Funding
The NIH Specialized Cooperative Centers Program in Reproduction and Infertility Research, (P50HD055764-09), as a part of the Specialized Cooperative Centres Programme in Reproduction and Infertility Research; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and NIH R01 (GM097165) and (GM116926); National Institute of General Medical Sciences (NIGMS to M.C.); Marie Curie International Outgoing Fellowships for Career Development (FP7-PEOPLE-2013-IOF GA 624 874), MateRNA; and EU Programmes to F.F.
Conflicts of interest
None.
References
- Ali A, Sirard MA. Protein kinases influence bovine oocyte competence during short-term treatment with recombinant human follicle stimulating hormone. Reproduction 2005;130:303–310. [DOI] [PubMed] [Google Scholar]
- Almonacid M, Ahmed WW, Bussonnier M, Mailly P, Betz T, Voituriez R, Gov NS, Verlhac MH. Active diffusion positions the nucleus in mouse oocytes. Nat Cell Biol 2015;17:470–479. [DOI] [PubMed] [Google Scholar]
- Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Meiotic abnormalities of c-mos knockout mouse oocytes: activation after first meiosis or entrance into third meiotic metaphase. Biol Reprod 1996;55:1315–1324. [DOI] [PubMed] [Google Scholar]
- Arnold DR, Francon P, Zhang J, Martin K, Clarke HJ. Stem-loop binding protein expressed in growing oocytes is required for accumulation of mRNAs encoding histones H3 and H4 and for early embryonic development in the mouse. Dev Biol 2008;313:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff A, Ballantyne S, Wickens M. Meiotic maturation in Xenopus requires polyadenylation of multiple mRNAs. EMBO J 1998;17:3168–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell 2004;119:641–651. [DOI] [PubMed] [Google Scholar]
- Bellone M, Zuccotti M, Redi CA, Garagna S. The position of the germinal vesicle and the chromatin organization together provide a marker of the developmental competence of mouse antral oocytes. Reproduction 2009;138:639–643. [DOI] [PubMed] [Google Scholar]
- Bennabi I, Terret ME, Verlhac MH. Meiotic spindle assembly and chromosome segregation in oocytes. J Cell Biol 2016;215:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt ML, Kong BY, Kim AM, O’Halloran TV, Woodruff TK. A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes. Biol Reprod 2012;86:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa IR, Nishimura RC, Franco MM, Dode MA. Transcription profile of candidate genes for the acquisition of competence during oocyte growth in cattle. Reprod Domest Anim 2013;48:781–789. [DOI] [PubMed] [Google Scholar]
- Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szollosi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod 1999;60:580–587. [DOI] [PubMed] [Google Scholar]
- Brevini-Gandolfi TA, Favetta LA, Mauri L, Luciano AM, Cillo F, Gandolfi F. Changes in poly(A) tail length of maternal transcripts during in vitro maturation of bovine oocytes and their relation with developmental competence. Mol Reprod Dev 1999;52:427–433. [DOI] [PubMed] [Google Scholar]
- Brunet S, Maro B. Germinal vesicle position and meiotic maturation in mouse oocyte. Reproduction 2007;133:1069–1072. [DOI] [PubMed] [Google Scholar]
- Brunet S, Verlhac MH. Positioning to get out of meiosis: the asymmetry of division. Hum Reprod Update 2011;17:68–75. [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol 1990;138:16–25. [DOI] [PubMed] [Google Scholar]
- Bultman SJ, Gebuhr TC, Pan H, Svoboda P, Schultz RM, Magnuson T. Maternal BRG1 regulates zygotic genome activation in the mouse. Genes Dev 2006;20:1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak H, Franciosi F, Zamah AM, Cedars MI, Conti M. Dynamic secretion during meiotic reentry integrates the function of the oocyte and cumulus cells. Proc Natl Acad Sci USA 2016;113:2424–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco PG, Donahue RP, Szollosi D. Germinal vesicle breakdown in the mouse oocyte. J Cell Sci 1972;10:369–385. [DOI] [PubMed] [Google Scholar]
- Calhaz-Jorge C, de Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V et al. . Assisted reproductive technology in Europe, 2012: results generated from European registers by ESHRE. Hum Reprod 2016;31:1638–1652. [DOI] [PubMed] [Google Scholar]
- Cao Q, Richter JD. Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J 2002;21:3852–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capco DG, Gallicano GI, McGaughey RW, Downing KH, Larabell CA. Cytoskeletal sheets of mammalian eggs and embryos: a lattice-like network of intermediate filaments. Cell Motil Cytoskeleton 1993;24:85–99. [DOI] [PubMed] [Google Scholar]
- Chalupnikova K, Solc P, Sulimenko V, Sedlacek R, Svoboda P. An oocyte-specific ELAVL2 isoform is a translational repressor ablated from meiotically competent antral oocytes. Cell Cycle 2014;13:1187–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth A, Wilczynska A, Thampi P, Cox LL, MacNicol AM. Musashi regulates the temporal order of mRNA translation during Xenopus oocyte maturation. EMBO J 2006;25:2792–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth A, Yamamoto TM, Cook JM, Silva KD, Kotter CV, Carter GS, Holt JW, Lavender HF, MacNicol AM, Ying Wang Y et al. . Xenopus laevis zygote arrest 2 (zar2) encodes a zinc finger RNA-binding protein that binds to the translational control sequence in the maternal Wee1 mRNA and regulates translation. Dev Biol 2012;369:177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, Sette C, Blelloch R, Conti M. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev 2011;25:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Torcia S, Xie F, Lin CJ, Cakmak H, Franciosi F, Horner K, Onodera C, Song JS, Cedars MI et al. . Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol 2013;15:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Xiong W, Ferrell JE Jr, Solomon MJ. Identification and comparative analysis of multiple mammalian Speedy/Ringo proteins. Cell Cycle 2005;4:155–165. [DOI] [PubMed] [Google Scholar]
- Clarke HJ. Post-transcriptional control of gene expression during mouse oogenesis. Results Probl Cell Differ 2012;55:1–21. [DOI] [PubMed] [Google Scholar]
