Abstract
Background:
Stroke as a devastating condition is one of the major causes of death worldwide. It is accountable for long time disability with high personal and social cost in adults. There are several risk factors for stroke such as diabetes and hypertension. Alpha-lipoic acid (ALA) as an antioxidant can be a risk modifier in these patients. We designed this trial to scrutinize the possible effects of ALA consumption on some cardiovascular risk factors in patients experienced stroke.
Methods:
In this randomized, double-blind, placebo-controlled clinical trial, 67 patients experienced stroke were randomly allocated into two groups (taking a 600 mg ALA supplement or placebo daily for 12 weeks). Fasting blood sugar (FBS), fasting insulin and systolic (SBP), and diastolic blood pressure (DBP) were measured before and after intervention in this study. Statistical analyses were performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA) software.
Results:
Primary features were similar in the intervention and placebo groups (P > 0.05). After the intervention period, SBP (P < 0.001), DBP (P < 0.001) and FBS (P < 0.001) reduced in ALA group compared with placebo group, significantly. No significant change was seen in insulin level (P = 0.82).
Conclusions:
Results of this trial indicated that 12 weeks supplementation with 600 mg ALA has beneficial effects on SBP, DBP, and FBS but has no effect on insulin level.
Keywords: Blood glucose, blood pressure, insulin, thioctic acid
Introduction
Thiocitic acid called alpha-lipoic acid (ALA) is an eight-carbon compound containing sulfur. Conventionally, it is recognized as a cofactor in the multienzyme complexes that are responsible for the oxidative decarboxylation of α-ketoacids.[1] A general agreement exists about the antioxidant properties of ALA. It is thought to function by clearing free radicals directly, chelating metallic ions, enhancing intracellular glutathione (GSH), and activating endogenous antioxidant systems.[2,3] Besides the antioxidant properties of ALA, nitric oxide synthesis can be increased by ALA, which may improve endothelial function.[4] Several animal[5,6,7] and human[8,9,10] studies investigated the effect of ALA on blood pressure (BP) and some introduced it as a potential BP regulator. In addition, evidence suggests that ALA can reduce blood glucose by increasing glucose transporter-4 (GLUT-4) transportation to muscle and fat cell membranes and increasing glucose uptake.[11,12,13] Clinical studies reported that up to 1800 mg/day ALA is safe in human.[14,15]
Stroke as a devastating condition is one of the major causes of death worldwide. It is accountable for long time disability with high personal and social cost in adults.[16,17] The 2012 Behavioral Risk Factor Surveillance System (CDC) data indicated that history of stroke was seen in 2.9% of people ≥18 years of age. In addition, projections show that by 2030, stroke will be experienced by more people and a 20.5% increase will be observed in prevalence from 2012.[18] There are several risk factors for stroke such as diabetes, hypertension, and hyperlipidemia, which promote cerebral atherogenesis.[19,20]
Although studying the effect of ALA on cardiovascular risk factors such as fasting blood sugar (FBS) and BP is not novel, results in human are controversial, and to the best of our knowledge, the beneficial effects of ALA supplementation in patients with stroke have not been investigated by far. Thus, we designed this trial to scrutinize the possible effects of ALA consumption on some cardiovascular risks in patients experienced stroke.
Methods
Study design
Research ethics committee of Isfahan University of Medical Sciences (IUMS) approved the protocol of this randomized, double-blind, placebo-controlled clinical trial (code: IR.MUI.REC.1395.3.068). In addition, we registered this trial protocol in Iranian Registry of Clinical Trial (IRCT2016051811763N23).
Eighty patients with stroke who referred to Alzahra Hospital (a referral and governmental hospital affiliated with IUMS) and met the study criteria were enrolled in this trial (from May 2016 to December 2016).
We calculated sample size with power 80% and α = 5% with below formula. Thirty-three participants were required for each group, after considering 20% sample loss, 40 patients were enrolled in each group.