- Collier B, Gorgoni B, Loveridge C, Cooke HJ, Gray NK. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J 2005;24:2656–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combelles CM, Albertini DF, Racowsky C. Distinct microtubule and chromatin characteristics of human oocytes after failed in-vivo and in-vitro meiotic maturation. Hum Reprod 2003;18:2124–2130. [DOI] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard FJ, Shitsukawa K, Tsafriri A. Role of cyclic nucleotide phosphodiesterases in resumption of meiosis. Mol Cell Endocrinol 1998;145:9–14. [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol 2012;356:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coticchio G, Dal Canto M, Mignini Renzini M, Guglielmo MC, Brambillasca F, Turchi D, Novara PV, Fadini R. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update 2015;21:427–454. [DOI] [PubMed] [Google Scholar]
- Dalton CM, Carroll J. Biased inheritance of mitochondria during asymmetric cell division in the mouse oocyte. J Cell Sci 2013;126:2955–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg JH, David G, Zhong S, van der Torre J, Wong WH, Depinho RA. mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev 2005;19:1581–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente R. Chromatin modifications in the germinal vesicle (GV) of mammalian oocytes. Dev Biol 2006;292:1–12. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol 2001;229:224–236. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, O’Brien MJ, Eppig JJ. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod 1999;14:3060–3068. [DOI] [PubMed] [Google Scholar]
- De Leon V, Johnson A, Bachvarova R. Half-lives and relative amounts of stored and polysomal ribosomes and poly(A) + RNA in mouse oocytes. Dev Biol 1983;98:400–408. [DOI] [PubMed] [Google Scholar]
- de Vantery C, Stutz A, Vassalli JD, Schorderet-Slatkine S. Acquisition of meiotic competence in growing mouse oocytes is controlled at both translational and posttranslational levels. Dev Biol 1997;187:43–54. [DOI] [PubMed] [Google Scholar]
- Debey P, Szollosi MS, Szollosi D, Vautier D, Girousse A, Besombes D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev 1993;36:59–74. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. Am J Hum Genet 2004;75:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, Einaudi S, Radetti G, Russo G, Sacco M et al. . Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab 2006;91:1976–1979. [DOI] [PubMed] [Google Scholar]
- Dieci C, Lodde V, Franciosi F, Lagutina I, Tessaro I, Modina SC, Albertini DF, Lazzari G, Galli C, Luciano AM. The effect of cilostamide on gap junction communication dynamics, chromatin remodeling, and competence acquisition in pig oocytes following parthenogenetic activation and nuclear transfer. Biol Reprod 2013;89:68. [DOI] [PubMed] [Google Scholar]
- DiLuigi A, Weitzman VN, Pace MC, Siano LJ, Maier D, Mehlmann LM. Meiotic arrest in human oocytes is maintained by a Gs signaling pathway. Biol Reprod 2008;78:667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 1996;383:531–535. [DOI] [PubMed] [Google Scholar]
- Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol 1998;12:1809–1817. [DOI] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature 1965;208:349–351. [DOI] [PubMed] [Google Scholar]
- Egbert JR, Shuhaibar LC, Edmund AB, Van Helden DA, Robinson JW, Uliasz TF, Baena V, Geerts A, Wunder F, Potter LR et al. . Dephosphorylation and inactivation of NPR2 guanylyl cyclase in granulosa cells contributes to the LH-induced decrease in cGMP that causes resumption of meiosis in rat oocytes. Development 2014;141:3594–3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbert JR, Uliasz TF, Shuhaibar LC, Geerts A, Wunder F, Kleiman RJ, Humphrey JM, Lampe PD, Artemyev NO, Rybalkin SD et al. . Luteinizing hormone causes phosphorylation and activation of the cGMP phosphodiesterase PDE5 in rat ovarian follicles, contributing, together with PDE1 activity, to the resumption of meiosis. Biol Reprod 2016;94:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn SW, Subtelny AO, Kronja I, Kwasnieski JC, Orr-Weaver TL, Bartel DP. mRNA poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila oocytes and early embryos. Elife 2016;5:e16955. doi:10.7554/eLife.16955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996;8:485–489. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction 2001;122:829–838. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod 1989;41:268–276. [DOI] [PubMed] [Google Scholar]
- Escrich L, Grau N, de los Santos MJ, Romero JL, Pellicer A, Escriba MJ. The dynamics of in vitro maturation of germinal vesicle oocytes. Fertil Steril 2012;98:1147–1151. [DOI] [PubMed] [Google Scholar]
- Esposito G, Vitale AM, Leijten FP, Strik AM, Koonen-Reemst AM, Yurttas P, Robben TJ, Coonrod S, Gossen JA. Peptidylarginine deiminase (PAD) 6 is essential for oocyte cytoskeletal sheet formation and female fertility. Mol Cell Endocrinol 2007;273:25–31. [DOI] [PubMed] [Google Scholar]
- Fabritius AS, Ellefson ML, McNally FJ. Nuclear and spindle positioning during oocyte meiosis. Curr Opin Cell Biol 2011;23:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair T. Follicular oocyte growth and acquisition of developmental competence. Anim Reprod Sci 2003;78:203–216. [DOI] [PubMed] [Google Scholar]
- Farrell JA, O’Farrell PH. From egg to gastrula: how the cell cycle is remodeled during the Drosophila mid-blastula transition. Annu Rev Genet 2014;48:269–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng R, Sang Q, Kuang Y, Sun X, Yan Z, Zhang S, Shi J, Tian G, Luchniak A, Fukuda Y et al. . Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med 2016;374:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE., Jr Building a cellular switch: more lessons from a good egg. Bioessays 1999. a;21:866–870. [DOI] [PubMed] [Google Scholar]
- Ferrell JE., Jr Xenopus oocyte maturation: new lessons from a good egg. Bioessays 1999. b;21:833–842. [DOI] [PubMed] [Google Scholar]
- Feuer SK, Camarano L, Rinaudo PF. ART and health: clinical outcomes and insights on molecular mechanisms from rodent studies. Mol Hum Reprod 2016;19:189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemr M, Moravec M, Libova V, Sedlacek R, Svoboda P. Lin28a is dormant, functional, and dispensable during mouse oocyte-to-embryo transition. Biol Reprod 2014;90:131. [DOI] [PubMed] [Google Scholar]
- Foygel K, Choi B, Jun S, Leong DE, Lee A, Wong CC, Zuo E, Eckart M, Reijo Pera RA, Wong WH et al. . A novel and critical role for Oct4 as a regulator of the maternal-embryonic transition. PLoS One 2008;3:e4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragouli E, Bianchi V, Patrizio P, Obradors A, Huang Z, Borini A, Delhanty JD, Wells D. Transcriptomic profiling of human oocytes: association of meiotic aneuploidy and altered oocyte gene expression. Mol Hum Reprod 2010;16:570–582. [DOI] [PubMed] [Google Scholar]
- Franciosi F, Lodde V, Goudet G, Duchamp G, Deleuze S, Douet C, Tessaro I, Luciano AM. Changes in histone H4 acetylation during in vivo versus in vitro maturation of equine oocytes. Mol Hum Reprod 2012;18:243–252. [DOI] [PubMed] [Google Scholar]
- Franciosi F, Manandhar S, Conti M. FSH regulates mRNA translation in mouse oocytes and promotes developmental competence. Endocrinology 2016;157:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freour T, Vassena R. Transcriptomics analysis and human preimplantation development. J Proteomics 2017;162:135–140. [DOI] [PubMed] [Google Scholar]
- Freudzon L, Norris RP, Hand AR, Tanaka S, Saeki Y, Jones TL, Rasenick MM, Berlot CH, Mehlmann LM, Jaffe LA. Regulation of meiotic prophase arrest in mouse oocytes by GPR3, a constitutive activator of the Gs G protein. J Cell Biol 2005;171:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway SM, Gregan SM, Wilson T, McNatty KP, Juengel JL, Ritvos O, Davis GH. Bmp15 mutations and ovarian function. Mol Cell Endocrinol 2002;191:15–18. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Meseguer M, Rubio C, Treff NR. Diagnosis of human preimplantation embryo viability. Hum Reprod Update 2015;21:727–747. [DOI] [PubMed] [Google Scholar]
- Gebauer F, Xu W, Cooper GM, Richter JD. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J 1994;13:5712–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon E, Plaks V, Dekel N. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol 2008;282:18–25. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB. Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev 2011;23:23–31. [DOI] [PubMed] [Google Scholar]
- Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 2008;14:159–177. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Kushnir VA, Albertini DF, Barad DH. Improvements in IVF in women of advanced age. J Endocrinol 2016;230:F1–F6. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Hall MN. Nutrient sensing and TOR signaling in yeast and mammals. EMBO J 2017;36:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosden R, Lee B. Portrait of an oocyte: our obscure origin. J Clin Invest 2010;120:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondahl ML, Yding Andersen C, Bogstad J, Nielsen FC, Meinertz H, Borup R. Gene expression profiles of single human mature oocytes in relation to age. Hum Reprod 2010;25:957–968. [DOI] [PubMed] [Google Scholar]
- Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG et al. . The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 2011;477:606–610. [DOI] [PubMed] [Google Scholar]
- Gui L, Homer H. Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development 2012;139:1941–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi CJ, Sands AT, Zambrowicz BP, Turner TK, Demers DA, Webster W, Smith TW, Imbalzano AN, Jones SN. Disruption of Ini1 leads to peri-implantation lethality and tumorigenesis in mice. Mol Cell Biol 2001;21:3598–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Lalioti MD, Aydiner F, Sasson I, Ilbay O, Sakkas D, Lowther KM, Mehlmann LM, Seli E. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J 2012;446:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 2004;13:2263–2278. [DOI] [PubMed] [Google Scholar]
- Han SJ. The Translation of Cyclin B1 and B2 is Differentially Regulated during Mouse Oocyte Reentry into the Meiotic Cell Cycle. Scientific reports, 2017. [DOI] [PMC free article] [PubMed]
- Han SJ, Chen R, Paronetto MP, Conti M. Wee1B is an oocyte-specific kinase involved in the control of meiotic arrest in the mouse. Curr Biol 2005;15:1670–1676. [DOI] [PubMed] [Google Scholar]
- Han SJ, Martins JPS, Yang Y, Kang MK, Daldello EM, Conti M. The translation of Cyclin B1 and B2 is differentially regulated during mouse oocyte reentry into the meiotic cell cycle. Sci Rep 2017;7:14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2001;2:280–291. [DOI] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 1996;382:420–425. [DOI] [PubMed] [Google Scholar]
- Hendrickson PG, Dorais JA, Grow EJ, Whiddon JL, Lim JW, Wike CL, Weaver BD, Pflueger C, Emery BR, Wilcox AL et al. . Conserved roles of mouse DUX and human DUX4 in activating cleavage-stage genes and MERVL/HERVL retrotransposons. Nat Genet 2017;49:925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert M, Kalleas D, Cooney D, Lamb M, Lister L. Meiosis and maternal aging: insights from aneuploid oocytes and trisomy births. Cold Spring Harb Perspect Biol 2015;7:a017970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 2005;287:249–261. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG, Ivanov IP, Sonenberg N. Translational control by 5’-untranslated regions of eukaryotic mRNAs. Science 2016;352:1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature 2010;463:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgman R, Tay J, Mendez R, Richter JD. CPEB phosphorylation and cytoplasmic polyadenylation are catalyzed by the kinase IAK1/Eg2 in maturing mouse oocytes. Development 2001;128:2815–2822. [DOI] [PubMed] [Google Scholar]
- Holubcova Z, Blayney M, Elder K, Schuh M. Human oocytes. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science 2015;348:1143–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YS, Kan MC, Lin CL, Richter JD. CPEB3 and CPEB4 in neurons: analysis of RNA-binding specificity and translational control of AMPA receptor GluR2 mRNA. EMBO J 2006;25:4865–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarte J, Belin D, Vassalli JD. Plasminogen activator in mouse and rat oocytes: induction during meiotic maturation. Cell 1985;43:551–558. [DOI] [PubMed] [Google Scholar]
- Huarte J, Belin D, Vassalli A, Strickland S, Vassalli JD. Meiotic maturation of mouse oocytes triggers the translation and polyadenylation of dormant tissue-type plasminogen activator mRNA. Genes Dev 1987;1:1201–1211. [DOI] [PubMed] [Google Scholar]
- Igea A, Mendez R. Meiosis requires a translational positive loop where CPEB1 ensues its replacement by CPEB4. EMBO J 2010;29:2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Shen L, Matoba S, Zhang Y. Haploinsufficiency, but not defective paternal 5mC oxidation, accounts for the developmental defects of maternal Tet3 knockouts. Cell Rep 2015;10:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova I, Much C, Di Giacomo M, Azzi C, Morgan M, Moreira PN, Monahan J, Carrieri C, Enright AJ, O’Carroll D. The RNA m6A reader YTHDF2 is essential for the post-transcriptional regulation of the maternal transcriptome and oocyte competence. Mol Cell 2017;67:1059–1067. e1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivshina M, Lasko P, Richter JD. Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu Rev Cell Dev Biol 2014;30:393–415. [DOI] [PubMed] [Google Scholar]