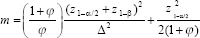
The inclusion criteria included filling informed consent, experienced thrombotic and embolic stroke, Body mass index (BMI) = 18.5–35, age 30–70 years, lack of malignancies and specific diseases such as liver disease, kidney disease, and cancer-based on self-reports, no vitamin, antioxidant and omega-3 supplementation. Exclusion criteria included no collaboration, failure to follow the program of the trial (compliance <80%), death, and recurrent stroke. We assessed participants’ compliance by checking their remaining capsules at the end of the study.
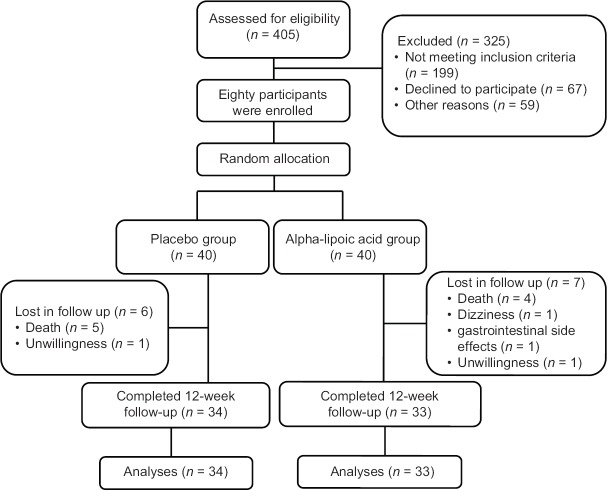
We allocated participants randomly into two quantitatively equal groups (using simple randomization method, from the randomized number in an 80-person list in a double-blind, parallel manner). ALA and placebo groups were taking a 600 mg ALA supplement and similar placebo capsule (containing wheat flour) every day for 12 weeks, respectively. ALA powder was prepared from Karen Company and capsulated in School of Pharmacy, IUMS. We prepared similar placebo capsule containing wheat flour in School of Pharmacy, IUMS. We excluded thirteen patients during intervention period due to different reasons; finally, remained 33 and 34 patients in ALA and placebo groups, respectively [Figure 1]
Figure 1.
Follow chart of participants throughout the intervention
For blinding the trial, ALA and placebo capsules were completely similar. A person, who was not in trial process, packed capsules and encoded them into A and B. Neither researchers nor participants knew about packets content up to the end of the study.
Procedures and assessment of variables
At the beginning of the study, we obtained written consent from all volunteers. Trained personnel performed all data collection and measurements. We measured body weight with minimal clothing to the nearest 0.1 kg by a digital scale. To measure height, a seca stadiometer was used and in the case of not being able to stand up for measuring height; we determined knee height and the following formula was used for calculating their height:
Height in centimeters (for men) = 64.19 – (0.04 × age) + (2.02 × knee height in centimeters).
We calculated BMI for each patient (BMI = weight in kg/height2 in meters). We measured waist circumference at the level of the iliac crest by an Ergonomic Circumference Measuring Tape (model 201; Seca GmbH & Co, KG, Hamburg, Germany). Food intakes were collected by 24-h food recall by a nutritionist then dietary data were analyzed by Nutritionist IV software (version 4.1, First Databank Division, The Hearst Corporation, San Bruno, CA, USA). We measured systolic and diastolic blood pressure (SBP and DBP) by a mercury sphygmomanometer after 5 min of sitting rest.
We collected venous blood samples after 12 h overnight fasting, and after centrifugation, the serum samples were frozen and stored at −70°C. We performed all biochemical measurements in the Laboratory of Biochemistry, Azahra Hospital, IUMS. FBS and fasting insulin were measured using spectrophotometer and ELISA methods, respectively.
Statistical analysis
All statistical analyses were performed using SPSS (version 16; SPSS Inc., Chicago, IL, USA). Quantitative data are presented as mean ± SD. Kolmogorov–Smirnov test was used for checking data normality. In the case of normal distribution of data, we used paired t-test to compare variables before and after the intervention within groups. Comparing the variables after the intervention, adjusting for baseline values and energy intake was performed by analysis of covariance (ANCOVA). All tests were two-sided. The values of P < 0.05 were considered statistically significant.