- Jang JK, Rahman T, Kober VS, Cesario J, McKim KS. Misregulation of the kinesin-like protein Subito induces meiotic spindle formation in the absence of chromosomes and centrosomes. Genetics 2007;177:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez R, Melo EO, Davydenko O, Ma J, Mainigi M, Franke V, Schultz RM. Maternal SIN3A regulates reprogramming of gene expression during mouse preimplantation development. Biol Reprod 2015;93:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GM, Cram DS, Song B, Magli MC, Gianaroli L, Lacham-Kaplan O, Findlay JK, Jenkin G, Trounson AO. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum Reprod 2008;23:1138–1144. [DOI] [PubMed] [Google Scholar]
- Juengel JL, McNatty KP. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum Reprod Update 2005;11:143–160. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Cheng Y, Kawamura N, Takae S, Okada A, Kawagoe Y, Mulders S, Terada Y, Hsueh AJ. Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum Reprod 2011;26:3094–3101. [DOI] [PubMed] [Google Scholar]
- Kim B, Cooke HJ, Rhee K. DAZL is essential for stress granule formation implicated in germ cell survival upon heat stress. Development 2012;139:568–578. [DOI] [PubMed] [Google Scholar]
- Kim JK, Huh SO, Choi H, Lee KS, Shin D, Lee C, Nam JS, Kim H, Chung H, Lee HW et al. . Srg3, a mouse homolog of yeast SWI3, is essential for early embryogenesis and involved in brain development. Mol Cell Biol 2001;21:7787–7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Kan R, Anguish L, Nelson LM, Coonrod SA. Potential role for MATER in cytoplasmic lattice formation in murine oocytes. PLoS One 2010;5:e12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL. Controls of germline stem cells, entry into meiosis, and the sperm/oocyte decision in Caenorhabditis elegans. Annu Rev Cell Dev Biol 2007;23:405–433. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K, Agerholm IE, Ingerslev HJ. Time-lapse monitoring as a tool for clinical embryo assessment. Hum Reprod 2012;27:1277–1285. [DOI] [PubMed] [Google Scholar]
- Kolano A, Brunet S, Silk AD, Cleveland DW, Verlhac MH. Error-prone mammalian female meiosis from silencing the spindle assembly checkpoint without normal interkinetochore tension. Proc Natl Acad Sci USA 2012;109:E1858–E1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovanci E, Rohozinski J, Simpson JL, Heard MJ, Bishop CE, Carson SA. Growth differentiating factor-9 mutations may be associated with premature ovarian failure. Fertil Steril 2007;87:143–146. [DOI] [PubMed] [Google Scholar]
- Labrecque R, Lodde V, Dieci C, Tessaro I, Luciano AM, Sirard MA. Chromatin remodelling and histone m RNA accumulation in bovine germinal vesicle oocytes. Mol Reprod Dev 2015;82:450–462. [DOI] [PubMed] [Google Scholar]
- Labrecque R, Sirard MA. The study of mammalian oocyte competence by transcriptome analysis: progress and challenges. Mol Hum Reprod 2014;20:103–116. [DOI] [PubMed] [Google Scholar]
- Ladstatter S, Tachibana-Konwalski K. A surveillance mechanism ensures repair of DNA lesions during zygotic reprogramming. Cell 2016;167:1774–1787. e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoud MH, Ristevski S, Venter DJ, Jermiin LS, Bertoncello I, Zavarsek S, Hasthorpe S, Drago J, de Kretser D, Hertzog PJ et al. . Gene targeting of Desrt, a novel ARID class DNA-binding protein, causes growth retardation and abnormal development of reproductive organs. Genome Res 2001;11:1327–1334. [DOI] [PubMed] [Google Scholar]
- Lamb JD, Shen S, McCulloch C, Jalalian L, Cedars MI, Rosen MP. Follicle-stimulating hormone administered at the time of human chorionic gonadotropin trigger improves oocyte developmental competence in in vitro fertilization cycles: a randomized, double-blind, placebo-controlled trial. Fertil Steril 2011;95:1655–1660. [DOI] [PubMed] [Google Scholar]
- Lane SI, Yun Y, Jones KT. Timing of anaphase-promoting complex activation in mouse oocytes is predicted by microtubule-kinetochore attachment but not by bivalent alignment or tension. Development 2012;139:1947–1955. [DOI] [PubMed] [Google Scholar]
- Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, Giraldez AJ. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature 2013;503:360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 2008;10:42–52. [DOI] [PubMed] [Google Scholar]
- LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metaphase/anaphase transition: a mechanism of meiotic nondisjunction in mammalian females. J Cell Biol 1997;139:1611–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol 2013;14:141–152. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Conti M, Ramalho-Santos M. Histone variant H3.3 maintains a decondensed chromatin state essential for mouse preimplantation development. Development 2013;140:3624–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CJ, Koh FM, Wong P, Conti M, Ramalho-Santos M. Hira-mediated H3.3 incorporation is required for DNA replication and ribosomal RNA transcription in the mouse zygote. Dev Cell 2014;30:268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa LA, Bordignon V, Seneda MM. Immunolocalization of BRG1-SWI/SNF protein during folliculogenesis in the porcine ovary. Zygote 2012;20:243–248. [DOI] [PubMed] [Google Scholar]
- Liu H, Aoki F. Transcriptional activity associated with meiotic competence in fully grown mouse GV oocytes. Zygote 2002;10:327–332. [DOI] [PubMed] [Google Scholar]
- Liu X, Morency E, Li T, Qin H, Zhang X, Zhang X, Coonrod S. Role for PADI6 in securing the mRNA-MSY2 complex to the oocyte cytoplasmic lattices. Cell Cycle 2017;16:360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xie F, Zamah AM, Cao B, Conti M. Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol Reprod 2014;91:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodde V, Modina S, Galbusera C, Franciosi F, Luciano AM. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence. Mol Reprod Dev 2007;74:740–749. [DOI] [PubMed] [Google Scholar]