Results
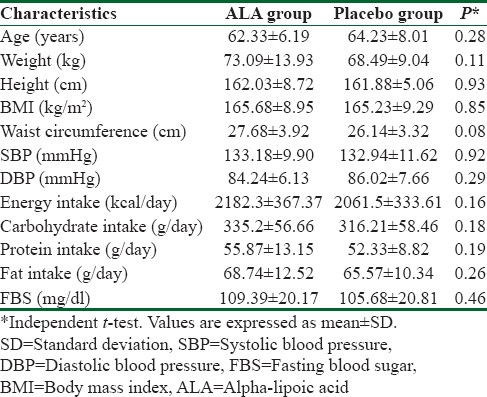
Primary characteristics were the same in the ALA and placebo groups [Table 1]. We found no statistically significant differences in age, weight, height, BMI, Waist circumference, BP, energy and macronutrients intake and FBS between 2 groups before the intervention (P > 0.05).
Table 1.
Primary characteristics of trials participants before the intervention (receiving 600 mg alpha-lipoic acid or placebo)
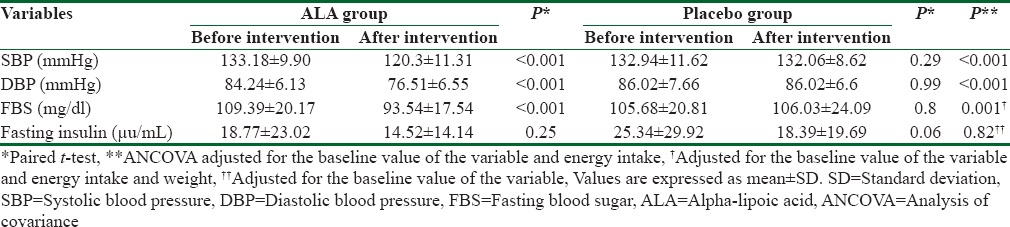
Based on within group analysis, no significant changes were observed in placebo group for all studied variables (P > 0.05) while SBP (P < 0.001), DBP (P < 0.001), and FBS (P < 0.001) decreased significantly within ALA group.
We observed statistically significant reductions in SBP (P < 0.001), DBP (P < 0.001) and FBS (P < 0.001) within ALA group, but there was no significant change in insulin level within this group. No variable changes in the placebo group were statistically significant (P > 0. 05).
Based on ANCOVA results, statistically significant reductions were observed for SBP (P < 0.001), DBP (P < 0.001), and FBS (P = 0.001) in ALA supplementation group compared with placebo group, but no significant change was seen in Insulin level, neither within ALA group nor between two groups [Table 2].
Table 2.
Cardiovascular risk factors of subjects of the trial before and after intervention in both groups
Discussion
This study results indicate that 12 weeks consumption of 600 mg ALA can improve SBP, DBP and FBS but cannot change insulin level in patients experienced stroke . According to our knowledge, present trial is the first study, which investigated the effect of ALA consumption on some cardiovascular risks in patients experienced stroke.