- Lodde V, Modina S, Maddox-Hyttel P, Franciosi F, Lauria A, Luciano AM. Oocyte morphology and transcriptional silencing in relation to chromatin remodeling during the final phases of bovine oocyte growth. Mol Reprod Dev 2008;75:915–924. [DOI] [PubMed] [Google Scholar]
- Lowther KM, Mehlmann LM. Embryonic poly(A)-binding protein is required during early stages of mouse oocyte development for chromatin organization, transcriptional silencing, and meiotic competence. Biol Reprod 2015;93:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano AM, Lodde V. Changes of large-scale chromatin configuration during mammalian oocyte differentiation In: Coticchio G, Albertini D, De Santis L (eds).. Oogenesis. London: Springer-Verlag, 2013;93–108. [Google Scholar]
- Luksza M, Queguigner I, Verlhac MH, Brunet S. Rebuilding MTOCs upon centriole loss during mouse oogenesis. Dev Biol 2013;382:48–56. [DOI] [PubMed] [Google Scholar]
- Ma P, de Waal E, Weaver JR, Bartolomei MS, Schultz RM. A DNMT3A2-HDAC2 complex is essential for genomic imprinting and genome integrity in mouse oocytes. Cell Rep 2015. a;13:1552–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Flemr M, Stein P, Berninger P, Malik R, Zavolan M, Svoboda P, Schultz RM. MicroRNA activity is suppressed in mouse oocytes. Curr Biol 2010;20:265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Flemr M, Strnad H, Svoboda P, Schultz RM. Maternally recruited DCP1A and DCP2 contribute to messenger RNA degradation during oocyte maturation and genome activation in mouse. Biol Reprod 2013. a;88:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Fukuda Y, Schultz RM. Mobilization of dormant Cnot7 mRNA promotes deadenylation of maternal transcripts during mouse oocyte maturation. Biol Reprod 2015. b;93:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JY, Li M, Luo YB, Song S, Tian D, Yang J, Zhang B, Hou Y, Schatten H, Liu Z et al. . Maternal factors required for oocyte developmental competence in mice: transcriptome analysis of non-surrounded nucleolus (NSN) and surrounded nucleolus (SN) oocytes. Cell Cycle 2013. b;12:1928–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNicol MC, Cragle CE, MacNicol AM. Context-dependent regulation of Musashi-mediated mRNA translation and cell cycle regulation. Cell Cycle 2011;10:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNicol AM, Wilczynska A, MacNicol MC. Function and regulation of the mammalian Musashi mRNA translational regulator. Biochem Soc Trans 2008;36:528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, Kodanaka I, Ichisaka T, Kawamura Y, Mochizuki H, Goshima N, Yamanaka S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 2011;474:225–229. [DOI] [PubMed] [Google Scholar]
- Mak W, Fang C, Holden T, Dratver MB, Lin H. An important role of Pumilio 1 in regulating the development of the mammalian female germline. Biol Reprod 2016;94:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen S, Soderstrom-Anttila V, Vainio J, Suikkari AM, Tuuri T. Does long in vitro culture promote large for gestational age babies? Hum Reprod 2013;28:828–834. [DOI] [PubMed] [Google Scholar]
- Maro B, Verlhac MH. Polar body formation: new rules for asymmetric divisions. Nat Cell Biol 2002;4:E281–E283. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BA, Albertini DF. Oogenesis: chromatin and microtubule dynamics during meiotic prophase. Mol Reprod Dev 1990;25:374–383. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ. Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 2002;296:2178–2180. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Hsueh AJ. Genomic analyses facilitate identification of receptors and signalling pathways for growth differentiation factor 9 and related orphan bone morphogenetic protein/growth differentiation factor ligands. Hum Reprod Update 2006;12:373–383. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Klein C, Roh J, Kaivo-Oja N, Mottershead DG, Korchynskyi O, Ritvos O, Hsueh AJ. Growth differentiation factor-9 signaling is mediated by the type I receptor, activin receptor-like kinase 5. Mol Endocrinol 2004;18:653–665. [DOI] [PubMed] [Google Scholar]
- McNally J, Callan D, Andronicos N, Bott N, Hunt PW. DNA-based methodology for the quantification of gastrointestinal nematode eggs in sheep faeces. Vet Parasitol 2013;198:325–335. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Moore LG, Hudson NL, Quirke LD, Lawrence SB, Reader K, Hanrahan JP, Smith P, Groome NP, Laitinen M et al. . The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction 2004;128:379–386. [DOI] [PubMed] [Google Scholar]
- Medvedev S, Pan H, Schultz RM. Absence of MSY2 in mouse oocytes perturbs oocyte growth and maturation, RNA stability, and the transcriptome. Biol Reprod 2011;85:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 2005;130:791–799. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 2004;306:1947–1950. [DOI] [PubMed] [Google Scholar]
- Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol 2001;2:521–529. [DOI] [PubMed] [Google Scholar]
- Merriman JA, Whittingham DG, Carroll J. The effect of follicle stimulating hormone and epidermal growth factor on the developmental capacity of in-vitro matured mouse oocytes. Hum Reprod 1998;13:690–695. [DOI] [PubMed] [Google Scholar]
- Modina S, Abbate F, Germana GP, Lauria A, Luciano AM. Beta-catenin localization and timing of early development of bovine embryos obtained from oocytes matured in the presence of follicle stimulating hormone. Anim Reprod Sci 2007;100:264–279. [DOI] [PubMed] [Google Scholar]
- Montag M, Liebenthron J, Koster M. Which morphological scoring system is relevant in human embryo development? Placenta 2011;32:S252–S256. [DOI] [PubMed] [Google Scholar]
- Morgan M, Much C, DiGiacomo M, Azzi C, Ivanova I, Vitsios DM, Pistolic J, Collier P, Moreira PN, Benes V et al. . mRNA 3’ uridylation and poly(A) tail length sculpt the mammalian maternal transcriptome. Nature 2017;548:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munne S, Chen S, Colls P, Garrisi J, Zheng X, Cekleniak N, Lenzi M, Hughes P, Fischer J, Garrisi M et al. . Maternal age, morphology, development and chromosome abnormalities in over 6000 cleavage-stage embryos. Reprod Biomed Online 2007;14:628–634. [DOI] [PubMed] [Google Scholar]
- Munne S, Tomkin G, Cohen J. Selection of embryos by morphology is less effective than by a combination of aneuploidy testing and morphology observations. Fertil Steril 2009;91:943–945. [DOI] [PubMed] [Google Scholar]
- Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. Critical roles for Dicer in the female germline. Genes Dev 2007;21:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka SI, Hassold TJ, Hunt PA. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 2012;13:493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira D, Ron-El R, Friedler S, Schachter M, Raziel A, Cortvrindt R, Smitz J. Meiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacity of human oocytes and allows subsequent embryonic development. Biol Reprod 2006;74:177–184. [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 2008;135:3229–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, Wang H, Ke H, Nikolaev VO, Jaffe LA. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development 2009;136:1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Han SJ, Conti M. Wee1B, Myt1, and Cdc25 function in distinct compartments of the mouse oocyte to control meiotic resumption. J Cell Biol 2010;188:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. Timely translation during the mouse oocyte-to-embryo transition. Development 2000;127:3795–3803. [DOI] [PubMed] [Google Scholar]
- Padmanabhan K, Richter JD. Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation. Genes Dev 2006;20:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Ma P, Zhu W, Schultz RM. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol 2008;316:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, O’Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev Biol 2005;286:493–506. [DOI] [PubMed] [Google Scholar]
- Pasternak M, Pfender S, Santhanam B, Schuh M. The BTG4 and CAF1 complex prevents the spontaneous activation of eggs by deadenylating maternal mRNAs. Open Biol 2016;6:160184. doi:10.1098/rsob.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynton BV, Rempel R, Bachvarova R. Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev Biol 1988;129:304–314. [DOI] [PubMed] [Google Scholar]
- Pfeiffer MJ, Siatkowski M, Paudel Y, Balbach ST, Baeumer N, Crosetto N, Drexler HC, Fuellen G, Boiani M. Proteomic analysis of mouse oocytes reveals 28 candidate factors of the ‘reprogrammome’. J Proteome Res 2011;10:2140–2153. [DOI] [PubMed] [Google Scholar]
- Philipps DL, Wigglesworth K, Hartford SA, Sun F, Pattabiraman S, Schimenti K, Handel M, Eppig JJ, Schimenti JC. The dual bromodomain and WD repeat-containing mouse protein BRWD1 is required for normal spermiogenesis and the oocyte-embryo transition. Dev Biol 2008;317:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus G, Enzmann EV. The comparative behavior of mammalian eggs in vivo and in vitro: I. The activation of ovarian eggs. J Exp Med 1935;62:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pique M, Lopez JM, Foissac S, Guigo R, Mendez R. A combinatorial code for CPE-mediated translational control. Cell 2008;132:434–448. [DOI] [PubMed] [Google Scholar]
- Pirino G, Wescott MP, Donovan PJ. Protein kinase A regulates resumption of meiosis by phosphorylation of Cdc25B in mammalian oocytes. Cell Cycle 2009;8:665–670. [DOI] [PubMed] [Google Scholar]
- Prasad CK, Mahadevan M, MacNicol MC, MacNicol AM. Mos 3’ UTR regulatory differences underlie species-specific temporal patterns of Mos mRNA cytoplasmic polyadenylation and translational recruitment during oocyte maturation. Mol Reprod Dev 2008;75:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update 2015;21:787–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racki WJ, Richter JD. CPEB controls oocyte growth and follicle development in the mouse. Development 2006;133:4527–4537. [DOI] [PubMed] [Google Scholar]
- Ramos SB, Stumpo DJ, Kennington EA, Phillips RS, Bock CB, Ribeiro-Neto F, Blackshear PJ. The CCCH tandem zinc-finger protein Zfp36l2 is crucial for female fertility and early embryonic development. Development 2004;131:4883–4893. [DOI] [PubMed] [Google Scholar]
- Reis A, Chang HY, Levasseur M, Jones KT. APCcdh1 activity in mouse oocytes prevents entry into the first meiotic division. Nat Cell Biol 2006;8:539–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JM, Ross PJ. Cytoplasmic polyadenylation in mammalian oocyte maturation. Wiley Interdiscip Rev RNA 2016;7:71–89. [DOI] [PubMed] [Google Scholar]
- Richani D, Ritter LJ, Thompson JG, Gilchrist RB. Mode of oocyte maturation affects EGF-like peptide function and oocyte competence. Mol Hum Reprod 2013;19:500–509. [DOI] [PubMed] [Google Scholar]
- Richter JD. CPEB: a life in translation. Trends Biochem Sci 2007;32:279–285. [DOI] [PubMed] [Google Scholar]
- Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N. Ovarian folliculogenesis. Results Probl Cell Differ 2016;58:167–190. [DOI] [PubMed] [Google Scholar]
- Robert C. Microarray analysis of gene expression during early development: a cautionary overview. Reproduction 2010;140:787–801. [DOI] [PubMed] [Google Scholar]
- Rosario R, Adams IR, Anderson RA. Is there a role for DAZL in human female fertility? Mol Hum Reprod 2016;22:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoevers EJ, Kidson A, Verheijden JH, Bevers MM. Effect of follicle-stimulating hormone on nuclear and cytoplasmic maturation of sow oocytes in vitro. Theriogenology 2003;59:2017–2028. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Tennier MT, Boatman DE, Bavister BD. Chromatin configurations and meiotic competence of oocytes are related to follicular diameter in nonstimulated rhesus monkeys. Biol Reprod 1993;48:349–356. [DOI] [PubMed] [Google Scholar]
- Schroeder AC, Downs SM, Eppig JJ. Factors affecting the developmental capacity of mouse oocytes undergoing maturation in vitro. Ann N Y Acad Sci 1988;541:197–204. [DOI] [PubMed] [Google Scholar]
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007;130:484–498. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Siatkowski M, Pfeiffer MJ, Baeumer N, Drexler HC, Wang B, Fuellen G, Boiani M. Maternal age effect on mouse oocytes: new biological insight from proteomic analysis. Reproduction 2014;148:55–72. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 2006;147:2280–2286. [DOI] [PubMed] [Google Scholar]
- Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci USA 2005;102:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson AF, von Dassow G, Bowerman B. Oocyte meiotic spindle assembly and function. Curr Top Dev Biol 2016;116:65–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev 2004;25:72–101. [DOI] [PubMed] [Google Scholar]
- Shu YM, Zeng HT, Ren Z, Zhuang GL, Liang XY, Shen HW, Yao SZ, Ke PQ, Wang NN. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum Reprod 2008;23:504–513. [DOI] [PubMed] [Google Scholar]