This study results show a significant reduction in SBP and DBP in ALA group compare with placebo. Several mechanisms have been proposed for the effect of ALA on BP. It is suggested to work by elevating nitric oxide production in endothelial cells, increasing reduced GSH levels in tissues, and affecting GSH peroxidase activity.[2,4,21] Several animal[5,6,7] and human[8,9,10,22,23] studies have supported our finding even though none of them has evaluated the effect of ALA consumption on BP in patients experienced stroke. Consistent with this trial Mohammadi et al.[8] examined the effect of 12-weeks supplementation with 600 mg ALA on BP in men with chronic spinal cord injury and reported a significant reduction in SBP and DBP within ALA group and between two groups. Mazloom and Ansar in a randomized clinical trial, in type II diabetes patients, reported that 8 weeks supplementation with 300 mg ALA improve SBP and DBP significantly.[10] In another clinical trial, a significant reduction in SBP and no change in DBP were observed following 12 weeks administration of combined ALA (800 mg) and pyridoxine (80 mg) in patients with diabetic nephropathy.[9] There are two studies with contrasting findings to our results. In one of them, Sola et al. investigated the effect of ALA, Irbesartan and their combination versus placebo on BP in patients with metabolic syndrome and observed no significant effect.[24] In another study, researchers examined the impact of ALA in quinapril-treated diabetic patients with stage I hypertension and found no significant effect on BP.[25]
We found that 12 weeks supplementation with ALA could reduce FBS in patients experienced stroke but could not change insulin level significantly. Evidence proposes which this effect on FBS may be mediated by increasing glucose uptake through enhancing GLUT-4 transportation to fat and muscle cell membranes.[11] In a study by Jacob et al. an intravenous injection of 1000 mg LA improved insulin sensitivity and insulin-stimulated metabolic clearance rate in patients with type II diabetes.[13] Another study in patients with type II diabetes indicated that oral administration of supplemental lipoic acid had a positive effect on insulin sensitivity and metabolic clearance rate.[26] In another randomized, double-blind, placebo-controlled clinical trial, in patients with type II diabetes, authors noted that supplementation with 300 mg ALA for 8 weeks could improve FBS and insulin resistance. In addition, Mohammadi et al.[8] examined the effect of 12 weeks supplementation with 600 mg ALA on FBS in men with chronic spinal cord injury and reported a significant reduction in FBS.
We can mention several limitations for this trial, which is better to be in counted in the interpretation of our results, including restricted duration of the study. In addition, we were not able to measure lipid particles number and apolipoproteins or oxidized particles and total antioxidant capacity as cardiovascular risks. We did not include patients with hemorrhagic stroke. It seems to be a need for further studies. Beyond these weaknesses, this is the first randomized, double-blind, placebo-controlled clinical trial investigating the effects of ALA on cardiovascular risks in patients experienced stroke.
Conclusions
In conclusion, based on this trial results, ALA consumption (600 mg, 12 weeks) has beneficial effects on SBP, DBP, and FBS and could be a risk modifier for patients experienced stroke.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Packer L, Witt EH, Tritschler HJ. Alpha-lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–50. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 2.Packer L. Alpha-lipoic acid: A metabolic antioxidant which regulates NF-kappa B signal transduction and protects against oxidative injury. Drug Metab Rev. 1998;30:245–75. doi: 10.3109/03602539808996311. [DOI] [PubMed] [Google Scholar]
- 3.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22:359–78. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 4.Heitzer T, Finckh B, Albers S, Krohn K, Kohlschütter A, Meinertz T. Beneficial effects of alpha-lipoic acid and ascorbic acid on endothelium-dependent, nitric oxide-mediated vasodilation in diabetic patients: Relation to parameters of oxidative stress. Free Radic Biol Med. 2001;31:53–61. doi: 10.1016/s0891-5849(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 5.de Queiroz TM, Xia H, Filipeanu CM, Braga VA, Lazartigues E. α-Lipoic acid reduces neurogenic hypertension by blunting oxidative stress-mediated increase in ADAM17. Am J Physiol Heart Circ Physiol. 2015;309:H926–34. doi: 10.1152/ajpheart.00259.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micili SC, Ergur BU, Ozogul C, Sarioglu S, Bagriyanik HA, Tugyan K, et al. Effects of lipoic acid in an experimentally induced hypertensive and diabetic rat model. Clin Exp Hypertens. 2013;35:373–81. doi: 10.3109/10641963.2012.732647. [DOI] [PubMed] [Google Scholar]
- 7.Vasdev S, Gill VD, Parai S, Gadag V. Effect of moderately high dietary salt and lipoic acid on blood pressure in Wistar-Kyoto rats. Exp Clin Cardiol. 2007;12:77–81. [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi V, Khalili M, Eghtesadi S, Dehghani S, Jazayeri S, Aghababaee SK, et al. The effect of alpha-lipoic acid (ALA) supplementation on cardiovascular risk factors in men with chronic spinal cord injury: A clinical trial. Spinal Cord. 2015;53:621–4. doi: 10.1038/sc.2015.35. [DOI] [PubMed] [Google Scholar]
- 9.Noori N, Tabibi H, Hosseinpanah F, Hedayati M, Nafar M. Effects of combined lipoic acid and pyridoxine on albuminuria, advanced glycation end-products, and blood pressure in diabetic nephropathy. Int J Vitam Nutr Res. 2013;83:77–85. doi: 10.1024/0300-9831/a000147. [DOI] [PubMed] [Google Scholar]
- 10.Mazloom Z, Ansar H. The effect of alpha-lipoic acid on blood pressure in type 2 diabetics. Iran J Endocrinol Metab. 2009;11:245–50. [Google Scholar]
- 11.Singh U, Jialal I. Alpha-lipoic acid supplementation and diabetes. Nutr Rev. 2008;66:646–57. doi: 10.1111/j.1753-4887.2008.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Evans JL, Goldfine ID. Alpha-lipoic acid: A multifunctional antioxidant that improves insulin sensitivity in patients with type 2 diabetes. Diabetes Technol Ther. 2000;2:401–13. doi: 10.1089/15209150050194279. [DOI] [PubMed] [Google Scholar]
- 13.Jacob S, Henriksen EJ, Schiemann AL, Simon I, Clancy DE, Tritschler HJ, et al. Enhancement of glucose disposal in patients with type 2 diabetes by alpha-lipoic acid. Arzneimittelforschung. 1995;45:872–4. [PubMed] [Google Scholar]
- 14.Goraca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid-Biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849–58. doi: 10.1016/s1734-1140(11)70600-4. [DOI] [PubMed] [Google Scholar]
- 15.Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of α-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur J Endocrinol. 2012;167:465–71. doi: 10.1530/EJE-12-0555. [DOI] [PubMed] [Google Scholar]
- 16.Berressem D, Koch K, Franke N, Klein J, Eckert GP. Intravenous treatment with a long-chain omega-3 lipid emulsion provides neuroprotection in a murine model of ischemic stroke-A pilot study. PLoS One. 2016;11:e0167329. doi: 10.1371/journal.pone.0167329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics-2013 update: A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profile from the Framingham study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 20.MacMahon S, Rodgers A. Blood pressure, antihypertensive treatment and stroke risk. J Hypertens Suppl. 1994;12:S5–14. [PubMed] [Google Scholar]
- 21.Hagen TM, Moreau R, Suh JH, Visioli F. Mitochondrial decay in the aging rat heart: Evidence for improvement by dietary supplementation with acetyl-L-carnitine and/or lipoic acid. Ann N Y Acad Sci. 2002;959:491–507. doi: 10.1111/j.1749-6632.2002.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi V, Dehghani S, Askari G. Does alpha-lipoic acid supplement regulate blood pressure? A systematic review of randomized, double-blind placebo-controlled clinical trials. Int J Prev Med. 2016;7:122. doi: 10.4103/2008-7802.206138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Midaoui A, de Champlain J. Prevention of hypertension, insulin resistance, and oxidative stress by alpha-lipoic acid. Hypertension. 2002;39:303–7. doi: 10.1161/hy0202.104345. [DOI] [PubMed] [Google Scholar]
- 24.Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: Results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–8. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 25.Rahman ST, Merchant N, Haque T, Wahi J, Bhaheetharan S, Ferdinand KC, et al. The impact of lipoic acid on endothelial function and proteinuria in quinapril-treated diabetic patients with stage I hypertension: Results from the QUALITY study. J Cardiovasc Pharmacol Ther. 2012;17:139–45. doi: 10.1177/1074248411413282. [DOI] [PubMed] [Google Scholar]
- 26.Jacob S, Ruus P, Hermann R, Tritschler HJ, Maerker E, Renn W, et al. Oral administration of RAC-alpha-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: A placebo-controlled pilot trial. Free Radic Biol Med. 1999;27:309–14. doi: 10.1016/s0891-5849(99)00089-1. [DOI] [PubMed] [Google Scholar]