- Shuhaibar LC, Egbert JR, Edmund AB, Uliasz TF, Dickey DM, Yee SP, Potter LR, Jaffe LA. Dephosphorylation of juxtamembrane serines and threonines of the NPR2 guanylyl cyclase is required for rapid resumption of oocyte meiosis in response to luteinizing hormone. Dev Biol 2016;409:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuhaibar LC, Egbert JR, Norris RP, Lampe PD, Nikolaev VO, Thunemann M, Wen L, Feil R, Jaffe LA. Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc Natl Acad Sci USA 2015;112:5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirard MA, Picard L, Dery M, Coenen K, Blondin P. The time interval between FSH administration and ovarian aspiration influences the development of cattle oocytes. Theriogenology 1999;51:699–708. [DOI] [PubMed] [Google Scholar]
- Slaidina M, Lehmann R. Translational control in germline stem cell development. J Cell Biol 2014;207:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc P, Saskova A, Baran V, Kubelka M, Schultz RM, Motlik J. CDC25A phosphatase controls meiosis I progression in mouse oocytes. Dev Biol 2008;317:260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa Martins JP, Liu X, Oke A, Arora R, Franciosi F, Viville S, Laird DJ, Fung JC, Conti M. DAZL and CPEB1 regulate mRNA translation synergistically during oocyte maturation. J Cell Sci 2016;129:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online 2007;14:700–708. [DOI] [PubMed] [Google Scholar]
- Stoop D, Ermini B, Polyzos NP, Haentjens P, De Vos M, Verheyen G, Devroey P. Reproductive potential of a metaphase II oocyte retrieved after ovarian stimulation: an analysis of 23 354 ICSI cycles. Hum Reprod 2012;27:2030–2035. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol 2007;302:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtelny AO, Eichhorn SW, Chen GR, Sive H, Bartel DP. Poly(A)-tail profiling reveals an embryonic switch in translational control. Nature 2014;508:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura S, Kobayashi S, Hashiyada Y, Ohtake M, Kaneda M, Yamanouchi T, Matsuda H, Aikawa Y, Watanabe S, Nagai T et al. . Follicular growth-stimulated cows provide favorable oocytes for producing cloned embryos. Cell Reprogram 2012;14:29–37. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O’Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 2007;134:2593–2603. [DOI] [PubMed] [Google Scholar]
- Suh N, Baehner L, Moltzahn F, Melton C, Shenoy A, Chen J, Blelloch R. MicroRNA function is globally suppressed in mouse oocytes and early embryos. Curr Biol 2010;20:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susor A, Jansova D, Anger M, Kubelka M. Translation in the mammalian oocyte in space and time. Cell Tissue Res 2016;363:69–84. [DOI] [PubMed] [Google Scholar]
- Susor A, Jansova D, Cerna R, Danylevska A, Anger M, Toralova T, Malik R, Supolikova J, Cook MS, Oh JS et al. . Temporal and spatial regulation of translation in the mammalian oocyte via the mTOR-eIF4F pathway. Nat Commun 2015;6:6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Franke V, Schultz RM. Sculpting the transcriptome during the oocyte-to-embryo transition in mouse. Curr Top Dev Biol 2015;113:305–349. [DOI] [PubMed] [Google Scholar]
- Szollosi D, Calarco P, Donahue RP. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci 1972;11:521–541. [DOI] [PubMed] [Google Scholar]
- Tay J, Hodgman R, Richter JD. The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev Biol 2000;221:1–9. [DOI] [PubMed] [Google Scholar]
- Toftager M, Bogstad J, Lossl K, Praetorius L, Zedeler A, Bryndorf T, Nilas L, Pinborg A. Cumulative live birth rates after one ART cycle including all subsequent frozen-thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod 2017;32:556–567. [DOI] [PubMed] [Google Scholar]
- Touati SA, Wassmann K. How oocytes try to get it right: spindle checkpoint control in meiosis. Chromosoma 2016;125:321–335. [DOI] [PubMed] [Google Scholar]
- Trapphoff T, Heiligentag M, Dankert D, Demond H, Deutsch D, Frohlich T, Arnold GJ, Grummer R, Horsthemke B, Eichenlaub-Ritter U. Postovulatory aging affects dynamics of mRNA, expression and localization of maternal effect proteins, spindle integrity and pericentromeric proteins in mouse oocytes. Hum Reprod 2016;31:133–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay K, Vigneault C, McGraw S, Sirard MA. Expression of cyclin B1 messenger RNA isoforms and initiation of cytoplasmic polyadenylation in the bovine oocyte. Biol Reprod 2005;72:1037–1044. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun SY, Zhang R, Hsueh AJ, Conti M. Oocyte maturation involves compartmentalization and opposing changes of cAMP levels in follicular somatic and germ cells: studies using selective phosphodiesterase inhibitors. Dev Biol 1996;178:393–402. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Pomerantz SH. Oocyte maturation inhibitor. Clin Endocrinol Metab 1986;15:157–170. [DOI] [PubMed] [Google Scholar]
- Tung JY, Rosen MP, Nelson LM, Turek PJ, Witte JS, Cramer DW, Cedars MI, Pera RA. Variants in Deleted in AZoospermia-Like (DAZL) are correlated with reproductive parameters in men and women. Hum Genet 2006;118:730–740. [DOI] [PubMed] [Google Scholar]
- Twisk M, Mastenbroek S, van Wely M, Heineman MJ, Van der Veen F, Repping S. Preimplantation genetic screening for abnormal number of chromosomes (aneuploidies) in in vitro fertilisation or intracytoplasmic sperm injection. Cochrane Database Syst Rev 2006;CD005291. [DOI] [PubMed] [Google Scholar]
- Vaccari S, Weeks JL 2nd, Hsieh M, Menniti FS, Conti M. Cyclic GMP signaling is involved in the luteinizing hormone-dependent meiotic maturation of mouse oocytes. Biol Reprod 2009;81:595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valve E, Penttila TL, Paranko J, Harkonen P. FGF-8 is expressed during specific phases of rodent oocyte and spermatogonium development. Biochem Biophys Res Commun 1997;232:173–177. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondrial function in the human oocyte and embryo and their role in developmental competence. Mitochondrion 2011;11:797–813. [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol 1990;140:307–317. [DOI] [PubMed] [Google Scholar]
- Velasquez C, Cheng E, Shuda M, Lee-Oesterreich PJ, Pogge von Strandmann L, Gritsenko MA, Jacobs JM, Moore PS, Chang Y. Mitotic protein kinase CDK1 phosphorylation of mRNA translation regulator 4E-BP1 Ser83 may contribute to cell transformation. Proc Natl Acad Sci USA 2016;113:8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T, Speed RM, Ross A, Cooke HJ. Partial rescue of the Dazl knockout mouse by the human DAZL gene. Mol Hum Reprod 2002;8:797–804. [DOI] [PubMed] [Google Scholar]
- Wang X, He C. Reading RNA methylation codes through methyl-specific binding proteins. RNA Biol 2014;11:669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Pfeiffer MJ, Drexler HC, Fuellen G, Boiani M. Proteomic analysis of mouse oocytes identifies PRMT7 as a reprogramming factor that replaces SOX2 in the induction of pluripotent stem cells. J Proteome Res 2016;15:2407–2421. [DOI] [PubMed] [Google Scholar]
- Wang HL, Sui HS, Liu Y, Miao DQ, Lu JH, Liang B, Tan JH. Dynamic changes of germinal vesicle chromatin configuration and transcriptional activity during maturation of rabbit follicles. Fertil Steril 2009;91:1589–1594. [DOI] [PubMed] [Google Scholar]
- Webster A, Schuh M. Mechanisms of aneuploidy in human eggs. Trends Cell Biol 2017;27:55–68. [DOI] [PubMed] [Google Scholar]
- Wells D, Alfarawati S, Fragouli E. Use of comprehensive chromosomal screening for embryo assessment: microarrays and CGH. Mol Hum Reprod 2008;14:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D, Patrizio P. Gene expression profiling of human oocytes at different maturational stages and after in vitro maturation. Am J Obstet Gynecol 2008;198:455 e451–455 e459. ; discussion 455 e459–411. [DOI] [PubMed] [Google Scholar]
- Wen D, Banaszynski LA, Liu Y, Geng F, Noh KM, Xiang J, Elemento O, Rosenwaks Z, Allis CD, Rafii S. Histone variant H3.3 is an essential maternal factor for oocyte reprogramming. Proc Natl Acad Sci USA 2014;111:7325–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe D, Albertini DF. Centrosome phosphorylation and the developmental expression of meiotic competence in mouse oocytes. Dev Biol 1992;152:62–74. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol 1991;143:162–172. [DOI] [PubMed] [Google Scholar]
- Wilde A, Zheng Y. Stimulation of microtubule aster formation and spindle assembly by the small GTPase Ran. Science 1999;284:1359–1362. [DOI] [PubMed] [Google Scholar]
- WHO. 2007; http://www.who.int/reproductivehealth/topics/infertility/burden/en/. [Google Scholar]
- Wu G, Scholer HR. Role of Oct4 in the early embryo development. Cell Regen (Lond) 2014;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Viveiros MM, Eppig JJ, Bai Y, Fitzpatrick SL, Matzuk MM. Zygote arrest 1 (Zar1) is a novel maternal-effect gene critical for the oocyte-to-embryo transition. Nat Genet 2003. a;33:187–191. [DOI] [PubMed] [Google Scholar]
- Wu X, Wang P, Brown CA, Zilinski CA, Matzuk MM. Zygote arrest 1 (Zar1) is an evolutionarily conserved gene expressed in vertebrate ovaries. Biol Reprod 2003. b;69:861–867. [DOI] [PubMed] [Google Scholar]
- Xu Y, Shi Y, Fu J, Yu M, Feng R, Sang Q, Liang B, Chen B, Qu R, Li B et al. . Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet 2016;99:744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto TM, Cook JM, Kotter CV, Khat T, Silva KD, Ferreyros M, Holt JW, Knight JD, Charlesworth A. Zar1 represses translation in Xenopus oocytes and binds to the TCS in maternal mRNAs with different characteristics than Zar2. Biochim Biophys Acta 2013;1829:1034–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto TM, Iwabuchi M, Ohsumi K, Kishimoto T. APC/C-Cdc20-mediated degradation of cyclin B participates in CSF arrest in unfertilized Xenopus eggs. Dev Biol 2005;279:345–355. [DOI] [PubMed] [Google Scholar]
- Yan C, Pendola FL, Jacob R, Lau AL, Eppig JJ, Matzuk MM. Oosp1 encodes a novel mouse oocyte-secreted protein. Genesis 2001;31:105–110. [DOI] [PubMed] [Google Scholar]
- Yang J, Medvedev S, Yu J, Tang LC, Agno JE, Matzuk MM, Schultz RM, Hecht NB. Absence of the DNA-/RNA-binding protein MSY2 results in male and female infertility. Proc Natl Acad Sci USA 2005;102:5755–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yang CR, Han SJ, Daldello EM, Cho A, Martins JPS, Xia G, Conti M. Maternal mRNAs with distinct 3’ UTRs define the temporal pattern of Ccnb1 synthesis during mouse oocyte meiotic maturation. Genes Dev 2017;31:1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yartseva V, Giraldez AJ. The maternal-to-zygotic transition during vertebrate development: a model for reprogramming. Curr Top Dev Biol 2015;113:191–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol 2010;224:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Ji SY, Sha QQ, Dang Y, Zhou JJ, Zhang YL, Liu Y, Wang ZW, Hu B, Sun QY et al. . BTG4 is a meiotic cell cycle-coupled maternal-zygotic-transition licensing factor in oocytes. Nat Struct Mol Biol 2016;23:387–394. [DOI] [PubMed] [Google Scholar]
- Yurttas P, Vitale AM, Fitzhenry RJ, Cohen-Gould L, Wu W, Gossen JA, Coonrod SA. Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo. Development 2008;135:2627–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Cui XS, Kim NH. Molecular characterization and polyadenylation-regulated expression of cyclin B1 and Cdc2 in porcine oocytes and early parthenotes. Mol Reprod Dev 2010. a;77:38–50. [DOI] [PubMed] [Google Scholar]
- Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010. b;330:366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Patel B, McMenamin M, Paprocki AM, Schramm RD, Nagl NG Jr., Wilsker D, Wang X, Moran E, Latham KE. Expression of genes encoding chromatin regulatory factors in developing rhesus monkey oocytes and preimplantation stage embryos: possible roles in genome activation. Biol Reprod 2004;70:1419–1427. [DOI] [PubMed] [Google Scholar]
- Zhu J, Lin S, Li M, Chen L, Lian Y, Liu P, Qiao J. Effect of in vitro culture period on birthweight of singleton newborns. Hum Reprod 2014;29:448–454. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Giorgi Rossi P, Martinez A, Garagna S, Forabosco A, Redi CA. Meiotic and developmental competence of mouse antral oocytes. Biol Reprod 1998;58:700–704. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Merico V, Bellone M, Mulas F, Sacchi L, Rebuzzini P, Prigione A, Redi CA, Bellazzi R, Adjaye J et al. . Gatekeeper of pluripotency: a common Oct4 transcriptional network operates in mouse eggs and embryonic stem cells. BMC Genomics 2011;12:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti M, Piccinelli A, Giorgi Rossi P, Garagna S, Redi CA. Chromatin organization during mouse oocyte growth. Mol Reprod Dev 1995;41:479–485. [DOI] [PubMed] [Google Scholar]